Abstract
Membrane proteins account for 70–80% of all pharmaceutical targets emphasizing their clinical relevance. Identification of new, differentially expressed membrane proteins reflecting distinct disease properties is thus of high importance. Unfortunately, isolation and analysis of membrane-bound proteins is hampered by their relative low abundance in total cell lysates, their frequently large size and their hydrophobic properties. We thus aimed to identify protocols that allow for highly efficient isolation and purification of membrane-bound proteins for subsequent protein profiling. We present a comparative study of different membrane protein extraction methods that vary in total protein yield between 0.02 and 4.8 mg using constant cell pellets of the colorectal carcinoma cell line SW620. We also demonstrate by means of polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot analysis that the majority of commercial membrane extraction kits harbor a substantial cytosolic contamination of their membranous fraction. Based on purity of membranous fraction, protein yield, time and costs, we show superiority of two commercial extraction kits for downstream proteome analyses of membrane proteins.
Keywords: Extraction techniques, Membrane proteins, Commercial kits, Sub-cellular fractions, Contamination, Protein yield
Introduction
Membrane proteins are critical for normal cellular differentiation and diverse functions (Sanders and Myers 2004). Alterations in protein patterns often lead to cell dysfunctions and disease phenotypes (Carter et al. 2004). The importance of membrane proteins for clinical application is underlined by the fact that they account for 70–80% of all drug targets. Furthermore, it is estimated that about two-thirds of all future drugs will target membrane proteins (Hopkins et al. 2006; Overington et al. 2006). Thus, identification of new, differentially expressed membrane proteins reflecting certain disease properties has the potential of providing novel biomarkers and therapeutic targets. The detection of membrane-bound proteins by standard proteomic methods, however, is challenging due to their relative low abundance in total cell lysates, their frequently large size and most notably their hydrophobic characteristics (Santoni et al. 2000; Wallin and von Heijne 1998). We thus aimed to identify protocols that allow for highly efficient isolation and purification of membrane-bound proteins for subsequent protein profiling. The traditional method for extraction of membrane proteins is a multistep detergent extraction combined with gradient-based ultracentrifugation (Helenius and Simons 1975; Tanford and Reynolds 1976). This approach is however time-consuming with up to 6 h processing time and requires an ultracentrifuge and sonication equipment (Zhang et al. 1994; Ellis et al. 1992). A potentially faster and easier way to isolate membrane proteins from mammalian cells or tissue might be provided by commercial membrane protein extraction kits. In this report, we present a comparative analysis of five commercial extraction kits regarding protein yield, membranous fraction purity, time and costs. Hereby we provide evidence that the majority of commercial kits harbor a substantial cytosolic contamination of the membranous fraction. We further identify two commercial extraction kits that prove to be superior for downstream proteome analyses of membrane-bound proteins.
Materials and methods
Samples
The colorectal adenocarcinoma cell line SW620 was obtained from ATCC (accession no. CCL-227™, Manassas, VA, USA). Cells were cultured in T175 flasks in Leibovitz L15 medium (PAA Laboratories, Pasching, Austria), supplemented with 10% (v/v) unheated fetal bovine serum (FBS, PAA, Laboratories, Pasching, Austria) and 1% (v/v) antibiotics (penicillin and streptomycin) in a humidified incubator without CO2 at 37 °C. The cells were grown to about 80% confluence and harvested by scraping in phosphate buffered saline or kit buffers, depending on the different extraction protocols provided by the kit suppliers.
Protein extraction
The five commercial kits were utilized each for extraction of 8–10 × 107 colon cancer cells. The extractions were performed according to the manufacturer’s instructions. All extractions were performed as three replicates. Each of the commercial kits yielded a cytosolic-soluble and one membranous protein fraction simultaneously.
Both sub-cellular fractions were further quantified and evaluated for cross contamination in parallel for each kit.
Protein quantification
Protein concentrations of both sub-cellular fractions were quantified using either the Micro-BCA™ Protein Assay Reagent Kit (Pierce, Rockford, IL, USA) or the Bradford method (Bradford 1976) depending on kit detergents and provider-advices. Fraction V, protease-free bovine serum albumin ((BSA), Roche Applied Science, Mannheim, Germany) was used as the protein standard.
SDS–PAGE and Western blotting
Protein samples were separated first using 7.5% (kit I–IV) or 16% (kit V) Tris–glycine SDS–PAGE gels and visualized by Commassie™ Brilliant Blue R-250 (Bio-Rad Laboratories, Hercules, CA, USA) staining to show differences in protein pattern between the cytosolic and membranous protein fractions. In order to check kit’s sub-cellular fractionation potential and purity of obtained fractions, 10 μg per lane of each cytosolic and membranous protein fractions were blotted to a Polyvinylidenfluorid membrane (PVDF, Carl Roth GmbH, Karlsruhe, Germany). For kit V, only 8 μg per lane could be used due to the low concentrations of both fractions. The blots were blocked in 5% (w/v) Blocking buffer (5 g non-fat dry milk (# 170-6404, Bio-Rad Laboratories, Hercules, CA, USA) in 100 mL 1x Tris-Buffered Saline with 0.1% (w/v) Tween-20 (#9997, Cell Signaling, Danvers, MA, USA) after probing with specific primary and horseradish peroxidase (HPR)-conjugated secondary anti mouse IgG antibody. Detection was performed with Immobilon Western chemiluminescence HRP Substrate (Millipore Corporation, MA, USA) followed by 30–300 s exposure to a 16-bit-charge-coupled device (CCD) camera of the imaging system ChemiDoc XRS+ (Bio-Rad Laboratories, Hercules, CA, USA). Specific marker-protein-antibodies for each fraction were selected using GAPDH (0.05–0.5 μg/mL ab37187) and AnnexinV (3 μg/mL, ab54775) for cytosolic fractions and panCadherin (3 μg/mL, ab22744), sodium potassium ATPase pump (0.4 μg/mL ab7671) (all of the above named were of Abcam plc, Cambridge, UK) and Calnexin (0.3 μg/mL, GTX22798, GeneTex, CA, USA) for membranous protein fractions.
Costs
The cost per extraction was calculated for each commercially available kit by dividing the cost by the number of extractions that could be performed with the kit.
Statistical analysis
Reproducibility of kits and variability of protein yield was determined by the coefficient of variation (CV).
Results and discussion
Comparison of selectivity and purity of sub-cellular fractions
According to the protocols supplied by the manufacturers, all five extraction kits yielded two sub-cellular fractions simultaneously: one cytosolic and one membranous fraction. Only Kit V yielded two cytosolic fractions (C1, C2) due to a recurring separation step in the protocol.
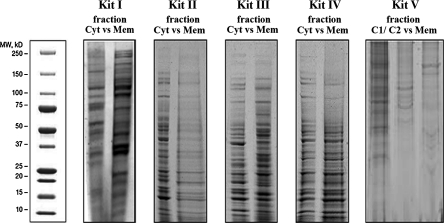
First, assessment of overall differences in protein patterns was performed by means of SDS–PAGE analysis. Hereby, cytosolic and membranous fractions of each kit were compared with each other. The membrane protein patterns (Mem) were distinct from the patterns of the corresponding cytosolic fractions (Cyt) particularly for kits I, III and V (Fig. 1). The membranous fractions of these three kits showed different protein bands as compared to their corresponding cytosolic fraction. In contrast, hardly any differences in the overall protein patterns could be detected between the sub-cellular fractions of kit II and IV (Fig. 1). Therefore, SDS–PAGE analysis presented kit I, III and V to show the most pronounced differences in overall protein patterns between the membranous and the corresponding cytosolic fractions. It is noteworthy though, that in the membranous fraction of Kit V not many distinct protein bands could be detected as compared to the corresponding two cytosolic fractions and in comparison to the membranous fractions of Kit I and III. The latter two kits thus seem to be superior in terms of membrane protein extraction selectivity and protein yield.
Fig. 1.
Comparison of overall protein patterns of membranous and cytosolic fractions. Extracted cytosolic (Cyt) and membranous (Mem) fractions of colon cancer cells separated using SDS–PAGE and visualized by Coomassie staining in order to show overall differences in protein patterns between both fractions. Kit V yielded two cytosolic fractions (C1, C2) due to repeated separation steps. Cyt cytosolic fraction, Mem membranous fraction, vs versus. The membranous fractions of kits I, III and V showed different protein bands as compared to their corresponding cytosolic fraction. In contrast, hardly any differences in the overall expression patterns could be detected between the sub-cellular fractions of kit II and IV. Reproducibility of replicates was between 67% (Kit I) and 100% (Kit II, III, IV and V)
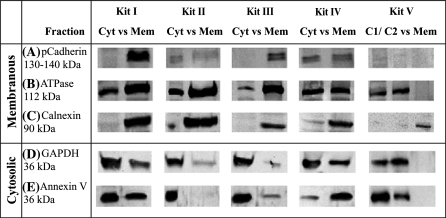
In order to further test the purity of the two sub-cellular fractions obtained by each kit, we performed Western blotting for all 11 sub-cellular fractions in three replicates using three membrane and two cytosolic protein markers. Reproducibility of replicates was between 73% (Kit IV), 80% (Kit I and II), 87% (Kit III) and 100% (Kit V). The membrane protein markers have been chosen as well known tumor biomarkers and proving potential as pharmaceutical targets, e.g. for prostate cancer (panCadherin—cell membrane receptor), melanomas (Calnexin—integral ER-membrane), and other malignancies (ATPase—multi-pass integral membrane protein) (Chen et al. 2006; Mijatovic et al. 2008; Dissemond et al. 2004; Wehbi et al. 2002). The two cytosolic protein markers (AnnexinV, GAPDH) were selected due to their well known cytosolic location and their potential for anti-cancer treatment response monitoring (Corsten et al. 2006; Kenis et al. 2007).
Analysis of Kit I revealed that two of the three membrane protein markers (panCadherin, Calnexin) showed expression only in the membranous fraction (M) (Fig. 2). The third membrane protein, ATPase, could also be detected in the cytosolic fraction. The two cytosolic marker proteins (GAPDH, Annexin V) could be observed in both, the cytosolic and the membranous fraction. They were however less intensely stained in the membranous fraction. In summary, Kit I retains all three membrane proteins and shows a fairly good separation of those compared to the cytosolic fraction. However, the membranous fraction shows substantial contamination with both cytosolic proteins.
Fig. 2.
Comparison of the purity of membranous and cytosolic fractions by Western blotting of fractionated colon cancer cells using specific antibodies to each sub-cellular fraction. (A − C) membrane-specific and (D + E) cytosol specific antibodies. Cyt cytosolic fraction, Mem membranous fraction, vs versus. Kit I retains all three membrane proteins and shows a fairly good separation of those compared to the cytosolic fraction. However, the membranous fraction shows substantial contamination with both cytosolic proteins. Kit II only retains two of three membrane proteins and one of them is also detectable in the corresponding cytosolic fraction. In contrast, the cytosolic proteins are well separated. Kit III shows an almost perfect separation of both, the membranous and cytosolic fraction with minimal cross-contamination. Kit IV shows overall poor separation characteristics with strong cross-contamination of both sub-cellular fractions. Kit V poorly retains proteins of the membranous fraction. Both cytosolic proteins could be well separated with negligible cross-contamination of the membranous fraction. Reproducibility of replicates was between 73% (Kit IV), 80% (Kit I and II), 87% (Kit III) and 100% (KitV)
Kit II showed a good separation only for one of the three membrane proteins (Calnexin) (Fig. 2). The remaining two membrane proteins are present at fairly similar concentrations in both sub-cellular fractions: panCadherin is present at very low concentrations overall whereas ATPase is strongly expressed in both fractions. For Kit II, only the cytosolic marker proteins were separated clearly between both fractions with the stronger staining intensity being visible in the cytosolic fraction. In summary, Kit II only retains two of three membrane proteins and one of them is also detectable in the corresponding cytosolic fraction. In contrast, the cytosolic proteins are well separated.
Kit III very well maintained all three membrane proteins in the membranous fraction and presented an overall negligible contamination of the cytosolic fraction (Fig. 2). Both cytosolic proteins were strongly retained within their fraction and well separated from the membranous fraction. In summary, Kit III shows an almost perfect separation of both, the membranous and cytosolic fraction with minimal cross-contamination.
Kit IV retains all three membrane proteins in their fraction, however, shows strong cross-contamination in the cytosolic fraction for two of them (Fig. 2). Both cytosolic proteins show strong contamination of the membranous fraction and only one of them is also retained sufficiently in the cytosolic fraction. In summary, Kit IV shows overall poor separation characteristics with strong cross-contamination of both sub-cellular fractions.
Kit V maintains only one (Calnexin) of three proteins in the membranous fraction (Fig. 2). The remaining two membrane proteins are present at weak (panCadherin) and strong concentration (ATPase) in the cytosolic fractions only. Overall good separation was achieved for the two cytosolic proteins. In summary, Kit V poorly retains proteins of the membranous fraction. Both cytosolic proteins could be well separated with negligible cross-contamination of the membranous fraction.
In order to compare the performance of the five kits in between each other we allocated for each kit protein scoring points a) in the membranous fraction if the membrane-bound protein was retained and/or well separated from the cytosolic fraction and b) in the cytosolic fraction if the cytosolic protein was retained and no cross-contamination of the membranous fraction could be detected. We considered a good separation in between sub-cellular fractions important for downstream analysis so that the majority of membrane-bound proteins were contained in the membranous fraction and that this fraction was not compromised by contamination of cytosolic proteins. According to Table 1 it becomes obvious that Kit III obtained the highest possible score due to almost perfect separation within both sub-cellular fractions while retaining all proteins in the desired fractions. Kit IV showed the poorest score particularly due to overall low separation performance. Also Kit V showed a low score due to poor separation and low recovery of membrane-bound proteins. Kit I and II both obtained the second highest score: Kit II proved low performance in retaining membrane-bound proteins but almost perfect separation of cytosolic proteins and hence almost no contamination of the membranous fraction. In contrast, Kit I retained all membrane-bound proteins but showed high contamination of cytosolic proteins in the membranous fraction. According to the above evaluated parameters we therefore strongly suggest Kit III to be used for membrane-bound protein extractions for downstream analyses whereas Kit I and II qualify as second choice.
Table 1.
Scoring of extraction performance for subsequent membrane protein profiling
| Scoring parameters | Kit I | Kit II | Kit III | Kit IV | Kit V | |
|---|---|---|---|---|---|---|
| Membrane protein fraction | Retaining membrane-bound proteins | +++ | ++ | +++ | +++ | + |
| Well separated from cytosolic fraction | ++ | + | +++ | + | + | |
| Points | 5 | 3 | 6 | 4 | 2 | |
| Cytosolic protein fraction | Retaining cytosolic proteins | ++ | ++ | ++ | + | ++ |
| No cross contamination of membranous fraction | ++ | ++ | ++ | |||
| Points | 2 | 4 | 4 | 1 | 4 | |
| Score sum | 7 | 7 | 10 | 5 | 6 |
In order to compare the performance of the five kits in between each other we allocated scoring points (a) in the membranous fraction if the membrane-bound protein was retained and/or well separated from the cytosolic fraction and (b) in the cytosolic fraction if the cytosolic protein was retained and no cross-contamination of the membranous fraction could be detected. Description of the Scoring: (+++) excellent, (++) good, (+) poor. According to the evaluated parameters we strongly suggest Kit III to be used for membrane-bound protein extractions for downstream analyses whereas Kit I and II qualify as second choice
Comparison of protein yield, recovery volume, processing time and costs
Even though purity and selectivity are the most important characteristics for choosing a certain sub-cellular extraction method, additional factors may influence the choice of product: protein yield, recovery volume, processing time and costs impact on the workflow for downstream analyses and the overall project design.
The protein yield fluctuated in a range of 0.02–4.8 mg for the membranous fraction depending on the kit used (Table 2). Interestingly, the two kits with the lowest total protein yield were the ones that showed weak staining overall on the SDS–PAGE (Fig. 1) and that obtained only low recovery of membrane-bound proteins (Fig. 2, Table 1): Kit II showed a total membrane protein yield of 0.02–0.03 mg, and Kit V only of 0.3–0.5 mg. While Kit V had been observed to be of inferior quality regarding purity and separation, Kit II had obtained the second best score in this respect. However, the lowest yield of membrane-bound proteins across all kits declassifies Kit II as a suitable alternative. Kit IV presented with the second highest membrane protein yield with 0.5–1.7 mg. However, this kit was not considered as a true alternative extraction method due to its poor purity and separation characteristics (Table 1). Kit I and III had reached the two best scores regarding purity and separation and also proved to obtain the highest protein yields for the membranous fraction overall: Kit I presented the highest yield with 0.3–10 mg, and Kit III showed the third highest protein yield with 0.3–1.3 mg. However, it needs to be acknowledged that despite the relatively high, overall protein yield, the yield of certain proteins could be low.
Table 2.
Comparison of sample volume, protein yield, time and costs of each kit
| Fraction | Kit I | Kit II | Kit III | Kit IV | Kit V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cyt | Mem | Cyt | Mem | Cyt | Mem | Cyt | Mem | Cyt | Mem | |
| Total protein yield (mg) | 0.2–0.6 | 0.3–10 | 0.1–0.2 | 0.01–0.03 | 3.4–6.8 | 0.3–1.3 | 1.5–3 | 0.5–1.7 | 1.3–2.8 | 0.3–0.5 |
| Mean of protein yield in mg (CV) | 0.47 (0.2) | 4.8 (5.1) | 0.15 (0.08) | 0.02 (0.01) | 4.6 (2.7) | 0.86 (0.5) | 2.0 (0.8) | 0.88 (0.75) | 1.9 (0.7) | 0.4 (0.1) |
| Obtained volume (mL) | 2 | 2 | 1 | 1 | 8 | 4 | 0.5 | 0.25 | 5 | 1 |
| Quantification method | Bradford | BCA | BCA | BCA | Bradford | |||||
| Representativity of Western blot replicates | 80% | 80% | 87% | 73% | 100% | |||||
| Required time | 1.5 h | 1.5 h | <1 h | 1 h | >2 h | |||||
| Costs/sample | 26 €/ ~ 38 $ | 14 €/ ~ 20 $ | 41 €/ ~ 61 $ | 15 €/ ~ 22 $ | 39 €/ ~ 58 $ | |||||
Euro/US Dollar conversion rate of 2009–11; representativity of Western blot results was calculated by the % of replicates that did show the same results regarding purity and comparison of sub-cellular fraction per kit
Cyt cytosolic fraction, mem membranous fraction
A low recovery volume of the membranous fraction was considered beneficial regarding downstream analyses. Unfortunately, Kit III—showing the best performance—gives the highest recovery volume (4 mL) of the membranous fraction. However, all other parameters (purity, separation, protein yield) do outweigh this drawback and justify a likely concentration step before downstream analyses can be performed. In line, Kit I—presenting the second best performance—gives 2 mL recovery volume and also seems to require concentration before downstream analyses.
Concerning required processing time, Kit III seemed to offer the fastest protocol with less than 1 h. Kit I required about 1.5 h—the overall average processing time. The most superior Kit III is also the most costly with € 41 per sample extraction (Table 2). However, its performance characteristics prevail over the costs. Kit I offers the second best performance and ranks third cost-wise with € 26 per sample extraction.
Conclusions
In this report we compared commercially available membrane protein extraction kits regarding their specificity, purity of sub-cellular fractions, protein yield, costs and processing time. The aim hereby was to identify the optimal extraction methodology to obtain membrane-bound proteins for downstream analyses. Ideally, the isolation process should be mild yet rapid and inexpensive. The extraction method of choice should be compatible with downstream applications like protein determination assays, Western blotting and subsequent proteomic analyses such as 2-DE, SELDI, and/or mass spectrometry. None of the kits evaluated required the use of equipment other than a bench-top centrifuge.
All necessary chemicals and reagents were supplied by the kit manufacturers. Unfortunately, one common drawback of all kits was that manufacturers did not provide information on the elution buffer-ingredients. This can not only cause difficulties regarding compatibility to downstream analyses but different separation procedures may also adversely affect biological activity of certain proteins. In addition, specific separation approaches may be optimal with specific cell types only. Here, we identified the need to optimize technologies and exhibited generic means for doing so. Considering all parameters evaluated we showed kit III (Calbiochem ProteoExtract Native Membrane protein Extraction Kit, #444810, Merck, Nottingham, UK) and kit I (Qproteome Cell Compartment Kit, #37502, Qiagen, Hilden, Germany) to be superior for subsequent membrane protein analyses. While kit III presented an even higher purity than kit I, the latter one obtained a slightly higher protein yield of the membranous fraction.
Acknowledgments
We’d like to thank Dr. Britta Fritzsche and M.Sc. Timo Gemoll for experimental advice and Katja Klempt-Gießing for technical assistance.
Competing interests statement The authors declare no competing interests.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter P, Smith L, Ryan M. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocr Relat Cancer. 2004;11:659–687. doi: 10.1677/erc.1.00766. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Contreras RG, Wang R, Fernandez SV, Shoshani L, Russo LH, Cereijido M, Russo J. Sodium/potassium ATPase (Na + , K +-ATPase) and ouabain/related cardiac glycosides: a new paradigm for development of anti- breast cancer drugs? Breast Cancer Res Treat. 2006;96:1–15. doi: 10.1007/s10549-005-9053-3. [DOI] [PubMed] [Google Scholar]
- Corsten MF, Hofstra L, Narula J, Reutelingsperger CP. Counting heads in the war against cancer: defining the role of annexin A5 imaging in cancer treatment and surveillance. Cancer Res. 2006;66:1255–1260. doi: 10.1158/0008-5472.CAN-05-3000. [DOI] [PubMed] [Google Scholar]
- Dissemond J, Busch M, Kothen T, Mors J, Weimann TK, Lindeke A, Goos M, Wagner SN. Differential downregulation of endoplasmic reticulum-residing chaperones calnexin and calreticulin in human metastatic melanoma. Cancer Lett. 2004;203:225–231. doi: 10.1016/j.canlet.2003.09.036. [DOI] [PubMed] [Google Scholar]
- Ellis JA, Jackman MR, Luzio JP. The post-synthetic sorting of endogenous membrane proteins examined by the simultaneous purification of apical and basolateral plasma membrane fractions from Caco-2 cells. Biochem J. 1992;283(Pt 2):553–560. doi: 10.1042/bj2830553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Mason JS, Overington JP. Can we rationally design promiscuous drugs? Curr Opin Struct Biol. 2006;16:127–136. doi: 10.1016/j.sbi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Kenis H, Hofstra L, Reutelingsperger CP. Annexin A5: shifting from a diagnostic towards a therapeutic realm. Cell Mol Life Sci. 2007;64:2859–2860. doi: 10.1007/s00018-007-7297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic T, Ingrassia L, Facchini V, Kiss R. Na +/K + -ATPase alpha subunits as new targets in anticancer therapy. Expert Opin Ther Targets. 2008;12:1403–1417. doi: 10.1517/14728222.12.11.1403. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Sanders CR, Myers JK. Disease-related misassembly of membrane proteins. Annu Rev Biophys Biomol Struct. 2004;33:25–51. doi: 10.1146/annurev.biophys.33.110502.140348. [DOI] [PubMed] [Google Scholar]
- Santoni V, Molloy M, Rabilloud T. Membrane proteins and proteomics: un amour impossible? Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tanford C, Reynolds JA. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976;457:133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Wallin E, Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbi NK, Dugger AL, Bonner RB, Pitha JV, Hurst RE, Hemstreet GP., 3rd Pan-cadherin as a high level phenotypic biomarker for prostate cancer. J Urol. 2002;167:2215–2221. doi: 10.1016/S0022-5347(05)65131-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ashendel CL, Becker GW, Morre DJ. Isolation and characterization of the principal ATPase associated with transitional endoplasmic reticulum of rat liver. J Cell Biol. 1994;127:1871–1883. doi: 10.1083/jcb.127.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]