Abstract
Primary melanoma can recur at the excision site if not excised with a safety margin of surrounding uninvolved skin. To characterize the nature of residual melanoma in the skin surrounding primary tumors targeted by safety margins, we used array comparative genomic hybridization and fluorescent in situ hybridization to detect and spatially map aberrations in the skin adjacent to acral melanomas. Melanocytic cells with genetic amplifications in histopathologically normal skin (field cells) were detected exclusively in the epidermis in 84% of 19 cases, with a mean extension of 6.1 mm (in situ melanomas) and 4.5 mm (invasive melanomas) beyond the histopathological margin. Genetic profiling of these field cells indicated that they represent an early phase of disease preceding melanoma in situ. The extent of field cells did not correlate with tumor depth or diameter, indicating that tumor depth is not suited to predict the extent of field cells. These results demonstrate that, on acral sites, melanoma field cells extend significantly into seemingly normal skin. These field cells provide a plausible explanation for the tendency of certain melanoma types to recur locally despite apparently having undergone complete excision.
INTRODUCTION
The primary treatment of melanoma is surgical excision. However, when not excised with sufficient safety margins of apparently uninvolved skin, melanoma can regrow at the primary excision site despite the absence of histologically recognizable melanoma at surgical margins. Even when employing standard safety margins, disease still recurs in a small proportion of cases. Local recurrences are proposed to be of two types, each with different prognostic implications (Treidman and McNeer, 1963; Olsen, 1970). In the first, melanoma recurs after an apparently complete excision because a histologically unrecognizable form of melanoma extending beyond the surgical margin remains. In this case, the recurrent melanoma typically has an intraepidermal component and develops through the same evolutionary stages as the original tumor, progressing from in situ to invasive growth. Alternatively, melanoma can recur locally from discontinuous, that is, metastatic, spread of the invasive component of the primary melanoma. This distinction is clinically important. Removal of persistent melanoma of the first type before it had evolved to a state capable of metastasis would be curative for the patient. By contrast, widening the margins to capture local metastatic deposits is less likely to provide lasting benefit, because local evidence of metastasis is often a marker of the widespread dissemination of potentially lethal cells.
Recently, we reported the finding of genetically abnormal melanocytes in histologically normal epidermis adjacent to melanomas on the non-hair-bearing skin of the palms and soles, that is, acral melanomas (AM) (Bastian et al., 2000a). These cells could be recognized using fluorescent in situ hybridization (FISH) because their genomes contained the characteristic high level of DNA amplifications we had found in the genomes of invasive AMs. They were termed “field cells” (Bastian et al., 2000a). Even after identification of field cells by their genetic abnormalities, we could not develop histopathologic criteria to recognize them because they were not sufficiently increased in number, abnormally distributed, or markedly atypical compared to normal melanocytes. FISH analysis of one melanoma that had recurred at the primary site repeatedly after multiple excisions with histologically negative surgical margins showed field cells at the excision margin. This suggested that field cells may be a form of occult melanoma that, if left behind, leads to local recurrence through the first aforementioned mechanism, that is, a subtle contiguous extension of the primary melanoma. This form of recurrence has been termed “local persistence” to distinguish it from the second type, “local metastasis” (Heenan and Ghaznawie, 1999; MacCormack et al., 2004). In this study, we provide further evidence that field cells most likely represent a subtle, early stage of melanoma in situ from which visible lesions can arise. We also demonstrate that the extent of the field effect is unrelated to the thickness of the primary tumor, the parameter currently used to determine the width of safety margins in melanoma.
RESULTS
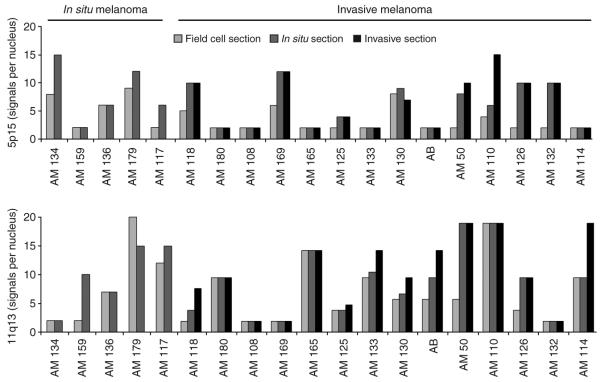
Of the 23 cases meeting inclusion criteria, 19 yielded adequate FISH signals, permitting analysis for the presence of field cells. The salient case characteristics are summarized in Table 1. Fourteen were invasive and five were in situ melanomas. 11q13 amplifications were found in 10 of 14 (71%) invasive and 4 out of 5 (80%) in situ tumors, and 5p15 was amplified in 7 of 14 (50%) invasive and 4 out of 5 (80%) in situ tumors. A total of 16 out of the 19 (84%) cases had detectable field cells as defined by the criteria above. Field cells were seen in both invasive (12 of 14 cases) and in situ categories (4 of 5 cases) (Figure 1). The average distance, which field cells extended beyond the histologically detectable intraepidermal component of the melanoma, was 6.1 mm (range 1.5–12.5 mm) for melanomas that were entirely in situ and 4.5 mm (range 2.0–9.0 mm) for melanomas that had an invasive component. There was no correlation between the extent of field cells and the tumor thickness or diameter of the melanomas (R2 0.001 and 0.086, respectively).
Table 1. Case characteristics.
| Case no. | Age | Sex | Site | Tumor depth |
Histologic width |
FC width beyond H&E |
FC at margin |
Patient follow-up after excision |
|---|---|---|---|---|---|---|---|---|
| AM 134 | 85 | F | Sole | In situ | 32.5 mm | 1.5 mm | − | No recurrence 60 months |
| AM 159 | 66 | F | Sole | In situ | 16.0 mm | — | − | No recurrence 57 months |
| AM 136 | 75 | F | Sole | In situ | 24.0 mm | 3.0 mm | − | No recurrence 52 months |
| AM 179 | —1 | —1 | —1 | In situ | 25.5 mm | 6.0, 6.52 | + | No recurrence 38 months |
| AM 117 | 40 | M | Sole | In situ | 40.0 mm | 3.0 mm | + | No recurrence 94 months |
| AM 118 | 73 | M | Sole | 0.4 mm | 28.0 mm | 10.5, 12.52 | + | Regional+systemic metastasis 54 months, died 57 months |
| AM 180 | —1 | —1 | —1 | 1.6 mm | 14.0 mm | 4.0 mm | − | Recurrence 32 months, dead 39 months |
| AM 108 | 63 | M | Sole | 1.8 mm | 17.0 mm | — | − | No recurrence 68 months |
| AM 169 | 66 | M | Sole | 2.3 mm | 39.5 mm | 4.5 mm | + | Dead 47 months (stroke), no recurrence |
| AM 165 | 74 | F | Sole | 2.5 mm | 30.5 mm | 2.5 mm | − | No recurrence 60 months |
| AM 125 | 83 | M | Sole | 2.7 mm | 9.5 mm | 6.5 mm | − | Inguinal skin metastasis 9 months, still alive 58 months |
| AM 133 | 84 | F | Sole | 2.8 mm | 14.5 mm | 2.5 mm | − | No recurrence 60 months |
| AM 130 | 69 | F | Sole | 2.9 mm | 12.0 mm | 3.0 mm | − | No recurrence 56 months |
| AB 1 | 84 | F | Palm | 3.0 mm | 10.0 mm | 9.0, 5.02 | − | No recurrence 23 months |
| AM 50 | —1 | M | Sole | 3.2 mm | 24.0 mm | 4 mm | − | No recurrence 12 months |
| AM 110 | 63 | M | Sole | 3.5 mm | 18.0 mm | 2 mm | − | No recurrence 88 months |
| AM 126 | 77 | M | Toe nail | 4.1 mm | 22.5 mm | 4.5 mm | − | Inguinal LN metastasis 25 months, died 32 months |
| AM 132 | 57 | M | Toe nail | 4.8 mm | 15.5 mm | — | − | No recurrence 65 months |
| AM 114 | 77 | M | Sole | 9.5 mm | 42.5 mm | 6.5 mm | + | Incomplete operation/stage 4, died 12 months |
FC, field cell; LN, lymph node.
Data not available.
Field cells extended past the histologic margin to both sides of the melanoma; distances in mm.
Figure 1. Amplification of 5p15 and 11q13 in AM as detected by FISH.
In all cases, the amplification levels either increased (15/19) or remained stable (4/19), moving from the peripheral field cells toward the center of the lesions (Figure 1). In five cases, we observed amplification of both 11q13 and 5p15 in the invasive portion, but amplification of only one locus in field cells (Figure 1). Thus, either a higher copy number or amplification of a second locus was present in the invasive melanoma but not in the field cells. These observations strongly suggest that field cells correspond to an early stage in tumor progression, rather than escapees from more advanced areas of the tumor. If field cells were migratory tumor cells originating from the advanced areas of the melanoma, one would expect that they would have similar or even more complex aberration patterns. Furthermore, field cells were distributed asymmetrically, mostly found immediately adjacent to the in situ portion, rather than adjacent to the invasive portion. If the cells derived from the invasive tumor, one would expect that they would be more symmetrically distributed around the recognizable parts of the tumor. In summary, the pattern of aberrations and the distribution of field cells indicate that these tumor precursors progress to tumor stage through the acquisition of additional genetic aberrations.
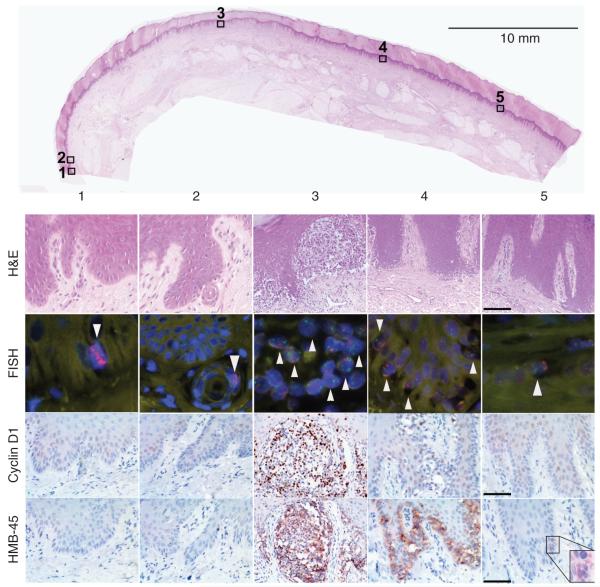
Concordantly, the protein levels of cyclin D1, the candidate oncogene residing at the 11q13 locus (Sauter et al., 2002; Curtin et al., 2005), increased from the periphery toward the invasive portions of the melanomas (Figure 2). Cyclin D1 was overexpressed in 19% (3/16) of field cell areas, 84% (16/19) of in situ areas, and 93% (13/14) of invasive areas. The observation that cyclin D1 expression was not found to be overexpressed in all areas colonized by field cells, even when FISH detected increased copy number, could be due to insufficient sensitivity of immunohistochemistry, altered function of regulators of protein expression in these cells, or because other genes of the 11q13 locus are also relevant in early stages of melanoma progression. HMB-45, an antibody against a melanosomal antigen frequently upregulated in melanoma, was detected in 56% (9/16) of field cell areas and 100% of the in situ and invasive areas (Figure 2). HMB-45 expression levels also increased from field cells to conventional melanoma in situ to invasive melanoma.
Figure 2. Case AM179.
(1) L margin, field cells present; FISH-11q13 (red) amplification (arrow head), 5p15 (green) normal. No atypical melanocytes in H&E-stained sections; cyclin D1 and HMB45 not expressed strongly; (2) field cell in eccrine duct near L margin; (3) superficially invasive melanoma with 11q13 (red) and 5p15 (green) amplification: cyclin D1 and HMB45 strongly positive; (4) melanoma in situ with 11q13 and 5p15 amplification; few cyclin D1-positive cells and robust expression of HMB-45; (5) R margin field cells; increased copy of 11q13 and 5p15. Weak expression of HBM45 (inset). Bars = 100 μm (H&E), 50 μm (HMB-45, cyclin D1).
Because field cells appear to represent an early form of melanoma in situ, the time to progression to clinically overt melanoma may be long. During the limited follow-up time (mean 51 months; range 12–94 months), none of our cases showed clinical evidence of local persistence and regrowth (Table 1). In 5 of the 19 cases, we found field cells extending to the surgical margin (Table 1, Figure 2 margin). Three of these patients died: two from metastatic melanoma and one from stroke (Table 1). However, the potential of field cells to develop into clinically overt melanoma is illustrated in the following case.
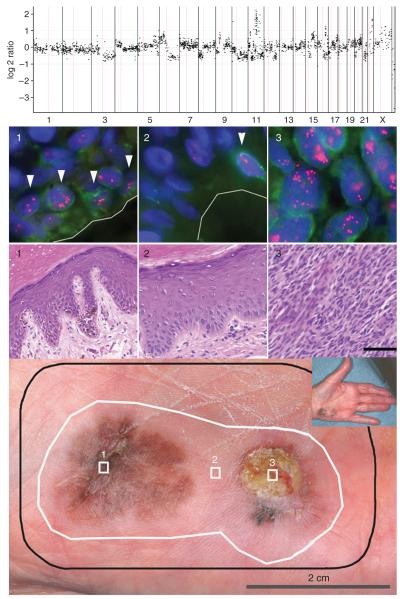
An 84-year-old Caucasian female presented with two pigmented lesions on her left hypothenar eminence separated by ~1 cm of clinically and dermoscopically normal skin (Figure 3, bottom panel). A superficial biopsy of the ulcerated lesion had shown invasive melanoma. The entire area including both lesions and a margin of clinically uninvolved skin was excised en block (Figure 3, bottom panel, black line). Histopathological examination showed two melanomas, one 3.0 mm in thickness with ulceration (Figure 3, area 3) and the other entirely in situ (Figure 3, area 1). The clinically and dermoscopically normal-appearing skin separating the two lesions was normal microscopically and showed no evidence of regression (Figure 3, area 2).
Figure 3. Case AB.
Bottom panel: (1) melanoma in situ; (2) clinically, dermoscopically, and microscopically normal skin; (3) invasive melanoma of 3.0 mm thickness. Black line: surgical margin; white line: field cell margin identified by amplification of 11q13. Top panel: array CGH profile of area 3. A log 2 ratio of 0 indicates normal copy number, and narrow spikes of increased copy number indicate regions of amplification that include 11q13. Middle panel: H&E and FISH for areas 1–3. FISH images represent one plane, so not all signals are visible. A green immunofluorescent stain to Melan-A was used to aid in identifying basal melanocytes. Cells with amplification of 11q13 are highlighted by arrowheads for areas 1 and 2. Bars (H&E) = 60 μm.
Array comparative genomic hybridization (CGH) analysis of DNA extracted from the invasive melanoma showed numerous aberrations including amplification of the cyclin D1 locus on 11q13 (Figure 3, top panel). FISH analysis revealed a mean copy number of 15 per nucleus for 11q13 in the invasive melanoma and 11 in the separate area of melanoma in situ (Figure 3, FISH panel). The epidermis in between these two lesions as well as in a significant area of the surrounding skin (Figure 3, bottom panel, white line) had field cells with a mean copy number of six signals per nucleus. The progressive increase of 11q13 copy number from the field cell areas to the in situ portion to the invasive melanoma suggests that increased cyclin-D1 gene dosage confers a growth advantage that manifests itself during tumor progression. Additional genetic aberrations likely occurred during the progression from field cells to invasive melanoma, some of which are among the chromosomal aberrations detected by CGH (Figure 3, top panel).
The presence of a common genetic aberration in the invasive tumor, the nearby in situ lesion, and the “normal” melanocytes in the intervening and surrounding skin indicates the presence of a clonally related population of melanocytes. These field cells populated a sizable area of skin (Figure 3, white outline) within which progression to overt melanoma proceeded at different rates resulting in two seemingly separate lesions. This case illustrates the potential of field cells to progress to melanoma and hints at their potential to lead to persistent melanoma if not completely excised.
DISCUSSION
In this study, we examined in detail the “fields” of genetically abnormal intraepidermal melanocytes that escape detection in melanomas on acral sites using current clinical and histopathological methods (Bastian et al., 2000a). The genetic data we present showing differences in copy-number levels and aberration patterns between field cells and the histologically recognizable melanoma strongly support our earlier hypothesis that these field cells represent the earliest form of melanoma yet to be identified. Despite their histologically benign appearance, the amplifications present in field cells represent severe genomic instability. Gene amplification requires recurrent double-stranded DNA breaks. Our observation that the amplifications are present in most of the basal melanocytes indicates that the amplifications at 11q13 have been selected for in a clonal outgrowth. The presence of these amplifications indicates that there are likely to be other aberrations in the cells, at least sufficient to cause failure of the checkpoints that normally prevent amplification.
Although our study does not provide direct evidence that field cells give rise to melanoma, the case described in Figure 3 demonstrates the probable progression of field cells to overt melanoma at different rates at separate locations within the field. The idea that field cells are an occult early stage of melanoma in situ provides a plausible mechanism for regrowth of melanoma at previous primary sites despite previous excision with histologically clear margins. It also explains why recurrences can arise after highly variable latent periods, ranging from months to up to 15 years, and at locations that are not immediately contiguous with the primary excision scar (Dong et al., 2000; Wildemore et al., 2001).
It has not yet been established whether field cells exist in other types of melanomas, as these tumors do not usually have characteristic high-level DNA copy-number changes that facilitate their detection in tissue sections. Gene amplifications occur in a small fraction of other melanoma subtypes such as superficial spreading, lentigo maligna, and nodular melanoma (Bastian et al., 2003; Curtin et al., 2005), but they are typically present in the invasive rather than the in situ component, indicating that they occur later in progression (B.C. Bastian, unpublished observation). However, local persistence is not restricted to AM. It also occurs in other types that have a lentiginous growth pattern, such as lentigo maligna and mucosal melanomas. We therefore consider it likely that the phenomenon of field cells as an early stage of melanoma progression will be found in at least some of the other melanoma types as well.
The finding of field cells offers a basis for a more rational design of excision margins in melanoma, as it offers a measurable mechanism for local persistence. Currently recommended surgical safety margins are based on tumor thickness. Many centers use 0.5 cm for melanoma in situ, 1 cm for melanomas <2 mm in Breslow thickness, and 2 cm for thicker melanomas, with deep margins down to the fascia. These recommendations are based on clinical trials that did not distinguish between the two proposed mechanisms for local recurrence, that is, persistence and local metastasis. Our study suggests that, for the specific purpose of preventing local persistence and regrowth, tumor thickness may not be a suitable parameter on which to base excision margins. In fact, the thinner tumors that we studied tended to have a larger extent of field cells than did the thicker tumors (Table 1). Furthermore, our study suggests that these cells responsible for local persistence and regrowth reside within the epidermis and in adnexal structures such as hair follicles and eccrine sweat ducts (Figure 2, area 2), suggesting that excisions to the level of the fascia may be excessive.
We believe that complete removal of the melanoma, including any field around it, is the appropriate goal of surgery at the primary site. The delineation of the extent of field cells would also enable a more thoughtful decision regarding the extent of surgery when a “standard” margin would encroach on a structure of cosmetic or functional importance. In most cases, field cells would be expected to take considerable time to form a new lesion if left behind, making narrower safety margins with frequent, long-term clinical follow-up a possible acceptable alternative. Also, because of this significant time delay in progression from field cells to melanoma, their removal would be of secondary concern for patients in whom metastases were already present at the time of diagnosis. Wider margins are unlikely to be beneficial for patients who develop local recurrence due to metastasis. Such recurrences indicate a poor prognosis (Kelly et al., 1984) and typically occur after a shorter time interval than persistent melanoma (Crowley and Seigler, 1992; Dong et al., 2000; Wildemore et al., 2001). The association of local metastases as a harbinger of fatal disease suggests that metastasis is not confined to the immediate vicinity of the primary.
Thus, the patients who might potentially benefit from improved definition of excisional safety margins are those with nonmetastasizing primary melanomas, in which field cells can be removed. However, due to the observational nature of this study, we are limited in drawing definite conclusions in the relationship of field cells and local persistence. Further case-controlled and prospective studies are needed to confirm the relationship between field cells and melanoma recurrence before definite recommendations for surgical margins can be formulated.
MATERIALS AND METHODS
Analysis of tumors
We reviewed hematoxylin and eosin (H&E)-stained sections of 182 cases of AMs from the archives of the Department of Pathology, University of California San Francisco (UCSF) and the Department of Dermatology, Kumamoto University, Japan. Unequivocal melanomas in sections flanked by histologically normal-appearing epidermis of at least >8 mm in width were tested for the presence of the most commonly amplified genomic regions in AM, chromosomes 5p15 and 11q13, by FISH in the tissue sections or by CGH employing DNA extracted from the tumor (Bastian et al., 2000a, 2003; Curtin et al., 2005). Amplifications were defined by CGH as distinct segments of a chromosomal arm with a tumor/reference fluorescence log 2 intensity ratio greater than 0.9 or by FISH as tumors in which the majority of cells showed a copy number of 11q13 or 5p15 greater than 2.5 times the copy number of a reference locus. Based on these criteria, 23 cases were included in the analyses for the presence of field cells. The described studies were approved by UCSF’s research ethical committee and conducted in accordance with the Declaration of Helsinki Principles.
Histopathology and FISH for analysis of field cells
Consecutive sections were analyzed using conventional histopathology, FISH, and immunohistochemistry. The transition point between in situ melanoma and uninvolved epidermis for each case was determined independently by two dermatopathologists (PEL and BCB). The distance between transition points on both sides of the melanoma was used as the diameter of the lesion. Dual-color FISH for chromosomes 11q13 and 5p15 was performed on 6 μm paraffin sections as described previously (Bastian et al., 2000a). Probes were labeled directly with Cy3 (Amersham, Arlington Heights, IL) or indirectly with digoxigenin (Boehringer Mannheim, Indianapolis, IN), which was detected with a FITC-labeled antibody (Roche, Indianapolis, IN). Hybridization conditions were optimized on a case-by-case basis by varying the time of the pretreatment (2–4 minutes in 1 m NaSCN 80°C bath and 4–7 minutes in a pepsin (4 mg ml−) 37°C bath) to achieve maximum hybridization efficiency. If a case did not yield acceptable hybridization signals with four different conditions, it was excluded from the study (4 cases). Signal counts for each region examined were obtained by averaging FISH signals from 10 distinct melanocyte nuclei. Field cells were defined as melanocytes that had at least twice the copy number of signals with probes for either 11q13 or 5p15 when compared with surrounding keratinocytes and that were found in an area extending over several rete ridges in the histopathologically uninvolved epidermis adjacent to the melanoma. In cases in which basal melanocytes were difficult to recognize, an FITC-labeled antibody Melan-A was used in combination with FISH.
Immunohistochemistry
Immunohistochemistry was performed using standard protocols with 3-amino-9-ethylcarbazole as a chromagen following the manufacturer’s specifications as described previously (Bastian et al., 2000b). The following antibodies were used: Cyclin D1, monoclonal antibody AM29 (Zymed, cat no. 18-0220; 1:200), HMB45 antibody (Enzo Life Sciences Inc., Farmingdale, NY, cat. no. 30930-6 ml), and Melan-A antibody (BioCare Medical, Walnut Creek, CA, cat no. CM165B).
Array CGH
DNA for CGH was extracted from tumor-bearing tissue as published previously (Bastian et al., 1998). Array CGH hybridization was carried out on 2 μg of genomic DNA, labeled by random priming, as described previously (Curtin et al., 2005).
ACKNOWLEDGMENTS
We thank Susan Charzan for excellent technical assistance. This work was supported by a grant from the National Cancer Institute P01 CA025874.
Abbreviations
- AM
acral melanoma
- CGH
comparative genomic hybridization
- FISH
fluorescent in situ hybridization
- H&E
hematoxylin and eosin
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000a;60:1968–73. [PubMed] [Google Scholar]
- Bastian BC, Leboit PE, Hamm H, Brocker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–5. [PubMed] [Google Scholar]
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000b;157:967–72. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003;163:1765–70. doi: 10.1016/S0002-9440(10)63536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NJ, Seigler HF. Relationship between disease-free interval and survival in patients with recurrent melanoma. Arch Surg. 1992;127:1303–8. doi: 10.1001/archsurg.1992.01420110045011. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Dong XD, Tyler D, Johnson JL, DeMatos P, Seigler HF. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer. 2000;88:1063–71. doi: 10.1002/(sici)1097-0142(20000301)88:5<1063::aid-cncr17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Heenan PJ, Ghaznawie M. The pathogenesis of local recurrence of melanoma at the primary excision site. Br J Plast Surg. 1999;52:209–13. doi: 10.1054/bjps.1998.3050. [DOI] [PubMed] [Google Scholar]
- Kelly JW, Sagebiel RW, Calderon W, Murillo L, Dakin RL, Blois MS. The frequency of local recurrence and microsatellites as a guide to reexcision margins for cutaneous malignant melanoma. Ann Surg. 1984;200:759–63. doi: 10.1097/00000658-198412000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCormack MA, Cohen LM, Rogers GS. Local melanoma recurrence: a clarification of terminology. Dermatol Surg. 2004;30(12 Part 2):1533–8. doi: 10.1111/j.1524-4725.2004.30562.x. [DOI] [PubMed] [Google Scholar]
- Olsen G. Some views on the treatment of melanomas of the skin. Arch Chir Neerl. 1970;22:79–90. [PubMed] [Google Scholar]
- Sauter ER, Yeo UC, von Stemm A, Zhu W, Litwin S, Tichansky DS, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–6. [PubMed] [Google Scholar]
- Treidman L, McNeer G. Prognosis with local metastasis and recurrence in malignant melanoma. Ann N Y Acad Sci. 1963;100:123–30. doi: 10.1111/j.1749-6632.1963.tb57117.x. [DOI] [PubMed] [Google Scholar]
- Wildemore JK, Schuchter L, Mick R, Synnestvedt M, Elenitsas R, Bedrosian I, et al. Locally recurrent malignant melanoma characteristics and outcomes: a single-institution study. Ann Plast Surg. 2001;46:488–94. doi: 10.1097/00000637-200105000-00006. [DOI] [PubMed] [Google Scholar]