Abstract
Greater phosphorus intake has been associated with lower levels of blood pressure in cross-sectional studies. This association, however, has not been assessed prospectively. We studied 13444 participants from the Atherosclerosis Risk in Communities cohort and the Multi-Ethnic Study of Atherosclerosis, with diet assessed at baseline using validated food frequency questionnaires. Blood pressure and use of antihypertensive medication were determined at baseline and during follow-up visits. Compared to individuals in the lowest quintile of phosphorus intake at baseline, those in the highest quintile had lower baseline systolic and diastolic blood pressure after adjustment for dietary and non-dietary confounders (−2.0 mmHg, 95% confidence interval −3.6, −0.5; p for trend=0.01; and −0.6, 95% confidence interval −1.6, +0.3, p for trend=0.20, respectively). During an average 6.2 years of follow-up, 3345 cases of hypertension were identified. Phosphorus intake was associated with the risk of hypertension (hazard ratio 0.80, 95% confidence interval 0.80-1.00, comparing extreme quintiles; p for trend=0.02) after adjustment for non-dietary factors, but not after additional adjustment for dietary variables (hazard ratio 1.01, 95% confidence interval 0.82-1.23, p for trend=0.88). Phosphorus from dairy products but not from other sources was associated with lower baseline blood pressure and reduced risk of incident hypertension. Hazard ratios (95% confidence interval) comparing extreme quintiles were 0.86 (0.76-0.97), p for trend=0.01, for phosphorus from dairy foods and 1.04 (0.93-1.17), p for trend=0.48, for phosphorus from other foods. These findings could indicate an effect of phosphorus in conjunction with other dairy constituents or of dairy itself without involvement of phosphorus.
Keywords: Phosphorus, Cohort, Dairy product, Epidemiology, Blood pressure, Hypertension
INTRODUCTION
Substantial evidence demonstrates that dietary factors affect the risk of hypertension.1 A diet rich in potassium, fruits, vegetables, and low-fat dairy products, and low in sodium and alcohol intake has been consistently associated with reduced levels of blood pressure (BP).2, 3 The effect of other nutrients, such as calcium, magnesium, fiber, protein, or phosphorus is less clear.1
A recent analysis from the International Study of Macro- and Micro-Nutrients and Blood Pressure (INTERMAP), an international cross-sectional study including 4680 individuals, showed that a higher phosphorus intake, independently of other nutrients, was associated with lower BP levels.4 Similarly, in the US National Health and Nutrition Examination Survey (NHANES), phosphorus intake presented a weak inverse association with systolic and diastolic BP.5 No prospective studies, however, have examined whether phosphorus intake might be associated with a reduced risk of hypertension.
Determining the relationship between dietary phosphorus and the risk of incident hypertension is particularly relevant, since phosphorus in the diet could affect levels of serum phosphorus, as shown in some studies 6-8 (though not in others).9-11 Elevated serum phosphorus levels, in turn, could increase the risk of cardiovascular events.12-14 Additionally, the intake of phosphorus in the US population has increased considerably in the last decades, particularly due to the important amount of phosphorus food additives in processed foods.15 Therefore, assessing the overall cardiovascular effect of dietary phosphorus, whether beneficial or deleterious, is a crucial public health need.
We examined the association of phosphorus intake with BP levels and risk of hypertension in two population-based studies in the US: the Atherosclerosis Risk in Communities (ARIC) Study and the Multi-Ethnic Study of Atherosclerosis (MESA). Additionally, we assessed whether phosphorous from different dietary sources was equally related to hypertension.
METHODS
Study population
The ARIC Study is a population-based cohort study aimed to identify risk factors for cardiovascular diseases in 4 US communities.16 Between 1987 and 1989, 15,792 men and women aged 45-64 were recruited from Washington County, MD, Forsyth County, NC, Jackson, MS, and suburbs of Minneapolis, MN. Participants underwent a baseline clinical examination. Follow-up examinations of the cohort occurred three times, at intervals of roughly three years. The sample included 27% African-Americans and 73% whites.
From July 2000 to September 2002, MESA recruited 6814 men and women, aged 45-84 and free of cardiovascular disease, from six different populations in the US: Baltimore, MD, Chicago, IL, St. Paul, MN, Forsyth County, NC, New York, NY, and Los Angeles, CA. 17 MESA conducted three follow-up examinations between 2002 and 2007. The race/ethnicity distribution was 41% white, 26% African-American, 21% Hispanic, and 12% Chinese-American.
Study participants who at baseline had diabetes or cardiovascular disease, or were taking antihypertensive medication were excluded from all analyses. We also excluded individuals with missing values in any of the covariates or who provided insufficient or implausible dietary information (missing diet data or extreme energy intake, as described elsewhere).18, 19 In the longitudinal analysis, we additionally excluded those who did not attend any follow-up visit or had systolic BP≥140 or diastolic BP≥90 at the baseline exam. Finally, in ARIC we excluded a small number of individuals who were not white in the Minnesota and Washington County site, or not white or black in the Forsyth County site; these exclusions were done to allow multivariate adjustment for race and study site. Response rates among survivors for the successive examinations were 93%, 86%, and 80% in ARIC, and 91%, 87%, and 85% in MESA.
Institutional Review Boards from all participating institutions approved the study; study participants provided written informed consent.
Diet assessment
In ARIC, diet was assessed at baseline using a 66-item food frequency questionnaire (FFQ) based on the 61-item FFQ developed by Willett et al.20 Participants reported usual frequency of food consumption in 9 categories, from never or less than once a month to 6 or more times per day. Average daily intake of nutrients was calculated by multiplying the frequency of consumption of each food item by its nutrient content and adding up the nutrient intake for all items.
Dietary data in MESA was assessed at baseline using a 120-item FFQ, developed according to the validated Block format.21 The MESA FFQ was adapted from the Insulin Resistance Atherosclerosis Study FFQ, modified to include unique Chinese foods and culinary practices. Information from the food-specific portion of the questionnaire was converted to approximate daily intake amounts of the micronutrients in the food using the Nutrition Data Systems for Research database (NDS-R, Nutrition Coordinating Center University of Minnesota; Minneapolis, MN).22
Nutrient intake was adjusted for total energy intake using the residual method, and categorized in sex-specific quintiles.23
Blood pressure measurement
At the ARIC baseline and follow-up visits, BP was measured by certified technicians with a random-zero sphygmomanometer. The second and third of 3 measurements were averaged to estimate systolic and diastolic BP. In MESA, BP was also measured at baseline and at each follow-up examination. Three seated BP measurements were taken 5 minutes apart by using an automated device (Dinamap Pro 100). The mean of the last 2 measurements was considered for analysis. Use of antihypertensive medication was assessed by questionnaire at all visits in both ARIC and MESA. A study participant was labeled as hypertensive (prevalent or incident) if she or he had systolic BP ≥140 mmHg, or diastolic BP ≥90 mmHg, or was currently using antihypertensive medication.
Assessment of other covariates
Measurement of other covariates is detailed in an online supplement. Please see http://hyper.ahajournals.org
Statistical analysis
Analyses were conducted separately in each cohort. Heterogeneity between cohorts was assessed with the Cochran’s Q statistic.24 If no evidence of heterogeneity was apparent, cohort-specific results were pooled, weighted by the inverse of their variance.
To study the cross-sectional association between baseline phosphorus intake and BP levels, we fitted separate multiple linear regression models for systolic and diastolic BP. An initial model included quintiles of phosphorus intake, as a categorical variable, age, sex, and race as independent variables. In a subsequent model, we added the following potential confounders: study site, body mass index (considered as a continuous variable), waist circumference (continuous), education (ordinal), income (ordinal), physical activity (continuous), eGFR (continuous), cigarette smoking (current, former, never), alcohol intake (continuous), and energy intake (continuous). Finally, a third model additionally adjusted for quintiles of other nutrients and foods: calcium, potassium, magnesium, sodium, vitamin D (only in ARIC), daily servings of fruits and vegetables, and daily serving of whole grains.
In the longitudinal analysis, we estimated hazard ratios (HR) of incident hypertension and their 95% confidence intervals (CI) by quintiles of phosphorus intake using Cox proportional hazards regression, with the lowest quintile as reference category. The main independent variable was time from baseline exam to the exam date in which hypertension was first diagnosed. Study participants were censored if they did not have hypertension by the time of their last study visit. As with the cross-sectional analysis, we ran different models including increasing number of potential confounders. Trends across quintiles of phosphorus intake were estimated assigning the quintile-specific median intake of phosphorus to all individuals in that quintile, and including the resulting value as a continuous variable in regression models.
If a nutrient is related to an outcome, the association should be seen independently from the food which it is derived. Because dairy products are the main source of dietary phosphorus and given the known inverse association between dairy product intake and risk of hypertension, we conducted separate analyses using dietary phosphorus from dairy products, and dietary phosphorus from other sources as main exposure variables. Phosphorus intake from different sources was calculated with a cohort-specific multivariable regression model, with daily phosphorus intake as the dependent variable, and daily servings of each food item in the FFQ as independent variables. The coefficients obtained from this model were multiplied by the average number of servings per day for each food item, providing the average daily phosphorus intake from the corresponding food.
In an online supplement, we have included additional details on sensitivity and stratified analyses (please see http://hyper.ahajournals.org).
All statistical analyses were conducted using SAS v 9.2 (SAS Inc, Cary, NC).
RESULTS
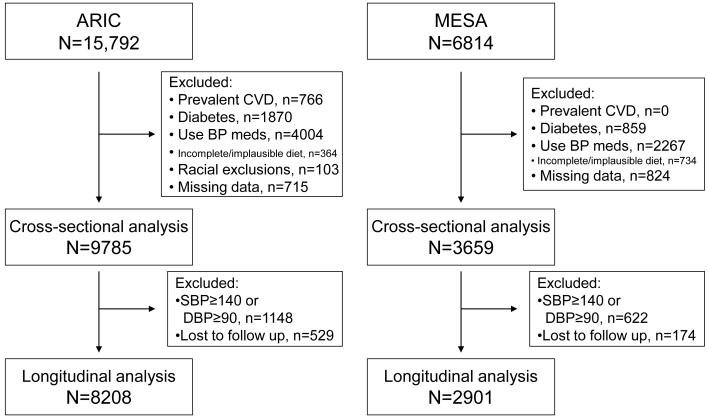
From the initial 15,792 ARIC and 6814 MESA participants, 9785 from ARIC and 3659 from MESA met inclusion criteria for the cross-sectional analysis. Of these, 8208 ARIC and 2901 MESA participants were also included in the longitudinal analysis. Figure 1 presents a flow diagram of participants in ARIC and MESA, and the different reasons for exclusion. In both studies, the main reason for exclusion was the use of antihypertensive medications or high blood pressure levels at baseline.
Figure 1.
Participation flow diagram, ARIC and MESA.
Tables 1 and 2 report selected characteristics of ARIC and MESA participants by sex-specific quintiles of phosphorus intake. Average phosphorus intake was similar in both cohorts (1084 mg/day in ARIC, 1103 mg/day in MESA). Individuals with higher phosphorus intake were older, more likely to be educated, and had an overall healthier lifestyle profile than those with low phosphorus intake. Thus, they were more likely to be non-smokers and physically active, had lower alcohol intake, and higher intake of calcium, potassium, magnesium, sodium, fruits and vegetables, and whole grains. Whites had higher phosphorus intake and, in MESA, African-Americans had lower phosphorus intake than Chinese-Americans and Hispanics. Correlations between phosphorus and other dietary minerals were relatively strong: 0.80 and 0.92 with calcium, 0.66 and 0.68 with potassium, 0.66 and 0.69 with magnesium, in ARIC and MESA respectively.
Table 1.
Selected characteristics of the study sample (mean or prevalence) by sex-specific quintile of phosphorus intake at baseline, ARIC, 1987-1989
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend* |
|---|---|---|---|---|---|---|
| N | 1956 | 1957 | 1957 | 1957 | 1958 | |
| Phosphorus intake (mg/day) | 739 | 947 | 1066 | 1194 | 1472 | <0.0001 |
| Range† | 0-929 | 839- 1061 |
964- 1179 |
1083- 1332 |
1234- 2856 |
|
| Age | 52.8 | 53.4 | 53.5 | 53.9 | 53.7 | <0.0001 |
| African-American, % | 33.1 | 24.5 | 16.8 | 13.3 | 9.2 | <0.0001 |
| Education, % | <0.0001 | |||||
| Less than completed high school | 29.1 | 22.2 | 17.2 | 13.2 | 11.5 | |
| High school completed | 45.9 | 42.7 | 42.6 | 41.1 | 36.3 | |
| More than high school | 25.0 | 35.2 | 40.2 | 45.7 | 52.2 | |
| BMI (kg/m2) | 26.5 | 26.7 | 26.6 | 26.7 | 26.8 | 0.04 |
| Weight (kg) | 75.2 | 76.0 | 75.7 | 76.5 | 76.8 | 0.0007 |
| Waist circumference (cm) | 94.1 | 94.3 | 93.9 | 94.6 | 94.5 | 0.23 |
| Systolic blood pressure (mmHg) | 119.7 | 118.6 | 117.1 | 116.7 | 115.3 | <0.0001 |
| Diastolic blood pressure (mmHg) | 73.3 | 72.8 | 72.0 | 71.2 | 70.9 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 96.8 | 93.8 | 92.3 | 91.6 | 91.9 | <0.0001 |
| Current smokers, % | 37.0 | 28.7 | 25.9 | 21.7 | 19.6 | <0.0001 |
| Physical activity (sports index) | 2.3 | 2.4 | 2.5 | 2.6 | 2.7 | <0.0001 |
| Alcohol (g/day) | 9.7 | 6.7 | 5.7 | 5.3 | 4.7 | <0.0001 |
| Energy intake (Kcal/day) | 1851 | 1511 | 1504 | 1549 | 1806 | 0.24 |
| Vitamin D intake (IU/day) | 115 | 167 | 203 | 248 | 358 | <0.0001 |
| Calcium intake (mg/day) | 396 | 533 | 624 | 726 | 1047 | <0.0001 |
| Potassium intake (mg/day) | 2066 | 2445 | 2637 | 2843 | 3231 | <0.0001 |
| Sodium intake (mg/day) | 1249 | 1424 | 1491 | 1566 | 1687 | <0.0001 |
| Magnesium intake (mg/day) | 198 | 235 | 254 | 277 | 315 | <0.0001 |
| Coffee intake (cups/day) | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 0.71 |
| Fruit and vegetables intake (servings/day) |
3.1 | 3.2 | 3.4 | 3.7 | 4.3 | <0.0001 |
| Whole grain intake (servings/day) | 0.7 | 0.9 | 1.2 | 1.5 | 2.0 | <0.0001 |
eGFR: estimated glomerular filtration rate
Chi-square (categorical variables) or linear regression (continuous variables)
Because cutoff points are sex-specific, overlap exists between quintiles
Table 2.
Selected characteristics of the study sample (mean or prevalence) by sex-specific quintile of phosphorus intake at baseline, MESA, 2000-2002
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend* |
|---|---|---|---|---|---|---|
| N | 731 | 732 | 732 | 732 | 732 | |
| Phosphorus intake (mg/day) | 787 | 968 | 1063 | 1171 | 1526 | <0.0001 |
| Range† | 50- 985 |
845- 1092 |
950- 1185 |
1040- 1324 |
1183- 3570 |
|
| Age (years) | 58.1 | 60.0 | 60.4 | 60.9 | 61.6 | <0.0001 |
| Race/ethnicity, % | <0.0001 | |||||
| White | 36.5 | 41.8 | 42.4 | 49.3 | 57.9 | |
| African-American | 28.9 | 21.6 | 18.6 | 13.8 | 12.2 | |
| Hispanic/Latino | 24.1 | 20.5 | 21.6 | 21.6 | 22.3 | |
| Chinese-American | 10.5 | 16.1 | 17.5 | 15.3 | 7.7 | |
| Education, % | <0.0001 | |||||
| Grade 11 or less | 16.0 | 17.6 | 16.8 | 14.8 | 13.1 | |
| High school completed | 48.8 | 46.3 | 44.4 | 38.9 | 40.9 | |
| Bachelors degree or more | 35.2 | 36.1 | 38.8 | 46.3 | 46.0 | |
| BMI (kg/m2) | 28.4 | 27.1 | 27.0 | 27.0 | 27.3 | 0.0002 |
| Weight (kg) | 79.9 | 75.3 | 75.1 | 75.5 | 77.0 | 0.004 |
| Waist circumference (cm) | 97.4 | 94.5 | 94.1 | 94.5 | 95.8 | 0.04 |
| Systolic blood pressure (mmHg) | 122.6 | 120.6 | 120.8 | 120.7 | 120.1 | 0.02 |
| Diastolic blood pressure (mmHg) | 72.4 | 70.8 | 70.7 | 70.0 | 69.7 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 81.6 | 81.0 | 79.4 | 78.8 | 79.0 | <0.0001 |
| Current smokers, % | 18.1 | 16.1 | 11.3 | 11.8 | 10.0 | 0.0003 |
| Physical activity (MET-min/wk) | 1428 | 1520 | 1582 | 1730 | 1984 | <0.0001 |
| Alcohol (g/day) | 8.2 | 6.3 | 4.2 | 5.1 | 4.8 | <0.0001 |
| Energy intake (Kcal/day) | 2118 | 1558 | 1438 | 1520 | 1929 | <0.0001 |
| Calcium intake (mg/day) | 422 | 612 | 702 | 821 | 1331 | <0.0001 |
| Potassium intake (mg/day) | 2176 | 2500 | 2658 | 2877 | 3378 | <0.0001 |
| Sodium intake (mg/day) | 2274 | 2447 | 2513 | 2484 | 2475 | <0.0001 |
| Magnesium intake (mg/day) | 211 | 246 | 263 | 286 | 333 | <0.0001 |
| Coffee intake (cups/day) | 1.4 | 1.3 | 1.1 | 1.3 | 1.5 | 0.94 |
| Fruit and vegetables intake (servings/day) |
4.1 | 3.9 | 3.9 | 4.3 | 4.5 | <0.0001 |
| Whole grain intake (servings/day) | 0.5 | 0.5 | 0.5 | 0.6 | 0.8 | <0.0001 |
eGFR: estimated glomerular filtration rate
Chi-square (categorical variables) or linear regression (continuous variables)
Because cutoff points are sex-specific, overlap exists between quintiles
In the baseline cross-sectional analysis, higher phosphorus intake was associated with lower levels of systolic and diastolic BP in both cohorts, even after adjustment for potential confounders (table 3; models 1 and 2). In models adjusted for sociodemographic variables and the main risk factors for high BP, systolic BP in ARIC participants in the highest quintile of phosphorus intake was 2.3 mmHg lower (95% CI, 1.3, 2.3) than in those in the lowest quintile. Among MESA participants, results were similar (difference in systolic BP between extreme quintiles: −2.3 mmHg, 95% CI, −4.2, −0.5). Smaller differences were observed for diastolic BP (ARIC: −0.9 mmHg, 95% CI −1.5, −0.3; MESA: −1.4 mmHg, 95% CI −2.3, −0.4, comparing extreme quintiles). Cochran’s Q statistic did not provide evidence of heterogeneity. Subsequent adjustment for other dietary factors attenuated the associations between phosphorus intake and BP. Still, the pooled analysis showed an association of phosphorus intake with systolic BP (table 3, model 3: −2.0 mmHg, 95% CI −3.6, −0.5, comparing extreme quintiles).
Table 3.
Cross-sectional difference (95% confidence intervals) in systolic and diastolic blood pressure by quintiles of phosphorus intake at baseline, ARIC, 1987-89, and MESA, 2000-2002
| ARIC | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
|---|---|---|---|---|---|---|
| SBP | ||||||
| Model 1 | Ref | −0.6 (−1.6, +0.4) | −1.3 (−2.4, −0.3) | −1.8 (−2.8, −0.8) | −2.6 (−3.6, −1.5) | <0.0001 |
| Model 2 | Ref | −0.4 (−1.4, +0.6) | −1.0 (−2.0, +0.0) | −1.5 (−2.5, −0.5) | −2.3 (−3.3, −1.3) | <0.0001 |
| Model 3 | Ref | −0.1 (−1.2, +1.0) | −0.6 (−1.9, +0.7) | −1.1 (−2.6, +0.3) | −2.3 (−4.0, −0.5) | 0.01 |
| DBP | ||||||
| Model 1 | Ref. | +0.2 (−0.4, +0.8) | +0.1 (−0.5, +0.7) | −0.5 (−1.1, +0.2) | −0.4 (−1.1, +0.2) | 0.04 |
| Model 2 | Ref. | +0.1 (−0.5, +0.7) | −0.1 (−0.7, +0.6) | −0.7 (−1.3, −0.1) | −0.9 (−1.5, −0.3) | 0.0004 |
| Model 3 | Ref. | +0.2 (−0.5, +0.9) | +0.2 (−0.6, +1.0) | −0.4 (−1.3, +0.5) | −0.5 (−1.6, +0.6) | 0.24 |
| MESA | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
|---|---|---|---|---|---|---|
| SBP | ||||||
| Model 1 | Ref | −2.7 (−4.6, −0.9) | −2.7 (−4.5, −0.8) | −2.9 (−4.8, −1.1) | −3.9 (−5.8, −2.1) | 0.0002 |
| Model 2 | Ref | −2.1 (−3.9, −0.2) | −1.6 (−3.5, +0.2) | −1.3 (−3.2, +0.6) | −2.3 (−4.2, −0.5) | 0.05 |
| Model 3 | Ref | −1.4 (−3.5, +0.7) | −0.7 (−3.1, +1.7) | −0.1 (−2.8, +2.6) | −1.2 (−4.5, +2.2) | 0.66 |
| DBP | ||||||
| Model 1 | Ref. | −1.3 (−2.3, −0.4) | −1.2 (−2.2, −0.3) | −1.7 (−2.6, −0.7) | −1.8 (−2.8, −0.9) | 0.0004 |
| Model 2 | Ref. | −1.1 (−2.1, −0.2) | −0.9 (−1.9, +0.0) | −1.3 (−2.2, −0.3) | −1.4 (−2.3, −0.4) | 0.01 |
| Model 3 | Ref. | −0.9 (−2.0, +0.1) | −0.7 (−1.9, +0.6) | −0.8 (−2.2, +0.6) | −1.0 (−2.7, +0.8) | 0.39 |
| Pooled | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
|---|---|---|---|---|---|---|
| SBP | ||||||
| Model 1 | Ref | −1.1 (−2.0, −0.2) | −1.6 (−2.5, −0.8) | −2.0 (−2.9, −1.1) | −2.9 (−3.8, −2.0) | <0.0001 |
| Model 2 | Ref | −0.8 (−1.7, +0.1) | −1.1 (−2.0, −0.2) | −1.4 (−2.3, −0.5) | −2.3 (−3.2, −1.4) | <0.0001 |
| Model 3 | Ref | −0.4 (−1.4, +0.6) | −0.6 (−1.7, +0.5) | −0.9 (−2.2, +0.3) | −2.0 (−3.6, −0.5) | 0.01 |
| DBP | ||||||
| Model 1 | Ref. | −0.2 (−0.8, +0.3) | −0.3 (−0.8, +0.2) | −0.8 (−1.4, −0.3) | −0.9 (−1.4, −0.3) | 0.0001 |
| Model 2 | Ref. | −0.3 (−0.8, +0.2) | −0.3 (−0.8, +0.2) | −0.9 (−1.4, −0.3) | −1.0 (−1.6, −0.5) | <0.0001 |
| Model 3 | Ref. | −0.1 (−0.7, +0.5) | −0.1 (−0.7, +0.6) | −0.5 (−1.3, +0.2) | −0.6 (−1.6, +0.3) | 0.20 |
SBP: systolic blood pressure; DBP: diastolic blood pressure
Model 1: Linear regression model adjusted for age, race, and sex. Model 2: Same as model 1, adjusted additionally for body mass index, waist circumference, eGFR, education, income, physical activity, cigarette smoking, study site, alcohol intake, and energy intake; Model 3: Same as model 2, adjusted additionally for calcium, vitamin D (only in ARIC), sodium, potassium, magnesium, fruits and vegetables, and whole grains intake.
During an average follow-up of 6.2 years (7.1 in ARIC, 3.8 in MESA), 3345 incident cases of hypertension (2400 in ARIC, 945 in MESA) were identified. Individuals in the top quintile of phosphorus intake had approximately a 10% lower risk of hypertension than those in the lowest, after adjustment for potential confounders (table 4, models 1 and 2). HRs (95% CI) of hypertension comparing extreme quintiles were 0.89 (0.78, 1.03) in ARIC, 0.90 (0.73, 1.10) in MESA, and 0.89 (0.80, 1.00) in the pooled analysis. The association disappeared after adjustment for other dietary factors (table 4, model 3). Results were virtually identical when we used Poisson regression instead of Cox models (data not shown). Restricting the definition of incident hypertension to systolic BP ≥140 or diastolic ≥90, without considering use of antihypertensive medications, or including only participants with systolic and diastolic BP <130/80 at baseline did not appreciably change the results (data not shown). Similarly, analyses restricted to never smokers, or adjusting additionally for pack-years of smoking, season of baseline BP measurement, or weight provided similar results.
Table 4.
Hazard ratios (95% confidence intervals) of hypertension by quintiles of phosphorus intake, ARIC, 1987-1998, and MESA, 2000-2007
| ARIC | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
|---|---|---|---|---|---|---|
| N | 1528 | 1618 | 1667 | 1701 | 1694 | |
| Cases | 466 | 527 | 490 | 484 | 433 | |
| Model 1 | 1 (ref.) | 1.09 (0.97, 1.24) | 0.98 (0.86, 1.11) | 0.95 (0.84, 1.08) | 0.86 (0.75, 0.98) | 0.003 |
| Model 2 | 1 (ref.) | 1.12 (0.99, 1.27) | 1.01 (0.89, 1.16) | 0.99 (0.87, 1.13) | 0.89 (0.78, 1.03) | 0.02 |
| Model 3 | 1 (ref.) | 1.14 (0.99, 1.32) | 1.05 (0.89, 1.24) | 1.03 (0.85, 1.24) | 0.97 (0.77, 1.24) | 0.63 |
| MESA | ||||||
|---|---|---|---|---|---|---|
| N | 555 | 591 | 585 | 588 | 582 | |
| Cases | 194 | 192 | 188 | 179 | 192 | |
| Model 1 | 1 (ref.) | 0.83 (0.68, 1.01) | 0.85 (0.69, 1.04) | 0.77 (0.63, 0.95) | 0.83 (0.68, 1.02) | 0.12 |
| Model 2 | 1 (ref.) | 0.87 (0.70, 1.06) | 0.90 (0.73, 1.11) | 0.82 (0.66, 1.02) | 0.90 (0.73, 1.10) | 0.38 |
| Model 3 | 1 (ref.) | 0.89 (0.71, 1.13) | 0.94 (0.72, 1.23) | 0.87 (0.64, 1.19) | 1.10 (0.75, 1.61) | 0.58 |
| Pooled | ||||||
|---|---|---|---|---|---|---|
| Model 1 | 1 (ref.) | 1.01 (0.91, 1.13) | 0.94 (0.84, 1.05) | 0.90 (0.80, 1.00) | 0.85 (0.76, 0.95) | 0.0008 |
| Model 2 | 1 (ref.) | 1.04 (0.94, 1.16) | 0.99 (0.88, 1.10) | 0.94 (0.84, 1.05) | 0.89 (0.80, 1.00) | 0.02 |
| Model 3 | 1 (ref.) | 1.07 (0.94, 1.21) | 1.02 (0.88, 1.17) | 0.98 (0.83, 1.15) | 1.01 (0.82, 1.23) | 0.88 |
Model 1: Cox proportional hazards model adjusted for age, race, and sex. Model 2: Same as model 1, adjusted additionally for body mass index, waist circumference, eGFR, education, income, physical activity, cigarette smoking, study site, alcohol intake, and energy intake. Model 3: Same as model 2, adjusted additionally for calcium, vitamin D (only in ARIC), sodium, potassium, magnesium, fruits and vegetables, and whole grains intake.
In analyses stratified by sex, greater phosphorus intake was similarly associated with lower risk of hypertension in men and women. The HR (95% CI) of hypertension comparing extreme quintiles of phosphorus intake was 0.86 (0.72, 1.03) in men and 0.93 (0.80, 1.09) in women (tables S1 and S2; please see http://hyper.ahajournals.org). Results stratified by race/ethnicity did not provide strong evidence of interaction, though the limited sample size among non-whites precluded drawing any meaningful conclusion (tables S3 and S4; please see http://hyper.ahajournals.org). Finally, no evidence of interaction by renal function was apparent (data not shown).
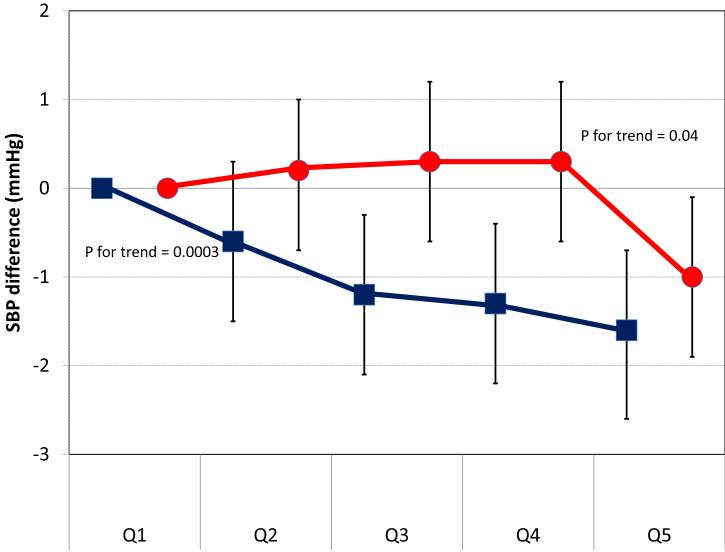
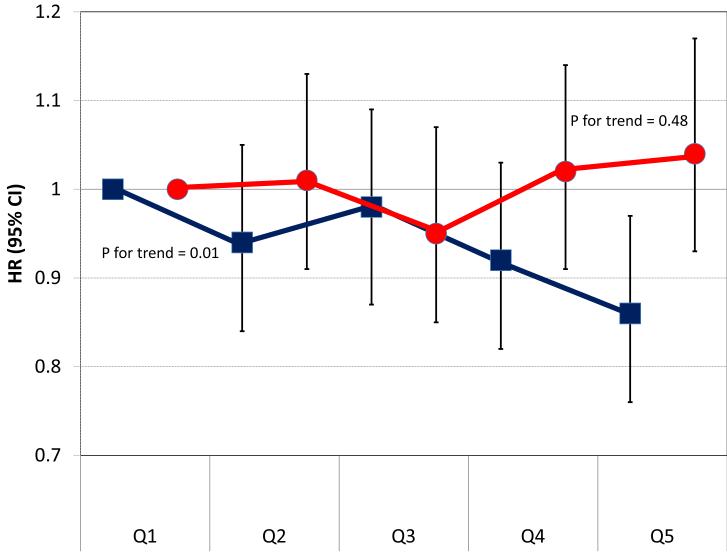
The main source of dietary phosphorus in both cohorts was dairy products (31% in ARIC, 29% in MESA), followed by fish (7%), red meat (7%), poultry (5%) and whole-grain bread (5%) in ARIC, and by white bread (6%), red meat (5%), whole grain bread (5%) and legumes (5%) in MESA. We estimated separately the association of phosphorus intake from dairy and non-dairy sources with cross-sectional BP levels and the risk of incident hypertension. Results are shown in figures 2 and 3. Overall, only higher phosphorus intake from dairy products, but not from other dietary sources, was consistently associated with lower levels of systolic BP and lower risk of hypertension.
Figure 2.
Cross-sectional difference in systolic BP (SBP) levels (and 95% confidence intervals) by quintiles of phosphorus from dairy products (black squares) and phosphorus from non-dairy foods (dark grey circles). Pooled results from ARIC and MESA. Linear regression model adjusted for age, race, sex, body mass index, waist circumference, eGFR, education, income, physical activity, cigarette smoking, study site, alcohol intake, and energy intake.
Figure 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of hypertension by quintiles of phosphorus from dairy products (black squares) and phosphorus from non-dairy foods (dark grey circles). Pooled results from ARIC and MESA. Cox proportional hazard model adjusted for age, race, sex, body mass index, waist circumference, eGFR, education, income, physical activity, cigarette smoking, study site, alcohol intake, and energy intake.
We conducted an additional analysis correcting diet measurement error using regression calibration.25 A 500 mg/day higher phosphorus intake was associated with 1.1 mmHg lower systolic BP (95% CI 0.5, 1.8) in the uncorrected analysis and 2.1 (95% CI 1.1, 3.0) in the corrected analysis. HR (95% CI) of hypertension associated with the same difference in phosphorus intake was 0.93 (0.87, 1.00) in the uncorrected analysis, and 0.88 (0.79, 0.99) in the corrected (detailed results are presented in tables S5 and S6, please see http://hyper.ahajournals.org).
DISCUSSION
In this analysis of two large population-based cohorts, we have found that phosphorus intake was inversely associated with systolic blood pressure levels, even after adjustment for confounders and highly correlated nutrients. Additionally, we observed a lower risk of hypertension among individuals with a higher phosphorus intake, but this association was considerably attenuated after adjusting for other dietary factors. More importantly, we found that these associations were only present for dietary phosphorus from dairy products but not for phosphorus from other dietary sources. As a whole, our results suggest that dairy foods, but not phosphorus per se, might have a beneficial effect on BP.
A few previous epidemiologic studies have assessed the association of phosphorus intake with BP levels. In the INTERMAP study, which included 4680 individuals from Japan, China, US, and the United Kingdom whose diet was assessed with multiple 24-hour recalls, increments of 2 standard deviations in phosphorus intake were associated with 2.2 mmHg and 1.7 mmHg lower systolic and diastolic BP in multivariable analysis.4 Similar results were found among 615 men of Japanese ancestry living in Hawaii, with those in the upper quintile of phosphorus intake, measured with one 24-hour recall, having 3.2mmHg lower systolic BP and 2.0 mmHg lower diastolic BP than those in the lowest quintile, after adjusting for age and body mass index.26 Finally, a cross-sectional analysis of 4519 NHANES 1999-2004 participants found that higher phosphorus intake, also assessed with one 24-hour recall, was weakly associated with lower levels of BP (−0.04 and −0.03 per each 100 mg phosphorus/day),5 though results in the opposite direction were found in NHANES I (1971-1975).27 Overall, existing data, including our results, suggests that higher phosphorus intake is associated with lower BP in cross-sectional analysis. Previous studies, however, did not explore whether this association varied according to dietary source of phosphorus.
We have found that only phosphorus from dairy products was associated with lower BP and decreased risk of hypertension in ARIC and MESA. A growing body of evidence suggests that higher consumption of dairy products is associated with lower risk of hypertension. Low-fat dairy products were a substantive part of the combination diet in the Dietary Approaches to Stop Hypertension (DASH) trial (the so-called DASH diet). The DASH diet was more effective in reducing BP than a diet rich in fruits and vegetables or a control diet.2 A number of prospective cohort studies have found lower risk of hypertension in individuals with higher dairy product intake (particularly low-fat dairy),28-33 including a recent analysis from the ARIC cohort.34 Parallel to our results, calcium from dairy products but not from other sources was associated with lower risk of hypertension in another prospective study.30 Our results highlight, once more, the importance of focusing on foods, in addition to nutrients, in nutritional epidemiology;35 and offer additional evidence of the potential beneficial effect of dairy foods on BP.
No convincing biological mechanism has been provided to explain how dietary phosphorus could reduce BP. Though dietary phosphorus might increase serum phosphorus6-8 and this, in turn, could be associated with lower BP,36 other studies have shown that dietary phosphorus has a limited impact on serum phosphorus levels.9-11 In addition, dietary phosphorus might have deleterious effects on BP. For example, dietary phosphorus loading has been associated with impaired endothelium-dependent vasodilation.37 Also, higher phosphorus intake could lead to lower levels of circulating 1-25-dihydroxy vitamin D,6 with lower serum levels of vitamin D associated with higher BP levels.38 Alternatively, it might be other nutrients in dairy foods, such as calcium, magnesium, potassium and lactopeptides, or their combination, that is effective in reducing BP levels and the risk of hypertension.39 The present analysis supports the latter explanation.
Our study has valuable strengths. We have analyzed data from two large cohorts, recruited in different areas of the US, and including a multi-ethnic population. The prospective design allowed us to estimate incidence rates of hypertension across categories of phosphorus intake. Additionally, BP measurements were performed following cohort-specific standardized protocols, reducing measurement error. We also accounted in the analysis for important potential confounders, particularly multiple lifestyle-related behaviors. Finally, losses to follow-up in both cohorts were limited, reducing the risk for selection bias.
As with other studies in nutritional epidemiology, one main limitation of the present analysis is the potential for measurement error in the dietary assessment, particularly relevant for some nutrients such as sodium. Food questionnaires used in both ARIC and MESA have been previously validated,20, 21 but certain degree of non-differential misclassification is unavoidable. We performed a regression-calibration analysis to partially remediate this problem. Results did not appreciably change after measurement error correction, suggesting that measurement error is unlikely to explain the observed associations.
Another major limitation is the high correlation of phosphorus with other nutrients potentially associated with BP, such as calcium, magnesium or potassium. High correlations lead to collinearity in multivariable models and, consequently, problems in the estimation of associations. This explains, in part, the large confidence intervals in the association estimates adjusted for other nutrients. High between-nutrient correlation, also, limits the ability of observational studies to assess the health effects of individual nutrients.35 Finally, unmeasured or residual confounding is an additional threat. Individuals with low or high phosphorus intake were substantially different in their lifestyles and other potential risk factors for high BP. Even though we have controlled for the major risk factors for hypertension, our results might overestimate the true association if healthier individuals had higher phosphorus intake.
PERSPECTIVES
We have shown that, in two diverse populations, higher phosphorus intake is associated with lower BP levels and a lower risk of hypertension, but these potential benefits seem to be restricted to phosphorus obtained through the intake of dairy products. This finding could be indicative of an effect of phosphorus in conjunction with other dairy constituents, or of dairy itself, even without involvement of phosphorus. Additional research is required to determine if other nutrients in dairy foods, or their combination, are responsible for this association.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the other investigators, staff and participants of the ARIC and MESA studies for their important contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
SOURCES OF FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. MESA was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
CONFLICTS OF INTEREST
Bryan Kestenbaum has received modest honoraria from Shire Inc and Genzyme Inc. David R. Jacobs has received significant funding from the National Institutes of Health, some of them focused on nutritional epidemiology. Additionally, he is unpaid scientific advisor for the California Walnut Commission.
References
- 1.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, Karanja N, Lin P-H. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 4.Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J, INTERMAP Cooperative Research Group Dietary phosphorus and blood pressure. International Study of Macro- and Micro-nutrients and Blood Pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr. 2008;87:1914–1925. doi: 10.1093/ajcn/87.6.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portale AA, Halloran BP, Murphy MM, Morris RC., Jr. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986;77:7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portale AA, Halloran BP, Morris RC., Jr. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus: implications for the renal production of 1,25-Dihydroxyvitamin D. J Clin Invest. 1987;80:1147–1154. doi: 10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemi VE, Karkkainen MUM, Lamberg-Allardt CJE. High phosphorus intakes acutely and negatively affect calcium and bone metabolism in a dose-dependent manner in healthy young females. Br J Nutr. 2006;96:545–552. [PubMed] [Google Scholar]
- 9.Mataix J, Aranda P, López-Jurado M, Sánchez C, Planells E, Llopis J. Factors influencing the intake and plasma levels of calcium, phosphorus and magnesium in southern Spain. Eur J Nutr. 2006;45:349–354. doi: 10.1007/s00394-006-0605-z. [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Rue T, Kestenbaum B. Serum phosphate concentrations in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr., Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, C, Recurrent Events Trial Investigators Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 14.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Calvo MS, Park YK. Changing phosphorus content of the U.S. diet: potential for adverse effects on bone. J Nutr. 1996;126:1168S–1180. doi: 10.1093/jn/126.suppl_4.1168S. [DOI] [PubMed] [Google Scholar]
- 16.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities Study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 19.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 22.HHHQ-DietSys Analysis Software [computer program]. Version 4.0. 1999. [Google Scholar]
- 23.Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 24.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 25.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65:1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 26.Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: the Honolulu heart study. Am J Clin Nutr. 1987;45:469–475. doi: 10.1093/ajcn/45.2.469. [DOI] [PubMed] [Google Scholar]
- 27.Harlan WR, Hull AL, Schmouder RL, Landis JR, Thompson FE, Larkin FA. Blood pressure and nutrition in adults: the National Health and Nutrition Examination Survey. Am J Epidemiol. 1984;120:17–28. doi: 10.1093/oxfordjournals.aje.a113870. [DOI] [PubMed] [Google Scholar]
- 28.Moore LL, Singer MR, Bradlee ML, Djoussé L, Proctor MH, Cupples LA, Ellison RC. Intake of fruits, vegetables, and dairy products in early childhood and subsequent blood pressure change. Epidemiology. 2005;16:4–11. doi: 10.1097/01.ede.0000147106.32027.3e. [DOI] [PubMed] [Google Scholar]
- 29.Pereira MA, Jacobs DR, Jr., Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults. The CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 30.Alonso A, Beunza JJ, Delgado-Rodríguez M, Martínez JA, Martínez-González MA. Low-fat dairy consumption and reduced risk of hypertension: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2005;82:972–979. doi: 10.1093/ajcn/82.5.972. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Manson JE, Buring JE, Lee I-M, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1–7. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 32.Engberink MF, Hendriksen MAH, Schouten EG, van Rooij FJA, Hofman A, Witteman JCM, Geleijnse JM. Inverse association between dairy intake and hypertension: the Rotterdam Study. Am J Clin Nutr. 2009;89:1877–1883. doi: 10.3945/ajcn.2008.27064. [DOI] [PubMed] [Google Scholar]
- 33.Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR., Jr. Associations of plant food, dairy product, and meat intakes with 15-year incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2005;82:1169–1177. doi: 10.1093/ajcn/82.6.1169. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A, Steffen LM, Folsom AR. Dairy intake and changes in blood pressure over 9 years: the ARIC study. Eur J Clin Nutr. 2009;63:1272–1275. doi: 10.1038/ejcn.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs DR, Jr., Tapsell LC. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev. 2007;65:439–450. doi: 10.1111/j.1753-4887.2007.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 36.Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure: Belgian Interuniversity Research on Nutrition and Health. Hypertension. 1988;12:589–593. doi: 10.1161/01.hyp.12.6.589. [DOI] [PubMed] [Google Scholar]
- 37.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Pfeuffer M, Schrezenmeir J. Milk and the metabolic syndrome. Obesity Reviews. 2006;8:109–118. doi: 10.1111/j.1467-789X.2006.00265.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.