Abstract
Cryopreservation introduces extreme temperature and osmolality changes that impart lethal and sublethal effects on spermatozoa survival. Additionally, evidence indicates that the osmotic stress induced by cryopreservation causes oxidative stress to spermatozoa as well. Our objective was to determine the effect of reactive oxygen species (ROS) on rhesus macaque (Macaca mulatta) sperm function and to determine whether osmotic stress elicits the production of ROS. In the first experiment, the xanthine-xanthine oxidase (X-XO) system was used to generate the ROS superoxide anion (O2−·) and hydrogen peroxide (H2O2) in the presence or absence of the ROS scavengers superoxide dismutase and catalase, respectively. In the second experiment, osmotic stress was introduced by incubation of spermatozoa in a series of anisosmotic media ranging from 100 to 1000 mOsmol/kg in the presence or absence of the antioxidant alpha-tocopherol. Treatment with the X-XO system resulted in a significant increase in the generation of O2−· and H2O2 that was detectable using flow cytometry. The ROS generated by the X-XO system was dose dependent, and as the concentration of ROS increased, motility decreased and lipid peroxidation increased while no affect was observed on viability. Incubation of spermatozoa in anisosmotic media also resulted in an increase in O2−· generation and lipid peroxidation that was significantly decreased in the presence of the powerful antioxidant alpha-tocopherol. These results clearly indicate that osmotic stress causes oxidative stress in rhesus macaque spermatozoa, which strongly supports the hypothesis that cryopreservation-induced osmotic stress may lead to oxidative cell damage.
Keywords: cryopreservation, gamete biology, nonhuman primate, osmotic stress, oxidative stress, rhesus macaque, sperm, spermatozoa, superoxide anion
Demonstration of a direct link between cryopreservation, osmotic stress, and reactive oxygen species in sperm and indications of a mechanism whereby these stressors affect sperm function.
INTRODUCTION
Because of their physiological and genetic similarities to humans, rhesus macaques have become the most widely used nonhuman primate (NHP) for the study of human disease. The ability to efficiently cryopreserve semen from NHP not only would provide an important resource for biomedical research but also would contribute to preserving colonies of NHP species that are endangered as a result of habitat destruction and hunting [1, 2]. Although many advances have been made over the past 30 years to enhance the efficiency of NHP sperm cryopreservation, a substantial amount of sublethal and lethal damage still results from exposure to the extreme temperature and osmolality effects that cryopreservation introduces [1, 3, 4]. As the cell is exposed to plunging temperatures, extracellular ice crystallization begins, which results in concentration of the surrounding solutes in the unfrozen aqueous channels between ice crystals [3, 5, 6]. In response to the developing hyperosmolal environment, cells lose water and shrink in volume until the intracellular and extracellular solute concentrations equilibrate [6]. During the thawing process, cells are subjected to a hypotonic environment that will cause an increase in cell volume in an effort to again equilibrate the intracellular and extracellular solute concentrations. If cell volume excursion during cryopreservation or thawing is driven beyond tolerance limits, damage will occur [2, 7, 8]. The tolerance of sperm to osmotic challenge varies among species, but the following factors remain consistent in determining the cellular response to volume changes: membrane phospholipid composition, water permeability, lipid phase transition temperature, Na+/K+-ATPase activity, water channels, and ion channels [8, 9]. Understanding how these mechanisms work is extremely important in determining the most efficient sperm cryopreservation protocol.
Not only does cryopreservation introduce an osmotic stress to cells, substantial evidence indicates that cryopreservation causes oxidative damage to sperm as well [10–16]. From a physiological standpoint, the generation of low levels of reactive oxygen species (ROS) by spermatozoa plays an important role in the cascade of events controlling sperm capacitation [17–21], acrosome reaction [19, 22, 23], hyperactivation [17, 18], and sperm-oocyte fusion [23]. In contrast, excessive generation of ROS by defective spermatozoa [24–28], contaminating leukocytes [25, 29–32], or cryopreservation [13, 14, 27, 33] can have a detrimental effect on sperm function. Numerous free radicals are generated during normal cellular respiration, but the most common ROS generated by spermatozoa is the superoxide anion (O2−·), which can then spontaneously or enzymatically (via superoxide dismutase) dismutate to hydrogen peroxide (H2O2) [34]. Although O2−· is capable of producing radicals of higher toxicity than H2O2, the half-life is very short (1 msec), and membrane permeability is limited [27, 29, 35–37]. A limited ability to store antioxidant enzymes combined with a membrane rich in unsaturated fatty acids makes spermatozoa particularly susceptible to oxidative stress and peroxidative attack by these ROS [38, 39]. Specifically, high levels of ROS have been associated with extensive cell damage, including morphological defects [40], lipid peroxidation and DNA fragmentation [41], decreased ability to acrosome react or fuse with the oocyte [42], and compromised pregnancy after in vitro fertilization [43, 44].
The objective of the present study was to determine how rhesus macaque spermatozoa respond to high levels of ROS and whether osmotic stress alone is enough to elicit an oxidative response. First, we wanted to determine the effect of excessive ROS production on rhesus macaque sperm motility, viability, and membrane lipid peroxidation. Second, using osmotic stress as an experimental model for cryopreservation, we wanted to determine whether osmotic stress alone is capable of introducing oxidative stress. Our overall goal is to investigate the role of oxidative stress during cryopreservation; however, in the present study, we focused on the interaction that oxidative stress may have with osmotic stress (separated from low-temperature stress).
MATERIALS AND METHODS
Superoxide dismutase (SOD), xanthine oxidase (XO), catalase (CAT), and α-tocopherol (type IV, from vegetable oil) were obtained from Sigma Chemical Co. Xanthine (X) was purchased from Calbiochem. Dihydroethidium (DHE), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), SYTOX Green, 4,4-difluro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (BODIPY581/591), and the LIVE/DEAD sperm viability kit (propidium iodide [PI] and SYBR-14) were obtained from Invitrogen. To minimize premature oxidation, DHE and H2DCFDA were initially solubulized in dimethyl sulfoxide, held under N2, and stored at −80°C until needed.
Experiment 1
Experiment 1 studied use of the xanthine-xanthine oxidase (X-XO) ROS-producing system to evaluate use of DHE and H2DCFDA for the detection of O2−· and H2O2, respectively, in rhesus macaque sperm and to determine the effects of ROS on motility and membrane characteristics. Semen samples were obtained by electroejaculation from five male rhesus macaques (Macaca mulatta) as previously described [45]. Animals were housed at the California National Primate Research Center (UC Davis) and maintained according to Institutional Animal Care and Use Committee protocols at the University of California. Semen samples were collected directly into 50-ml centrifuge tubes containing 5 ml of Hepes-Biggers, Whitten, and Whittingham (BWW) media [46]. Following semen collection, samples were diluted in 5 ml of Hepes-BWW containing 2 mg/ml of polyvinyl alcohol (PVA) for a final concentration of 1 mg/ml of PVA. The samples were gently rocked for 5 min, after which the coagulum was removed and the sample was maintained at room temperature for 10 min to allow any ejaculate particulates to settle to the bottom. The upper 9.5 ml of semen were then transferred into a separate tube for determination of initial motility. A total of 2.5 ml of semen were layered over 3 ml of 80% buffered Percoll and centrifuged at 300 × g for 25 min as described by Baumber and Meyers [47]. Following centrifugation, the supernatant was removed and the pellet washed twice in Hepes-BWW plus 1 mg/ml of PVA (300 × g, 5 min). After two washes, the spermatozoa were resuspended in BWW plus 1 mg/ml of PVA to a final concentration of 25 × 106 sperm/ml in 500-μl aliquots. Pretreatment motility was evaluated, and samples were then incubated at 37°C (5% CO2 in air) for 60 min according to the following treatments: 1) spermatozoa alone, 2) spermatozoa plus 1 mM xanthine, 3) spermatozoa plus 0.1 U/ml of XO, 4) spermatozoa plus 200 U/ml of SOD, 5) spermatozoa plus 200 U/ml of CAT, 6) spermatozoa + 0.1 mM X-0.01 U/ml of XO, 7) spermatozoa plus 0.3 mM X-0.025 U/ml of XO, 8) spermatozoa plus 0.6 mM X-0.05 U/ml of XO, and 9) spermatozoa plus 1 mM X-0.1 U/ml of XO [48]. A room-temperature control was also included. After the 60-min treatment, motility was evaluated, and the samples were processed for determination of viability, lipid peroxidation, O2−· production, and H2O2 production as further described below.
Experiment 2
Experiment 2 studied the effect of osmotic stress on ROS production by rhesus macaque spermatozoa and the subsequent effect on motility and membrane characteristics. Semen was processed exactly as described for experiment 1 except that spermatozoa were incubated in the following anisosmotic treatments: 100, 200, 300, 400, 500, 600, or 1000 mOsmol/kg of Dulbecco PBS (DPBS) plus 1 mg/ml of PVA for 30 min at room temperature [1]. After the hypotonic (100 and 200 mOsmol/kg), isotonic (300 mOsmol/kg), or hypertonic (400-1000 mOsmol/kg) incubations, motility was evaluated, and samples were centrifuged at 300 × g for 5 min and returned to 300 mOsmol/kg of DPBS at a concentration of 25 × 106 sperm/ml. Five minutes after return to an isotonic environment, motility was evaluated, and cells were processed for determination of viability, lipid peroxidation, O2−· production, and H2O2 production.
Additionally, the effect of α-tocopherol during osmotic stress was evaluated. Spermatozoa were incubated in 100, 300, 600, and 1000 mOsmol/kg of DPBS plus 1 mg/ml of PVA in the presence of 100 μM α-tocopherol or vehicle (0.5% ethanol). Samples were processed as described above for determination of O2−· and lipid peroxidation.
Motility
Sperm motility was evaluated by means of computer-assisted sperm analysis with HTM Ceros, version 12.2 g (Hamilton Thorne Biosciences, Inc.). At least 200 cells in a minimum of four fields were evaluated for each treatment. The following instrument settings were used for computed-assisted sperm analysis: frame rate, 60 Hz; frames acquired, 30; minimum contrast, 80; minimum cell size, four pixels; static average velocity of the smoothed cell path (VAP) cutoff, 20 μm/sec; static average velocity measured in a straight line from the beginning to the end of the track cutoff (VSL), 10 μm/sec; progressive VAP threshold, 25 μm/sec; progressive average value of the ratio VSL/VAP threshold (STR; measures the departure of the cell path from a straight line), 80%; static intensity limits, 0.6–1.4; static size limits, 0.6–2.31; and static elongation limits, 0–80. Percentage total motility and percentage progressive (forward) motility were determined at all time points.
Viability
SYBR-14 and PI were added to 500 μl of each sample for a final concentration of 0.1 and 12 μM, respectively. Spermatozoa were incubated for 10 min at room temperature, diluted to 1 × 106 sperm/ml, and analyzed by flow cytometry. SYBR-14 is a membrane-permeant nucleic acid stain and will stain all cells, whereas PI is membrane-impermeant dye that binds to DNA. Therefore, SYBR-14-labeled cells (green) were scored as “viable,” and SYBR-14/PI-labeled cells (green and red) were scored as “nonviable.” The excitation wavelength used for both fluorochromes was 488 nm, and the emission maxima of SYBR-14 and PI was 516 and 617 nm, respectively.
DHE Assay
The oxidation of DHE by the superoxide anion within a cell produces the DNA-sensitive fluorochromes, Et+ and 2OHEt+, that together generate a red nuclear fluorescence in cells that excites at 488 nm, and it has an emission of 630 nm. DHE is an excellent probe for measuring intracellular O2−· generation [49–51] but does not distinguish between live and dead cells; therefore, a vitality probe must be used in combination with DHE to account for nonviable cells. SYTOX Green, which is a nuclear chromosome counterstain that is impermeant to live cells and, thus, only stains dead/nonviable cells, was used as the vitality probe. In preliminary experiments, we validated that SYTOX Green was an accurate viability probe for rhesus spermatozoa by using it in sperm cell populations that were 100% live, 75% live, 50% live, and 100% dead. To obtain 100% dead cells, an aliquot of live cells was submerged in LN2 and thawed (37°C water bath) three times for 2 min each time. The dead cells were then mixed with the live cells in the above-described proportions and run on our FACScan flow cytometer to determine that the cell populations appeared in the correct quadrants.
Dihydroethidium (2 μM) and SYTOX Green (0.05 μM) were added together for 15 min before the end of treatment incubation [51]. Then, cells were diluted to 1 × 106 cells/ml and analyzed by flow cytometry. DHE was effectively used for the evaluation of O2−· by rhesus spermatozoa using the ROS-generating system (X-XO with or without varying levels of the O2−· scavenger SOD). Comparison of DHE fluorescence in control samples to that in samples treated with X-XO allowed the establishment of a threshold for identification of positively DHE-labeled cells and, thus, cells producing O2−·.
H2DCFDA Assay
The production of H2O2 was evaluated using the cell-permeant probe H2DCFDA. This reduced fluorescein is freely permeable across cell membranes, but once inside the cell, cellular esterases remove the acetate groups, resulting in the impermeant product H2DCF. Oxidation of H2DCF by H2O2 yields dichlorofluorescein (DCF), which has an excitation/emission spectra of 488/530 nm. Preliminary experiments were performed to determine the optimal concentration, temperature, and time for loading spermatozoa with H2DCFDA for maximum assay sensitivity. The efficiency of this probe was tested by incubating spermatozoa in 1 mM to 25 μM H2O2 with and without CAT, testing both incubation time and temperature.
Fifteen minutes before the end of the treatment incubation, 50 μM H2DCFDA and 5 μM PI were added. Spermatozoa were then diluted to 1 × 106 sperm/ml and analyzed by flow cytometry. Inclusion of the viability stain PI allowed the differentiation between live and dead cells in the production of H2O2. Comparison of DCF fluorescence in control samples to those treated with H2O2 allowed the establishment of a threshold for identification of positively DCF-labeled cells and, thus, cells producing H2O2.
BODIPY581/591C-11 Assay
Lipid peroxidation was evaluated using the fluorescence lipid probe BODIPY581/591C-11 (4,4-difluro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid). When this probe becomes incorporated into the sperm membrane, it fluoresces red until lipid radicals peroxidize the membrane, at which time the probe undergoes an emission shift to green (red, nonperoxidized; green, peroxidized). Before experimental treatment, spermatozoa were incubated in 1 μM BODIPY 581/591 C-11 for 30 min at room temperature to load the probe into the cell membranes. Spermatozoa were then washed two times (300 × g, 5 min) to remove excess probe and resuspended to 25 × 106 sperm/ml in their respective treatments in the presence or absence of the lipid peroxidation promoters ferrous sulfate (1 μM) and sodium ascorbate (5 μM). Because it is possible for dead cells to undergo lipid peroxidation, the vitality probe PI (final concentration, 12 μM) was added during the last 5 min of treatment incubation so that dead lipid-peroxidized cells could be distinguished from live lipid-peroxidized cells. Spermatozoa were then diluted to 1 × 106 sperm/ml and analyzed by flow cytometry. During preliminary experiments, the ideal BODIPY 581/591 C-11 and ferrous sulfate/sodium ascorbate concentrations were determined. To verify that lipid peroxidation was working through ferrous ion promotion, 100 μM ethylenediaminetetra-acetic acid (EDTA) was added to determine if lipid peroxidation could be decreased through the chelation of iron. Comparison of the fluorescence in control samples to those treated with X-XO in the presence of ferrous sulfate and sodium ascorbate allowed the establishment of a threshold for identification of positively BODIPY 581/591 C-11-labeled cells and, thus, cells that were undergoing lipid peroxidation.
Flow Cytometric Analysis
Flow cytometry was performed using a FACScan flow cytometer (Becton, Dickinson and Company) equipped with a 488-nm excitation laser, and data were analyzed using CellQuest software (Becton, Dickinson and Company). SYBR-14, SYTOX Green, and DCF were measured with a 530/30 bandpass filter, whereas PI was measured with a 585/42 bandpass filter and DHE with a 650LP bandpass filter. Adjustments were made to address and eliminate fluorochrome spectral overlap so that each cell population was seen as distinct. To limit the evaluation of DHE, DCF, and BODIPY 581/591 C-11 fluorescence to viable spermatozoa, only the subpopulation outside of SYTOX Green- or PI-positive cells, respectively, were included in the evaluation. A total of 10 000 gated events were analyzed per sample.
Statistical Analysis
Repeated-measures ANOVA was used to determine the significance of treatment and whether treatment elicited a dose response. The same statistical method was used to look at the effect treatment had on sperm motility, viability, and lipid peroxidation. When the latter repeated-measures ANOVA was significant, post-hoc comparisons were performed to determine which treatments were significantly different from each other. A P value of less than 0.05 was considered to be statistically significant.
All X-XO treatments were compared to control (37°C), and 0.6 mM X-0.05 U/ml of XO plus SOD or CAT was compared to 0.6 mM X-0.05 U/ml of XO alone. All osmotic treatments were compared to control (300 mOsmol/kg).
RESULTS
Experiment 1
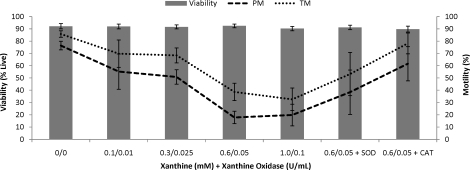
Experiment 1 studied use of the X-XO ROS-producing system to evaluate use of DHE and H2DCFDA for the detection of O2−· and H2O2, respectively, in rhesus macaque sperm and to determine the effects of ROS on motility and membrane characteristics. The addition of increasing levels of ROS over a time period of 60 min had no effect on viability but negatively affected motility (Fig. 1). A decrease was found in total (P < 0.005) and progressive (P < 0.01) motility with increasing concentrations of X-XO. The addition of CAT was able to increase both total (X-XO, 39%; X-XO + CAT, 78%; control, 86%; P < 0.05) and progressive (X-XO, 18%; X-XO + CAT, 62%; control, 76%; P < 0.05) motility to near control levels. However, SOD was not as effective and increased total (X-XO, 39%; X-XO + SOD, 53%; control, 86%; P = 0.38) and progressive (X-XO, 18%; X-XO + SOD, 38%; control, 76%; P = 0.26) motility by a much smaller margin.
FIG. 1.
Treatment with xanthine-xanthine oxidase (X-XO) resulted in a decrease in total motility (P < 0.01) and progressive motility (P < 0.01), whereas the addition of catalase (CAT, 200 U/ml) improved both total motility (P < 0.05) and progressive motility (P < 0.05) to near control levels. Superoxide dismutase (SOD, 200 U/ml) improved motility, but this improvement was not significant. Data were analyzed using ANOVA with repeated measures, and values represent the mean ± SEM (n = 5).
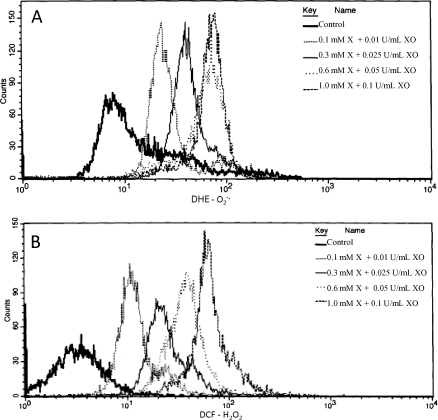
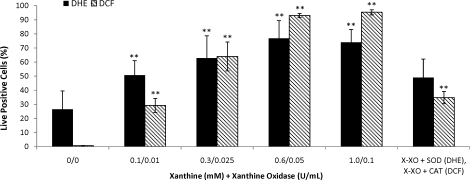
Incubation in the presence of the X-XO system exhibited a significant and dose-dependent increase in O2−· (P < 0.01) (Figs. 2A and 3) and H2O2 production (P < 0.0001) (Figs. 2B and 3) that was sensitive to the presence of SOD (Fig. 3) and CAT (Fig. 3), respectively.
FIG. 2.
Flow cytometric histograms showing the distribution of dihydroethidium (DHE; A) and 2′,7′–dichlorodihydrofluorecein diacetate (DCF; B) fluorescence in rhesus spermatozoa incubated with and without xanthine-xanthine oxidase (X-XO). DHE measures superoxide (O2−·) production, whereas DCF measures hydrogen peroxide (H2O2) production. Data were analyzed using ANOVA with repeated measures, and values represent the mean ± SEM (n = 5).
FIG. 3.
Treatment with varying levels of xanthine-xanthine oxidase (X-XO) significantly increased superoxide (O2−·; DHE positive) and hydrogen peroxide (H2O2; DCF positive) production, whereas the addition of superoxide dismutase (SOD; 200 U/ml of SOD + 0.6 mM xanthine and 0.05 U/ml of XO) and catalase (CAT: 200 U/ml of CAT + 0.6 mM xanthine and 0.05 U/ml of XO) were able to decrease O2−· and H2O2, respectively. Data were analyzed using ANOVA with repeated measures, and values represent the mean ± SEM (n = 5). **P < 0.01 vs. control.
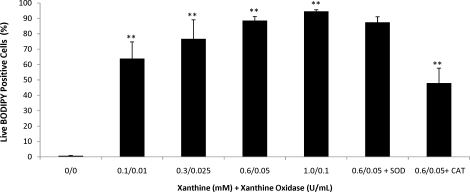
In accordance with increased ROS production, high levels of lipid peroxidation were observed during incubation with the X-XO system. As the level of X-XO increased, an increase in the level of lipid peroxidation was observed (P < 0.005) (Fig. 4). Although the response was not affected by the addition of SOD, it was significantly decreased by the addition of CAT (P < 0.01), which supports the fact that H2O2, as opposed to O2−·, is a major contributor to the lipid peroxidation of sperm cell membranes.
FIG. 4.
The lipid peroxidation response of rhesus spermatozoa to increasing concentration of xanthine-xanthine oxidase (X-XO) and the sensitivity to the antioxidants superoxide dismutase (SOD; 200 U/ml) and catalase (CAT; 200 U/ml). Treatment with X-XO significantly increased lipid peroxidation (BODIPY 581/591 C-11 positive), whereas only CAT was able to significantly decrease the observed increase in lipid peroxidation. All lipid peroxidation assays were conducted in the presence of 1 μM ferrous sulfate and 5 μM sodium ascorbate. Data were analyzed using ANOVA with repeated measures, and values represent the mean ± SEM (n = 5). **P < 0.01 vs. control.
Experiment 2
Experiment 2 studied the effect of osmotic stress on ROS production by rhesus macaque spermatozoa and the subsequent effect on motility and membrane characteristics. In agreement with earlier work from our laboratory [1], both total and progressive motility significantly decreased when exposed to anisotonic conditions (P < 0.0001 and P < 0.0001, respectively), with 0% total motility observed at 600-1000 mOsmol/kg (data not shown). Viability was also significantly decreased by osmotic stress, with the larger effect observed in hypertonic conditions (data not shown; P < 0.0005).
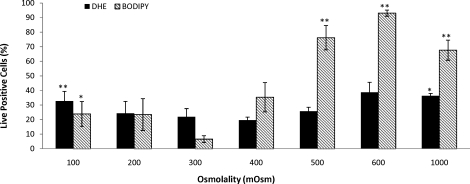
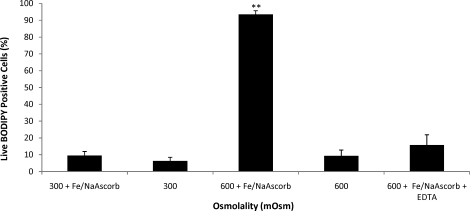
When spermatozoa were subjected to osmotic stress, extremely hypotonic (100 mOsmol/kg) or extremely hypertonic (1000 mOsmol/kg) conditions, a significant increase in O2−· generation was observed (P < 0.01 and P < 0.05, respectively) (Fig. 5). Although we were unable to detect significant H2O2 production, lipid peroxidation was significantly high in both hypotonic (100 mOsmol/kg; P < 0.05) and hypertonic (500, 600, and 1000 mOsmol/kg; P < 0.0005, P < 0.0001, and P < 0.005, respectively) treatments (Fig. 5). The addition of ferrous sulfate and sodium ascorbate were required to produce a measurable increase in lipid peroxidation, and this effect was counteracted by the addition of the iron chelator EDTA (Fig. 6). Taken together, these results indicate that low but significant levels of H2O2 were present and that the observed peroxidative damage was caused by ferrous ion-promoted H2O2 generation of the hydroxyl radical.
FIG. 5.
The effect of osmotic stress on rhesus sperm superoxide (O2−·) production and lipid peroxidation. Exposure to anisotonic conditions resulted in a significant increase in superoxide (O2−·) production (DHE positive) as well as a significant increase in lipid peroxidation (BODIPY 581/591 C-11 positive). All lipid peroxidation assays were conducted in the presence of ferrous sulfate (1 μM) and sodium ascorbate (5 μM). Data were analyzed using ANOVA with repeated measures with orthogonal contrasts, and values represent the mean ± SEM (n = 5). *P < 0.05, **P < 0.01 vs. control.
FIG. 6.
The effect of osmotic stress on rhesus sperm lipid peroxidation in the presence or absence of ferrous sulfate (1 μM) and sodium ascorbate (5 μM). In the absence of ferrous ion promoters, very low levels of lipid peroxidation (BODIPY 581/591 C-11 positive) were observed under hypertonic conditions, which were significantly different from what was seen in the presence of ferrous ion promotion. Exposing cells to hypertonic conditions in the presence of ferrous ion promoters as well as 100 μM EDTA resulted in lipid peroxidation levels comparable to those in the absence of ion promoters. Values represent the mean ± SEM (paired t-test, n = 4). **P < 0.01 vs. control.
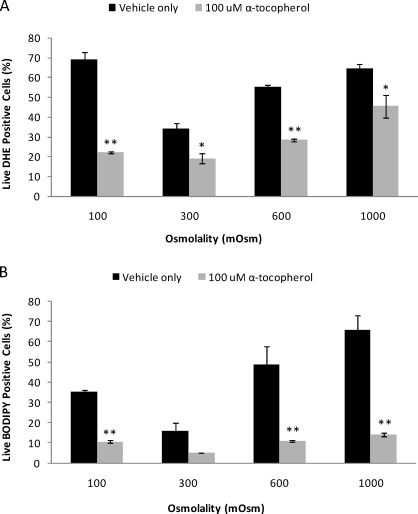
Because of the high levels of O2−· and lipid peroxidation observed under osmotic stress, a brief experiment was conducted in which rhesus sperm were subjected to 100, 300, 600, and 1000 mOsmol/kg conditions in the presence or absence of the powerful antioxidant α-tocopherol. The addition of 100 μM α-tocopherol or vehicle (0.5% ethanol) had no significant affect on viability or motility (total/progressive; data not shown), but α-tocopherol had a profound effect on O2−· production and lipid peroxidation. A significant decrease in O2−· production was observed under all conditions (100 mOsmol/kg, P < 0.01; 300 mOsmol/kg, P < 0.05; 600 mOsmol/kg, P < 0.005; 1000 mOsmol/kg, P < 0.05) (Fig. 7A), and lipid peroxidation was significantly reduced in both hypotonic (100 mOsmol/kg, P < 0.01) and hypertonic (600 mOsmol/kg, P < 0.01; 1000 mOsmol/kg, P < 0.01) (Fig. 7B) conditions.
FIG. 7.
A) The antioxidant α-tocopherol (100 μM) significantly decreased superoxide (O2−·) production (DHE positive) in anisotonic as well as isotonic conditions. B) Lipid peroxidation (BODIPY 581/591 C-11 positive) was also significantly reduced in the presence of α-tocopherol under anisotonic conditions, with no significant decrease observed under isotonic conditions. Data were analyzed using ANOVA with repeated measures with orthogonal contrasts, and values represent the mean ± SEM (n = 4). *P < 0.05, **P < 0.01 vs. control.
DISCUSSION
In the present study, we determined that DHE and H2DCFDA, used in conjunction with either SYTOX Green or PI to exclude dead cells, are reliable probes for the detection of intracellular ROS in rhesus spermatozoa. Incubation with X-XO elicited a dose-dependent production of O2−· and H2O2 that appeared to level out at our highest concentrations, indicating a saturation effect in this enzyme system. Interestingly, the observed increase in O2−· production by rhesus spermatozoa is similar to observations in equine spermatozoa [36] but different than that reported for porcine spermatozoa [52], suggesting species differences in ROS production induced by X-XO. Porcine spermatozoa exhibited a mild increase in O2−·, but the majority of the response was observed in H2O2 generation [52]. The high levels of ROS introduced by X-XO had no affect on rhesus sperm viability but did negatively affect both total and progressive motility. In the presence of CAT, however, motility was returned to near control levels, suggesting that H2O2 is the main ROS affecting rhesus sperm motility and supporting previous reports for other species [53–57].
In addition to negatively affecting motility, ROS have long been implicated in the lipid peroxidation of sperm membranes from both fresh and cryopreserved semen because of the relatively high polyunsaturated fatty acid content of sperm membranes [24, 37, 38, 58]. In the present study, rhesus spermatozoa incubated in the presence of X-XO exhibited significant membrane lipid peroxidation, which was localized primarily in the midpiece. This is consistent with previous reports [35, 39, 59, 60], but low levels were observed in the posterior region of the head as well. The exact mechanism for ROS-induced lipid peroxidation is unknown, but it has been shown that ROS generated by treatment with X-XO affects a key sperm metabolism enzyme, glucose-6-phosphate-dehydrogenase (G6PD) [61]. It is believed that diffusion of H2O2 across the cell membrane may cause the inhibition of G6PD that subsequently leads to a decrease in the availability of NADPH. A decrease in NADPH may then lead to an accumulation of glutathione and reduced glutathione, leaving spermatozoa vulnerable to ROS attack because of a compromised antioxidant defense system [60, 62].
It is clear from the present study that rhesus spermatozoa are susceptible to oxidative stress, and it also is clear that osmotic stress alone can illicit oxidative stress as well, a direct link that to our knowledge has not been shown previously in rhesus spermatozoa. Incubation of rhesus spermatozoa under anisotonic conditions resulted in a significant increase in O2−· production, which interestingly has just been observed with equine spermatozoa as well [63]. The exact mechanism to explain the increased generation of this ROS in response to osmotic stress remains unclear; however, work with other cell types suggests that hyperosmotic-induced cell swelling may activate the membrane-associated phospholipase A2. Activation of phospholipase A2 may then lead to an increase in arachidonic acid, which subsequently activates the NADPH oxidase complex, resulting in increased production of O2−· [64, 65]. Even in the absence of a clear explanation for osmotic-induced ROS production, it is clear from the present study and from previous work that an interaction occurs between the two stresses [64, 66, 67].
The high levels of lipid peroxidation observed in rhesus spermatozoa under osmotic stress were not surprising. A significant level of membrane stretching occurs under osmotic stress, resulting in a decrease in lateral packing of lipids [68] and reorganization of the actin cytoskeleton [4], leaving the cell membrane vulnerable to ROS attack. Surprisingly, H2O2 was not detected during anisotonic conditions, which was unexpected, especially in the presence of ferrous ion promotion. Consequently, it is quite possible that low levels of H2O2 were present and that our assay unfortunately lacked the sensitivity to measure this ROS. The probe used to detect lipid peroxidation, BODIPY 581/591 C-11, only responds to changes in chain-propagating species (alkoxyl-RO. and peroxyl radicals ROO.) and does not allow detection of the specific ROS (i.e., O2−· or H2O2) that participate in the first chain initiation of the lipid peroxidation cascade [69]. Ferrous sulfate was added in these experiments to accelerate the lipid peroxidation cascade and allow a more direct study of the effects on sperm function [70, 71]. When ferrous sulfate is added in the presence of ROS, the initiation of a lipid peroxidation cascade, termed the Fenton reaction, begins [72]. In the presence of O2−· (or sodium ascorbate), the ferric ion (Fe3+) is reduced to form the ferrous ion (Fe2+) and oxygen (O2); this step is termed the Haber-Weiss reaction [73]. The second step (i.e., the Fenton reaction) occurs when Fe2+ is oxidized by H2O2 to form Fe3+, the hydroxyl anion (OH−), and the hydroxyl radical (OH.). It is now the chain-propagating species, OH., that will cause lipid peroxidation of the sperm membrane and, thus, a shift in BODIPY 581/591 C-11 fluorescence from red to green. Although undetectable, it is possible that low levels of H2O2 were present, and when combined with high levels of O2−·, a significant level of cell membrane lipid peroxidation resulted.
In an effort to protect rhesus spermatozoa from the deleterious effects of osmotic-induced oxidative stress, the enzymatic antioxidants CAT and SOD were added but, unfortunately, proved to be unsuccessful. This may have been a result of the method in which rhesus spermatozoa were exposed to osmotic stress, because the method used did not allow pretreatment incubation with either antioxidant. Alternatively, the nonenzymatic antioxidant α-tocopherol was used with much better success. α-Tocopherol is a powerful lipophilic antioxidant that can intercept lipid peroxyl radicals and break the chain of lipid peroxidation [37], which was clearly observed when this antioxidant was added to rhesus spermatozoa in the presence of ROS. Under both hypotonic and hypertonic conditions, addition of 100 μM α-tocopherol significantly reduced both O2−· production and lipid peroxidation. It is not known if the addition of α-tocopherol during rhesus sperm cryopreservation could improve postthaw quality. No advantage was observed by adding this antioxidant during equine sperm cryopreservation [74], although one of the hypothesized reasons for this failure was a sensitivity to the ethanol vehicle used in that study. Our present study had no indication of vehicle effect with α-tocopherol treatment.
These results clearly indicate, to our knowledge for the first time, that osmotic stress directly causes oxidative stress in rhesus macaque spermatozoa and strongly suggests that osmotic changes associated with cryopreservation lead to oxidative cell damage. Because osmotic and oxidative changes are major components of the cryopreservation process, these data support a very strong interaction between the two components and suggest that this interaction likely is a significant mechanism for cryoinjury to a number of cellular compartments during cryopreservation.
Acknowledgments
The authors gratefully acknowledge Dr. Barry Ball, Department of Population Health and Reproduction, School of Veterinary Medicine, UC-Davis, for his advice and technical assistance during this project.
Footnotes
Supported by a grant from the National Center for Research Resources (NIH 2 R01 RR016581-05A1).
REFERENCES
- Rutllant J, Pommer AC, Meyers SA.Osmotic tolerance limits and properties of rhesus monkey (Macaca mulatta) spermatozoa. J Androl 2003; 24: 534–541. [DOI] [PubMed] [Google Scholar]
- Agca Y, Mullen S, Liu J, Johnson-Ward J, Gould K, Chan A, Critser J.Osmotic tolerance and membrane permeability characteristics of rhesus monkey (Macaca mulatta) spermatozoa. Cryobiology 2005; 51: 1–14. [DOI] [PubMed] [Google Scholar]
- Pommer AC, Rutllant J, Meyers SA.The role of osmotic resistance on equine spermatozoal function. Theriogenology 2002; 58: 1373–1384. [DOI] [PubMed] [Google Scholar]
- Correa LM, Thomas A, Meyers SA.The macaque sperm actin cytoskeleton reorganizes in response to osmotic stress and contributes to morphological defects and decreased motility. Biol Reprod 2007; 77: 942–953. [DOI] [PubMed] [Google Scholar]
- Mazur P.Studies on rapidly frozen suspensions of yeast cells by differential thermal analysis and conductometry. Biophys J 1963; 3: 323–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P.Principles of Cryobiology. Boca Raton, FL:CRC Press;2004: 2–65. [Google Scholar]
- de la Cueva FI, Rigau T, Bonet S, Miro J, Briz M, Rodriguez-Gil JE.Subjecting horse spermatozoa to hypo-osmotic incubation: effects of ouabain. Theriogenology 1997; 47: 765–784. [DOI] [PubMed] [Google Scholar]
- Meyers SA.Spermatozoal response to osmotic stress. Anim Reprod Sci 2005; 89: 57–64. [DOI] [PubMed] [Google Scholar]
- Si W, Benson JD, Men H, Critser JK.Osmotic tolerance limits and effects of cryoprotectants on the motility, plasma membrane integrity and acrosomal integrity of rat sperm. Cryobiology 2006; 53: 336–348. [DOI] [PubMed] [Google Scholar]
- Thuwanut P, Chatdarong K, Techakumphu M, Axner E.The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology 2008; 70: 233–240. [DOI] [PubMed] [Google Scholar]
- Watson PF.The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci 2000; 60–61: 481–492. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT.Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 1992; 13: 232–241. [PubMed] [Google Scholar]
- Wang AW, Zhang H, Ikemoto I, Anderson DJ, Loughlin KR.Reactive oxygen species generation by seminal cells during cryopreservation. Urology 1997; 49: 921–925. [DOI] [PubMed] [Google Scholar]
- Baumber J, Ball BA, Linfor JJ, Meyers SA.Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J Androl 2003; 24: 621–628. [DOI] [PubMed] [Google Scholar]
- Bell M, Wang R, Hellstrom WJ, Sikka SC.Effect of cryoprotective additives and cryopreservation protocol on sperm membrane lipid peroxidation and recovery of motile human sperm. J Androl 1993; 14: 472–478. [PubMed] [Google Scholar]
- O'Flaherty C, Beconi M, Beorlegui N.Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia 1997; 29: 269–275. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C.Human sperm hyperactivation in whole semen and its association with low superoxide scavenging capacity in seminal plasma. Fertil Steril 1993; 59: 1291–1295. [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C.Reactive oxygen species (ROS) and reproduction. Adv Exp Med Biol 1994; 366: 185–197. [DOI] [PubMed] [Google Scholar]
- Griveau JF, Renard P, Le Lannou D.An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int J Androl 1994; 17: 300–307. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C.Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med 1997; 22: 643–656. [DOI] [PubMed] [Google Scholar]
- Baumber J, Sabeur K, Vo A, Ball BA.Reactive oxygen species promote tyrosine phosphorylation and capacitation in equine spermatozoa. Theriogenology 2003; 60: 1239–1247. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Lamothe G.Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med 2009; 46: 502–510. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C.A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl 1993; 16: 21–25. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Clarkson JS, Fishel S.Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod 1989; 41: 183–197. [DOI] [PubMed] [Google Scholar]
- Aitken J, Krausz C, Buckingham D.Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev 1994; 39: 268–279. [DOI] [PubMed] [Google Scholar]
- Rao B, Soufir JC, Martin M, David G.Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res 1989; 24: 127–134. [DOI] [PubMed] [Google Scholar]
- Ball BA, Vo AT, Baumber J.Generation of reactive oxygen species by equine spermatozoa. Am J Vet Res 2001; 62: 508–515. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Gagnon C.Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992; 57: 409–416. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Buckingham DW, Brindle J, Gomez E, Baker HW, Irvine DS.Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum Reprod 1995; 10: 2061–2071. [DOI] [PubMed] [Google Scholar]
- Baumber J, Vo A, Sabeur K, Ball BA.Generation of reactive oxygen species by equine neutrophils and their effect on motility of equine spermatozoa. Theriogenology 2002; 57: 1025–1033. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, West KM.Analysis of the relationship between reactive oxygen species production and leucocyte infiltration in fractions of human semen separated on Percoll gradients. Int J Androl 1990; 13: 433–451. [DOI] [PubMed] [Google Scholar]
- Wolff H, Politch JA, Martinez A, Haimovici F, Hill JA, Anderson DJ.Leukocytospermia is associated with poor semen quality. Fertil Steril 1990; 53: 528–536. [PubMed] [Google Scholar]
- Michael A, Alexopoulos C, Pontiki E, Hadjipavlou-Litina D, Saratsis P, Boscos C.Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology 2007; 68: 204–212. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C.Reactive oxygen species and sperm physiology. Rev Reprod 1997; 2: 48–54. [DOI] [PubMed] [Google Scholar]
- Brouwers JF, Silva PF, Gadella BM.New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze-thawing. Theriogenology 2005; 63: 458–469. [DOI] [PubMed] [Google Scholar]
- Burnaugh L, Sabeur K, Ball BA.Generation of superoxide anion by equine spermatozoa as detected by dihydroethidium. Theriogenology 2007; 67: 580–589. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford:: Oxford University Press;; 1989. [Google Scholar]
- Baker MA, Aitken RJ.Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol 2005; 3: 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild DM, Brouwers JF, Colenbrander B, Aguero A, Gadella BM.Lipid peroxide formation in relation to membrane stability of fresh and frozen thawed stallion spermatozoa. Mol Reprod Dev 2005; 72: 230–238. [DOI] [PubMed] [Google Scholar]
- Aziz N, Saleh RA, Sharma RK, Lewis-Jones I, Esfandiari N, Thomas AJ, Jr, Agarwal A.Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril 2004; 81: 349–354. [DOI] [PubMed] [Google Scholar]
- Fraczek M, Kurpisz M.The redox system in human semen and peroxidative damage of spermatozoa [in Polish]. Postepy Hig Med Dosw (Online) 2005; 59: 523–534. [PubMed] [Google Scholar]
- Lemkecher T, Dartigues S, Vaysse J, Kulski O, Barraud-Lange V, Gattegno L, Wolf JP.Leucocytospermia, oxidative stress and male fertility: facts and hypotheses [in French]. Gynecol Obstet Fertil 2005; 33: 2–10. [DOI] [PubMed] [Google Scholar]
- Zorn B, Vidmar G, Meden-Vrtovec H.Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl 2003; 26: 279–285. [DOI] [PubMed] [Google Scholar]
- Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, Lorenz M, Schmidt W.Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and nonpregnant patients after IVF/ICSI. Reprod Biomed Online 2006; 13: 696–706. [DOI] [PubMed] [Google Scholar]
- Sarason RL, VandeVoort CA, Mader DR, Overstreet JW.The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol 1991; 20: 122–125. [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittingram DG.Methods in Mammalian Embryology. San Francisco:W.H. Freeman & Co. Ltd.;1971: 86–116. [Google Scholar]
- Baumber J, Meyers SA.Changes in membrane lipid order with capacitation in rhesus macaque (Macaca mulatta) spermatozoa. J Androl 2006; 27: 578–587. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I.The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 1968; 249: 391–399. [PubMed] [Google Scholar]
- Benov L, Sztejnberg L, Fridovich I.Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med 1998; 25: 826–831. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B.Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 2003; 34: 1359–1368. [DOI] [PubMed] [Google Scholar]
- De Iuliis GN, Wingate JK, Koppers AJ, McLaughlin EA, Aitken RJ.Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab 2006; 91: 1968–1975. [DOI] [PubMed] [Google Scholar]
- Awda BJ, Mackenzie-Bell M, Buhr MM.Reactive oxygen species and boar sperm function. Biol Reprod 2009; 81: 553–556. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C.Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl 1992; 13: 368–378. [PubMed] [Google Scholar]
- Aitken RJ, Buckingham D, Harkiss D.Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. J Reprod Fertil 1993; 97: 441–450. [DOI] [PubMed] [Google Scholar]
- Lapointe S, Sirard MA.Catalase and oviductal fluid reverse the decreased motility of bovine sperm in culture medium containing specific amino acids. J Androl 1998; 19: 31–36. [PubMed] [Google Scholar]
- Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC.The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl 2000; 21: 895–902. [PubMed] [Google Scholar]
- Guthrie HD, Welch GR.Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J Anim Sci 2006; 84: 2089–2100. [DOI] [PubMed] [Google Scholar]
- Jones R, Mann T, Sherins R.Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril 1979; 31: 531–537. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ.Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 2008; 93: 3199–3207. [DOI] [PubMed] [Google Scholar]
- Brouwers JF, Gadella BM.In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic Biol Med 2003; 35: 1382–1391. [DOI] [PubMed] [Google Scholar]
- Griveau JF, Dumont E, Renard P, Callegari JP, Le Lannou D.Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fertil 1995; 103: 17–26. [DOI] [PubMed] [Google Scholar]
- Saleh RA, Agarwal A.Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002; 23: 737–752. [PubMed] [Google Scholar]
- Burnaugh L, Ball BA, Sabeur K, Thomas AD, Meyers SA.Osmotic stress stimulates generation of superoxide anion by spermatozoa in horses. Anim Reprod Sci 2009; 117: 249–260. [DOI] [PubMed] [Google Scholar]
- Lambert IH.Reactive oxygen species regulate swelling-induced taurine efflux in NIH3T3 mouse fibroblasts. J Membr Biol 2003; 192: 19–32. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Pedersen SF, Poulsen KA.Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol (Oxf) 2006; 187: 75–85. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Fathallah L, Lang HJ.Interaction between osmotic and oxidative stress in diabetic precataractous lens: studies with a sorbitol dehydrogenase inhibitor. Biochem Pharmacol 1999; 58: 1945–1954. [DOI] [PubMed] [Google Scholar]
- Beffagna N, Buffoli B, Busi C.Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant Cell Physiol 2005; 46: 1326–1339. [DOI] [PubMed] [Google Scholar]
- Lehtonen JY, Kinnunen PK.Phospholipase A2 as a mechanosensor. Biophys J 1995; 68: 1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA.Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol Hum Reprod 2007; 13: 203–211. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Harkiss D, Buckingham DW.Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol Reprod Dev 1993; 35: 302–315. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Harkiss D, Buckingham D.Relationship between iron-catalyzed lipid peroxidation potential and human sperm function. J Reprod Fertil 1993; 98: 257–265. [DOI] [PubMed] [Google Scholar]
- Fenton H.Oxidation of tartaric acid in the presence of iron. J Chem Soc 1984; 65: 899–903. [Google Scholar]
- Haver F, Weiss J.On the catalysis of hydroperoxide. Naturwissenschaften 1932; 20: 948–950. [Google Scholar]
- Baumber J, Ball BA, Linfor JJ.Assessment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. Am J Vet Res 2005; 66: 772–779. [DOI] [PubMed] [Google Scholar]