Abstract
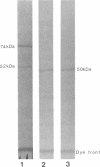
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease characterized by the presence of antimitochondrial antibodies in the serum. It is possible that the PBC-specific immunoreactive trypsin-sensitive antigens on the inner mitochondrial membrane, termed M2, are important in the pathogenesis of this autoimmune disease. We have previously shown that a major M2"a" antigen is the E2 component of the pyruvate dehydrogenase multienzyme complex located within mitochondria. Analysis of the primary structure of the E2 components of all three 2-oxo acid dehydrogenase complexes reveals a high degree of homology with a similar highly segmented structure including lipoyl domains, E3-binding domains, C-terminal catalytic domains, and interdomain linker sequences. Immunoblotting of PBC patients' sera against purified E2 protein from 2-oxoglutarate dehydrogenase complex and branched-chain 2-oxo acid dehydrogenase complex reveals that these polypeptides are also autoantigens in this disease. Sera from 29 of 40 (72.5%) PBC patients gave a positive response against bovine 2-oxoglutarate dehydrogenase complex E2 and from 25 of 40 (62.5%) PBC patients gave a positive response against bovine branched-chain 2-oxo acid dehydrogenase complex E2. All 40 PBC patients (100%) have autoantibodies directed against at least one of the E2 components of the family of 2-oxo acid dehydrogenase complexes. Identification of these M2 mitochondrial autoantigens and detailed knowledge of their structure will allow important questions concerning this autoimmune disease to be addressed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum H., Palmer C. The PBC-specific antigen. Mol Aspects Med. 1985;8(3):201–234. doi: 10.1016/0098-2997(85)90007-x. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R. Immunology of primary biliary cirrhosis. Baillieres Clin Gastroenterol. 1987 Jul;1(3):675–706. doi: 10.1016/0950-3528(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R., Lindenborn-Fotinos J. Antimitochondrial antibodies in primary biliary cirrhosis. J Hepatol. 1986;2(1):123–131. doi: 10.1016/s0168-8278(86)80015-0. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R. Molecular determination of the primary biliary cirrhosis-specific M2 antigen. Hepatology. 1988 Jan-Feb;8(1):200–201. doi: 10.1002/hep.1840080146. [DOI] [PubMed] [Google Scholar]
- Bismuth H., Castaing D., Ericzon B. G., Otte J. B., Rolles K., Ringe B., Sloof M. Hepatic transplantation in Europe. First Report of the European Liver Transplant Registry. Lancet. 1987 Sep 19;2(8560):674–676. doi: 10.1016/s0140-6736(87)92453-6. [DOI] [PubMed] [Google Scholar]
- Bleile D. M., Munk P., Oliver R. M., Reed L. J. Subunit structure of dihydrolipoyl transacetylase component of pyruvate dehydrogenase complex from Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4385–4389. doi: 10.1073/pnas.76.9.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A. P., Aitken A., Beg F., Cook K. G., Yeaman S. J. Amino acid sequence surrounding the lipoic acid cofactor of bovine kidney 2-oxoglutarate dehydrogenase complex. FEBS Lett. 1987 Sep 28;222(1):211–214. doi: 10.1016/0014-5793(87)80221-1. [DOI] [PubMed] [Google Scholar]
- Bradford A. P., Howell S., Aitken A., James L. A., Yeaman S. J. Primary structure around the lipoate-attachment site on the E2 component of bovine heart pyruvate dehydrogenase complex. Biochem J. 1987 Aug 1;245(3):919–922. doi: 10.1042/bj2450919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Bell R. L., Branchek T. Changes in order of migration of polypeptides in complex III and cytochrome C oxidase under different conditions of SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1977 Jan 24;74(2):425–433. doi: 10.1016/0006-291x(77)90321-7. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Reed L. J. Acyl group and electron pair relay system: a network of interacting lipoyl moieties in the pyruvate and alpha-ketoglutarate dehydrogenase complexes from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4223–4227. doi: 10.1073/pnas.74.10.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. G., Bradford A. P., Yeaman S. J. Resolution and reconstitution of bovine kidney branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1985 Feb 1;225(3):731–735. doi: 10.1042/bj2250731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcucci O., Lindsay J. G. Component X. An immunologically distinct polypeptide associated with mammalian pyruvate dehydrogenase multi-enzyme complex. Eur J Biochem. 1985 Jun 18;149(3):641–648. doi: 10.1111/j.1432-1033.1985.tb08972.x. [DOI] [PubMed] [Google Scholar]
- Doniach D., Roitt I. M., Walker J. G., Sherlock S. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other liver diseases and their clinical implications. Clin Exp Immunol. 1966 Jul;1(3):237–262. [PMC free article] [PubMed] [Google Scholar]
- Frazer I. H., Mackay I. R., Jordan T. W., Whittingham S., Marzuki S. Reactivity of anti-mitochondrial autoantibodies in primary biliary cirrhosis: definition of two novel mitochondrial polypeptide autoantigens. J Immunol. 1985 Sep;135(3):1739–1745. [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R., Sturgess A., Coppel R. L. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987 May 15;138(10):3525–3531. [PubMed] [Google Scholar]
- Guest J. R., Creaghan I. T. Gene-protein relationships of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: isolation and characterization of lipoamide dehydrogenase mutants. J Gen Microbiol. 1973 Mar;75(1):197–210. doi: 10.1099/00221287-75-1-197. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Lewis H. M., Graham L. D., Packman L. C., Perham R. N. Genetic reconstruction and functional analysis of the repeating lipoyl domains in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Mol Biol. 1985 Oct 20;185(4):743–754. doi: 10.1016/0022-2836(85)90059-2. [DOI] [PubMed] [Google Scholar]
- Hodgson J. A., De Marcucci O. G., Lindsay J. G. Lipoic acid is the site of substrate-dependent acetylation of component X in ox heart pyruvate dehydrogenase multienzyme complex. Eur J Biochem. 1986 Aug 1;158(3):595–600. doi: 10.1111/j.1432-1033.1986.tb09796.x. [DOI] [PubMed] [Google Scholar]
- Hodgson J. A., De Marcucci O. G., Lindsay J. G. Structure function studies on the lipoate-acetyltransferase--component-X-core assembly of the ox heart pyruvate dehydrogenase complex. Eur J Biochem. 1988 Feb 1;171(3):609–614. doi: 10.1111/j.1432-1033.1988.tb13831.x. [DOI] [PubMed] [Google Scholar]
- Hummel K. B., Litwer S., Bradford A. P., Aitken A., Danner D. J., Yeaman S. J. Nucleotide sequence of a cDNA for branched chain acyltransferase with analysis of the deduced protein structure. J Biol Chem. 1988 May 5;263(13):6165–6168. [PubMed] [Google Scholar]
- James O., Macklon A. F., Watson A. J. Primary biliary cirrhosis--a revised clinical spectrum. Lancet. 1981 Jun 13;1(8233):1278–1281. doi: 10.1016/s0140-6736(81)92457-0. [DOI] [PubMed] [Google Scholar]
- James S. P., Hoofnagle J. H., Strober W., Jones E. A. NIH conference: Primary biliary cirrhosis: a model autoimmune disease. Ann Intern Med. 1983 Oct;99(4):500–512. doi: 10.7326/0003-4819-99-4-500. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Primary biliary cirrhosis. N Engl J Med. 1987 Feb 26;316(9):521–528. doi: 10.1056/NEJM198702263160907. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Griffin T. A., Hu C. W., Chuang D. T. Conservation of primary structure in the lipoyl-bearing and dihydrolipoyl dehydrogenase binding domains of mammalian branched-chain alpha-keto acid dehydrogenase complex: molecular cloning of human and bovine transacylase (E2) cDNAs. Biochemistry. 1988 Mar 22;27(6):1972–1981. doi: 10.1021/bi00406a025. [DOI] [PubMed] [Google Scholar]
- Lawson R., Cook K. G., Yeaman S. J. Rapid purification of bovine kidney branched-chain 2-oxoacid dehydrogenase complex containing endogenous kinase activity. FEBS Lett. 1983 Jun 27;157(1):54–58. doi: 10.1016/0014-5793(83)81115-6. [DOI] [PubMed] [Google Scholar]
- Lindenborn-Fotinos J., Baum H., Berg P. A. Mitochondrial antibodies in primary biliary cirrhosis: species and nonspecies specific determinants of M2 antigen. Hepatology. 1985 Sep-Oct;5(5):763–769. doi: 10.1002/hep.1840050510. [DOI] [PubMed] [Google Scholar]
- Miles J. S., Guest J. R. Molecular genetic aspects of the citric acid cycle of Escherichia coli. Biochem Soc Symp. 1987;54:45–65. [PubMed] [Google Scholar]
- Munoz L. E., Thomas H. C., Scheuer P. J., Doniach D., Sherlock S. Is mitochondrial antibody diagnostic of primary biliary cirrhosis? Gut. 1981 Feb;22(2):136–140. doi: 10.1136/gut.22.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yamauchi T., Yamamoto I. Augmentation of the antibody response by lipoic acid in mice. II. Restoration of the antibody response in immunosuppressed mice. Jpn J Pharmacol. 1986 Oct;42(2):275–280. doi: 10.1254/jjp.42.275. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Packman L. C., Radford S. E. 2-Oxo acid dehydrogenase multi-enzyme complexes: in the beginning and halfway there. Biochem Soc Symp. 1987;54:67–81. [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Darlison M. G., Stephens P. E., Duckenfield I. K., Guest J. R. Nucleotide sequence of the sucB gene encoding the dihydrolipoamide succinyltransferase of Escherichia coli K12 and homology with the corresponding acetyltransferase. Eur J Biochem. 1984 Jun 1;141(2):361–374. doi: 10.1111/j.1432-1033.1984.tb08200.x. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley C. J., Perham R. N. Purification of 2-oxo acid dehydrogenase multienzyme complexes from ox heart by a new method. Biochem J. 1980 Oct 1;191(1):147–154. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. E., Darlison M. G., Lewis H. M., Guest J. R. The pyruvate dehydrogenase complex of Escherichia coli K12. Nucleotide sequence encoding the dihydrolipoamide acetyltransferase component. Eur J Biochem. 1983 Jul 1;133(3):481–489. doi: 10.1111/j.1432-1033.1983.tb07490.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H., Bleile D. M., Reed L. J. Lipoic acid content of dihydrolipoyl transacylases determined by isotope dilution analysis. Biochem Biophys Res Commun. 1980 May 14;94(1):78–84. doi: 10.1016/s0006-291x(80)80190-2. [DOI] [PubMed] [Google Scholar]
- Yamada G., Hyodo I., Tobe K., Mizuno M., Nishihara T., Kobayashi T., Nagashima H. Ultrastructural immunocytochemical analysis of lymphocytes infiltrating bile duct epithelia in primary biliary cirrhosis. Hepatology. 1986 May-Jun;6(3):385–391. doi: 10.1002/hep.1840060309. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Fussey S. P., Danner D. J., James O. F., Mutimer D. J., Bassendine M. F. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988 May 14;1(8594):1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]