Abstract
Background
Left atrial (LA) size has incremental value in risk stratification. We aim to assess feasibility and reproducibility of two quick measures of LA size by chest CT (axial LA area and LA anterior-posterior [AP] diameter) using contrast-enhanced and CT scans.
Methods
We measured LA size in 100 contrast-enhanced 64-slice MDCT scans (randomly selected from the ROMICAT collective) by 1) axial LA area at the level of the left ventricular outflow tract and the mitral valve leaflets, 2) AP-diameter in 3-chamber view, and 3) three-dimensional (3D) LA volume by Simpson's methods. We assessed inter- and intraobserver intraclass correlation coefficient (ICC) for axial LA area and AP-diameter as well as their correlation to 3D LA volume. For axial area, feasibility and reproducibility were also determined in 100 non-contrast MDCT scans, randomly selected from the Framingham Heart Offspring collective.
Results
In contrast-enhanced CT, both LA axial area and AP-diameter had excellent reproducibility (Interobserver: Axial area: ICC 0.96, mean relative difference 2.4±7.4%, AP-diameter: ICC 0.91, 3.6±7.2%; Intraobserver: Axial area: ICC 0.99, 0.4±5.2%, AP-diameter: ICC 0.94, 1.7±5.5%). Correlations with 3D volume were better for axial area (r=0.88) than for AP-diameter (r=0.67). In non-contrast images, axial area could be assessed with excellent reproducibility (Interobserver: ICC 0.96, 0.5±8.3%; Intraobserver: ICC 0.99, 0.01±4.4%).
Conclusion
Both AP-diameter and axial LA area permit quick and reproducible estimates of LA volume in contrast-enhanced and non-contrast ECG-gated chest CT. However, LA area should be used preferably over AP-diameter because of its better agreement to 3D LA volume.
Keywords: Left atrium, axial area, reproducibility, MDCT, EBCT
Left atrial (LA) size has been used to risk stratify subjects with and without known cardiovascular disease, as LA enlargement is an independent predictor of cardiovascular events such as congestive heart failure, myocardial infarction, atrial fibrillation, stroke, and cardiovascular death.1-11 In daily clinical practice, echocardiography is the standard imaging modality for LA size assessment. In addition, information on the LA is readily available in every chest computed tomography (CT), either from contrast-enhanced multidetector CT (MDCT) or from non-contrast MDCT or electron beam CT. CT scans of the chest are performed with increasing frequency 12 for a variety or reasons, including coronary calcium scoring and coronary CT angiography. Recently, we have shown that threshold-based three-dimensional LA volume is a reliable measure that can be obtained from contrast-enhanced CT images.13 Moreover, Christiaens et al. described a close correlation between LA volume by CT and 3D echocardiography.14 However, volumetric assessment of left atrial size requires specialized software and is relatively time consuming. Therefore it is rarely used in clinical practice.
The purpose of this study was to determine a simple measure of LA size that can be performed within seconds from any CT exam to be used in clinical practice and larger population-based CT studies such as the Framingham Heart MDCT Study. We aimed to assess inter- and intraobserver reproducibility of axial LA area and LA antero-posterior (AP)-diameter as quantified by contrast-enhanced MDCT and correlate these measures to 3 dimensional LA volume. For LA axial area, feasibility and reproducibility was assessed from non-contrast MDCT scans.
Methods
The study was approved by the institutional review boards of the Boston University Medical Center and the Massachusetts General Hospital. All subjects provided written informed consent.
Study Population for the Contrast-enhanced Images
We included 100 consecutive subjects who presented to the emergency department with the chief complaint of chest pain whose initial electrocardiogram and biomarkers were negative or inconclusive and who were awaiting hospital admission. The subjects underwent ECG-gated contrast-enhanced 64-slice MDCT as part of the ROMICAT study (Rule Out myocardial Infarction using Computed Assisted Tomography).15 Patients were excluded, if their serum creatinine was > 1.3 mmol/L or if they had known allergy to iodinated contrast, history of asthma, previous intolerance to a beta-blocker, metformin use, or referral for invasive coronary angiography.
Study Population for the Non-contrast Images
We randomly selected 100 study participants from the Framingham Heart Multi-detector Computed Tomography Study Offspring population, who underwent MDCT imaging between June 2002 and April 2005. Study design has been described previously.16, 17 Exclusion criteria were pregnancy or age <40 for females and age <35 for males as well as weight >350 pounds. Unlike the population that underwent contrast-enhanced CT, the Framingham cohort consists of asymptomatic subjects. Measurements of non-contrast enhanced CT scans were performed in 100 randomly selected asymptomatic participants from the Framingham Offspring cohort (mean age: 60 ± 13 years, 49% females).
64-slice Contrast-enhanced MDCT Imaging Protocol
Imaging was performed using a standard coronary artery 64-slice MDCT (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) imaging protocol that included the administration of intravenous beta-blocker (up to 20 mg of intravenous metoprolol) if the baseline heart rate was >60 beats per minute and sublingual nitroglycerin (0.6 mg), if contraindications were absent.
Images were acquired within a single inspiratory breath hold in helical mode with 330 ms rotation time, 32 × 0.6 mm collimation, a pitch of 0.2, tube voltage of 120 kVp, with maximum effective tube current-time product of 850 mAseff during a time interval from 470 ms to 140 ms before the next expected R-wave. ECG tube current modulation algorithm was implemented when possible. A test bolus protocol was used to determine optimal contrast timing. On average, 80 mL of iodinated contrast agent (Iodhexodol 320 g/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ), followed by 40 mL saline solution was injected at a rate of 5 mL/s.
8-slice Non-contrast MDCT Imaging Protocol
Participants underwent radiographic assessment of their chest in supine position utilizing an 8-slice MDCT-scanner (LightSpeed Ultra, General Electric Milwaukee, WI). The scan was preformed during inspiratory breath hold with an average scan time of 18 seconds (tube voltage 120 kVp, tube current 320 mA <220 lbs, 400 mA >220 lbs, gantry rotation time 500 ms, temporal resolution: 330 ms). Image acquisition was prospectively triggered with the center of the acquisition at 70% of the R-R-interval. Images were reconstructed with a slice thickness of 2.5 mm without overlap and a field of view of 25 cm. On average, 48 contiguous slices were taken for volume coverage from the carina to the diaphragm.
Quantitative Assessment of Left Atrial Dimensions in Contrast-enhanced Images
The measurements of the contrast-enhanced images were performed offline using a dedicated workstation (Leonardo, Siemens Medical Solutions, Forchheim, Germany). From the multiphase dataset, the reader qualitatively determined the ventricular end-systolic phase in which LA measurements were performed. For interobserver reproducibility assessment, independent readers performed all measurements on LA dimensions. For intra-observer reproducibility, one reader repeated the measurements of 20 randomly-selected cases.
LA axial area was assessed from a single axial slice. Therefore, the area of the LA was manually traced at the level of the left ventricular outflow tract and the height of the mitral valve leaflets, excluding the pulmonary veins (Figure 1). We determined the slice level by qualitatively visualizing the maximal LA area in the axial dataset. We selected this specific slice in consensus by a group of 4 highly experienced readers as it represents the largest LA area in the axial dataset, can be clearly defined by various distinct anatomic landmarks and allowing clear separation from the left ventricle, the left ventricular outflow tract and the aorta to ensure high reproducibility of this measurement.
Figure 1.

Axial area in contrast-enhanced MDCT images. The area of the left atrium was manually traced in the axial slice at the level left ventricular outflow tract and the mitral valve.
Anterior-posterior (AP) diameter of the LA was assessed from a three chamber view, reconstructed in multi-planar reformat, to adapt the parasternal long axis view from echocardiography (Figure 2). As recommended by the American Society of Echocardiography and the European Association of Echocardiography, the AP-diameter in parasternal long axis view is standard measure for quick assessment of LA dimensions by echocardiography.18 Both, axial area and AP-diameter were assessed using the Circulation software (Leonardo, Siemens Medical Solutions, Forchheim, Germany).
Figure 2.
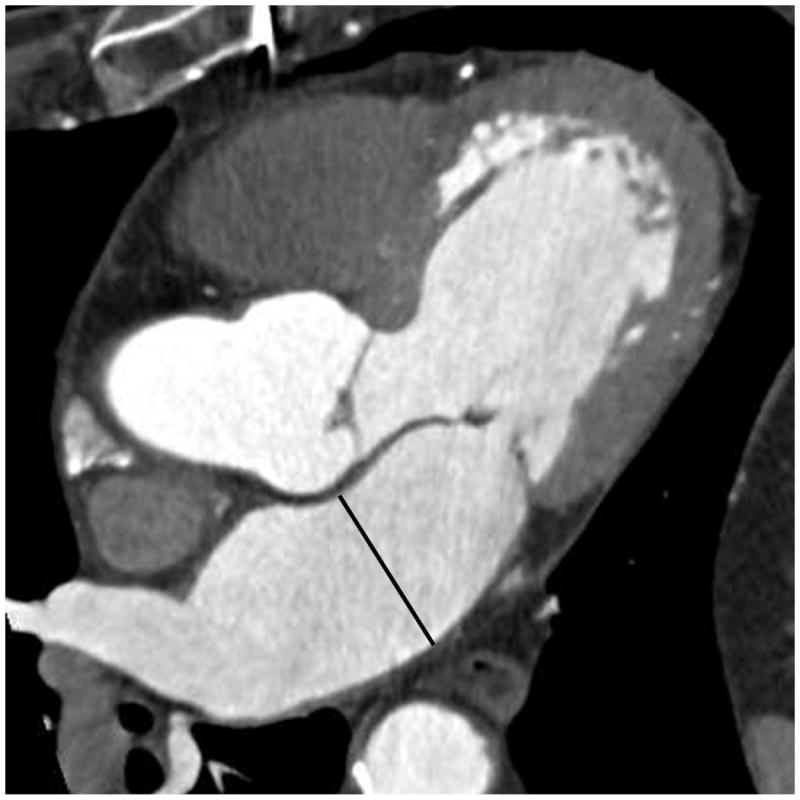
Anterior-posterior (AP) diameter in contrast enhanced MDCT images. The 3-chamber view was reconstructed using multiplanar reconstructions to adapt the parasternal long axis view from echocardiography.
LA volume was assessed using a three dimensional threshold-based volume measurements as previously described.13 Briefly, regions of interest were manually traced at the boundaries of the LA in each axial slice (slice thickness of 1.5 mm) and 3D volume was calculated by Simpson's rule.
Quantitative Assessment of Left Atrial Axial Area in Non-contrast Images
The measurements of the non-contrast images were performed offline using a dedicated workstation (Aquarius 3D Workstation, TeraRecon Inc., San Mateo, CA). To determine the interobserver reproducibility, two independent readers performed all measurements on LA axial area. For intraobserver reproducibility, one reader preformed repeated measurements of all 100 cases. Axial LA area was traced at the same slice location as in the contrast-enhanced images (Figure 3).
Figure 3.

Axial area in non-contrast MDCT image. Similar to the contrast-enhanced images, the area of the left atrium was manually traced in the axial slice at the level left ventricular outflow tract and the mitral valve.
Statistical Analysis
The distribution of continuous variables is presented as mean ± standard deviation and binary variables are given in percentages. We determined the inter- and intra-observer reproducibility using a single measure intraclass correlation coefficient (ICC) model for all measurements. Correlations of LA volume with axial area and AP-diameter were assessed using the Pearson correlation coefficient. A p-value of ≤ 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS 14.01 software (LEAD Technologies, New York).
Results
For LA evaluation using contrast-enhanced MDCT images, 100 subjects were included in this study (mean age: 53 ± 12 years, 47% females). LA volume, axial area, and AP-diameter could be assessed in all 100 contrast-enhanced scans. Mean values for LA size with each method are presented in Table 1. Using the contrast-enhanced dataset, inter- and intraobserver variability were excellent for both axial area and AP-diameter with a slight advantage for axial area (Table 2, Figure 4). No systematic bias was observed between the two observers' measurement by Bland-Altman analysis for both methods.
Table 1.
Distribution of axial area and anterior-posterior (AP) diameter in contrast-enhanced MDCT scans
| Axial area in contrast-enhanced scans (cm2) | AP-diameter (cm) | |
|---|---|---|
| Mean ± SD | 20.60 ± 5.08 | 3.87 ± 0.61 |
| Range (Min-Max) | 25.98 (11.21-39.19) | 3.44 (1.98-5.42) |
Max: maximum, Min: minimum, SD: standard deviation.
Table 2.
Intraclass correlation coefficient and relative difference for axial area and anterior-posterior (AP) diameter in contrast-enhanced and non-contrast CT scans
| Interobserver Variability | Intraobserver Variability | |||
|---|---|---|---|---|
| ICC | Rel Diff ± SD (%) | ICC | Rel Diff ± SD (%) | |
| Axial area in contrast-enhanced scans | 0.958* | 2.4 ± 7.4 | 0.988* | 0.4 ± 5.2 |
| AP-diameter | 0.910* | 3.6 ± 7.2 | 0.938* | 1.7 ± 5.5 |
| Axial area in non-contrast scans | 0.957* | 0.5 ± 8.3 | 0.988* | 0.01 ± 4.4 |
ICC: intraclass correlation coefficient, Rel Diff: relative difference, SD: standard deviation.
p<0.0001
Figure 4.

Bland-Altman Plot for the inter-observer variability of axial area and anterior-posterior (AP) diameter. The contiguous line represents the mean difference between both readers; the dashed lines are placed at ± two standard deviations.
Mean LA volume using 3D threshold-based MDCT was 91.6 ± 25.8 ml. There was moderate, positive correlation between AP-diameter and 3D volume (r=0.67, p<0.001) and very good correlation between axial area and LA volume (r=0.88, p<0.001; Figure 5). Among 100 asymptomatic subjects who underwent noncontrast MDCT (mean age 60 ± 13 years, 49% females), axial left atrial area could be assessed from all 100 non-contrast scans. Inter- and Intra-observer reproducibility was excellent (Table 2).
Figure 5.
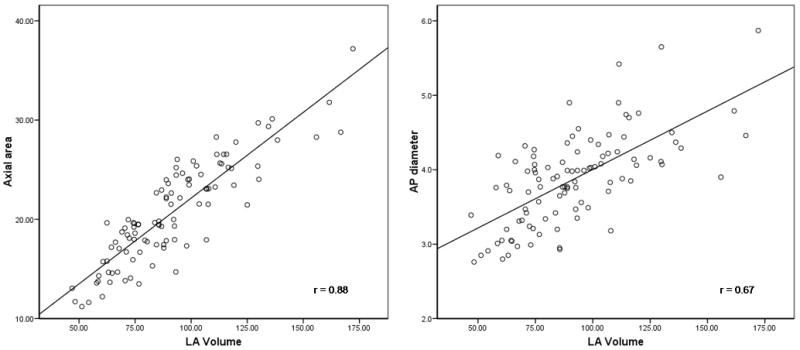
Correlation between 3-dimensional left atrial volume and axial area and anterior-posterior (AP) diameter, showing closer correlation for axial area to 3D volume than for AP-diameter.
Discussion
We demonstrate that axial area is a simple measurement of LA size that is quick to assess from contrast-enhanced and non-contrast images with excellent reproducibility. As compared to AP-diameter, axial area had a better correlation to 3D threshold-based volume.
Available data suggest that quantitative assessment of LA size has incremental value for cardiovascular risk stratification. 1-11, 19-21 Today, AP-diameter in the echocardiographic parasternal long-axis view is the most commonly assessed measure of LA size.18 However, evaluation of the LA in the AP dimension assumes that a consistent relationship is maintained between all LA dimensions as the atrium enlarges, which is often not the case.22 Maddukuri et al. showed that AP-diameter is unreliable in estimating true LA size, using 3D echocardiography as the gold standard.23 These findings are in accord with our findings of the AP-diameter being only moderately correlated with 3D volume of the LA. On the other hand, we found a high correlation between LA axial area with 3D LA volume. This suggests that axial LA area might be a helpful tool for an initial assessment of LA size in CT scans of the chest. Unlike LA AP-diameter for which a 3-chamber view needs to be reconstructed by the reader, axial LA area can be measured directly from the axial dataset, which makes it faster to assess and enables evaluation even from PAX systems without the need for 3D reconstruction software programs.
Implications
CT scans of the chest or the heart including evaluation of the coronary artery anatomy with contrast-enhanced MDCT scans or assessment of coronary artery calcification with non-contrast scans are prevalent radiologic examinations. Axial area of the LA can be assessed from every cardiac or ECG-gated chest CT without any additional contrast administration or radiation exposure. Furthermore, it can be easily measured within seconds and may be an alternative option to the currently established measures of LA size in clinical and research settings. An increasing number of scans are performed using prospective gating limited to diastole to spare radiation exposure to the patient. Therefore, normal CT based reference ranges of not only systolic, but also diastolic LA axial area need to be established.
Limitations
Our study is limited in that we did not compare our measurements to cardiac magnetic resonance imaging, the current gold standard for the assessment of LA size. Furthermore, we were unable to directly compare measurements derived from non-contrast CT scans to those obtained with contrast enhancement, since these were obtained in different subjects. However, we were able to report the reproducibility of LA measures performed in non-contrast scans. In addition, the population undergoing contrast enhanced CT consisted of subjects with the chief complaint of chest pain and the population undergoing non-contrast CT was asymptomatic. Additionally, LA size was assessed in end systole to achieve largest LA dimensions as a standard phase for LA evaluation in non-contrast enhanced scans, while the non-contrast scans were prospectively gated at 70% of the RR-interval.
Conclusion
Both AP-diameter and axial LA area permit facile and reproducible estimates of LA volume in both contrast-enhanced and non-contrast ECG gated chest CT. The measurement of LA area should be preferentially used over AP-diameter in CT because of its better correlation with 3D LA volume.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (N01-HC-25195) and by NIH (R01 HL080053). Amir A. Mahabadi is supported by a grant from the German National Academic Foundation. Quynh A. Truong has received support form NIH (T32HL076136 and L30HL093896). Christopher L. Schlett is supported by grants from the Federal Ministry of Education and Research, and Foundation of German Business, Berlin.
List of abbreviations
- LA
Left Atrium
- CT
Computed Tomography
- MDCT
Multidetector Computed Tomography
- AP
Anterior-Posterior
- ICC
Intraclass correlation coefficient
Footnotes
No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. Journal of the American College of Cardiology. 2003 Oct 1;42(7):1199–1205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 2.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) American heart journal. 2006 Feb;151(2):412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995 Aug 15;92(4):835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 4.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke; a journal of cerebral circulation. 1999 Oct;30(10):2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 5.Flaker GC, Fletcher KA, Rothbart RM, Halperin JL, Hart RG. Clinical and echocardiographic features of intermittent atrial fibrillation that predict recurrent atrial fibrillation. Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. The American journal of cardiology. 1995 Aug 15;76(5):355–358. doi: 10.1016/s0002-9149(99)80100-3. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994 Feb;89(2):724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 7.Modena MG, Muia N, Sgura FA, Molinari R, Castella A, Rossi R. Left atrial size is the major predictor of cardiac death and overall clinical outcome in patients with dilated cardiomyopathy: a long-term follow-up study. Clinical cardiology. 1997 Jun;20(6):553–560. doi: 10.1002/clc.4960200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM, Shelton BJ, Weiner DH. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. Journal of the American College of Cardiology. 2000 Apr;35(5):1237–1244. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 9.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? Journal of the American College of Cardiology. 2006 Mar 7;47(5):1018–1023. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 10.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) The American journal of cardiology. 2006 Jan 1;97(1):83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 11.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. Journal of the American College of Cardiology. 2003 Mar 19;41(6):1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 13.Mahabadi AA, Samy B, Seneviratne SK, Toepker MH, Bamberg F, Hoffmann U, Truong QA. Quantitative Assessment of Left Atrial Volume by ECG-gated Contrast-enhanced Multidetector Computed Tomography. Journal of Cardiovascular Computed Tomography. 2009 doi: 10.1016/j.jcct.2009.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiaens L, Lequeux B, Ardilouze P, Ragot S, Mergy J, Herpin D, Bonnet B, Allal J. A new method for measurement of left atrial volumes using 64-slice spiral computed tomography: Comparison with two-dimensional echocardiographic techniques. Int J Cardiol. 2008 Jan 3; doi: 10.1016/j.ijcard.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara SD, Shapiro M, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary Computed Tomography Angiography For Early Triage of Patients with Acute Chest Pain - The Rule Out Myocardial Infarction Using Computed Assisted Tomography (ROMICAT) Trial. Journal of the American College of Cardiology. 2009;53(18):1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963 May 22;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 17.Shurtleff D. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease. Washington, D.C.: 1973. Some characteristics related to the incidence of cardiovascular disease and death: Framingham study, 18 year follow-up. [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Alsaileek AA, Osranek M, Fatema K, McCully RB, Tsang TS, Seward JB. Predictive value of normal left atrial volume in stress echocardiography. Journal of the American College of Cardiology. 2006 Mar 7;47(5):1024–1028. doi: 10.1016/j.jacc.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 20.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003 May 6;107(17):2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 21.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. The American journal of cardiology. 2002 Dec 15;90(12):1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 22.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. The American journal of cardiology. 1999 Oct 1;84(7):829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 23.Maddukuri PV, Vieira ML, DeCastro S, Maron MS, Kuvin JT, Patel AR, Pandian NG. What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr. 2006 Aug;19(8):1026–1032. doi: 10.1016/j.echo.2006.03.011. [DOI] [PubMed] [Google Scholar]


