Abstract
Background
Cigarette smoking is a chronic, relapsing illness that is inadequately addressed in primary care practice.
Objective
To compare cessation rates among smokers receiving pharmacotherapy management alone or combined with either moderate- or high-intensity disease management that includes counseling and provider feedback.
Design
Randomized clinical trial from June 2004 to December 2007.
Setting
50 rural primary care practices.
Patients
750 patients smoking ≥ 10 cigarettes/day.
Intervention
Participants were randomized to one of three groups: pharmacotherapy management (n = 250); pharmacotherapy management supplemented with up to 2 counseling calls (moderate-intensity disease management (n = 249)); or pharmacotherapy management supplemented with up to 6 counseling calls (high-intensity disease management (n = 251)). Interventions were offered every six months for two years. All participants received offers of free pharmacotherapy; moderate-intensity and high-intensity disease management recipients had post-counseling progress reports faxed to their physicians. Participants and counselors were not blinded to treatment assignment.
Measurements
Self-reported point-prevalence smoking abstinence at 24 months (primary outcome) and overall (0 to 24 months) analyses of smoking abstinence, utilization of pharmacotherapy, and discussions about smoking with physicians (secondary outcomes). Research assistants, blinded to treatment assignment, conducted outcome assessments.
Results
Pharmacotherapy utilization was comparable across treatment groups, with 473 of 741 (63.8%), 302 of 739 (40.9%), 175 of 732 (23.9%), and 179 of 726 (24.7%) requesting pharmacotherapy during the 1st, 2nd, 3rd, and 4th 6-month cycles of treatment. Of participants that saw a physician during any given treatment cycle, 37.5 – 59.5% reported that they discussed smoking cessation with their physician, but this did not differ across the treatment groups. Abstinence rates increased throughout the 24-month study and overall (0 to 24 months) analyses demonstrated higher abstinence among recipients of high-intensity versus moderate-intensity disease management (OR, 1.43 [95% CI, 1.00 to 2.03]) and higher abstinence in the combined disease management groups compared to pharmacotherapy management alone (OR, 1.47 [95% CI, 1.08 to 2.00]). The primary outcome, self-reported abstinence at 24 months, was 68 of 244 (27.9%) and 56 of 238 (23.5%) in the high-intensity and moderate-intensity disease management groups, respectively (OR, 1.33 [95% CI, 0.88 to 2.02]) and 56 of 244 (23.0%) in the pharmacotherapy management group (OR, 1.12 [95% CI, 0.78 to 1.61] (combined disease management versus pharmacotherapy management alone)).
Limitations
The impact of pharmacotherapy management cannot be separated from the provision of free pharmacotherapy. Cessation was validated in only 58% of self-reported quitters.
Conclusion
Smokers are willing to make repeated pharmacotherapy-assisted quit attempts leading to progressively greater smoking abstinence. Although point-prevalence abstinence was not different at 24 months, analyses that incorporated assessments across the full 24 months of treatment suggest that higher intensity disease management is associated with increased abstinence.
Introduction
Cigarette smoking is a chronic illness characterized by repeated cycles of quit attempts and relapse. Most models for addressing smoking cessation are based on single, short-term interventions lasting only a few weeks or months (1). Although most smokers will not quit after a single intervention, few studies have addressed the chronic nature of nicotine dependence by providing systematic, repetitive treatment opportunities (1). The reach of current smoking cessation interventions is further limited by providing treatment only to smokers that are already prepared to quit (2). New models of chronic disease care might provide an alternative approach for expanding the reach and effectiveness of smoking cessation efforts (3).
Physicians are in direct contact with approximately 70% of smokers each year (4, 5). The potential role of physicians in promoting smoking cessation has been well delineated and incorporated into current clinical practice guidelines (1). With the development of new, more effective prescription pharmacotherapy for smoking cessation, the role of primary care practices in promoting smoking cessation is now more important than ever. Unfortunately, only half of smokers seeing their physicians are asked about their smoking (6), fewer receive advice from their health care provider to quit, and only a small subset receive pharmacotherapy or follow-up (4, 7). Smoking cessation counseling competes with other pressing clinical tasks, and beyond brief advice, many physicians feel they are too busy to routinely and repeatedly counsel patients who smoke (8–10).
To assist primary care physicians in the treatment of rural smokers, we developed KanQuit, a smoking cessation program based upon the Chronic Care Model (4) that integrates principles of disease management into the treatment of smokers seen in rural primary care. Our objective was to enroll smokers, regardless of their willingness to quit, into a disease registry and to compare cessation rates among smokers receiving pharmacotherapy management alone or when combined with either moderate-intensity or high-intensity disease management that includes counseling and provider feedback.
METHODS
Design Overview
This was a randomized, single-blinded trial of varying levels of disease management for smoking cessation. Patients smoking > 10 cigarettes/day were recruited from rural primary care clinics across the state of Kansas and randomly assigned to receive pharmacotherapy management alone, pharmacotherapy management supplemented by 1–2 counseling calls every 6 months (moderate-intensity disease management), or pharmacotherapy management supplemented by up to 6 counseling calls every 6 months (high-intensity disease management). For recipients of moderate-intensity and high-intensity disease management, periodic progress reports were faxed to the patient’s physician. All participants were offered free pharmacotherapy (either bupropion or transdermal nicotine patch) every 6 months. Participants were enrolled between June 2004 – October 2005 and followed for 24 months with follow-up completed in December 2007. All participants provided written, informed consent. The study was approved by the University of Kansas Medical Center’s Human Subjects Committee.
Setting and Participants
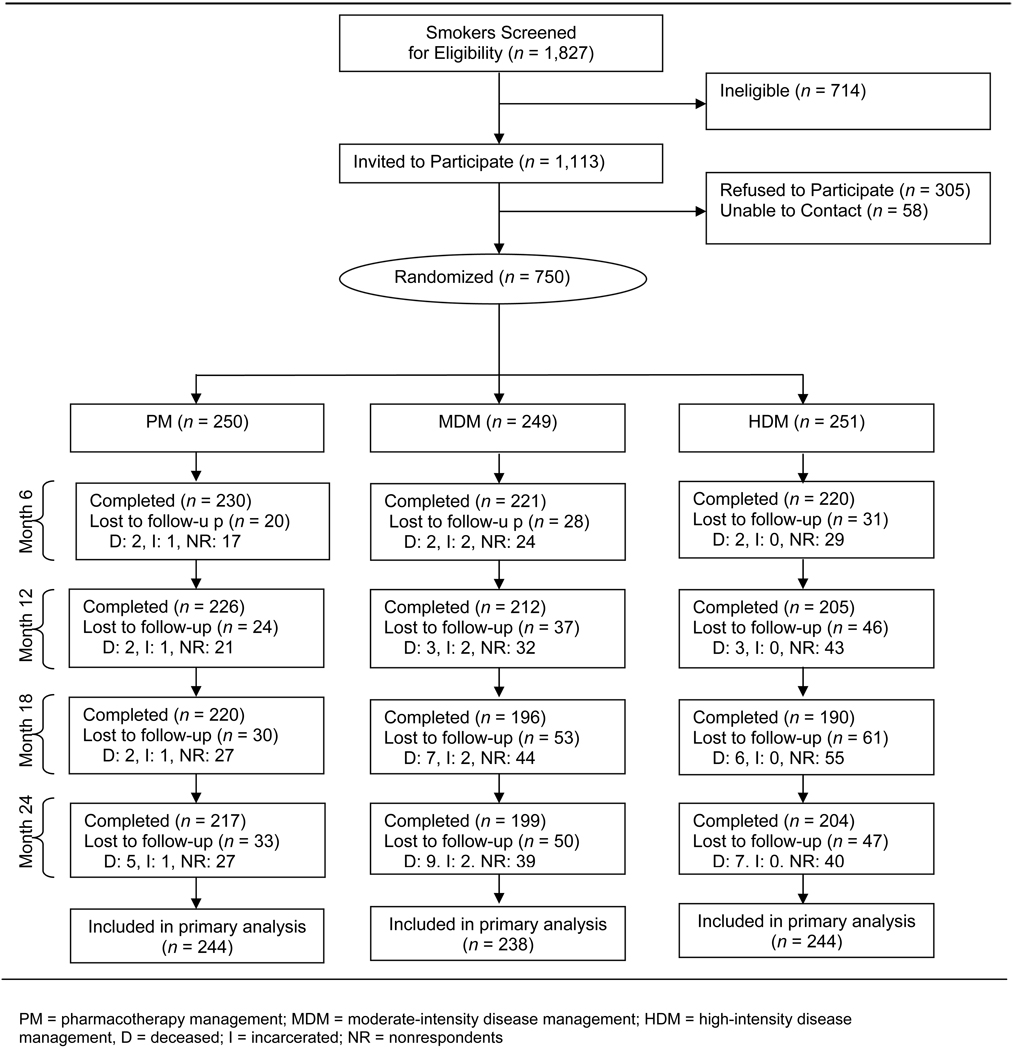
This study was conducted in 50 rural primary care practices in the Kansas Physicians Engaged in Prevention Research (KPEPR) network (11). As part of a rural primary care research experience, trained medical students systematically screened patients, identified smokers, and recruited them for this study, regardless of their interest in quitting (12). Smokers were eligible if they had a primary care physician participating in this study, and they were ≥ 18 years of age, smoked ≥ 10 cigarettes/day, smoked for at least one year, smoked at least 25 out of the past 30 days, spoke English, and had a telephone. Smokers were excluded if they were pregnant or planning to become pregnant, were planning to move out of the study area, displayed signs of dementia or mental illness that would preclude participation in the study, or lived with a smoker already enrolled in the study. Of 1,827 smokers screened, 61% met criteria for study entry (Figure 1); of these, 67% enrolled.
Figure 1.
Study flow diagram
Randomization and Interventions
Participant randomization
Randomization occurred at the patient level. A computer generated random numbers table was utilized to generate allocation cards in blocks of 24 with allocation equally distributed across treatment groups. To conceal allocation, these cards were placed in sequentially numbered, opaque, sealed envelopes. After research assistants verified patient eligibility and completed the baseline assessment, the project director opened the next sequential sealed envelope and determined the patient’s treatment allocation.
All interventions were conducted from a single central site by one of 9 counselors trained in smoking cessation and motivational interviewing (12). Participants were assigned to counselors without regard to practice site.
Pharmacotherapy Management
At baseline, all smokers received a health education mailing consisting of a welcome letter, information about the use of bupropion and the nicotine patch for smoking cessation, and copies of: You Can Quit Smoking (13) and When Smokers Quit (14). At months 0, 6, 12, and 18, participants also received a mailed offer for free pharmacotherapy consisting of either a 6-week course of 21 mg/day nicotine patch or a 7 week course of bupropion SR (150 mg twice daily). Participants interested in using either medication could return a postage-paid postcard or call a toll-free number. All participants requesting pharmacotherapy were screened for potential contraindications (15). Participants with absolute contraindications for a given drug were ineligible to receive that drug but were offered the option of receiving the other drug. Participants with contraindications to both drugs were not eligible to receive medication from the study, but could participate in all other aspects of the intervention. For all participants requesting bupropion and for participants with relative contraindications to the nicotine patch, research staff faxed a prescription request to the participant’s primary care physician. This prescription request delineated any relative contraindications or potential drug interactions. For these participants, their physician made the final assessment of the appropriateness of the bupropion or the patch. For participants without contraindications to the nicotine patch or upon receipt of a faxed, signed prescription, the bupropion or patches were mailed to the participant along with instructions for use.
Disease Management
In addition to the pharmacotherapy management, the moderate-intensity and high-intensity disease management groups received educational support, telephone counseling, and periodic progress reports with counseling suggestions faxed to their physician. Every 6 months they received a KanQuit newsletter addressing tips on quitting smoking, talking with their doctor about smoking, and using pharmacotherapy for cessation. The newsletters were personalized to include study updates, counselor photographs, physician feature stories, and testimonials of participants who had quit smoking.
Moderate-intensity disease management participants were offered up to 2 telephone-based counseling sessions every 6 months (one session to promote a quit attempt and one additional follow-up session for those who made a quit attempt). Participants in high-intensity disease management were offered up to 6 counseling calls every 6 months to either promote quitting or to prevent relapse. Calls were scheduled at the participant’s convenience and varied according to the participant’s quit plan, but followed a rough schedule of calls at 1, 3, 6, 9, and 16 weeks after the onset of each 6-month treatment cycle. Counselors utilized motivational interviewing techniques following a semi-structured protocol to promote a cessation attempt or, for abstinent smokers, to encourage relapse prevention. During counseling calls, case managers reminded participants about the availability of pharmacotherapy and, for interested participants, provided immediate support for acquiring either the nicotine patch or bupropion as described above.
Personalized progress reports with suggestions for interventions were faxed to the participant’s physician after the first counseling call (both moderate-intensity and high-intensity disease management participants) and after the last counseling call (high-intensity disease management participants only) during each 6-month cycle of the study. Additional progress reports were faxed to the participant’s physician whenever the moderate-intensity or high-intensity participant set a quit date.
Outcomes, Measurements, and Follow-up
Research assistants, blinded to treatment group assignment, conducted assessments via telephone at baseline, 6, 12, 18, and 24 months.
Primary Outcome
The primary outcome measure was self-reported 7-day abstinence at 24-months defined as not having smoked a cigarette during the prior 7 days. Although self-reported abstinence has been considered sufficient for population-based smoking cessation studies (16), to test for a reporting bias between treatment groups, we validated self-reported abstinence at months 12 and 24 by mailed salivary cotinine analysis (< 15 ng/ml) (17). Due to high resistance of participants to providing salivary samples at month 12, we also conducted validation via proxy report from a significant other at month 24 for quitters who did not return a salivary sample (18).
Secondary Outcomes
Secondary outcomes included self-reported 7-day abstinence at 6, 12, and 18 months. We assessed utilization of pharmacotherapy based on whether or not the participant requested bupropion or the transdermal nicotine patch during any given 6-month treatment cycle. At the conclusion of each 6-month cycle, we also asked participants if they had seen their physician in the prior 6 months and, if so, if they had discussed smoking cessation.
Other Measures
At baseline, we assessed age, gender, education level, and major comorbid conditions. Smoking history included number of cigarettes smoked per day, previous bupropion use, previous nicotine replacement use, and stage of readiness to stop smoking (19). We assessed nicotine dependence using the Fagerström Test for Nicotine Dependence (20). Importance and confidence in quitting were assessed separately using an 11-point Likert scale ranging from no importance or confidence (0) to extreme importance or confidence (10).
Monitoring Procedures
The Data Safety and Monitoring Plan was approved by the Kansas University Medical Center’s Institutional Review Board. Although we did not explicitly ask participants about adverse effects, spontaneous reports of adverse events were recorded by counselors during intervention calls and by research assistants during semi-annual assessments and reported to the Kansas University Medical Center Human Subjects Committee. Data quality monitoring efforts included dual data entry, examination of frequency distributions and range checks, and identification and verification of missing values.
Statistical Analysis
Power calculations indicated that 250 participants per arm would have 80% power to compare high-intensity disease management versus moderate-intensity disease management and 95% power to compare the combined high-intensity disease management and moderate-intensity disease management versus pharmacotherapy management based on self-reported quit rates of 10%, 15%, and 25% for pharmacotherapy management, moderate-intensity disease management, and high-intensity disease management, respectively.
All data analyses (except where specified) were conducted using SAS version 9.1, SAS Institute Inc., Cary, North Carolina. Descriptive statistics for baseline measures were generated to assess imbalance across treatment arms. For the primary endpoint, self-reported abstinence at 24 months, overall (0 to 24 months) self-reported abstinence, pharmacotherapy use, and patient/physician discussions about smoking, we used generalized linear mixed models (GLMMs) (21). The two primary tests of the KanQuit study were comparisons of self-reported cessation rates between high-intensity disease management versus moderate-intensity disease management and high-intensity disease management and moderate-intensity disease management combined versus pharmacotherapy management alone. In addition, we considered two sensitivity analyses that (1) imputed missing data as smokers and (2) imputed missing data as quit. GLMMs included terms for main effects of treatment arm and time and their interaction. Models were fit using PROC NLMIXED. For the sensitivity analyses, data were not imputed for participants known to have expired or been incarcerated. We averaged our GLMM estimates over the subject random effects to arrive at marginal estimates of the effects of intervention (22) (21). Validated abstinence at12 and 24 months were compared using unconditional logistic regression models.
Although the intervention was administered from a central location, we assessed for clustering of treatment effects with the participating primary care practices. A GLMM was used to test for this clustering using a logistic regression model with a random intercept term to allow for facility effects (21). Based on the 24 month outcome, we found no evidence of a clustering effect (23).
We computed costs from a provider perspective, i.e., including only direct, variable costs associated with executing each intervention. Principal intervention costs were associated with counseling and pharmacotherapy. Counselor time and time associated with pharmacotherapy management were recorded through a computerized tracking log and valued at local hourly wages plus fringe. Pharmacotherapy was valued at prevailing on-line prices plus mailing costs. Telephone and FAX charges were valued at long-distance rates. Costs were valued in 2005 dollars to reflect when the intervention began.
Role of the funding source
This study was funded by the National Cancer Institute (R01-101963). GlaxoSmithKline provided study medication. The funding sources were not involved in the design, conduct or analysis of this study or the decision to submit the study for publication.
RESULTS
Randomization resulted in groups with similar baseline characteristics (Table 1). In addition to smoking, 427 participants (57%) had at least one other major risk factor for cardiovascular disease. Participants reported seeing their physician a median of 3.5 times in the twelve months prior to the study. Participants smoked on average 24 cigarettes per day; 30.4% were at the preparation stage of quitting, 60.9% at the contemplation stage, and 8.7% at the precontemplation stage.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Total (n = 750) |
PM (n = 250) |
MDM (n = 249) |
HDM (n = 251) |
|---|---|---|---|---|
| Age, mean (SD), y | 47.2 (13.1) | 47.1 (13.4) | 48.2 (12.4) | 46.4 (13.5) |
| Female, No. (%) | 439 (58.5) | 144 (57.6) | 144 (57.8) | 151 (60.2) |
| ≤High school graduate, No. (%) | 385 (51.3) | 128 (51.2) | 129 (51.8) | 128 (51.0) |
| No. of cigarettes smoked per day, mean (SD) | 23.7 (10.4) | 24.3 (11.0) | 23.8 (10.3) | 22.9 (10.0) |
| Fagerström score, mean (SD)* | 5.2 (2.2) | 5.2 (2.2) | 5.2 (2.2) | 5.0 (2.1) |
| Previous use of bupropion, No. (%) | 245 (32.7) | 83 (33.2) | 74 (29.7) | 88 (35.1) |
| Previous use of nicotine replacement, No. (%) | 397 (52.9) | 128 (51.2) | 128 (51.4) | 141 (56.2) |
| Other smokers in household, No. (%) | 345 (46.0) | 119 (47.6) | 116 (46.6) | 110 (43.8) |
| Motivation to quit, mean (SD)† | 8.7 (2.1) | 8.7 (2.0) | 8.6 (2.2) | 8.6 (2.0) |
| Confidence to quit, mean (SD)† | 6.1 (2.7) | 5.9 (2.7) | 6.1 (2.8) | 6.3 (2.6) |
| Precontemplation stage of change, No. (%) | 65 (8.7) | 22 (8.8 | 20 (8.0) | 23 (9.2) |
| Contemplation stage of change, No. (%) | 457 (60.9) | 158 (63.2) | 153 (61.5) | 146 (58.2) |
| Preparation stage of change, No. (%) | 228 (30.4) | 70 (28.0) | 76 (30.5) | 82 (32.7) |
| Hypertension, No. (%) | 258 (34.4) | 95 (38.0) | 78 (31.3) | 85 (33.9) |
| Hyperlipidemia, No. (%) | 269 (35.9) | 91 (36.4) | 86 (34.5) | 92 (36.7) |
| Diabetes, No. (%) | 101 (13.5) | 43 (17.2) | 30 (12.1) | 28 (11.2) |
| Chronic lung disease, No. (%) | 202 (26.9) | 65 (26.0) | 73 (29.3) | 64 (25.5) |
| Heart disease, No. (%) | 73 (9.7) | 24 (9.6) | 29 (11.7) | 20 (8.0) |
| History of depression, No. (%) | 304 (40.5) | 107 (42.8) | 96 (38.6) | 101 (40.2) |
PM = pharmacotherapy management; MDM = moderate-intensity disease management; HDM = high-intensity disease management.
Fagerström test score for nicotine dependence ranges from 0 to 10. Scores of 6 or higher indicate greater levels of nicotine dependence.
Motivation and confidence to quit smoking scores range from 0 to 10
Prior to the 24 month follow-up, 24 participants died or were incarcerated. Of the 750 participants, loss to follow-up due to non-response varied by treatment group (Figure 1) with 22.0% of the pharmacotherapy management group, 31.3% of the moderate-intensity disease management group, and 31.1% of the high-intensity disease management group lost to follow-up during one or more of the assessment periods (p = 0.03).
Utilization of counseling
During the course of the 24 month intervention, high-intensity disease management participants completed an average of 8.2 counseling calls (range 0 –24) and moderate-intensity disease management participants completed an average of 3.6 calls (range 0 – 7). Engagement in counseling declined during the course of the intervention with 90.9%, 67.9%, 57.3%, and 54.3% of high-intensity disease management participants participating in at least one counseling session, and 90.0%, 68.2%, 60.3%, and 59.2% of moderate-intensity disease management participants participating in at least one counseling session during the 1st, 2nd, 3rd, and 4th cycles of treatment, respectively, (Table 2). The average number of calls completed was 3.2, 1.8, 1.6, and 1.7 for high-intensity disease management recipients and 1.3, 0.9, 0.7, and 0.7 for moderate-intensity recipients during the 1st, 2nd, 3rd, and 4th cycles of treatment, respectively.
Table 2.
Number of Counseling Calls Completed Among Recipients of Moderate-Intensity Disease Management (MDM), or High-Intensity Disease Management (HDM)
| Number of completed counseling calls - n(%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| n* | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Cycle 1 | ||||||||
| MDM | 249 | 25 (10.0) | 133 (53.4) | 91 (36.5) | --- | --- | --- | --- |
| HDM | 251 | 23 (9.1) | 34 (13.5) | 42 (16.7) | 37 (14.7) | 44 (17.5) | 40 (15.9) | 31 (12.3) |
| Cycle 2 | ||||||||
| MDM | 245 | 78 (31.8) | 123 (50.2) | 44 (17.9) | --- | --- | --- | --- |
| HDM | 249 | 80 (32.1) | 54 (21.6) | 27 (10.8) | 41 (16.4) | 21 (8.4) | 20 (8.0) | 6 (2.4) |
| Cycle 3 | ||||||||
| MDM | 244 | 97 (39.7) | 120 (49.1) | 27 (11.1) | --- | --- | --- | --- |
| HDM | 248 | 106 (42.7) | 38 (15.3) | 27 (10.8) | 35 (14.1) | 29 (11.6) | 6 (2.4) | 7 (2.8) |
| Cycle 4 | ||||||||
| MDM | 240 | 98 (40.8) | 112 (46.6) | 30 (12.5) | --- | --- | --- | --- |
| HDM | 245 | 112 (45.7) | 29 (11.8) | 27 (11.0) | 22 (8.9) | 20 (8.1) | 21 (8.5) | 14 (5.7) |
Cycle 1 = months 0–6; Cycle 2 = months 6–12; Cycle 3 = months 12–18; Cycle 4 = months 18–24.
The n changes over time due to the drop out of ‘deceased’ and ‘incarcerated’ participants
Utilization of pharmacotherapy
We identified a treatment-by-time interaction in utilization of pharmacotherapy (likelihood ratio chi-square tests df=6, p < 0.01). Thus, comparisons between treatment groups in use of pharmacotherapy were derived at each survey period. These results (Table 3) found differences in pharmacotherapy uptake in the pooled disease management arms compared to pharmacotherapy management, with an increase in uptake during the first 6-month period (OR,1.53 [95% CI, 1.10 to 2.13]), but lower uptake in the disease management arms during the following 6-month period (OR, 0.71 [95% CI, 0.51 to 0.99]). Requests for pharmacotherapy declined following the first 6-month study period. Among all participants, 63.8%, 40.9%, 23.9%, and 24.7% requested pharmacotherapy during the 1st, 2nd, 3rd, and 4th cycles of treatment, respectively. By the conclusion of the study, 23%, 33%, 23%, 12%, and 9% of participants had requested a total of 0, 1, 2, 3, or 4 cycles of pharmacotherapy, respectively. Overall, 41.1% of pharmacotherapy treatment cycles utilized bupropion and 58.9% utilized the nicotine patch with no difference in choice of pharmacotherapy between the treatment groups.
Table 3.
Utilization of Pharmacotherapy (nicotine patch or bupropion) by Treatment Period Among Recipients of Pharmacotherapy Management (PM), Moderate-Intensity Disease Management (MDM), or High-Intensity Disease Management (HDM)
| Patients n/n* (%) | Odds Ratio (95% CI)† | ||||
|---|---|---|---|---|---|
| Months | PM | MDM | HDM | HDM vs. MDM | (HDM or MDM) vs. PM |
| 0–6 | 142/ 247(57.5) | 160/245 (65.3) | 171/249 (68.7) | 1.19 (0.81–1.76) | 1.53 (1.10–2.13) |
| 6–12 | 114/247 (46.2) | 93/244 (38.1) | 95/248 (38.3) | 1.01 (0.69–1.48) | 0.71 (0.51–0.99) |
| 12–18 | 65/247 (26.3) | 49/240 (20.4) | 61/245 (24.9) | 1.28 (0.83–1.96) | 0.82 (0.57–1.17) |
| 18–24 | 55/244 (22.5) | 65/238 (27.3) | 59/244 (24.2) | 0.84 (0.55–1.28) | 1.18 (0.82–1.70) |
The denominator changes over time due to the drop out of ‘deceased’ and ‘incarcerated’ participants.
Marginal odds ratios estimated from a GLMM, which indicated the presence of a treatment-by-time interaction (df=6, p<0.01).
Support from health care providers
In the three treatment groups combined, 635 (84.7%) of participants reported one or more office visits with their physician with a median of 5 visits over the course of the two year study. Of participants that saw a physician during any given treatment cycle, the proportion reporting that they discussed smoking cessation with their physician ranged from 37.5 – 59.5% (Table 4) and did not vary between treatment arms (OR, 1.03 for high-intensity versus moderate-intensity disease management [95% CI, 0.75 to 1.41] and OR, 0.92 for the pooled disease management groups versus pharmacotherapy management alone [95% CI, 0.70 to 1.19]) (Table 4).
Table 4.
Frequency of Patient/Physician Smoking Related Discussions by Treatment Cycle Among Recipients of Pharmacotherapy Management (PM), Moderate-Intensity Disease Management (MDM) and High-Intensity Disease Management*
| No. / Total seen by physician (%) | |||
|---|---|---|---|
| Months | PM | MDM | HDM |
| 0–6 | 97/171 (56.7) | 84/164 (51.2) | 94/158 (59.5) |
| 6–12 | 75/155 (48.4) | 70/148 (47.3) | 63/137 (46.0) |
| 12–18 | 67/156 (43.0) | 55/124 (44.4) | 54/130 (41.5) |
| 18–24 | 61/141 (43.3) | 51/136 (37.5) | 47/125 (37.6) |
GLMM for having a patient-physician smoking related discussion did not provide evidence for a treatment-by-time interaction (df=6, p=0.87). Estimated marginal odds ratios from the GLMM were 1.03 (95% CI 0.75 to 1.41) for HDM vs. MDM, and 0.92 (95% CI 0.70 to 1.19) for HDM and MDM vs. PM.
Smoking cessation
Throughout the study, smokers moved through different transitional states of smoking status (Table 5). In our primary, 24 month analysis, the7-day, point prevalence self-reported abstinence was 27.9% and 23.5% in the high-intensity and moderate-intensity disease management groups, respectively (OR, 1.33 [95% CI, 0.88 to 2.02]) (missing = smoking) (Table 6). The cessation rate in these disease management groups combined was similar to the 23.0% self-reported cessation rate in the pharmacotherapy management group (OR, 1.12 [95% CI, 0.78 to 1.61]). Sensitivity analysis showed these effects to be similar across other methods of handling missing data except in the case where non-response was imputed as quit.
Table 5.
Changes in Smoking Status of Participants Over Time Among Recipients of Pharmacotherapy Management (PM), Moderate-intensity Disease Management (MDM), and High-intensity Disease Management (HDM).
| Status at beginning of cycle |
PM, n (row %) | Status at Completion of the Cycle MDM, n (row %) |
HDM, n (row %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Smoke | Quit | NR | D/I | n | Smoke | Quit | NR | D/I | n | Smoke | Quit | NR | D/I | |
| Month 6 | |||||||||||||||
| Month 0 | |||||||||||||||
| Smoke | 250 | 204 (81.6) | 26 (10.4) | 17 (6.8) | 3 (1.2) | 249 | 185 (74.3) | 36 (14.5) | 24 (9.6) | 4 (1.6) | 251 | 179 (71.3) | 41 (16.3) | 29 (11.6) | 2 (0.8) |
| Month 12 | |||||||||||||||
| Month 6 | |||||||||||||||
| Smoke | 204 | 172 (84.3) | 21 (10.3) | 11 (5.4) | --- | 185 | 154 (83.2) | 16 (8.7) | 14 (7.6) | 1 (0.5) | 179 | 130 (72.6) | 27 (15.1) | 21 (11.7) | 1 (0.6) |
| Quit | 26 | 9 (34.6) | 16 (61.5) | 1 (3.9) | --- | 36 | 2 (5.6) | 33 (91.7) | 1 (2.8) | --- | 41 | 9 (22.0) | 31 (75.6) | 1 (2.4) | --- |
| NR | 17 | 7 (41.2) | 1 (5.9) | 9 (52.9) | --- | 24 | 7 (29.2) | --- | 17 (70.8) | --- | 29 | 6 (20.7) | 2 (6.9) | 21 (72.4) | --- |
| Month 18 | |||||||||||||||
| Month 12 | |||||||||||||||
| Smoke | 188 | 163 (86.7) | 15 (8.0) | 10 (5.3) | --- | 163 | 132 (81.0) | 10 (6.1) | 17 (10.4) | 4 (2.5) | 145 | 107 (73.8) | 22 (15.2) | 14 (9.7) | 2 (1.4) |
| Quit | 38 | 16 (42.1) | 18 (47.4) | 4 (10.5) | ---- | 49 | 15 (30.6) | 30 (61.2) | 4 (8.2) | --- | 60 | 14 (23.3) | 37 (61.7) | 8 (13.3) | 1 (1.7) |
| NR | 21 | 6 (28.6) | 2 (9.5) | 13 (61.9) | --- | 32 | 9 (28.1) | --- | 23 (71.9) | --- | 43 | 10 (23.3) | --- | 33 (76.7) | --- |
| Month 24 | |||||||||||||||
| Month 18 | |||||||||||||||
| Smoke | 185 | 151 (81.6) | 26 (14.1) | 7 (3.8) | 1 (0.5) | 156 | 120 (76.9) | 21 (13.5) | 13 (8.3) | 2 (1.3) | 131 | 109 (83.2) | 17 (13.0) | 5 (3.8) | --- |
| Quit | 35 | 4 (11.4) | 28 (80.0) | 1 (2.9) | 2 (5.7) | 40 | 8 (20.0) | 32 (80.0) | --- | --- | 59 | 13 (22.0) | 44 (74.6) | 1 (1.7) | 1 (1.7) |
| NR | 27 | 6 (22.2) | 2 (7.4) | 19 (70.4) | --- | 44 | 15 (34.1) | 3 (6.8) | 26 (59.1) | --- | 55 | 14 (25.5) | 7 (12.7) | 34 (61.8) | --- |
D/I = died or incarcerated; NR = nonrespondents
Table 6.
Self-Reported and Validated Abstinence Among Recipients of Pharmacotherapy Management (PM), Moderate-Intensity Disease Management (MDM) and High-Intensity Disease Management
| Table 6a 7-Day Point Prevalence Abstinence Self-Report and Validated* | |||
|---|---|---|---|
| Outcomes | PM | MDM | HDM |
| 7-Day Point Prevalence, N (%) | |||
| Month 6 | 26/247 (10.5) | 36/245 (14.7) | 41/249 (16.5) |
| Month 12 | 38/247 (15.4) | 49/244 (20.1) | 60/248 (24.2) |
| Month 18 | 35/247 (14.2) | 40/240 (16.7) | 59/245 (24.1) |
| Month 24 | 56/244 (23.0) | 56/238 (23.5) | 68/244 (27.9) |
| Validated 7-Day Point Prevalence, N (%) | |||
| Month 12† | 13/247 (5.3) | 24/244 (9.8) | 28/248 (11.3) |
| Month 24‡ | 33/244 (13.5) | 35/238 (14.7) | 36/244 (14.8) |
| Table 6b Statistical Comparisons | ||||
|---|---|---|---|---|
| HDM vs. MDM | (HDM & MDM) vs. PM | |||
| Analysis | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value |
| Point Prevalence Estimates | ||||
| 24 month, (no imputation; n = 708)§ | 1.31 (0.85–2.02) | 0.22 | 1.19 (0.82–1.73) | 0.35 |
| 24 month, (missing=smoker; n = 741)§ |
1.33 (0.88–2.02) | 0.18 | 1.12 (0.78–1.61) | 0.54 |
| 24 month, (missing=quit; n = 741)§ | 1.23 (0.84–1.66) | 0.30 | 1.42 (1.01–1.99) | 0.04 |
| 12 month validated (n = 643) | 1.24 (0.69–2.22) | 0.47 | 2.33 (1.24–4.38) | 0.01 |
| 24 month validated (n = 620) | 1.00 (0.60–1.68) | 0.99 | 1.19 (0.76–1.87) | 0.44 |
| Overall (0 to 24 months; no imputation; n = 708)§ |
1.43 (1.00–2.03) | 0.05 | 1.47 (1.08–2.00) | 0.02 |
All missing values were classified as smoking
Validated by salivary cotinine (< 15 ng/ml).
Validated by salivary cotinine (< 15 ng/ml) or significant other.
GLMM for self-reported abstinence did not provide evidence for a treatment-by-time interaction (df=6, p=0.31).
Saliva samples were not provided by 33.3%, 34.7%, and 31.6% of the self-reported quitters at 12 months and by 39.7%, 33.9%, and 30.3% at 24 months in the HDM, MDM and PM groups, respectively.
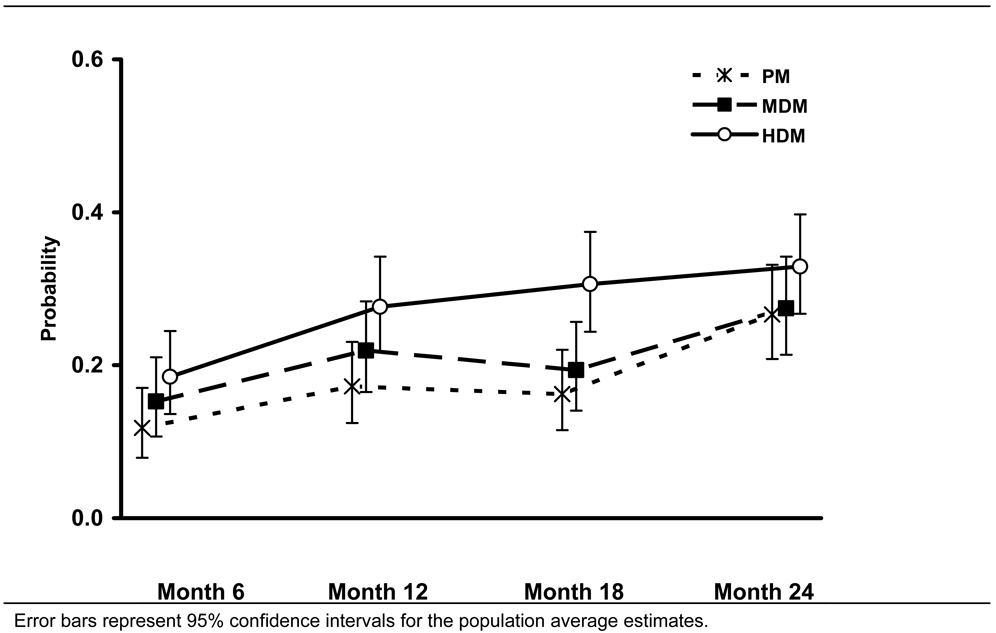
Secondary, overall (0 to 24 months) analyses, however, showed that self-reported abstinence rates were higher in the high-intensity than in the moderate-intensity disease management group over the course of the study (Table 6, Figure 2) (OR, 1.43 [95% CI, 1.00 to 2.03]) (no imputation)) and higher in the combined disease management groups than in the pharmacotherapy management group (Table 6, Figure 2) (OR, 1.47 [95% CI, 1.08 to 2.00] (no imputation)). Comparable findings were seen from the sensitivity analysis, except when all missing values were classified as smoking.
Figure 2.
Population-averaged Estimates of the Probability of Quitting Smoking Among Recipients of Pharmacotherapy Management (PM), Moderate-intensity Disease Management (MDM), or High Intensity Disease Management (HDM) (no imputation)
The rate of validation of self-reported abstinence was comparable in the three treatment groups at both 12 and 24 months, indicating that there was no apparent bias in self-reported abstinence associated with more intensive treatment. Cotinine-confirmed abstinence rates at 12 months were 11.3%, and 9.8% in the high-intensity and moderate intensity disease management groups, respectively (OR, 1.24 [95% CI, 0.69 to 2.22]) (Table 6). The cotinine-confirmed abstinence rate in the pooled disease management groups was higher than the 5.3% abstinence rate seen in the pharmacotherapy management group (OR, 2.33 [95% CI, 1.24 to 4.38]). Confirmed abstinence rates at 24 months, using either cotinine validation or proxy, were similar among the three treatment groups (Table 6).
Cost Analysis
There were no significant differences in pharmacotherapy costs across the three treatment groups ($209 per participant for pharmacotherapy management and moderate-intensity disease management and $225 for high-intensity disease management, p = 0.64). Over the course of the 24 month study, time devoted to pharmacotherapy and disease management was 0.5, 3.8, and 7.7 hours/participant in the pharmacotherapy management, moderate-intensity, and high-intensity disease management arms, respectively. These differences in counselor time led to significant differences between the three arms in total intervention costs per participant ($231 ± 222 for pharmacotherapy management, $348 ± 236 for moderate-intensity disease management, and $460 ± 289 for high-intensity disease management, p < 0.001).
DISCUSSION
This study demonstrates the feasibility of treating smoking as a chronic disease by following a group of smokers and offering them repeated interventions to support quit attempts and treat relapses. In KanQuit, a large proportion of rural smokers were willing to participate in a 24-month smoking cessation program. For these smokers, offers of free pharmacotherapy, accompanied by a modest effort to coordinate prescriptions through primary care physicians, resulted in high levels of pharmacotherapy utilization and repeated pharmacotherapy-assisted quit attempts in all three treatment groups. Although abstinence at 24 months was not different between the treatment groups, overall (0 to 24 months) data analyses suggest that more intensive disease management was associated with higher rates of smoking abstinence throughout the study period.
In KanQuit, more intensive counseling was not consistently associated with higher utilization of pharmacotherapy, and faxes to physicians that provided participant-specific counseling and treatment suggestions were not associated with more frequent discussions between doctor’s and participants about smoking cessation. Thus the impact of more intensive disease management on smoking cessation during the first 18 months of this intervention appears to be a direct result of the counseling itself and not mediated through greater utilization of pharmacotherapy or more frequent discussions between doctors and patients.
One of the most critical elements of a successful clinical intervention is the ability to reach the population at risk (2). While many interventions for smoking cessation have resulted in high abstinence rates, participation in these programs is often as low as 1–10% (24). Even if programs result in high smoking cessation rates (e.g. 30%), these programs may ultimately impact less than 3% of the total population of smokers. While free pharmacotherapy can substantially increase the number of smokers willing to make a quit attempt (25), passive offers of free pharmacotherapy such as those offered by state quitlines still only reach about 3% of eligible smokers (26, 27). In KanQuit, the creation of a ‘disease registry’ allowed enrollment of smokers into a smoking cessation intervention regardless of their immediate interest in quitting. Indeed, the high rates of participation in this study suggest that this approach could reach up to two-thirds of the smokers encountered in primary care practices. The proactive engagement of these smokers in KanQuit, even at the modest levels provided to the pharmacotherapy management group, was associated with utilization of pharmacotherapy by more than three-fourths of the smokers in the registry.
An English-language MEDLINE search through October 2008 reveals that only a few studies have followed smokers beyond 6–12 months and attempted to re-engage relapsed smokers in treatment. Although small early studies of recycling smokers were discouraging (28, 29), larger studies have demonstrated that active treatment of relapsed smokers with transdermal nicotine patch (30), nicotine lozenge (31), or bupropion (32) is associated with higher cessation rates. Our study extends these previous findings by showing progressively higher rates of abstinence among smokers that have been reengaged in treatment up to four times over a 2-year period.
Studies by Joseph et al and Fu et al have shown that the majority of smokers who fail a quit attempt are interested in trying again (33, 34). In a VA study by Partin et al, in which relapsed smokers received phone calls and their physicians received computerized reminders, 32% of the relapsed smokers took advantage of smoking cessation pharmacotherapy (35). Our study suggests that even more smokers can be engaged in pharmacological treatment for smoking cessation if proactive offers of treatment are repeated over time.
The strengths of this study include the randomized design, the population-based recruitment, the prolonged intervention and follow-up period, and the high rates of participant follow-up. This study is limited by the substantial variability in smoking cessation rates over time. The discrepancy between our overall (0–24 month) findings and 24-month point prevalence abstinence appears to be related to a large increase in abstinence reported by the pharmacotherapy management group between month 18 and 24. This sudden and unexplained increase in smoking cessation was not associated with increased use of pharmacotherapy or discussion of smoking cessation between patients and physicians. It might represent a delayed effect of the pharmacotherapy management intervention or it might be related to forces external to the study itself such as the release of varenicline in 2006 or new smoking restrictions in rural hospitals and rural communities. Nevertheless, it is hard to understand how these external forces would have uniquely affected the pharmacotherapy management group.
Additional limitations of this study include the lack of blinding of participants and the inability to isolate the effect of pharmacotherapy management from offers of free pharmacotherapy. Assessments were all conducted by telephone or mail, and we relied on mailed saliva samples for biochemical verification. Although we only validated self-reported smoking cessation in 58% of participants at 24 months, the rate of validation was similar in all three treatment arms suggesting that there was no bias in self-reported abstinence associated with treatment assignment. Our study relied on transdermal nicotine patch and bupropion; use of newer, more effective, agents might be associated with different results (36, 37). Finally, provision of free pharmacotherapy as part of this study limits the generalizability of these findings into current practice where financial support for pharmacotherapy is highly variable.
This study demonstrates that a disease management approach can reach a high proportion of smokers seen in primary care practice. Even smokers who are initially unwilling to quit are likely to engage in treatment during a 2 year follow-up period. In the presence of free pharmacotherapy and pharmacotherapy management, the majority of smokers will make one or more pharmacotherapy-assisted quit attempts. Although more intensive disease management was not associated with differences in smoking abstinence at 24 months, the overall (0 to 24 months) analyses illustrated an association between the intensity of the intervention and smoking cessation rates. To address this discrepancy, additional long-term studies on disease management for smoking cessation are needed.
Acknowledgment
The authors thank the research assistants and case managers Dr. Carla Berg, Genevieve Casey, Olivia Chang, Andrea Elyacher, Tresza Hutcheson, Dr. Shawn Jeffries, and Terri Tapp for their support with the design and implementation of this study. In addition, they thank Dr. Harry Lando, Division of Epidemiology and Community Health, University of Minnesota School of Public Health, for his scientific contributions to the study concept and design.
Grant Support: From the National Cancer Institute (R01- 101963). Study medication was provided by GlaxoSmithKline.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT00440115.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available, with institutional approval, from Dr. Ellerbeck (eellerbe@kumc.edu).
References
- 1.Fiore MCJC, Baker TB, et al. [Accessed May 28, 2008];Rockville, MD: US Dept of Health and Human Services; Treating Tobacco Use and Dependence: 2008 Update. 2008 May; http://www.ahrq.gov/path/tobacco.htm#Clinic.
- 2.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79(4):579–612. doi: 10.1111/1468-0009.00222. iv–v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Control CfD. Physician and other health-care professional counseling of smokers to quit--United States, 1991. MMWR. 1993;42(44):854–857. [PubMed] [Google Scholar]
- 5.Lopez-Quintero C, Crum RM, Neumark YD. Racial/ethnic disparities in report of physician-provided smoking cessation advice: analysis of the 2000 National Health Interview Survey. Am J Public Health. 2006;96(12):2235–2239. doi: 10.2105/AJPH.2005.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson MD, Laurent SL, Little JM., Jr Including smoking status as a new vital sign: it works! J Fam Pract. 1995;40(6):556–561. [PubMed] [Google Scholar]
- 7.Ellerbeck EF, Ahluwalia JS, Jolicoeur DG, Gladden J, Mosier MC. Direct observation of smoking cessation activities in primary care practice. J Fam Pract. 2001;50(8):688–693. [PubMed] [Google Scholar]
- 8.Jaen CR, Stange KC, Tumiel LM, Nutting P. Missed opportunities for prevention: smoking cessation counseling and the competing demands of practice. J Fam Pract. 1997;45(4):348–354. [PubMed] [Google Scholar]
- 9.Jaen CR, McIlvain H, Pol L, Phillips RL, Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859–863. [PubMed] [Google Scholar]
- 10.Holtrop JS, Malouin R, Weismantel D, Wadland WC. Clinician perceptions of factors influencing referrals to a smoking cessation program. BMC Fam Pract. 2008;9:18. doi: 10.1186/1471-2296-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KDHE. Primary care: Health professional underserved areas report. In: Health OLR, editor. Kansas Department of Health and Environment. Office of Local and Rural Health; 2005. [Google Scholar]
- 12.Cox LS, Cupertino AP, Mussulman LM, et al. Design and baseline characteristics from the KAN-QUIT disease management intervention for rural smokers in primary care. Prev Med. 2008;47(2):200–205. doi: 10.1016/j.ypmed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services; You can quit smoking consumer guideline. 2006
- 14.American Cancer Society I. When smokers quit. 2003. [Google Scholar]
- 15.Cupertino PA, Richter KP, Cox LS, et al. Smoking cessation pharmacotherapy preferences in rural primary care. Nicotine Tob Res. 2008;10(2):301–307. doi: 10.1080/14622200701825817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SRNT. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Richard RJ. Optimum cutoff points for biochemical validation of smoking status. Am J Public Health. 1988;78(5):574–575. doi: 10.2105/ajph.78.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Rennie DC, Dosman JA. The reliability of cigarette consumption reports by spousal proxies. Am J Public Health. 1995;85(12):1711–1712. doi: 10.2105/ajph.85.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59(2):295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 20.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 21.McCulloch CE, Searle SR. Generalized, linear, and mixed models. Chapter 8. New York: John Wiley & Sons; 2001. [Google Scholar]
- 22.Diggle P, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford, New York: Clarendon Press ;Oxford University Press; 1994. p. 142. [Google Scholar]
- 23.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 24.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med. 1998;339(10):673–679. doi: 10.1056/NEJM199809033391006. [DOI] [PubMed] [Google Scholar]
- 25.Miller N, Frieden TR, Liu SY, et al. Effectiveness of a large-scale distribution programme of free nicotine patches: a prospective evaluation. Lancet. 2005;365(9474):1849–1854. doi: 10.1016/S0140-6736(05)66615-9. [DOI] [PubMed] [Google Scholar]
- 26.Swartz SH, Cowan TM, Klayman JE, Welton MT, Leonard BA. Use and effectiveness of tobacco telephone counseling and nicotine therapy in Maine. Am J Prev Med. 2005;29(4):288–294. doi: 10.1016/j.amepre.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Cummings KM, Fix B, Celestino P, Carlin-Menter S, O'Connor R, Hyland A. Reach, efficacy, and cost-effectiveness of free nicotine medication giveaway programs. J Public Health Manag Pract. 2006;12(1):37–43. doi: 10.1097/00124784-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Tonnesen P, Mikkelsen K, Norregaard J, Jorgensen S. Recycling of hard-core smokers with nicotine nasal spray. Eur Respir J. 1996;9(8):1619–1623. doi: 10.1183/09031936.96.09081619. [DOI] [PubMed] [Google Scholar]
- 29.Tonnesen P, Norregaard J, Sawe U, Simonsen K. Recycling with nicotine patches in smoking cessation. Addiction. 1993;88(4):533–539. doi: 10.1111/j.1360-0443.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 30.Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311(7001):363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiffman S, Dresler CM, Rohay JM. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction. 2004;99(1):83–92. doi: 10.1111/j.1360-0443.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales DH, Nides MA, Ferry LH, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin Pharmacol Ther. 2001;69(6):438–444. doi: 10.1067/mcp.2001.115750. [DOI] [PubMed] [Google Scholar]
- 33.Joseph AM, Rice K, An LC, Mohiuddin A, Lando H. Recent quitters' interest in recycling and harm reduction. Nicotine Tob Res. 2004;6(6):1075–1077. doi: 10.1080/14622200412331324893. [DOI] [PubMed] [Google Scholar]
- 34.Fu SS, Partin MR, Snyder A, et al. Promoting repeat tobacco dependence treatment: are relapsed smokers interested? Am J Manag Care. 2006;12(4):235–243. [PubMed] [Google Scholar]
- 35.Partin MR, An LC, Nelson DB, et al. Randomized trial of an intervention to facilitate recycling for relapsed smokers. Am J Prev Med. 2006;31(4):293–299. doi: 10.1016/j.amepre.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 37.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]