Abstract
Phage-displayed random peptide libraries, in which high affinity phage peptides are enriched by repetitive selection (panning) on target antibody, provide a unique tool for identifying antigen specificity. This paper describes a new panning method that enables selection of peptides in 1 day as compared to about 6 days required in traditional panning to identify virus-specific epitopes. The method, termed ultra-fast selection of peptide (UFSP), utilizes phage produced by bacterial infection (phage amplification) directly for subsequent panning. Phage amplified in less than 1 h of infection in Escherichia coli are used for binding to target antibody pre-coated in the same wells of an ELISA plate, obviating the need for traditional large-scale amplification and purification. Importantly, phage elution at 37 °C was superior to that at room temperature, and phage amplification in a 150-μl volume of E. coli cells was superior to that in 250-μl volume. Application of UFSP to two monoclonal antibodies generated from clonally expanded plasma cells in subacute sclerosing panencephalitis (SSPE) brain identified high-affinity measles virus-specific-peptide epitopes. The UFSP panning methodology will expedite identification of peptides reacting with antibodies generated in other diseases of unknown antigenic specificity such as multiple sclerosis (MS), sarcoidosis and Behcet’s disease.
Keywords: Phage display, Random peptide library, Epitope, Mimotope, Measles virus
Phage-displayed random peptide libraries provide a unique approach to identify antigen specificity (Smith and Petrenko, 1997). The combinatorial nature of these libraries allows selection of target-specific peptides in an unbiased functional assay without preconceptions regarding the targets in disease (Mintz et al., 2003). Phage-displayed peptide libraries have been used successfully to map neutralizing antibodies to infectious agents such as the Puumala hantavirus (Heiskanen et al., 1999) and human immunodeficiency virus antigens (Ferrer et al., 1999), and to identify epitopes/mimotopes of measles virus using recombinant antibodies (rAbs) cloned from subacute sclerosing panencephalitis (SSPE) brain (Owens et al., 2006). This is important because in SSPE as well as other chronic central nervous system (CNS) infectious diseases, bands of oligoclonal IgG in the brain and cerebrospinal fluid (CSF) have been shown to be directed against the agent that causes disease, e.g., measles virus in SSPE (reviewed in Gilden, 2005). Multiple sclerosis (MS) is a chronic inflammatory CNS disease in which the agent/antigen against which the oligoclonal IgG is directed is unknown. Improved panning methods can potentially help to identify the unknown agent/antigen in various chronic inflammatory CNS disorders of unknown etiology, such as MS, sarcoidosis and Behcet’s disease.
Standard panning includes binding of phage-displayed random peptide libraries to antibodies, washing away unbound phage, and elution of specifically bound phage (the first pan). Eluted phage are then large-scale amplified by infecting Escherichia coli followed by purification and additional rounds of panning to enrich for specific peptides (Smith and Scott, 1993). Typically, three to five rounds are performed, a procedure that takes about 6 days.
This study describes a panning method, designated ultra-fast selection of peptides (UFSP), which utilizes phage that are quickly amplified in bacterial cultures in the presence of the selecting antibody for subsequent panning without phage purification. This brief E. coli infection/amplification step yields phage in amounts sufficient for repeated rounds of panning in the same day. The use of UFSP to pan two phage-displayed random peptide libraries on recombinant antibodies (rAbs) prepared from clonally expanded plasma cells from an SSPE brain (Owens et al., 2006) identified measles virus (MV)-specific peptide epitopes and mimotopes similar to those revealed by standard panning methods.
Two rAbs SSPE 2B4 and 3B were used. Details of rAb cloning, expression and IgG sequencing have been described (Burgoon et al., 1999, 2005; Owens et al., 2006).
For each panning experiment, four wells of a Reacti-Bind Protein A plate (Pierce) were coated with 50 μl of rAb (10 μg/ml) in TBS for 2 h at room temperature. Phage (2 × 1011) from the PhD.-12™ or PhD.-7™ phage-displayed-peptide libraries (New England BioLab) were added to the first well (for first pan) and incubated for 1 h at room temperature. After washing with TBST (0.05% Tween 20) 10 times for 2 min each time, bound phage were eluted with 50 μl of 0.2 M glycine (pH 2.2)/0.1% BSA for 10 min at room temperature or at 37 °C. In second pan, a mixture of 40 μl of eluted phage and 150 μl of E. coli 2738 cells (OD 0.5) was added to a second rAb-coated well and incubated at 37 °C for 50 min with shaking. Bound phage were washed 10 times and eluted as before. Two additional cycles of infection/amplification/binding were carried out (Fig. 1A). After each pan, 10–20 μl of phage eluate was plated on bacterial plates for titration analysis, and individual plaques from the titration plates were amplified in U96-Deepwell™ plates (NUNC) for determining phage specificity.
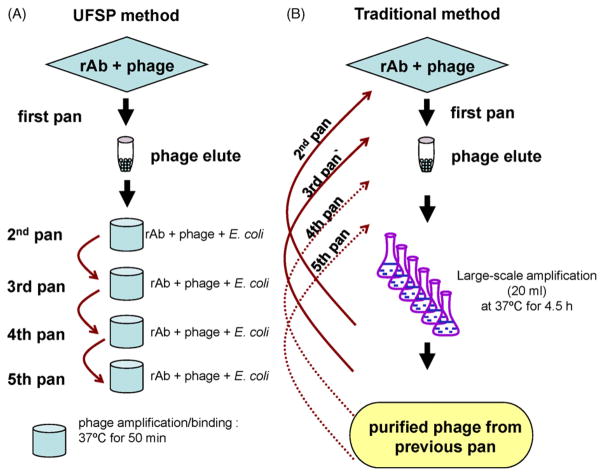
Fig. 1.
Comparison of UFSP to traditional method of phage panning. For the first pan in both methods, phage libraries are incubated with rAb-coated wells of Protein A plates, and bound phage are eluted with 0.2 M glycine buffer. UFSP: phage eluted from the first pan are used to infect mid-log phase E. coli in ELISA wells pre-coated with rAb for 50 min at 37 °C. Amplified phage that bind to rAb within the same well are eluted (second panning); a third pan reiterates the procedure by directly adding eluted phage from the second pan to wells pre-coated with rAb followed by incubation with phage-cell-rAb for 50 min. This procedure is repeated for subsequent pans as needed. Vigorous washing of unbound phage out of the ELISA wells was carried out during each pan. The complete panning takes only 1 day. Traditional panning: phage eluted from the first pan are amplified in large-scale 20 ml bacterial cultures for 4.5 h at 37 °C, and amplified phage are precipitated overnight at 4 °C, further purified and titered. For the second pan, amplified phage are added to wells coated with rAb and washed. Bound phage are eluted, amplified in large-scale cultures, purified and titered again for subsequent panning the next day. This procedure is repeated for subsequent pans as needed. The complete panning takes at least 6 days.
Phage eluted from each pan were mixed with 200 μl of E. coli 2738 cells and plated on LB top agar plates for overnight growth at 37 °C. Individual plaques were amplified in 500 μl of a 1:100 dilution of E. coli 2738 cells in U96-Deepwell plates (Yu et al., 2006a). For large-scale phage amplification, 5 μl of phage solution from 96-well amplification plates was added to 20 ml of a 1:100 dilution of overnight E. coli 2738 cells and incubated at 37 °C for 4.5 h followed by purification as described (Yu et al., 2006b).
For primary screening of phage peptides selected by panning, 50 μl of each phage amplified in U96-Deepwell plates was added to wells of ELISA plates coated with rAbs at 1 μg/ml (Yu et al., 2006a). Bound phage were detected after incubation with a 1:500 dilution of HRP-conjugated anti-M13 antibody for 1 h followed by color development in ABTS (Vector). Positive phage peptides were further confirmed by ELISA (in duplicate with a BSA negative control). Phage were considered positive when the ELISA OD value was at least three times that of the negative control. Dose responses of phage binding to rAbs were determined by adding serial 4-fold dilutions of purified phage to rAb-coated wells and detected as described above.
Single-stranded phage DNA was sequenced by the University of Colorado Cancer Center DNA Sequencing and Analysis Core (University of Colorado Denver, Aurora, CO). The deduced amino acid sequences were obtained and peptide sequences were identified (Yu et al., 2006b).
An ultra-fast method for panning phage peptides with rAbs was developed. Phage eluates after each round of panning were mixed with bacterial cells, and the mixture was added directly to microtiter plate wells pre-coated with panning rAb. After a short incubation time (50 min) that allowed phage to amplify and bind to rAb, bound phage were eluted and used for subsequent panning. Repeated rounds of panning were performed in the same day (Fig. 1A), obviating the large-scale amplification and purification used in traditional panning methods (Fig. 1B).
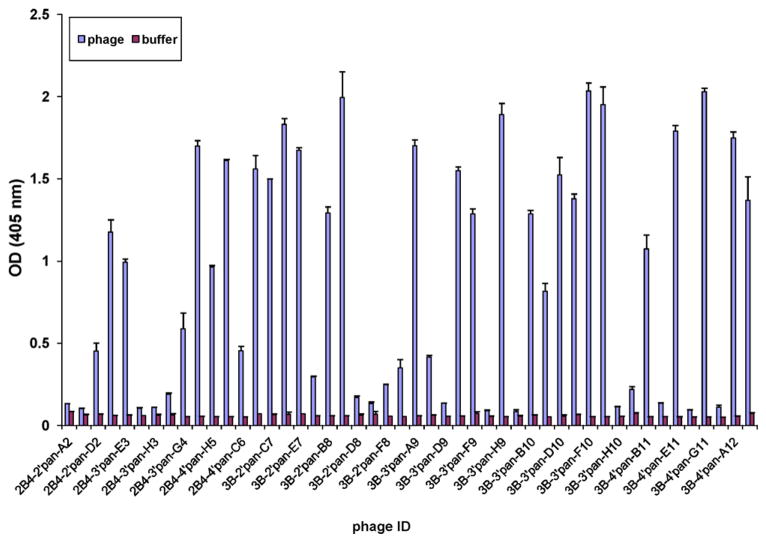
After four rounds of panning with two SSPE rAbs (2B4 and 3B), UFSP identified MV-specific peptides. Both rAb 2B4 and 3B selected positive phage clones after the second, third and fourth round of panning (Table 1). The highest rate of positive phage selection was 68.75% after the fourth pan with rAb 3B. Thirteen positive phage clones were identified by rAb 2B4 and 25 positives by rAb 3B (Fig. 2). Dose-dependent binding to rAb further confirmed the specificity of the selected phage clones (Fig. 3). In six to seven separate experiments, a phage elution temperature of 37 °C proved superior to elution at room temperature. In addition, amplification in a 150-μl volume of mid-log bacterial cells was superior to amplification in a 250-μl volume (Table 1).
Table 1.
Percentage of positive phage peptides identified by rAb 2B4 and 3B using different culture conditionsa.
| Second pan | Third pan | Fourth pan | |
|---|---|---|---|
| rAb 2B4 | |||
| Condition 1 | 6.25% | 0 | 4.50% |
| Condition 2 | 12.50% | 18.75% | 18.75% |
| rAb 3B | |||
| Condition 1 | 45.80% | 20.80% | 21% |
| Condition 2 | 37.50% | 62.50% | 68.75% |
After each pan, eluted phage were plated and phage plaques were amplified in U96-Deepwell plates. Phage were considered positive when the ELISA OD value was at least three times that of the negative control. Condition 1: 250 μl of E. coli cells used for amplification; elution at room temperature for 20 min. Condition 2: 150 μl of cells used for amplification; elution at 37 °C for 10 min.
Fig. 2.
Specific binding of phage peptides to two SSPE rAbs in ELISA. Individual phage plaques from the titration plates of second, third and fourth pans on either SSPE rAb were amplified in 500 μl of 1:100 dilutions of overnight E. coli 2738 cells for 4.5 h at 37 °C. Phage solutions of 50 μl (~5 × 108 pfu) were added to ELISA wells coated with panning rAb SSPE 2B4 or 3B. Bound phage were detected with HRP-conjugated anti-M13 antibody followed by ABTS substrate for color development. BSA-coated wells served as negative controls. Error bars represent standard deviation of each duplicate sample.
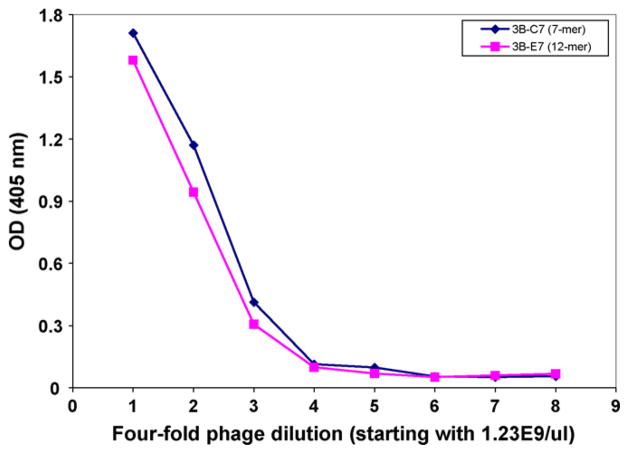
Fig. 3.
Dose response of purified phage panned by SSPE rAb 3B. Four-fold serial dilutions of large-scale purified phage 3B-C7 (selected from 7-mer library panning) and 3B-E7 (selected from 12-mer library panning) were added to rAb 3B-coated wells of ELISA plates; both phage peptides bound to rAb 3B in a dose-dependent manner, regardless of their peptide length.
Positive phage-peptide clones were purified and the peptides expressed were identified by DNA sequencing (Table 2). Peptides identified by either rAb 2B4 or 3B shared sequence homology with the MV N protein (data not shown). Peptide sequences obtained were similar to those identified by traditional panning methods (Owens et al., 2006). Use of rAb 2B4 identified 11 unique peptide sequences (Table 2). The first consensus sequence domain STWYD/EW (a MV mimotope) was identical to that previously reported; peptide sequence ISSPDMLLRAQA is similar to a MV epitope previously identified (NXLLRXQA) and the MV N sequence (amino acid: 491–500, MV Edmonston strain) (Owens et al., 2006). rAb 3B identified 22 unique sequences, of which all 7-mer peptides shared the consensus motif YNDXSLL previously described (Owens et al., 2006). The peptides selected by rAbs 2B4 and 3B were aligned as shown in Table 3. Peptide HTQPYAYEARDH, which aligns with the MV N protein, had not been previously identified (Table 2). UFSP using the two rAbs not only took less time, but also enriched equivalent amounts of phage-specific peptides compared to traditional panning (Tables 4 and 5).
Table 2.
Positive peptides identified by rAb 2B4 and 3Ba.
| Peptide panned by rAb 2B4 | Peptide panned by rAb 3B | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | S | N | W | I | T | W | Q | P | L | L | P | H | T | Q | P | Y | A | Y | E | A | R | D | H |
| S | T | S | P | W | V | Q | W | Q | P | M | R | H | T | Q | P | Y | A | Y | E | A | R | D | H |
| I | S | S | P | D | M | L | L | R | A | Q | A | H | T | Q | P | Y | A | Y | E | A | R | D | H |
| H | E | Q | P | H | W | L | L | W | Q | P | P | H | T | Q | P | Y | A | Y | E | A | R | D | H |
| S | S | T | W | N | T | W | Q | Y | F | P | L | T | E | T | P | Y | Y | T | D | Y | N | L | L |
| Q | A | T | W | Y | T | W | V | P | I | L | N | Q | N | P | T | A | L | L | |||||
| Q | A | T | W | Y | T | W | H | S | H | Q | V | F | N | L | L | S | L | L | |||||
| T | T | T | W | Y | S | W | T | Q | Y | T | A | Y | S | P | V | P | L | L | |||||
| T | T | T | W | Y | S | W | H | K | H | I | D | N | P | L | A | L | L | L | |||||
| K | T | L | W | W | Q | W | A | N | I | H | E | F | N | P | D | V | L | L | |||||
| T | S | T | W | L | Q | W | S | V | D | W | M | N | D | P | R | L | L | M | |||||
| T | V | T | W | W | H | F | F | H | P | Y | Y | N | P | I | G | L | L | H | |||||
| A | T | M | W | W | E | W | M | F | N | D | P | R | L | L | A | G | P | N | |||||
| H | N | D | P | L | L | L | |||||||||||||||||
| Y | N | Q | Q | S | L | L | |||||||||||||||||
| S | N | P | I | E | L | L | |||||||||||||||||
| Y | N | P | W | P | L | L | |||||||||||||||||
| S | N | P | L | P | L | L | |||||||||||||||||
| D | N | P | Q | H | L | L | |||||||||||||||||
| Y | N | N | P | G | L | L | |||||||||||||||||
| F | N | N | H | A | L | L | |||||||||||||||||
| F | N | S | L | T | L | L | |||||||||||||||||
| Y | N | P | T | Y | L | L | |||||||||||||||||
| W | N | N | T | A | L | L | |||||||||||||||||
| Y | N | M | P | A | L | L | |||||||||||||||||
After each pan beginning with second pan, phage DNA from each positive phage was purified and sequenced.
Table 3.
Multiple alignments of 7-mer peptides selected by rAb 2B4 and 3Ba.
| 2B4-3′pan-E3 | T | V | T | W | W | H | F | 7 |
| 2B4-4′pan-C6 | T | S | T | W | L | Q | W | 7 |
| 2B4-2′pan-F2 | T | T | T | W | Y | S | W | 7 |
| 2B4-4′pan-A6 | T | T | T | W | Y | S | W | 7 |
| 2B4-3′pan-H4 | Q | A | T | W | Y | T | W | 7 |
| 2B4-2′pan-D2 | K | T | L | W | W | Q | W | 7 |
| 2B4-4′pan-H5 | A | T | M | W | W | E | W | 7 |
| * | : | |||||||
| 3B-2′pan-D7 | S | N | P | I | E | L | L | 7 |
| 3B-3′pan-E9 | S | N | P | L | P | L | L | 7 |
| 3B-2′pan-E7 | Y | N | P | W | P | L | L | 7 |
| 3B-3′pan-F9 | D | N | P | Q | H | L | L | 7 |
| 3B-2′pan-C7 | Y | N | Q | Q | S | L | L | 7 |
| 3B-2′pan-B7 | H | N | D | P | L | L | L | 7 |
| 3B-2′pan-B8 | Y | N | N | P | G | L | L | 7 |
| 3B-2′pan-A9 | F | N | N | H | A | L | L | |
| * | * | * |
Symbol “*” indicates identical residues; symbol “:” indicates conserved residues. The consensus sequence contained the identical and conserved residues.
Table 4.
Panning output with rAb 2B4 using UFSP vs. traditional method.
| UFSP | Traditional | |
|---|---|---|
| Enrichment (3/1)a | 9.91E+04 | 5.6E+04 |
| Unique peptides | 11 | 13 |
| Measles virus epitopes | 1 | 1 |
| Time | 1 day | 6 days |
The ratio of output to input phage tiers after the third round of panning compared to the first panning.
Table 5.
Panning output with rAb 3B using UFSP vs. traditional method.
| UFSP | Traditional | |
|---|---|---|
| Enrichment (3/1)a | 8.25E+05 | 4.00E+05 |
| Unique peptides | 12 | 9 |
| Measles virus epitopes | 2 | 1 |
| Time | 1 day | 6 days |
The ratio of output to input phage tiers after the third round of panning compared to the first panning.
Typical panning of phage-displayed random peptide libraries includes binding of phage libraries to target antibody, removal of unbound phage by repeated washing, and elution of bound phage followed by large-scale amplification in bacterial cells and purification. Three to five rounds of screening are usually needed to enrich specific peptides. The most time-consuming part of peptide library panning is preparation of phage for subsequent panning rounds, which involves large-scale amplification, purification and titration of eluted phage. In the ultra-fast panning method, phage released after bacterial infection in less than 1 h are used directly as input phage for subsequent panning. Because input phage are produced in the presence of target antibody, this method eliminates time-consuming and expensive phage amplification, purification and titration.
UFSP using disease-relevant rAbs successfully panned two phage-peptide libraries that expressed 7-mer or 12-mer peptides and selected ELISA-positive peptides (Table 2). Positive phage peptides were identified by both rAbs from the second, third and fourth pan. Peptide sequences were similar to those identified by conventional panning methods (Owens et al., 2006). Importantly, optimization of conditions for infection/amplification and elution revealed that a total volume of 150 μl of E. coli cells for phage amplification and 37 °C for phage elution provided higher rates of positive phage selection (Table 2). The smaller volume of bacterial culture (150 μl) used to amplify eluted phage in rAb-coated wells likely enhances phage interaction with both cells and target antibody, while an incubation temperature of 37 °C with elution buffer may favor disruption of strong phage-antibody binding compared to incubation at room temperature.
Compared to conventional panning, UFSP is rapid (1 day), produces equivalent enrichment rates of positive peptides, and identifies similar numbers of unique high affinity peptides. The shorter amplification time and direct selection of released phage without purification is likely to reduce the growth competition of phage that may be encountered in larger-scale amplification method and may contribute to rapid selection of high affinity peptides. Others have found that phage-displayed peptides with good binding affinity may grow poorly and be overgrown by poor or non-specific binders (Rodi and Makowski, 1999; Wang and Yu, 2004). Elimination of large-scale phage amplification by using UFSP may also reduce the risk of contamination due to excessive numbers of phage amplification cycles and additional manipulation steps required in the conventional techniques. UFSP promises to replace standard panning methodologies by expediting identification of peptides reacting with antibodies generated in diseases such as multiple sclerosis in which antigenic specificities are not known.
Acknowledgments
This work was supported in part by Public Health Service Grants (Grant no. NS 32623) from the National Institutes of Health, and a research grant from the National Multiple Sclerosis Society (Grant no. RG 3934A1/1). We thank Marina Hoffman for editorial review and Cathy Allen for preparing the manuscript.
References
- Burgoon MP, Williamson RA, Owens GP, Ghausi O, Bastidas RB, Burton DR, Gilden DH. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles virus-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94:204–211. doi: 10.1016/s0165-5728(98)00243-4. [DOI] [PubMed] [Google Scholar]
- Burgoon MP, Keays KM, Owens GP, Ritchie AM, Rai PR, Cool CD, Gilden DH. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci USA. 2005;102:7245–7250. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M, Sullivan BJ, Godbout KL, Burke E, Stump HS, Godoy J, Golden A, Profy AT, van Schravendijk MR. Structural and functional characterization of an epitope in the conserved C-terminal region of HIV-1 gp120. J Pept Res. 1999;54:32–42. doi: 10.1034/j.1399-3011.1999.00082.x. [DOI] [PubMed] [Google Scholar]
- Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiskanen T, Lundkvist A, Soliymani R, Koivunen E, Vaheri A, Lankinen H. Phage-displayed peptides mimicking the discontinuous neutralization sites of puumala Hantavirus envelope glycoproteins. Virology. 1999;262:321–332. doi: 10.1006/viro.1999.9930. [DOI] [PubMed] [Google Scholar]
- Mintz PJ, Kim J, Do KA, Wang X, Zinner RG, Cristofanilli M, Arap MA, Hong WK, Troncoso P, Logothetis CJ, Pasqualini R, Arap W. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21:57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- Owens GP, Shearer AJ, Yu X, Ritchie AM, Keays KM, Bennett JL, Gilden DH, Burgoon MP. Screening random peptide libraries with subacute sclerosing panencephalitis brain-derived recombinant antibodies identifies multiple epitopes in the C-terminal region of the measles virus nucleocapsid protein 1. J Virol. 2006;80:12121–12130. doi: 10.1128/JVI.01704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodi DJ, Makowski L. Phage-display technology—finding a needle in a vast molecular haystack. Curr Opin Biotechnol. 1999;10:87–93. doi: 10.1016/s0958-1669(99)80016-0. [DOI] [PubMed] [Google Scholar]
- Smith GP, Scott JK. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- Smith GP, Petrenko VA. Phage display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Wang LF, Yu M. Epitope identification and discovery using phage display libraries: applications in vaccine development and diagnostics. Curr Drug Targets. 2004;5:1–15. doi: 10.2174/1389450043490668. [DOI] [PubMed] [Google Scholar]
- Yu X, Owens GP, Gilden DH. Rapid and efficient identification of epitopes/mimotopes from random peptide libraries. J Immunol Methods. 2006a;316:67–74. doi: 10.1016/j.jim.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yu X, Gilden DH, Ritchie AM, Burgoon MP, Keays KM, Owens GP. Specificity of recombinant antibodies generated from multiple sclerosis cerebrospinal fluid probed with a random peptide library. J Neuroimmunol. 2006b;172:121–131. doi: 10.1016/j.jneuroim.2005.11.010. [DOI] [PubMed] [Google Scholar]