Abstract
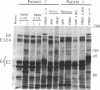
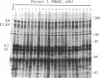
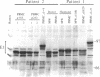
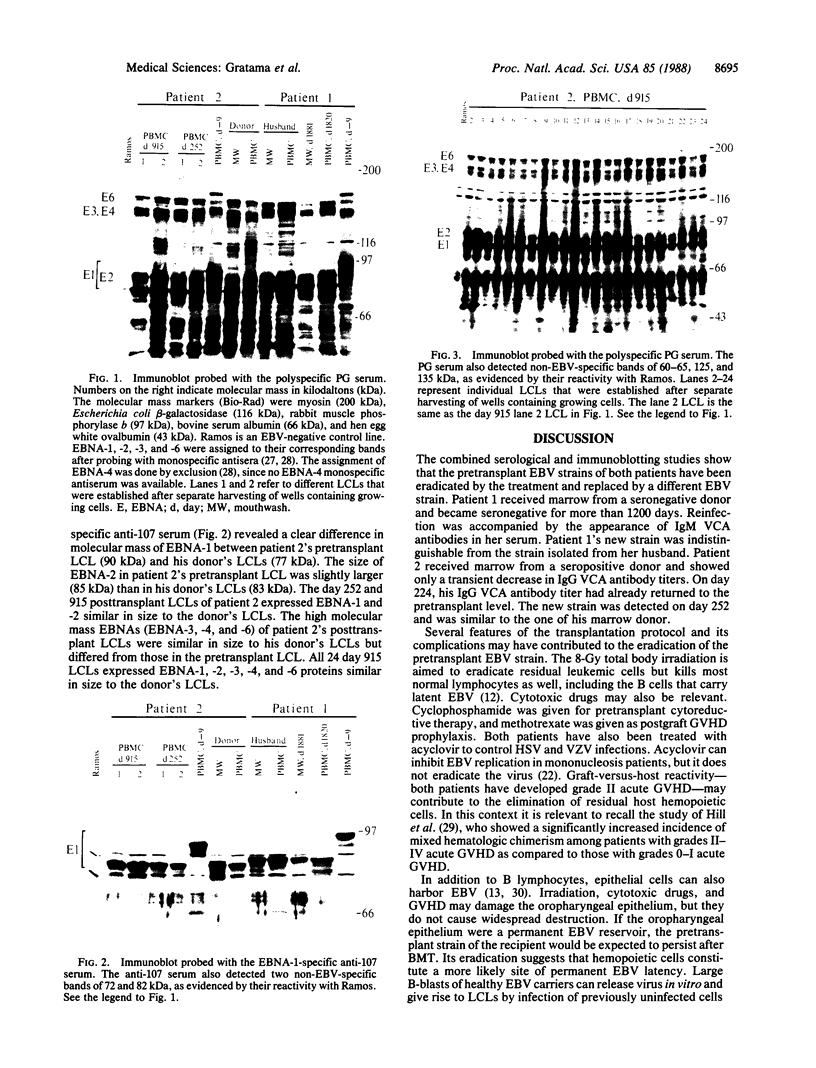
Wild-type strains of Epstein-Barr virus (EBV) can be distinguished on the basis of variations in the molecular weight of virus-encoded, growth transformation-associated proteins. This approach was used to study the persistence of EBV in two seropositive recipients of allogeneic bone marrow transplants. The first patient received marrow from her EBV-seronegative brother, became EBV seronegative after grafting, and remained so for greater than 1200 days. Subsequently, she became infected with a new EBV strain that differed from her pretransplant strain but was indistinguishable from the virus isolated from her husband. The second patient received marrow from his EBV-seropositive brother. This patient showed only a transient decrease in IgG antibodies to EBV capsid antigen. His pretransplant strain differed from the virus of his donor. On days 252 and 915 after transplantation, lymphoblastoid cell lines were grown from the peripheral blood of the patient and were found to carry exclusively the virus of the donor. These results suggest that the latently EBV-infected host cells reside in a cellular compartment that can be destroyed by graft-versus-host reactivity, irradiation, or cytotoxic drugs. Hemopoietic tissue is the most likely candidate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford D. H., Mulholland N., Iliescu V., Hawkins R., Powles R. Epstein-Barr virus infection and immunity in bone marrow transplant recipients. Transplantation. 1986 Jul;42(1):50–54. doi: 10.1097/00007890-198607000-00010. [DOI] [PubMed] [Google Scholar]
- Dillner J., Kallin B. The Epstein-Barr virus proteins. Adv Cancer Res. 1988;50:95–158. doi: 10.1016/s0065-230x(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Dillner J., Sternås L., Kallin B., Alexander H., Ehlin-Henriksson B., Jörnvall H., Klein G., Lerner R. Antibodies against a synthetic peptide identify the Epstein-Barr virus-determined nuclear antigen. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4652–4656. doi: 10.1073/pnas.81.15.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernberg I., Andersson J. Acyclovir efficiently inhibits oropharyngeal excretion of Epstein-Barr virus in patients with acute infectious mononucleosis. J Gen Virol. 1986 Oct;67(Pt 10):2267–2272. doi: 10.1099/0022-1317-67-10-2267. [DOI] [PubMed] [Google Scholar]
- Ernberg I., Kallin B., Dillner J., Falk K., Ehlin-Henriksson B., Hammarskjöld M. L., Klein G. Lymphoblastoid cell lines and Burkitt-lymphoma-derived cell lines differ in the expression of a second Epstein-Barr virus encoded nuclear antigen. Int J Cancer. 1986 Nov 15;38(5):729–737. doi: 10.1002/ijc.2910380517. [DOI] [PubMed] [Google Scholar]
- Finke J., Rowe M., Kallin B., Ernberg I., Rosén A., Dillner J., Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987 Dec;61(12):3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S. J., Sullivan J. L., Wright C., Ratech H., Racklin B., Blume K. G. Epstein-Barr-virus-related malignant B cell lymphoplasmacytic lymphoma following allogeneic bone marrow transplantation for aplastic anemia. Transplantation. 1987 Aug;44(2):244–249. doi: 10.1097/00007890-198708000-00014. [DOI] [PubMed] [Google Scholar]
- Greenspan J. S., Greenspan D., Lennette E. T., Abrams D. I., Conant M. A., Petersen V., Freese U. K. Replication of Epstein-Barr virus within the epithelial cells of oral "hairy" leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985 Dec 19;313(25):1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- Guiot H. F., van der Meer J. W., van Furth R. Selective antimicrobial modulation of human microbial flora: infection prevention in patients with decreased host defense mechanisms by selective elimination of potentially pathogenic bacteria. J Infect Dis. 1981 May;143(5):644–654. doi: 10.1093/infdis/143.5.644. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Andersson J., Ernberg I., Klein G., Horwitz C. A., Marklund G., Rymo L., Wellinder C., Straus S. E. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci U S A. 1987 Jan;84(2):570–574. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Hill R. S., Petersen F. B., Storb R., Appelbaum F. R., Doney K., Dahlberg S., Ramberg R., Thomas E. D. Mixed hematologic chimerism after allogeneic marrow transplantation for severe aplastic anemia is associated with a higher risk of graft rejection and a lessened incidence of acute graft-versus-host disease. Blood. 1986 Mar;67(3):811–816. [PubMed] [Google Scholar]
- Lange B., Henle W., Meyers J. D., Yang L. C., August C., Koch P., Arbeter A., Henle G. Epstein-Barr virus-related serology in marrow transplant recipients. Int J Cancer. 1980 Aug;26(2):151–157. doi: 10.1002/ijc.2910260205. [DOI] [PubMed] [Google Scholar]
- Lewin N., Aman P., Masucci M. G., Klein E., Klein G., Oberg B., Strander H., Henle W., Henle G. Characterization of EBV-carrying B-cell populations in healthy seropositive individuals with regard to density, release of transforming virus and spontaneous outgrowth. Int J Cancer. 1987 Apr 15;39(4):472–476. doi: 10.1002/ijc.2910390411. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Shulman H. M., Schubach W. H., Hansen J. A., Fefer A., Miller G., Thomas E. D. Fatal Epstein-Barr-virus-associated proliferation of donor B cells after treatment of acute graft-versus-host disease with a murine anti-T-cell antibody. Ann Intern Med. 1984 Sep;101(3):310–315. doi: 10.7326/0003-4819-101-3-310. [DOI] [PubMed] [Google Scholar]
- Morinet F., Icart J., Ruelle C., Gluckman E., Perol Y. Epstein-Barr virus serology in bone marrow transplantations: a one-year retrospective study with detection of EBV IgM-VCA-specific antibodies. J Med Virol. 1986 Apr;18(4):349–360. doi: 10.1002/jmv.1890180408. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Rickinson A. Epstein-Barr virus in epithelium. Nature. 1984 Jul 12;310(5973):99–100. doi: 10.1038/310099a0. [DOI] [PubMed] [Google Scholar]
- Ricksten A., Kallin B., Alexander H., Dillner J., Fåhraeus R., Klein G., Lerner R., Rymo L. BamHI E region of the Epstein-Barr virus genome encodes three transformation-associated nuclear proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(4):995–999. doi: 10.1073/pnas.85.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubach W. H., Hackman R., Neiman P. E., Miller G., Thomas E. D. A monoclonal immunoblastic sarcoma in donor cells bearing Epstein-Barr virus genomes following allogeneic marrow grafting for acute lymphoblastic leukemia. Blood. 1982 Jul;60(1):180–187. [PubMed] [Google Scholar]
- Sculley T. B., Moss D. J., Hazelton R. A., Pope J. H. Detection of Epstein-Barr virus strain variants in lymphoblastoid cell lines 'spontaneously' derived from patients with rheumatoid arthritis, infectious mononucleosis and normal controls. J Gen Virol. 1987 Aug;68(Pt 8):2069–2078. doi: 10.1099/0022-1317-68-8-2069. [DOI] [PubMed] [Google Scholar]
- Shapiro R. S., McClain K., Frizzera G., Gajl-Peczalska K. J., Kersey J. H., Blazar B. R., Arthur D. C., Patton D. F., Greenberg J. S., Burke B. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988 May;71(5):1234–1243. [PubMed] [Google Scholar]
- Shearer W. T., Ritz J., Finegold M. J., Guerra I. C., Rosenblatt H. M., Lewis D. E., Pollack M. S., Taber L. H., Sumaya C. V., Grumet F. C. Epstein-Barr virus-associated B-cell proliferations of diverse clonal origins after bone marrow transplantation in a 12-year-old patient with severe combined immunodeficiency. N Engl J Med. 1985 May 2;312(18):1151–1159. doi: 10.1056/NEJM198505023121804. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Nedrud J. G., Raab-Traub N., Hanes R. A., Pagano J. S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med. 1984 May 10;310(19):1225–1230. doi: 10.1056/NEJM198405103101905. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Wallen W. C., Johnson F. L. Epstein-Barr virus infection following bone-marrow transplantation. Int J Cancer. 1978 Aug 15;22(2):132–135. doi: 10.1002/ijc.2910220205. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Huang E. S., Miller M. J., Lin C. H., Ho W. G., Gale R. P., Champlin R. E. Molecular epidemiology of cytomegalovirus infections associated with bone marrow transplantation. Ann Intern Med. 1985 Jan;102(1):16–20. doi: 10.7326/0003-4819-102-1-16. [DOI] [PubMed] [Google Scholar]
- Wolf H., Haus M., Wilmes E. Persistence of Epstein-Barr virus in the parotid gland. J Virol. 1984 Sep;51(3):795–798. doi: 10.1128/jvi.51.3.795-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]