Abstract
Purpose
Current physical activity guidelines are based in part on studies of cardiorespiratory fitness, however, the effects of fitness may differ from activity. Analyses were undertaken to determine the dose–response relationships of fitness to incident hypertension, hypercholesterolemia, and diabetes independent of activity.
Methods
Self-reported physician-diagnosed incident diabetes, hypercholesterolemia, and hypertension were compared to baseline running distance in 29,139 men and 11,985 women followed prospectively for 7.7 and 7.4 yr, respectively, and compared to cardiorespiratory fitness (m·s−1 10-km performance) in 85% of men and 76% of women.
Results
During follow up, 2342 men (8.53%) and 499 women (4.26%) became hypertensive, 3330 men (12.2%) and 599 women (5.14%) became hypercholesterolemic, and 197 men (0.68%) and 28 women (0.23%) became diabetic. Longer baseline distance predicted lower incident hypertension (men, P < 0.0001; women, P = 0.08), hypercholesterolemia (men and women, P < 0.0001), and diabetes (men, P < 0.001; women, P < 0.01) during follow up. The odds for hypercholesterolemia decreased significantly with each 16 km·wk−1 increment in distance through 64 km·wk−1 in men and 48 km·wk−1 in women. Higher baseline fitness predicted significantly lower odds for incident hypertension (men, P < 0.0001; women, P < 0.001), hypercholesterolemia (men, P < 0.0001; women, P < 0.01), and diabetes (men, P < 0.001; women, P < 0.01), independent of distance. Compared to the least fit men, the fittest men had 62% lower odds for becoming hypertensive, 67% lower odds for becoming hypercholesterolemic, and 86% lower odds for becoming diabetic. When adjusted for BMI, greater fitness predicted significantly lower odds for hypertension, hypercholesterolemia, and diabetes in men.
Conclusions
Higher cardiorespiratory fitness reduces the odds for hypertension, hypercholesterolemia, and diabetes, independent of physical activity and is an important risk factor separate from physical activity.
INTRODUCTION
Current public health guidelines for the prevention of dislipidemia [27], hypertension [9], diabetes [25], and premature death [13,28] all recommend moderate amounts of moderately intense physical activity. Thirty minutes of brisk walking on most days of the week is one way to meet the recommendations [9,13,25,27,28]. Although many of these guidelines acknowledge that additional health benefits may accrue for larger doses of more intense activity, the paucity of relevant data requires their being vague on the benefits accrued.
Prospective epidemiological studies of cardiorespiratory fitness, particularly those of the Aerobic Center Longitudinal Study [4,5], were important to the formulation of current guidelines. They suggested that being unfit (< 20th percentile) significantly increases the risks for morbidity and mortality. Their incorporation into guideline recommendations is based on the premises that cardiorespiratory fitness is a more objective measure of physical activity than those obtained from questionnaires or logs, and that the dose–response relationships between disease and fitness are more informative than those between disease and self-reported physical activity per se [4,5,28]. This interpretation warrants scrutiny because the reductions in cardiovascular and total mortality are greater when plotted against fitness than when against physical activity [32]. The presumed benefits of physical activity will be overestimated if cardiorespiratory fitness reduces cardiovascular disease risk independent of physical activity. Moreover, inappropriately ascribing the health benefits of fitness exclusively to physical activity could lead public health guidelines to undervalue fitness as an important risk factor and ignore its etiology.
Diabetes, hypertension, and hypercholesterolemia are all established risk factors for total mortality and cardiovascular disease [9,25,27]. We have shown in a large cross-sectional sample of 107,332 runners that use of antidiabetic, antihypertensive, and LDL-cholesterol lowering medications were inversely related to the dose of physical activity [39]. The prevalence of these medications showed an even stronger inverse relationship with cardiorespiratory fitness, which remained significant when adjusted for weekly training distance [39]. These associations are suggestive of an effect of cardiovascular fitness beyond being a more objective measure of physical activity than physical activity recall. Cardiorespiratory fitness may reflect the intensity of training or represent, in part, innate genetic differences between individuals or differences in the ability of individuals to benefit cardiovascularly from training, which may also be genetic [6,12,21,26]. However, the cross-sectional basis of these associations limit the ability to draw a causal inference between the dose of exercise, the level of fitness, and lower prevalence for hypertension, hypercholesterolemia, or diabetes. Proof of their causal relationship has been provided by randomized clinical trials, but because of expense and the difficulty of getting sedentary individuals to exercise, these trials have achieved only modest improvements in physical activity and fitness over reasonably short durations [28,29].
Prospective epidemiological cohort studies provide evidence consistent with causality by comparing risk factor levels at baseline to incidence of disease during follow up. The National Runners’ Health Study is unique among population cohorts in its focus on the health impact of higher doses of vigorous physical activity [30,31,35–39]. Data from this cohort are used to: (1) assess the dose–response relationship between cardiorespiratory fitness and incident hypertension, hypercholesterolemia, and diabetes during 7 yr of follow up; (2) assess the dose–response relationship of physical activity to these metabolic conditions; and (3) assess whether lower incident rates of hypertension, hypercholesterolemia, and diabetes with greater fitness are independent of physical activity. In previous reports [35,37], we demonstrated that sustained vigorous physical activity attenuates age-related weight gain. Thus, a fourth objective is to assess whether body weight may mediate, in part, the reductions in hypertension, hypercholesterolemia, and diabetes associated with physical activity or fitness.
METHODS
The design and methods of the National Runners’ Health Study are described elsewhere [30,31,35–40]. Briefly, a two-page questionnaire, distributed nationally at races and to subscribers of the nation’s largest running magazine (Runner’s World, Emmaus, PA), solicited information on demographics (age, race, education), running history (age when began running at least 12 miles per week, average weekly mileage, number of marathons run over the preceding 5 yr, and best marathon and 10-km times), weight history (greatest and current weight, weight when began running, least weight as a runner, circumferences of the chest, waist, and hips), diet (vegetarianism and the current weekly intakes of alcohol, red meat, fish, fruit, vitamin C, vitamin E, and aspirin), current and past cigarette use, prior history of heart attacks and cancer, and medications for blood pressure, thyroid conditions, cholesterol levels, or diabetes. Recruitment took place between 1991 and 1994 (primarily 1993) and follow-up between 1999 and 2002.
Approximately 15% of the runners targeted for recruitment at baseline consented to participate (there is some uncertainty about the number of baseline questionnaires actually delivered). Eighty percent of the 54,956 participants of the National Runners’ Health Study provided follow-up information or were deceased. The study protocol was approved by the Committee for the Protection of Human Subjects, and all participants signed committee-approved informed consents.
Height and weight were determined by asking the participant, “What is your current height (in inches, without shoes)?” and “What is your current weight (pre-pregnancy weight if pregnant)?” Running distances were reported in miles run per week and body weights in pounds, which were converted to kilometers and kilograms for this report. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. Previously, we reported strong correlations between repeated questionnaires for self-reported running distances (r = 0.89) [35], between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [35], and for self-reported running distances versus self-reported BMI and body circumferences in cross-sectional analyses [30].
Participants reported whether a physician had told them they had high blood pressure, high cholesterol, or diabetes since their baseline questionnaire, and whether they took medications for these conditions at baseline and follow up. Incident diabetes, hypercholesterolemia, and hypertension were defined as physician diagnosis or starting medications for these conditions since their baseline questionnaire. Self-reported hypertension and high cholesterol have been demonstrated by others as generally reliable using repeated surveys and confirmed diagnosis from medical records [10] and are reported by the Nurses’ Health Study [16] and other major cohort studies [24]. Running distances were reported in usual miles run per week. Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) show running represents (±SD) 91.5 ± 19.1% and 85.2 ± 24.0% of all vigorously intense activity in men and women, respectively, and 73.5 ± 23.7% and 69.4% ± 25.7% of total leisure time physical activity, respectively.
For this report, baseline cardiorespiratory fitness was defined as speed in meters per second (m·s−1) of the participant’s best 10-km race during the previous 5 yr (reported as finish time in minutes). Published data support the use of running performance to estimate maximal oxygen consumption (VO2max) [2,11,14]. Balke and Ware [2] initially reported a positive correlation between walk-run endurance performance and aerobic capacity when they suggested relating laboratory-determined VO2max to the distance covered in a given time period or to the time required to run a given distance. Hellerstein [14] accurately estimated the time in minutes to complete a marathon race from 70% of the VO2max and published energy costs of progressive running speeds. Cooper [11] showed a correlation of r = 0.90 between VO2max from a laboratory treadmill test and 12-min walk–run test for distance.
Statistical analyses
Logistic regression was used to estimate the dose–response relationships of incident hypertension, high cholesterol, and diabetes to distances run and cardiorespiratory fitness. Reported weekly intakes of alcohol, meat, fish, and fruit, along with follow-up duration, age, and BMI were used as covariates, with quadratic terms for age and BMI because of their nonlinear relationships to running distance and each other [40]. In addition, odds ratios are presented that compare distance and fitness intervals to all higher activity and fitness levels and to the least fit and least active runners. For the analyses presented, the odds ratios were essentially the same as relative risks (agreement within ± 0.02, analyses not displayed).
RESULTS
Eighty percent of the initial cohort returned questionnaires or were known deceased. From the 29,726 men and 12,222 women who had submitted complete data on age, running distance, diet, and BMI, we excluded 410 men and 199 women who smoked and 177 men and 38 women who had preexisting diabetes at baseline, leaving 29,139 men and 11,985 women for analyses. Our analyses of incident hypertension excluded an additional 1680 men and 260 women for preexisting hypertension, and our analyses of incident high cholesterol excluded an additional 1823 men and 334 women for preexisting high cholesterol. A fitness subset consisted of 24,517 men (85%) and 9057 women (76%) who at baseline provided their best recent 10-km performance time during the previous 5 yr.
During follow-up, 2342 men (8.53%) and 499 women (4.26%) became hypertensive, 3330 men (12.2%) and 599 women (5.14%) became hypercholesterolemic, and 197 men (0.68%) and 28 women (0.23%) became diabetic. Table 1 presents the baseline characteristics of the runners by diagnosis. All three diagnoses were associated with older age, longer follow-up duration, higher BMI, shorter running distance, and a slower 10-km performance. Table 2 shows that slower 10-km performance was associated with older age, shorter weekly running distance, and greater body weight. When adjusted for age and diet, 10-km performance was only moderately correlated with weekly running distance (males, r = 0.43; females, r = 0.40). These correlations were slightly weakened when adjusted for BMI in addition to age and diet (males, r = 0.36; females, r = 0.35). On average, each 1 km·wk−1 increase in running distance was associated with a 0.0095 ± 0.0001 m·s−1 increment in 10-km performance in men, and a 0.0090 ± 0.0002 m·s−1 increment in performance in women.
TABLE 1.
Baseline characteristics (means ± SD) of runners by self-reported physician-diagnosed hypertension, high cholesterol, and diabetes status.
| Hypertension | High Cholesterol | Diabetes | ||||
|---|---|---|---|---|---|---|
| Diagnosed | Not Diag. | Diagnosed | Not Diag. | Diagnosed | Not Diag. | |
| Men | ||||||
| Sample (N) | 2342 | 25118 | 3330 | 23987 | 197 | 28943 |
| Age (yr) | 48.5 ± 9.2§ | 44.1 ± 10.3 | 47.7 ± 9.1§ | 44.1 ± 10.5 | 48.3 ± 9.0§ | 44.9 ± 10.4 |
| Education (yr) | 16.5 ± 2.5 | 16.5 ± 2.4 | 16.5 ± 2.5 | 16.5 ± 2.4 | 16.1 ± 2.4* | 16.5 ± 2.4 |
| Follow-up duration (yr) | 8.2 ± 1.7§ | 7.7 ± 1.8 | 8.2 ± 1.7§ | 7.7 ± 1.8 | 8.2 ± 1.8‡ | 7.7 ± 1.8 |
| BMI current (kg·m−2) | 24.9 ± 2.8§ | 23.7 ± 2.5 | 24.7 ± 2.7§ | 23.7 ± 2.5 | 27.1 ± 3.8§ | 23.8 ± 2.5 |
| Running distance (km·wk−1) | 35.5 ± 21.4§ | 38.3 ± 22.9 | 33.3 ± 19.6§ | 38.8 ± 23.2 | 31.3 ± 21.1‡ | 37.8 ± 22.7 |
| 10-km performance (m·s−1) | 3.8 ± 0.5§ | 4.0 ± 0.5 | 3.8 ± 0.5§ | 4.0 ± 0.5 | 3.5 ± 0.5§ | 3.9 ± 0.5 |
| Women | ||||||
| Sample (N) | 499 | 11225 | 599 | 11051 | 28 | 11956 |
| Age (yr) | 44.7 ± 10.0§ | 38.4 ± 9.9 | 44.2 ± 10.1§ | 38.4 ± 9.9 | 45.7 ± 12.4‡ | 38.9 ± 10.1 |
| Education (yr) | 15.8 ± 2.5 | 15.9 ± 2.4 | 15.6 ± 2.5‡ | 15.9 ± 2.4 | 14.9 ± 2.2* | 15.9 ± 2.4 |
| Follow-up duration (yr) | 8.1 ± 1.8§ | 7.4 ± 2.1 | 8.1 ± 1.8§ | 7.4 ± 2.1 | 8.4 ± 1.2† | 7.4 ± 2.1 |
| BMI current (kg·m−2) | 22.4 ± 3.2§ | 21.2 ± 2.3 | 22.1 ± 3.0§ | 21.2 ± 2.3 | 24.7 ± 4.6§ | 21.3 ± 2.4 |
| Running distance (km·wk−1) | 33.5 ± 21.0† | 36.4 ± 21.6 | 31.5 ± 20.1§ | 36.5 ± 21.7 | 25.4 ± 15.8† | 36.2 ± 21.6 |
| 10-km performance (m·s−1) | 3.3 ± 0.5§ | 3.5 ± 0.5 | 3.3 ± 0.5§ | 3.5 ± 0.5 | 3.0 ± 0.5§ | 3.5 ± 0.5 |
Significance levels for differences are designated by
for P < 0.05,
for P < 0.01,
for P < 0.001, and
for P < 0.0001.
TABLE 2.
Baseline characteristics (mean ± SE) of runners by cardiorespiratory fitness (10-km performance time).
| 10-km Performance (m·s−1) | |||||
|---|---|---|---|---|---|
| Fastest | Fast | Intermediate | Slow | Slowest | |
| Males | |||||
| N | 1980 | 5094 | 8617 | 6565 | 2262 |
| Age (yr) | 35.2 ± 0.2 | 40.9 ± 0.1 | 44.6 ± 0.1 | 48.2 ± 0.1 | 51.9 ± 0.2 |
| Education (yr) | 16.3 ± 0.1 | 16.5 ± 0.0 | 16.5 ± 0.0 | 16.5 ± 0.0 | 16.5 ± 0.0 |
| Running distance (km·wk−1) | 62.0 ± 0.7 | 48.4 ± 0.3 | 38.7 ± 0.2 | 31.2 ± 0.2 | 25.7 ± 0.3 |
| BMI (kg·m−2) | 21.9 ± 0.0 | 22.8 ± 0.0 | 23.7 ± 0.0 | 24.5 ± 0.0 | 25.4 ± 0.1 |
| Females | |||||
| N | 1343 | 2286 | 2961 | 1755 | 711 |
| Age (yr) | 34.4 ± 0.2 | 37.0 ± 0.2 | 39.6 ± 0.2 | 43.0 ± 0.2 | 45.4 ± 0.4 |
| Education (yr) | 16.2 ± 0.1 | 16.1 ± 0.0 | 15.9 ± 0.0 | 15.9 ± 0.1 | 15.8 ± 0.1 |
| Running distance (km·wk−1) | 54.4 ± 0.7 | 43.9 ± 0.4 | 36.1 ± 0.3 | 28.9 ± 0.4 | 25.9 ± 0.6 |
| BMI (kg·m−2) | 20.2 ± 0.1 | 20.6 ± 0.0 | 21.2 ± 0.0 | 21.8 ± 0.1 | 22.9 ± 0.1 |
Cardiovascular fitness categories are defined as slowest (males, m·s−1 < 3.25; females, m·s−1 < 2.8); slow (males, 3.25 ≤ m·s−1 < 3.75; females, 2.8 ≤ m·s−1 < 3.20); intermediate (males, 3.75 ≤ m·s−1≤ 4.25; females, 3.20 < m·s−1 ≤ 3.60); fast (males, 4.25 < m·s−1 ≤ 4.75; females, 3.6 < m·s−1 ≤ 4.00); fastest (males, 4.75 < m·s−1; females, 4.00 < m·s−1).
Hypertension
Table 3 shows that running longer weekly distances at baseline was associated with reductions in the odds of the men becoming hypertensive (P < 0.0001). The women’s decline in the odds per km run was similar to that of the men but was only marginally significant (P = 0.08). Adjustment for BMI eliminated the significant reduction in men’s hypertension per kilometer run.
TABLE 3.
Odds ratios (95% confidence intervals) of incident hypertension, high cholesterol, and diabetes in relation to physical activity and cardiorespiratory fitness.
| Males | Females | |||
|---|---|---|---|---|
| Physical Activity (Running Distance km·wk−1) | Cardiorespiratory Fitness (10-km Performance in m·s−1) | Physical Activity (Running Distance km·wk−1) | Cardiorespiratory Fitness (10-km Performance in m·s−1) | |
| Hypertension | ||||
| All | ||||
| BMI unadjusted | 0.996§ (0.993, 0.998) | 0.996 (0.991, 1.000) | ||
| BMI adjusted | 1.002 (1.000, 1.004) | 1.000 (0.996, 1.005) | ||
| Fitness subset | ||||
| BMI unadjusted | 0.997† (0.994, 0.999) | 0.996 (0.991, 1.001) | ||
| BMI unadjusted | 0.571§ (0.517, 0.631) | 0.640§ (0.509, 0.821) | ||
| BMI unadjusted | 1.003* (1.000, 1.005) | 0.546§ (0.489, 0.609) | 1.000 (0.994, 1.006) | 0.641‡ (0.500, 0.821) |
| BMI adjusted | 1.003† (1.001, 1.006) | 1.001 (0.995, 1.006) | ||
| BMI adjusted | 0.785§ (0.703, 0.876) | 0.802 (0.633, 1.017) | ||
| BMI adjusted | 1.006§ (1.003, 1.008) | 0.721§ (0.642, 0.810) | 1.002 (0.997, 1.008) | 0.775* (0.603, 0.997) |
| High Cholesterol | ||||
| All | ||||
| BMI unadjusted | 0.989§ (0.987, 0.991) | 0.989§ (0.985, 0.994) | ||
| BMI adjusted | 0.993§ (0.991, 0.995) | 0.992‡ (0.988, 0.996) | ||
| Fitness subset | ||||
| BMI unadjusted | 0.989§ (0.987, 0.992) | 0.987§ (0.982, 0.993) | ||
| BMI unadjusted | 0.583§ (0.535, 0.635) | 0.628§ (0.510, 0.774) | ||
| BMI unadjusted | 0.993§ (0.991, 0.996) | 0.653§ (0.594, 0.717) | 0.990‡ (0.985, 0.996) | 0.735† (0.587, 0.919) |
| BMI adjusted | 0.993§ (0.991, 0.995) | 0.990‡ (0.985, 0.995) | ||
| BMI adjusted | 0.698§ (0.636, 0.767) | 0.732† (0.588, 0.910) | ||
| BMI adjusted | 0.995§ (0.993, 0.997) | 0.749§ (0.679, 0.828) | 0.992† (0.986, 0.997) | 0.821 (0.653, 1.033) |
| Diabetes | ||||
| All | ||||
| BMI unadjusted | 0.986‡ (0.979, 0.994) | 0.972† (0.951, 0.994) | ||
| BMI adjusted | 1.003 (0.996, 1.011) | 0.986 (0.965, 1.008) | ||
| Fitness subset | ||||
| BMI unadjusted | 0.985‡ (0.976, 0.994) | 0.970* (0.946, 0.995) | ||
| BMI unadjusted | 0.231§ (0.167, 0.319) | 0.236‡ (0.099, 0.560) | ||
| BMI unadjusted | 1.000 (0.990, 1.009) | 0.232§ (0.164, 0.328) | 0.980 (0.954, 1.006) | 0.303† (0.118, 0.775) |
| BMI adjusted | 1.003 (0.995, 1.013) | 0.984 (0.960, 1.009) | ||
| BMI adjusted | 0.497‡ (0.341, 0.725) | 0.466 (0.180, 1.206) | ||
| BMI adjusted | 1.008 (0.999, 1.018) | 0.455§ (0.309, 0.671) | 0.988 (0.963, 1.015) | 0.528 (0.197, 1.416) |
Odds ratios adjusted to the mean age, follow-up duration, and intakes of red meat, fish, fruit, and alcohol. In addition, the coefficients were also adjusted for BMI and BMI2 as indicated. Significance levels are coded:
P < 0.05,
P < 0.01,
P < 0.001, and
P < 0.0001.
The fitness subset refers to the 85% of the men and 76% of the women who had reported at baseline completing a 10-km foot race during the previous 5 yr.
Faster 10-km performance predicted significantly lower odds for the men and women becoming hypertensive during follow up. Adding physical activity to the model had little effect on the fitness coefficients. BMI accounted for nearly one half of the reduction in hypertension associated with activity-adjusted performance. However, even when adjusted for both running distance and BMI, 10-km performance remained a significant predictor for decreased hypertension in men (P < 10−6) and women (P = 0.05).
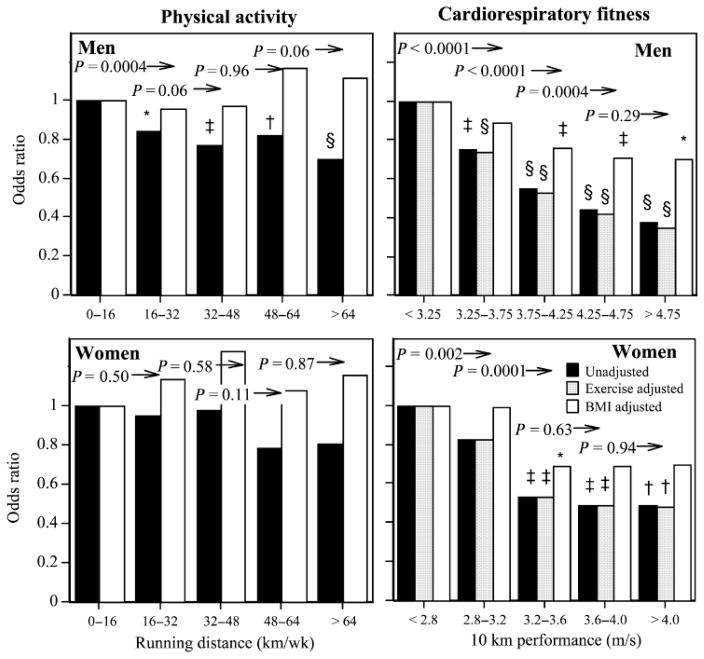
Figure 1 displays the reduction in odds for hypertension with running distance. Two types of comparisons are used to test the dose–response relationship. First, we compare whether the odds ratio is significant relative to the lowest-mileage runners. Second, we test at each running distance, whether there is a significant odds reduction by running further. The figure shows that men running > 16 km·wk−1 had lower odds for hypertension relative to running 0–16 km·wk−1 (P = 0.0004), and although the reduction becomes more significant by running further, there is no significant odds reduction running > 32 km·wk−1 versus 16 to 32 km·wk−1 (P = 0.06).
FIGURE 1.
Odds ratios for incident physician-diagnosed hypertension by physical activity and cardiorespiratory fitness in men and women followed prospectively for 7.7 and 7.4 yr, respectively. Significance levels for odds reductions from the least-fit or active men and women are coded: *P < 0.05, † P < 0.01, ‡ P < 0.001, and § P < 0.0001 by logistic regression. The significance level above each bar is for the reduction in odds between the fitness or distance category versus all greater values before the adjustment for exercise or BMI. All analyses were adjusted for age, follow-up duration, reported weekly intakes of meat, fish, fruit, and alcohol, and additional variables as designated in the legend.
Figure 1 also displays the dose–response relationship of incident hypertension versus 10-km performance. The cut points were chosen to include a reasonable sample size in each interval, and therefore were shifted towards a faster running pace in men vis-à-vis women. The men’s odds for becoming hypertensive declined linearly with faster 10-km performance, and declined significantly for every 0.5 m·s−1 increment in running velocity through 4.25 m·s−1. This relationship was independent of the quantity of running (km·wk−1) at baseline but could be attributed in part to BMI. However, even when adjusted for BMI, men who ran faster than 3.75 m·s−1 had a significantly lower odds ratio for developing hypertension relative to the least-fit men. Women who ran faster than 3.2 m·s−1 had significantly lower odds for becoming hypertensive than least-fit women, which was independent of their running distance, but not of their BMI.
High cholesterol
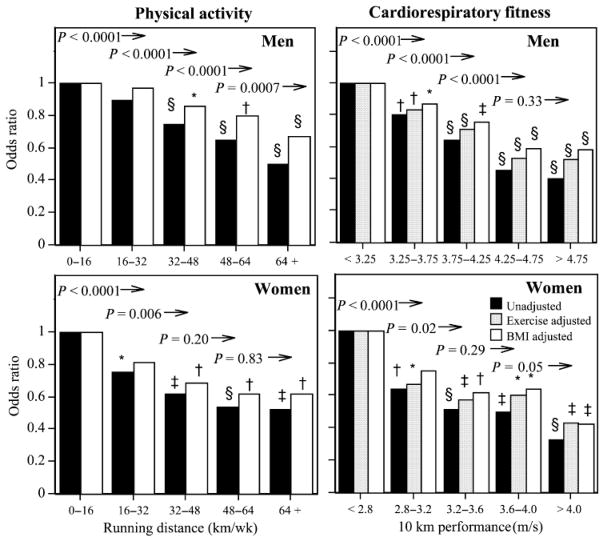
In both men and women, running further was associated with lower odds for incident physician-diagnosed high cholesterol (Table 3), which persisted even when adjusted for BMI (men, P < 10−10; women, P = 0.0005), 10-km performance (men, P < 10−10; women, P = 0.0004), and BMI and fitness simultaneously (men, P < 0.0001; women, P = 0.003). Figure 2 shows that the odds for developing high cholesterol declined significantly for each 16 km·wk−1 increment in running distance through 64 km·wk−1 in men and through 32 km·wk−1 in women. Some, but not all, of the odds reductions were attributable to the lower BMI of the longer-distanced runners.
FIGURE 2.
Odds ratio for incident physician-diagnosed hypercholesterolemia by physical activity and cardiorespiratory fitness (see Figure 1 for explanation).
The odds for incident high cholesterol were also significantly lower in men and women who were fitter at baseline, even when adjusted for exercise levels (Table 3). Adjustment for exercise increased the odds ratio for 10-km performance only slightly, and in both sexes faster race performance predicted significantly lower odds for developing high cholesterol when adjusted. Only a portion of the association between performance and incident hypercholesterolemia was attributable to BMI and the BMI-adjusted fitness effects remained significant. Simultaneous adjustment for both BMI and running distance further reduced the fitness effect, particularly in women, however, in men, faster 10-km performance lowered the odds for developing hypercholesterolemia when adjusted for both (P < 10−6).
Figure 2 shows that incident hypercholesterolemia declined linearly with 10-km performance, which was only slightly attenuated by adjusting for either running distance or BMI. The odds for hypercholesterolemia declined significantly with each 0.5 m·s−1 increment in men’s fitness through 4.25 m·s−1 and with each 0.4 increment in women’s fitness through 4 m·s−1 (except 3.2–3.6 vs. higher fitness).
Diabetes
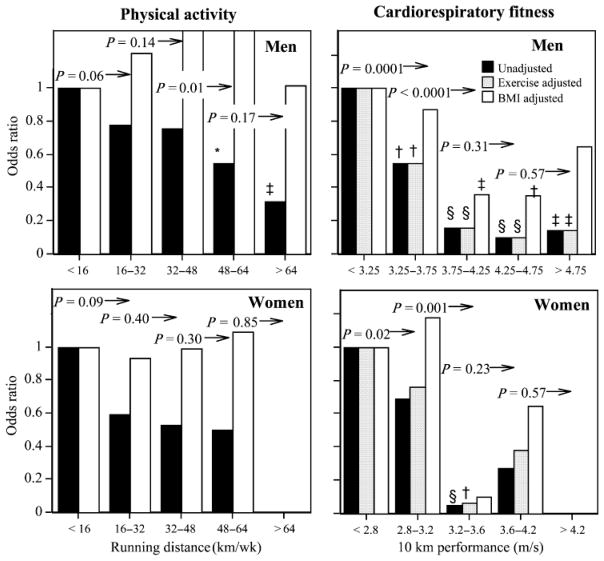
Table 2 shows that baseline running distance also predicted incident physician-diagnosed diabetes in both sexes, but this was attributed entirely to the leanness of the longer-distanced runners. In contrast, 10-km performance predicted incident diabetes even when adjusted for the initial leanness of fitter men at baseline. In both sexes, the odds ratios relating incident diabetes to baseline meters per second was affected negligibly by adjustment for distance and remained statistically significant (men, P < 10−10; women, P = 0.01). The odds ratios were doubled when adjusted for baseline BMI, but remained strongly significant in men (P = 0.0001). The women’s 10-km performance coefficient was consistent with that of the men (Table 3); however, only 28 women became diabetic during follow up, which limits the statistical power to test the effect. Figure 3 displays the similarity in the men’s and women’s declines in the odds for incident diabetes with increasing fitness. The odds for diabetes declined with each 0.5 m·s−1 increment through 3.75 m·s−1 in men and with each 0.4 m·s−1 increment through 3.2 m·s−1 in women.
FIGURE 3.
Odds ratios for incident physician-diagnosed diabetes by physical activity and cardiorespiratory fitness in men and women followed prospectively for 7.7 and 7.4 yr, respectively (see Figure 1 for explanation).
DISCUSSION
These prospective data strengthen considerably our initial cross-sectional assessment of the strong inverse relationship between cardiorespiratory fitness (10-km race performance in meters per second) and the prevalence of antidiabetic, antihypertensive, and cholesterol-lowering medications [39]. They show dose–response relationships of vigorous physical activity with diabetes, hypertension, and hypercholesterolemia that extend substantially beyond the minimum guideline levels [13,28]. Their strength derives from the measurement of physical activity and cardiorespiratory fitness before the diagnosis of disease, which is consistent with the premise that cause should precede effect.
Physical activity is defined as voluntary movements produced by skeletal muscles that result in energy expenditure, whereas cardiorespiratory fitness relates to the ability of circulation and respiration to supply oxygen during sustained physical activity [8,28]. The significant reductions in the odds for hypertension, hypercholesterolemia, and diabetes with increasing cardiorespiratory fitness were statistically significant before and after the adjustment for physical activity, and adjustment for physical activity produced small changes in the odds ratios for fitness (Table 3). This suggests that cardiorespiratory fitness is an independent determinant for these maladies for reasons not necessarily related to physical activity. Current public health guidelines do not distinguish between being physically more fit and physically more active [28]. Physical activity may be the appropriate treatment for the unfit, but inactivity may not be its principal cause. Cardiorespiratory fitness is said to be as informative a predictor for cardiovascular disease as are blood pressure, lipoproteins, or glucose-tolerance tests [25]. Our results suggest the importance of ascertaining fitness in routine clinical practice and of scientific investigations into the etiology of low fitness beyond inactivity.
Elsewhere we have used meta-analysis to show that reductions in cardiovascular disease risk differed in relation to cardiorespiratory fitness and physical activity [32]. We have also argued that cardiorespiratory fitness cannot be a better measure of physical activity than reported activity per se because estimates provided by physical activity questionnaires correlate more strongly with their repeated measurements than with cardiorespiratory fitness [32]. Simulation studies show that differences in cardiovascular-disease risk from purported changes in cardiorespiratory fitness could be an artifact of measurement error [33,34]. These observations, together with published contrary opinions [3,18], will be germane to the crafting of future recommendations.
We hypothesize that intrinsic differences across individuals, including genetic differences, account in part for individual differences in cardiorespiratory fitness. Intra-class correlations of estimated maximum aerobic uptake in Norwegian twins were 0.62 for monozygotics and 0.29 for dizygotics, suggesting heritability of over 60% [26]. This agrees with a 66% heritability estimate from a smaller study of twins in which VO2max was measured directly and adjusted for body weight, fat, and sports participation [12]. Genetic factors were estimated to account for approximately 40% of the variance in VO2max in family sets [21]. Fifty percent heritability was reported for VO2max in the sedentary state [6] and VO2max in response to training [7] in the HERITAGE family study.
Our analyses demonstrate statistically significant and clinically important health benefits to exceeding 30 min or more of moderate intensity physical activity over the course of most days of the week. Specifically, the logistic regression analyses of Table 3 demonstrate a significant dose– response relationship over a range of activity levels that exceeds guideline levels. One specific way to meet the guideline recommendation is to walk 2 miles briskly. A 2-mile brisk walk 5–7 d·wk−1 is the energy equivalent of running 10.9 to 15.2 km·wk−1 [1], which corresponds to our least-active distance category. Compared to the odds for men at this guideline level, Figures 1–3 show that the odds for hypercholesterolemia decreased significantly with every 16 km·wk−1 through 64 km·wk−1, and odds for hypertension decreased by running > 16 km·wk−1 and for diabetes by running > 48 km·wk−1. The odds for hypercholesterolemia in women also generally decreased with each 16 km·wk−1 increase through 48 km·wk−1. The trends for diabetes in women were similar to those displayed for men, but there was less statistical power to identify the women’s associations as statistically significant. Cross-sectional observations show fasting-plasma glucose levels, systolic and diastolic blood pressures, and plasma LDL-cholesterol levels all decline with running distance through at least 64 km·wk−1 [30], and that runners’ blood pressures are more strongly associated with 10-km performance times than with running distances [31]. Collectively, these findings suggest that the health benefits of physical activity continue to accrue through at least 64 km·wk−1.
The final report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) recommends a moderate amount of moderate intensity physical activity as part of a therapeutic lifestyle change to favorably affect metabolic syndrome, lower triglycerides, and increase high-density lipoproteins [27]. The report states that the efficacy of LDL-cholesterol lowering by exercise is limited to some individuals. This perception is in contradiction to the strong inverse association demonstrated in Figure 2 between hypercholesterolemia and distance run and the strong inverse association we reported cross-sectionally between LDL-cholesterol levels and running distances in men [30]. Running is a vigorous activity requiring between 7 and 16 METs, depending on intensity [1], and physical activity could need to be vigorous to prevent elevation of LDL or may require quantities greater than generally attained by moderately intense activity.
Adjustment for BMI eliminated the significant reduction in hypertension with running distance, which we interpret as evidence of the mediating effects of BMI, i.e., exercise attenuates age-related weight gain and thereby reduces the risks of diabetes, hypertension, and high cholesterol. In western societies, men and women usually gain weight with age [35]. We have shown that long-term runners experience less weight gain over time in proportion to their weekly distance run [35], but then are subject to accelerated weight gain when they cease running that is not simply lost when running resumes [37]. Self-selection based on pre-exercise weight accounts for 26% and 58% of the leanness associated with physical activity dose in the male and female runners, respectively, but all of the leanness associated with their 10-km performance [38].
The limitation of these analyses warrant acknowledgement. We studied runners specifically because cohorts recruited geographically, occupationally, clinically, or to be representative of the general population do not generally provide adequate statistical power to define the dose–response relationship between the dose of vigorous physical activity and health. For analyses of cardiovascular fitness, our sample was restricted somewhat further to subjects who had completed a 10-km foot race during the previous 5 yr (85% of men, 76% of women). Thus the associations reported here are relevant to a range of activities and fitness that are generally more active than reported by others [15,22]. However, our goals are to complement rather than to replicate previous reports by assessing the dose–response relationships over a range of fitness and physical activity levels poorly represented in other cohorts. We acknowledge despite their average of 4 yr of college education, self-reported incident hypertension, hypercholesterolemia, and diabetes may be subject to greater error than in cohorts of physicians or nurses [10]. Errors in reporting these outcomes will contribute to less statistical power to detect the association, but there is no a priori reason to assume that this would vary by the dose of physical activity or fitness level. We do not believe that the declining incidence of hypertension, hypercholesterolemia, and diabetes with running distance is because of the avoidance of opportunities for diagnosis in the more athletic men. The Health Professionals Study reported that their more vigorously active participants had more routine medical check-ups than less active men [19], and there was no difference in routine medical check-up by activity level in the Nurses’ Health Study [20]. The estimated risk reductions cited here and in other reports may underestimate the benefits of physical activity due to exercise recidivism during follow-up, e.g., the decline in the risk for diabetes per kilometer per week run for all male runners is 42% less than for men who maintained their exercise within ± 5 km·wk−1 between baseline and the conclusion of follow-up [36].
In summary, we have demonstrated that clinically important health improvements are likely to accrue at higher doses of physical activity than the minimum guideline levels. This is consistent with the greater emphasis given to prolonged vigorous exercise in the most recent update of the AHA and ACSM guidelines [13]. Although the Institute of Medicine advocates exercising more vigorously for longer durations to maintain healthy weight [17], the potential benefits of prolonged vigorous exercise have traditionally not been strongly emphasized by other public health policy statements [9,13,25,27,28]. Second, prolonged vigorous exercise may warrant greater recognition in the prevention of hypercholesterolemia. Third, high cardiorespiratory fitness reduces the risks for hypertension, hypercholesterolemia, and diabetes, independent of physical activity, and may be an important risk factor separate from physical activity. Our findings support the testing of cardiorespiratory fitness as part of routine clinical evaluations for assessing disease risk.
Acknowledgments
This study was supported in part by grants HL-45652, HL-072110, and DK-066738 from the National Heart Lung and Blood Institute, and was conducted at the Ernest Orlando Lawrence Berkeley Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
Footnotes
Results of the present study do not constitute endorsement by ACSM.
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:875–88. [PubMed] [Google Scholar]
- 3.Blair SN, Jackson AS. Physical fitness and activity as separate heart disease risk factors: a meta- analysis. Med Sci Sports Exerc. 2001;33(5):762–64. doi: 10.1097/00005768-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–98. [PubMed] [Google Scholar]
- 5.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for V O2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30(2):252–58. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 8.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Colditz G, Martin AP, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 11.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–4. [PubMed] [Google Scholar]
- 12.Fagard R, Bielen RE, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise. J Appl Physiol. 1991;70:357–62. doi: 10.1152/jappl.1991.70.1.357. [DOI] [PubMed] [Google Scholar]
- 13.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 14.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann NY Acad Sci. 1977;301:484–94. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Willett WC, Manson JE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–8. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: The National Academies Press; 2002. p. 936. [Google Scholar]
- 18.Jackson AS, Kampert JB, Barlow CE, Morrow JR, Jr, Church TS, Blair SN. Longitudinal changes in cardiorespiratory fitness: measurement error or true change? Med Sci Sports Exerc. 2004;36(7):1175–80. doi: 10.1249/01.mss.0000132269.26126.3b. [DOI] [PubMed] [Google Scholar]
- 19.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 21.Lortie G, Bouchard C, Leblanc C, et al. Familial similarity in aerobic power. Hum Biol. 1982;54:801–12. [PubMed] [Google Scholar]
- 22.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992;268:63–7. [PubMed] [Google Scholar]
- 23.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin- dependent diabetes mellitus in women. Lancet. 1991;338:774–8. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 24.Paffenbarger RS, Jr, Wing AL, Hyde RT, Jung DL. Physical activity and incidence of hypertension in college alumni. Am J Epidemiol. 1983;117:245–57. doi: 10.1093/oxfordjournals.aje.a113537. [DOI] [PubMed] [Google Scholar]
- 25.Sherwin RS, Anderson RM, Buse JB, et al. American Diabetes Association; National Institute of Diabetes and Digestive and Kidney Diseases. Prevention or delay of type 2 diabetes. Diabetes Care. 2004;27 (Suppl 1):S47–54. doi: 10.2337/diacare.27.2007.s47. [DOI] [PubMed] [Google Scholar]
- 26.Sundet JM, Magnus P, Tambs K. The heritability of maximal aerobic power: a study of Norwegian twins. Scand J Med Sci Sports. 1994;4:181–5. [Google Scholar]
- 27.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. pp. 1–276. [Google Scholar]
- 29.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 30.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch Intern Med. 1998;158:237–45. doi: 10.1001/archinte.158.3.237. [DOI] [PubMed] [Google Scholar]
- 32.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33(5):754–61. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams PT. The illusion of improved physical fitness and reduced mortality. Med Sci Sports Exerc. 2003;35(5):736–40. doi: 10.1249/01.MSS.0000064995.89335.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT. Longitudinal changes in cardiorespiratory fitness: measurement error or true change? Med Sci Sports Exerc. 2005;37(5):877–8. doi: 10.1249/01.mss.0000162620.45200.f0. [DOI] [PubMed] [Google Scholar]
- 35.Williams PT. Maintaining vigorous activity attenuates 7-year weight gain in 8,340 runners. Med Sci Sports Exerc. 2007;39(5):801–9. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PT. Changes in vigorous physical activity and incident diabetes in male runners. Diabetes Care. 2007;30:2838–42. doi: 10.2337/dc07-1189. [DOI] [PubMed] [Google Scholar]
- 37.Williams PT. Asymmetric weight gain and loss from increasing and decreasing exercise. Med Sci Sports Exerc. 2008;40(2):296–302. doi: 10.1249/mss.0b013e31815b6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obes Res. 2008;16:102–6. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 39.Williams PT, Franklin B. Vigorous exercise and diabetic, hypertensive, and hypercholesterolemia medication use. Med Sci Sports Exerc. 2007;39(11):1933–41. doi: 10.1249/mss.0b013e318145b337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams PT, Satariano WA. Relationships of age and weekly distance run to BMI and circumferences in 41,582 physically active women. Obes Res. 2005;13:1370–80. doi: 10.1038/oby.2005.166. [DOI] [PubMed] [Google Scholar]