Abstract
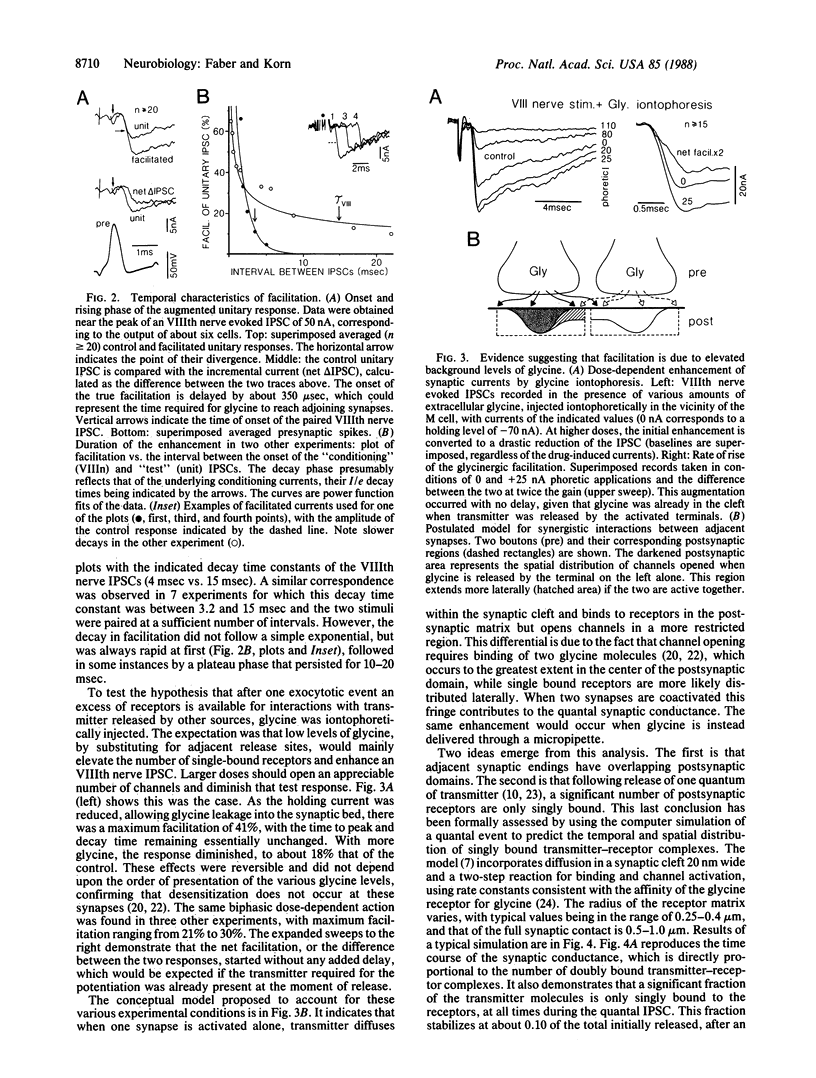
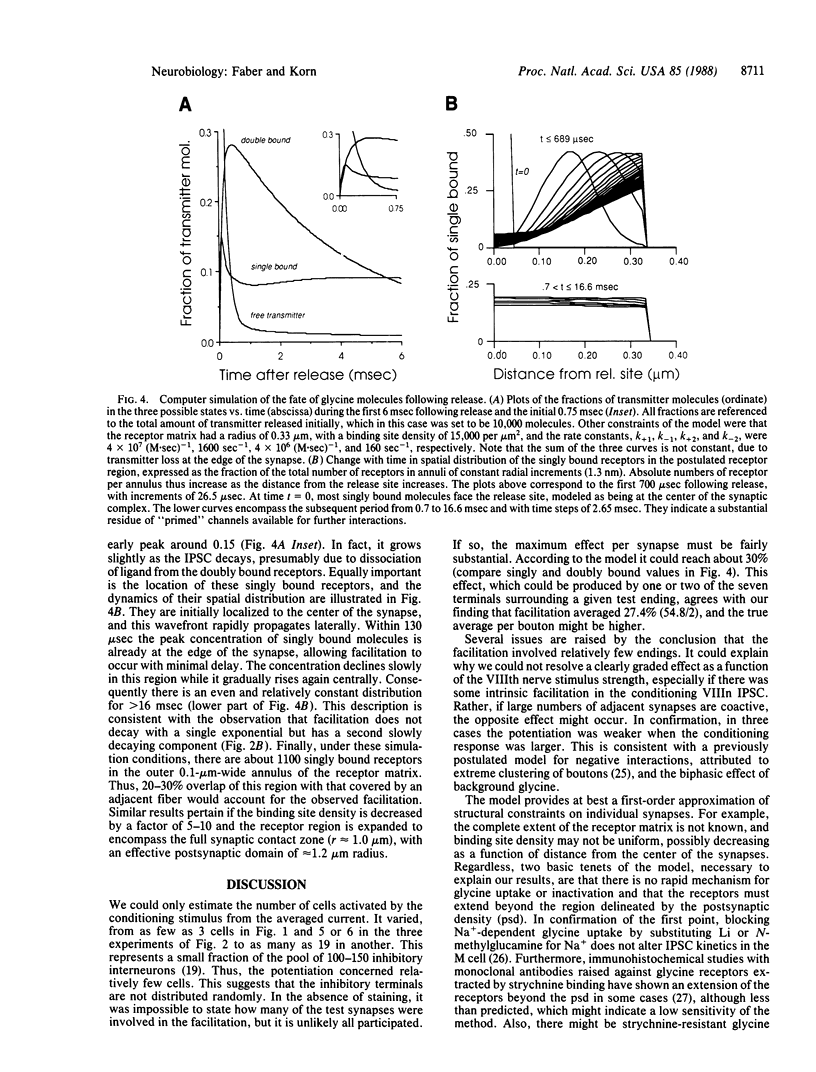
Recordings of inhibitory postsynaptic currents in the goldfish (Carassius auratus) Mauthner cell demonstrate nonlinear interactions when adjacent synapses are coactivated. Responses evoked by single presynaptic neurons were paired with those due to activation of a limited pool of similar inhibitory cells. In about 50% of the experiments the compound currents were substantially larger than the sum of their individual components. Potentiation was maximal when the two responses occurred nearly simultaneously, and its decay paralleled that of the conditioning current; it started with a delay of about 300 microsec, and calculations indicate that in this time transmitter molecules would diffuse laterally 0.5-1.1 micron, which equals the separation of adjacent synapses. Iontophoretically applied glycine produced a comparable enhancement of the eighth nerve evoked inhibitory current. When quantal inhibitory responses were simulated, taking into consideration glycine diffusion, transmitter-receptor interactions, and channel activation, with the latter requiring two binding steps, the results demonstrated that the facilitation when neighboring inputs are active is due to lateral diffusion of the transmitter, glycine, to fringe areas where single bound receptors are available for further interactions and channel opening.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Roper S. Analysis of Mauthner cell responses to iontophoretically delivered pulses of GABA, glycine and L-glutamate. J Physiol. 1973 Jul;232(1):113–128. doi: 10.1113/jphysiol.1973.sp010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Funch P. G., Korn H. Evidence that receptors mediating central synaptic potentials extend beyond the postsynaptic density. Proc Natl Acad Sci U S A. 1985 May;82(10):3504–3508. doi: 10.1073/pnas.82.10.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J Neurophysiol. 1982 Sep;48(3):654–678. doi: 10.1152/jn.1982.48.3.654. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J., Lebeda F. J. Role of uptake in gamma-aminobutyric acid (GABA)-mediated responses in guinea pig hippocampal neurons. Cell Mol Neurobiol. 1985 Dec;5(4):353–371. doi: 10.1007/BF00755401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Korn H., Burnod Y., Faber D. S. Spontaneous quantal currents in a central neuron match predictions from binomial analysis of evoked responses. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5981–5985. doi: 10.1073/pnas.84.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Faber D. S., Triller A. Probabilistic determination of synaptic strength. J Neurophysiol. 1986 Feb;55(2):402–421. doi: 10.1152/jn.1986.55.2.402. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S. Vertebrate central nervous system: same neurons mediate both electrical and chemical inhibitions. Science. 1976 Dec 10;194(4270):1166–1169. doi: 10.1126/science.186868. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A. Transformation of binomial input by the postsynaptic membrane at a central synapse. Science. 1984 Sep 14;225(4667):1157–1159. doi: 10.1126/science.6474167. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor site density affects the rising phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3736–3740. doi: 10.1073/pnas.77.6.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7200–7204. doi: 10.1073/pnas.78.11.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring R. H., Zigmond R. E. Ultrastructural distribution of 125I-toxin F binding sites on chick ciliary neurons: synaptic localization of a toxin that blocks ganglionic nicotinic receptors. J Neurosci. 1987 Jul;7(7):2153–2162. doi: 10.1523/JNEUROSCI.07-07-02153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Triller A., Korn H. Mise en évidence électrophysiologique et anatomique de neurones vestibulaires inhibiteurs commissuraux chez la tanche (Tinca tinca). C R Acad Sci Hebd Seances Acad Sci D. 1978 Jan;286(1):89–92. [PubMed] [Google Scholar]
- Triller A., Korn H. Transmission at a central inhibitory synapse. III. Ultrastructure of physiologically identified and stained terminals. J Neurophysiol. 1982 Sep;48(3):708–736. doi: 10.1152/jn.1982.48.3.708. [DOI] [PubMed] [Google Scholar]
- Triller A., Korn H. Variability of axonal arborizations hides simple rules of construction: a topological study from HRP intracellular injections. J Comp Neurol. 1986 Nov 22;253(4):500–513. doi: 10.1002/cne.902530407. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli S. J., Faber D. S. An identifiable class of statoacoustic interneurons with bilateral projections in the goldfish medulla. Neuroscience. 1980;5(7):1287–1302. doi: 10.1016/0306-4522(80)90201-8. [DOI] [PubMed] [Google Scholar]



