Abstract
Human immunodeficiency virus type 1-based defective lentiviral vectors (HIV-based vector) efficiently transduce a wide range of mammalian cell types, but little is known with respect to their utility for gene transfer applications involving primary mouse monocytes/macrophages. This may be important for preclinical development of a range of potential gene therapeutic modalities. Present study described the development of an optimized method for viral vector-mediated gene transfer into primary mouse monocytes/macrophages and the establishment of reproducible protocols for cell isolation/cultivation. It has been determined that bone marrow-derived monocytes/macrophages were consistently more susceptible to viral vector-mediated gene transduction, as compared to blood-derived cells. It has also been documented that the efficiency of transduction increased when cells were maintained in vitro, prior to exposure to vector virus. Finally, experiments were conducted to compare the efficiency of gene transfer mediated by HIV-based vectors to that achieved by other lentivirus or retrovirus vector systems. These studies showed that HIV-based vector system was consistently superior. Overall, these results establish a new and efficient method for gene transfer into primary mouse monocytes/macrophages. This may be of utility in the preclinical development of gene therapies that target this important cell type.
Keywords: Mouse, Blood- and bone marrow-derived monocytes and macrophages, Lentiviral vector, Transduction
1. Introduction
Monocytes are a readily available cell type, with a natural ability to penetrate otherwise inaccessible end organs such as the brain (Gartner et al., 1986; Zhu, 2000). As a result, it has been suggested that it may be possible to use genetically modified monocytes to carry exogenous genes of interest into the brain or other organs, for purposes of gene therapy (Collman et al., 1989; Burke et al., 2002). The evaluation of this novel approach to CNS gene therapy will require considerable pre-clinical analysis, but a critical first step lies in the development of efficient methods for ex vivo gene transfer into primary mouse monocytes/macrophages.
HIV-based defective lentiviral vectors are known to transduce both dividing and nondividing cells from a wide range of mammalian species (Verma and Somia, 1997; Mautino, 2002), and therefore they represent an obvious candidate vector system for this purpose. However, methods for viral vector-mediated gene transfer in primary mouse monocytes/macrophages have not been described and remain to be developed. Here, present report describes the development of an efficient method for the isolation, cultivation and ex vivo transduction of primary mouse monocytes/macrophages using HIV-based vectors. Current study also shows that the efficiency of gene transfer can be enhanced through (i) short-term in vitro culture of cells prior to exposure to viral vectors, (ii) increasing the multiplicity of infection, and (iii) use of bone marrow-derived monocytes/macrophages, as compared to blood-derived cells. Finally, a comparative test was conducted to assess the efficiency of gene transduction by HIV-based vectors to that achieved with other lentivirus or retrovirus vectors; the HIV-based vector system was found to be markedly superior. Overall, these studies establish an efficient method for ex vivo gene transfer into primary mouse monocytes/macrophages and lay the groundwork for future in vivo studies using genetically modified monocytes/macrophages as a potential gene delivery vehicle for the central nervous system.
2. Materials and methods
2.1. Vector production
HIV-based vector, feline immunodeficiency virus (FIV)-based Felix vectors, and murine leukemia virus (MLV)-based N2A vectors were produced by transient transfection of human embryonic kidney 293T cells, as described previously (Burns et al., 1993; Naldini et al., 1996; White et al., 1999; Curran et al., 2000; Kootstra and Verma, 2003). All vector viruses were pseudotyped with the vesicular stomatitis virus (VSV) G protein. The HIV-based vector used in this study is a recently constructed vector containing no structural genes (gag/pol/env) and accessory genes (Zeng et al., 2005). The FIV-based Felix vector plasmids were generously provided by Dr. G.P. Nolan, Stanford University Medical Center and the complete vector maps and sequences are available online at http://www.stanford.edu/group/nolan/retroviral_system/felix_map.html. The MLV-based N2A vector was a modification of a parental N2A construct that contained a SV40 ori fragment in order to permit plasmid replication in 293T cells (and thereby increase vector titers). All three vector viruses were concentrated by ultracentrifugation and titered on 293T or CEM cells.
2.2. Animals
Adult CD-1 mice (35 ± 5 g) were purchased from Charles River Laboratories, Inc. and kept at the University of Hawaii at Manoa School of Medicine Animal Facility until employed in the experiments; mice were used in experiments after at least an 1 week period of acclamation to the local animal facility. All animal procedures were approved by the animal care and use committee of the University of Hawaii at Manoa.
2.3. Cell culture
All mouse monocyte cultures were incubated at 37 °C with 5% CO2. The culture medium used for all the experiments was RPMI-1640 (Sigma) supplemented with 20% equine serum (ES, Hyclone) and 10% defined fetal bovine serum (FBS, Hyclone), 2 mM l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (Sigma).
2.4. Blood-derived monocytes/macrophages
Mouse blood-derived monocytes/macrophages were isolated according to previously described methods (Wirth et al., 1982; Schreurs et al., 1999) with some modifications. Briefly, experimental mice were euthanized in a CO2 chamber and peripheral blood was immediately collected by cardiac puncture and pooled together in BD K3EDTA vacutainer (BD Biosciences). Peripheral blood mononuclear cells (PBMC) were isolated by two cycles of gradient centrifugation after diluting blood with Dulbecco's phosphate buffer saline (DPBS) at a ratio of 1:2 (blood:DPBS). The first centrifugation was at 1000 × g for 30 min with Lympholyte-M gradient medium (Cedarlane, Canada) and the second one was at 820 × g for 20 min with Ficoll-Paque gradient solution (Pharmacia, Sweden). Cells were collected from the white layer at interface and further purified by washing with DPBS at 460 g for 15 min and 220 g for 8 min, respectively. Recovered PBMC were resuspended in growth medium, counted and then seeded into 96-well plates (Corning) at a concentration of 1 × 106 viable cells/well. Following 3- or 48-h incubation at 37 °C with 5% CO2, non-adherent cells were removed by washing with DPBS and adherent monocytes were cultured in growth medium until used.
2.5. Bone marrow-derived monocytes/macrophages
Preparation of primary mouse bone marrow-derived monocytes/macrophages was conducted according to the methods established by Lutz et al. (1999), Agger et al. (2000) and Young-Ik et al. (2002) with minor modification. In brief, experimental mice were sacrificed by CO2 chamber euthanasia and femurs were removed. After trimming off muscle, bone marrow cells were flushed with DPBS from the bone shafts. Cells were pipetted to break any cell aggregates and then subjected to Ficoll-Paque gradient centrifugation at 820 × g for 20 min. The white cell layer at the interface was recovered and then washed once with DPBS at 220 g for 8 min. The cell pellet was resuspended with growth medium, counted, and then cultured in precisely the same fashion as the blood-derived cells.
2.6. Immunochemistry
Expression of defined cell surface markers was evaluated by immunochemical staining using selected monoclonal antibodies conjugated with PE (PE-mAb) according to the manufacturer's recommendations, with minor modification (Leenen et al., 1994). Briefly, cells in 96-well plates were washed twice with DPBS containing 0.5% (w/v) bovine serum albumin (BSA) and then directly stained with appropriately diluted PE-conjugated rat monoclonal antibodies directed against mouse CD11b, CD14 and F4/80. After incubation for 1 h in a dark room, cells were washed with DPBS. PE-positive cells were visually detected and documented using an inverted fluorescence microscope (Olympus IX70) with a digital camera attachment.
2.7. Transduction of freshly isolated mouse monocytes
Blood- or bone marrow-derived monocytes/macrophages were adjusted to a concentration of 1 × 107 cells/ml, and HIV-based vector was diluted to 1 × 108 IU/ml (using RPMI-1640 medium containing no FBS). For gene transduction, triplicate wells of a 96-well plate were seeded with 0.1 ml/well of the cell suspension (1 × 106 cells) and 0.1 ml/well of diluted vector virus was added in the presence of 8 μg/ml polybrene (Sigma) (an multiplicity of infection of 10 was routinely used in most experiments, unless otherwise indicated in the pertaining figure legend). Cells were then incubated overnight with the vector virus (unless otherwise indicated in the pertaining figure legend), and residual vector virus and non-adherent cells were removed by washing the wells with DPBS. 0.2 ml/well of growth medium was then added, and the plate was returned to the 37 °C incubator. Five days following the initial addition of vector virus, transduction efficiency was determined by counting the number of GFP-positive cells in random microscope fields, using the fluorescence microscope.
2.8. Effect of in vitro culture time on the efficiency of DLV transduction
Primary mouse blood- and bone marrow-derived monocytes and macrophages were maintained in culture for between 2 and 11 days prior to exposure to HIV-based vector virus at an multiplicity of infection of 10, in the presence of polybrene (see above). Following adsorption over night at 37 °C, residual vector virus was removed by washing the cell monolayer with DPBS and then 0.2 ml of fresh growth medium was added. The number of green fluorescent protein (GFP)-positive cells was then enumerated at day 5 post-transduction by counting the number of GFP-positive cells.
2.9. Transduction of mouse monocytes with different vector viruses
HIV-based vector, FIV-based Felix and MLV-based N2A vector viruses were tested and compared for their efficiency in transducing primary cultures of mouse blood- and bone marrow-derived monocytes/macrophages at an multiplicity of infection of 10. The transduction protocol was the same as described above, and transduction efficiency was determined by counting GFP-positive cells at post-transduction day 5.
3. Results
3.1. Isolation and cultivation of primary mouse monocytes/macrophages
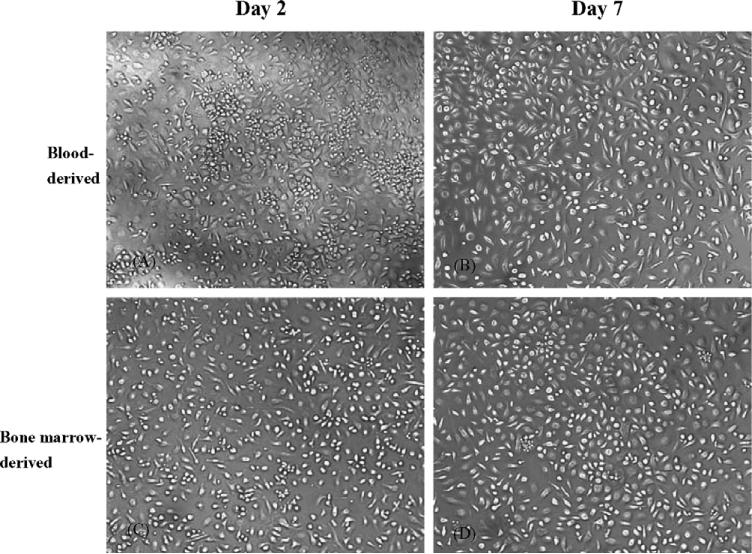
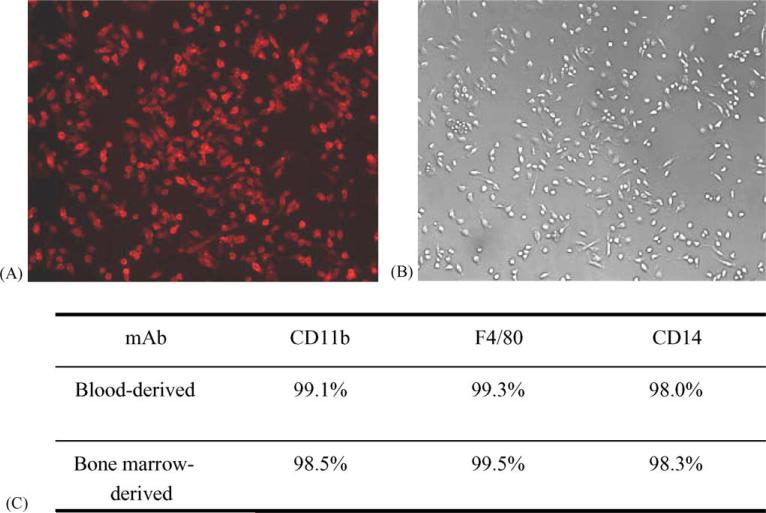
To determine if primary mouse monocytes/macrophages can be effectively transduced with viral vectors, it is essential to establish a reliable protocol for proficient isolation and cultivation of these cells. Using two cycles of gradient centrifugation with Lympholyte-M and Ficoll-Paque, respectively, mouse PBMC were effectively separated from plasma and red blood cells. Following several washing steps with DPBS and then allowing the cells to adhere to tissue culture plastic, viable and purified mouse monocytes/macrophage cultures were obtained (Fig. 1). For isolation of bone marrow-derived cultures, a very similar methodology was developed, although only a single Ficoll-Paque gradient was found to be necessary during the initial fractionation of mononuclear cells. Purity of the primary mouse blood- and bone marrow-derived monocytes/macrophages cell cultures was confirmed by immunochemistry staining showing over 98% of cells to be immunoreactive with PE-conjugated rat monoclonal antibodies directed against mouse monocyte/macrophage cellular proteins CD11b, F4/80 and CD14 (Fig. 2). As a negative control, PE-conjugated rat IgG isotype was also included in the test and the cells showed no specific immunoreactions to this conjugate. These immunological identities of primary mouse monocytes/macrophages remained no changes up to cultivation day 14 (data not shown).
Fig. 1.
Photomicrographs of primary cultures of mouse monocytes/macrophages at selected incubation times in vitro. (A and B) Mouse blood-derived monocytes and (C and D) bone marrow-derived monocytes.
Fig. 2.
In vitro primary cultures of mouse blood- and bone marrow-derived monocytes/macrophages at day 2 were stained with selected PE-conjugated monoclonal antibodies (mAbs). Results from monocytes/macrophages cultures that were stained with a CD11b-specific mAb and then visualized under fluorescent light (A) or normal light (B) are shown (magnification 100×). (C) Summary of immunostaining results for the indicated cultures, using a panel of mAbs directed against selected monocytes/macrophages cell surface markers.
3.2. Transduction of freshly isolated mouse monocytes
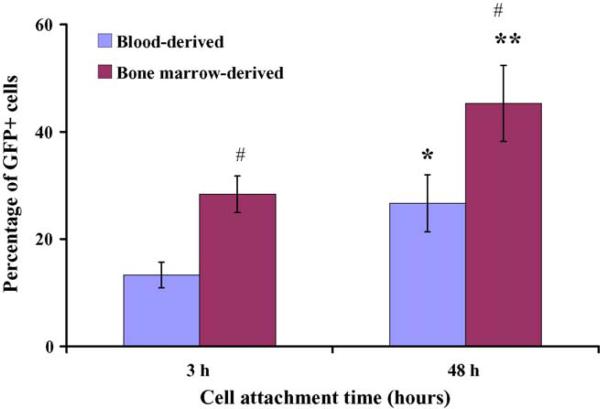
The freshly isolated mouse monocytes were highly susceptible to viral vector-mediated gene transfer. Using the HIV-based vector at an multiplicity of infection of 10, viral vector-mediated transduction efficiencies for freshly isolated blood- and bone marrow-derived cells were 14 ± 2% and 27 ± 5%, respectively, following 3 h vector virus infection and cell attachment. When vector virus was allowed to attach to cells for 48 h prior to washing, the transduction efficiency increased to 28 ± 3% and 45 ± 7%, respectively, for blood- and bone marrow-derived monocyte cultures. Present data revealed that increasing the incubation times from 3 to 48 h led to a significant elevation in the efficiency of viral vector-mediated gene transfer (paired t-test, p < 0.05). Experimental data also showed that monocytes isolated from bone marrow were significantly more sensitive to viral vector-mediated transduction than blood-derived cells (p < 0.05) (Fig. 3).
Fig. 3.
DLV-mediated transduction efficiency of freshly isolated blood- or bone marrow-derived mouse monocytes. In this experiment, vector and cells were incubated in suspension at 37 °C for either 3 or 48 h. After incubation, excess vector was removed by vigorous washing. Statistical significance was determined by paired t-test. * and ** denote statistical significance (p < 0.001 and p < 0.05, respectively, as compared to the 3 h incubation time); # denotes a statistically significant difference (p < 0.05) as compared to DLV-transduced blood-derived monocytes at the matched time point.
3.3. Prolonged time in culture results in more efficient gene transfer in primary mouse monocytes
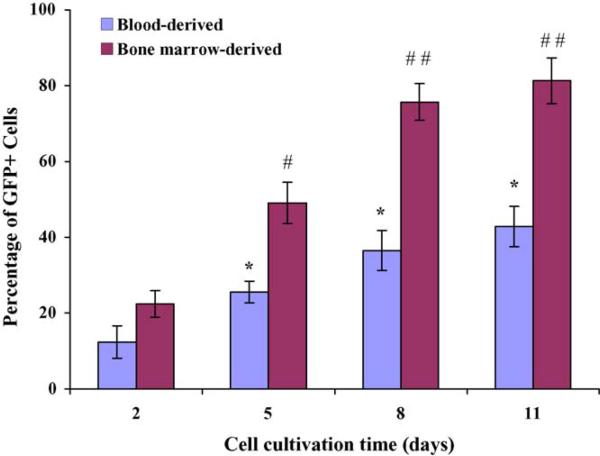
Primary cultures of blood- and bone marrow-derived monocytes were maintained in culture for time periods ranging from 2 to 11 days, prior to exposure to HIV-based vector. For blood-derived monocytes, transduction efficiency increased from 12 ± 4.3% for day 2 cultures to 37 ± 5.3% for day 8 cultures (Fig. 4). Viral vector-mediated transduction of bone barrow-derived monocytes increased from 22 ± 3.5% for day 2 cultures to 76 ± 4.6% for day 8 cultures (Fig. 4). In both cases, maintenance of cultures for longer time periods (11 days) failed to yield any further enhancement of gene transfer efficiency. Overall, these data suggest that the efficiency of DLV-mediated gene transduction in mouse monocytes may be influenced by the extent of cellular differentiation and/or by other changes associated with prolonged in vitro culture. This is consistent with similar observations using primary human monocytes/macrophage cell cultures (Neil et al., 2001; Zeng et al., 2005).
Fig. 4.
Effect of in vitro culture time on transduction of primary mouse blood and bone marrow-derived monocytes/macrophages. Transduction efficiency was determined by calculating the percentage of GFP+ cells within the culture. Statistical significance was determined by paired t-test. Transduction efficiency increased significantly from day 2 to day 5 (* and #p < 0.05) and from day 5 to day 8 (##p < 0.01), but there was no significant increase after day 8 (* and ##p > 0.05). Bone marrow-derived monocytes were more efficiently transduced than blood-derived monocytes at the 5, 8 and 11-day time points (#p < 0.05 and ##p < 0.01).
3.4. Effect of vector virus dose on transduction efficiency
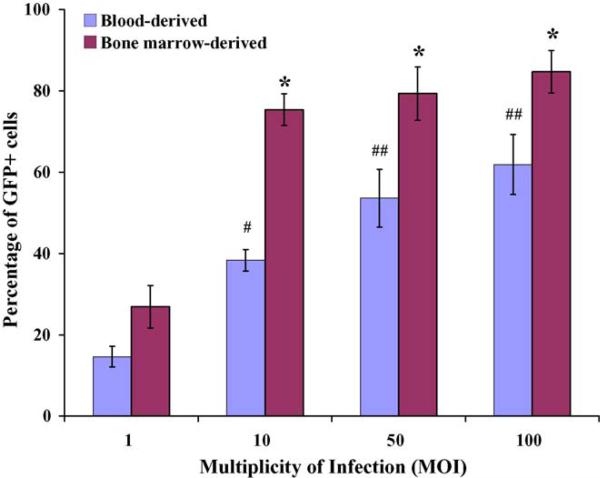
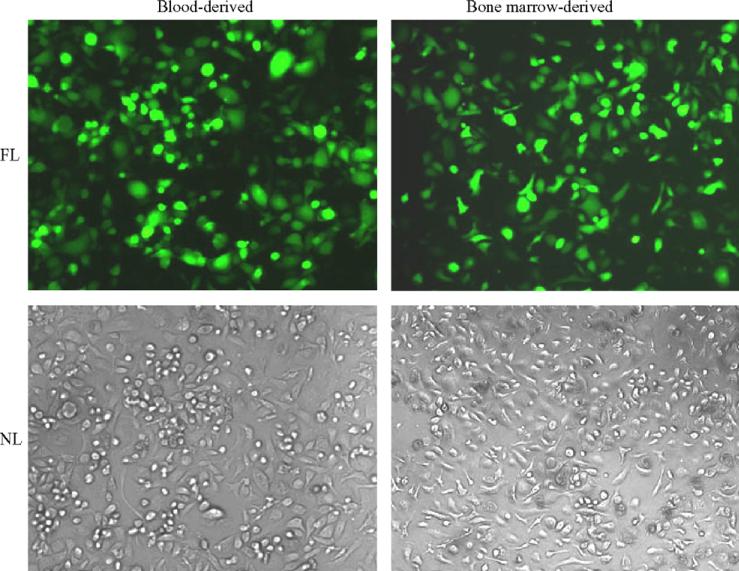
To evaluate the impact of vector virus concentrations on transduction efficiency four different doses of vector viruses (multiplicity of infection = 1, 10, 50, and 100) were tested and compared. As expected, the efficiency of HIV-based vector-mediated transduction of mouse monocytes/macrophages was dose-dependent. As shown in Fig. 5, transduction efficiency in blood-derived monocytes/macrophages increased from an initial level of 15 ± 2.6% of cells at an multiplicity of infection of 1 to 62 ± 7.4% of cells at the highest viral vector dose tested (multiplicity of infection of 100) (p < 0.001). A similar result was obtained for bone marrow-derived monocytes/macrophages, although the efficiency of gene transfer was consistently higher at all viral vector doses tested. The vector virus-transduced monocytes/macrophages with high infectious dose did not show apparent changes in cell morphology and growth kinetics, etc. (Fig. 6). In addition, evaluation of RNA isolated from viral vector-transduced mouse monocyte/macrophages by RT-PCR revealed that the expression of the GFP reporter gene remained stable in transduced cells for at least 14 days (data not shown).
Fig. 5.
BM-derived monocytes/macrophages are more efficiently transduced than blood-derived cells, at all multiplicities of infection (MOI) tested. Primary mouse blood- and bone marrow-derived monocytes/macrophages were exposed to DLV vectors at the indicated MOIs, and % GFP+ cell determined. Data represent the mean and standard deviation of multiple samples from at least three independent experiments for each data point. As expected, transduction efficiency increased significantly when a MOI of 10, 50 or 100 was used as compared to MOI of 1 (* and #p < 0.005 and ##p < 0.05; paired t-test).
Fig. 6.
Photomicrographs of transduced mouse monocytes/macrophages showing transgene (GFP) expression. Primary cultures of mouse monocytes/macrophages derived from blood and bone marrow sources at day 8 were infected with concentrated defective lentiviral vector (multiplicity of infection = 100) at post-transduction day 5. FL = fluorescent light and NL = normal light. Magnification 100×.
3.5. Transduction efficiency with different vector viruses
Since the HIV-based vector system was able to efficiently transduce primary mouse monocytes, it was important to know whether other related viral vector systems might also be capable of mediating efficient gene transfer into the cells. For this purpose, a test was conducted to evaluate two other viral vector systems that were also pseudotyped with VSV G coat protein: a replication-defective feline immunodeficiency virus (FIV)-based lentivirus vector and a replication-defective murine leukemia virus (MLV)-based retrovirus vector. As summarized in Table 1, the HIV-based vector considerably outperformed the other two viral vectors tested, when compared at an multiplicity of infection of 10.
Table 1.
Comparison of gene transfer efficiency in primary mouse monocytes/macrophages, using three different viral vector systems
| Vector | HIV-based | FIV Felix | N2A |
|---|---|---|---|
| Blood-derived (%) | 36 ± 5.2 | 5.4 ± 2.3 | 1.8 ± 0.4 |
| Bone marrow-derived (%) | 76 ± 4.8 | 9.1 ± 3.3 | 3.1 ± 0.9 |
Transduction was carried out on day 8 monocytes/macrophage cultures, at a multiplicity of infection of 10 with the indicated vectors, in the presence of 8 μg/ml of polybrene (HIV-based: replication-defective HIV-based lentivirus vector; FIV Felix: replication-defective feline immunodeficiency virus-based lentivirus vector; N2A: replication-defective murine leukemia virus-based retrovirus vector). Transduction efficiency was determined at day 5 post-transduction by counting the number of GFP-positive cells within the transduced cultures. Results denote mean and standard deviations for three replicate determinations from a single experiment that is representative of a total of two experiments that were performed.
4. Discussion
With the growing interest in the use of monocytes/macrophages as a novel cellular vehicle for gene therapy in the CNS, it is of interest and importance to develop optimized in vitro transfection techniques for genetic modification of primary monocytes/macrophages ex vivo. It has recently been demonstrated that primary monocyte cultures isolated from human blood can be efficiently transduced in vitro with HIV-based viral vector pseudotyped with VSV-G envelope and transduced cells maintained an active and long-term reporter gene expression (Zeng et al., 2005). To further investigate the use of the genetically modified monocytes/macrophages in gene therapy for the central nervous system, it is of importance to establish an experimental model system to test the efficacy of monocyte trafficking into the brain and the efficiency of target gene expression in vivo. Laboratory mice have proven to be invaluable to address the efficacy and safety of viral vectors in transferring genes of interest to the brain in vivo (Ikeda et al., 2002) and thus, represent an ideal animal model system for both in vitro and in vivo validation of potential use of monocytes as a noninvasive gene delivery system for the central nervous system.
Current in vitro studies on primary mouse monocytes and macrophages have been limited. There have been only a few reports related to the isolation and in vitro cultivation of primary mouse blood-derived monocytes/macrophages (Wirth et al., 1982; Grace et al., 1988; Agger et al., 2000), but no report for primary cultures of mouse bone marrow-derived monocytes/macrophages in literature. In addition, only sporadic reports have been documented in viral vectors-based gene transfer into mouse cells in vitro to date (Riviere et al., 2000; Hughes et al., 2001; Rossi et al., 2003). The main aims of the present study were to establish an optimized protocol for the isolation and culture of primary mouse monocytes, and to develop a method for efficient and stable gene transfer into these cells. Present report describes the development of simple and reproducible methods for the isolation of highly purified cultures of primary monocytes/macrophages from both mouse blood and bone marrow. In attempts to obtain pure PBMC from mouse blood it showed that an initial two cycles of gradient centrifugation with gradient media Lympholyte-M and Ficoll-Paque, respectively, are necessary since mouse blood is rich in red blood cells and platelets with a high viscosity. On the other hand, it is much easier to get pure monocytes cultures from bone marrow and it approved that one cycle centrifugation with Ficoll-Paque gradient is effective enough to remove the red blood cells and platelet contaminants. RPMI-1640 medium was chosen to support primary cultures of mouse monocytes in vitro because this medium has been frequently reported in literature as a basic medium for the isolation and cultivation of murine macrophages and dendritic cells (Lutz et al., 1999; Agger et al., 2000; Son et al., 2002; Rossi et al., 2003). Mouse serum was initially employed as a medium supplement to enhance the growth of mouse peritoneal macrophages (Grace et al., 1988) but recent studies have largely involved using fetal calf serum (FCS) or fetal bovine serum instead (Lutz et al., 1999; Agger et al., 2000; Son et al., 2002; Rossi et al., 2003). Present study has chosen to include 10% FBS and 20% ES in RPMI-1640 medium for the in vitro cultivation of primary mouse monocytes because of their ready availability in the laboratory. The experimental results obtained from this study showed that the medium are able to support the attachment, growth and maintaining of primary mouse monocytes/macrophages, proves especially useful in bulky isolation and cultivation of mouse monocytes. Comparative analysis showed that initial cell seeding density of isolated PBMC would also have a significant impact on subsequent attachment and growth of mouse monocytes. As shown in this study, healthy-appearing cell monolayer can be achieved when an optimal concentration of cells (1.1 ± 0.1 × 106 cells/well) is seeded in a 96-well plate. Seeding cells at either lower or higher density would lead to an insufficient cell attachment and poor cell growth (data not shown). The described protocol exhibited to be reliable which supports the good preparation of primary mouse monocyte culture, growth and maintaining in vitro, and for viral vector-mediated gene transduction.
With the establishment of the in vitro isolation and primary cultivation of mouse monocytes, it allowed to demonstrate that monocytes/macrophage cultures prepared from mouse bone marrow were significantly more susceptible to viral vector-mediated transduction than corresponding cultures that were established from mouse blood. This observation suggests that bone marrow-derived cells may be of particular value in future gene therapy studies using viral vector-mediated gene transfer. It also suggests that differences in the differentiation status (or other properties) of bone marrow- versus blood-derived monocytes/macrophages may have important effects on viral vector-mediated gene transfer. This might be consistent with the fact that extended in vitro culture of both the bone marrow- and blood-derived cell populations resulted in further improvement of gene transfer efficiency, and it suggests the need for future studies to explore this question further. At the same time, there may be inherent differences in the monocytes/macrophage populations isolated from bone marrow versus blood which also contribute to the efficiency of viral vector-mediated gene transfer. In this regard, it is notable that the bone marrow-derived cell population was consistently more susceptible to viral vector-mediated gene transfer, regardless of time in culture or infectious doses of vector viruses.
Among the viral vector systems tested in the experiments, the HIV-based vector emerged as the most efficient for transduction of primary mouse monocytes/macrophages. This is consistent with prior reports concerning the ability of VSV G-protein pseudotyped HIV-based vectors to transduce both dividing and non-dividing cells from a broad range of tissues and species (Ikeda et al., 2002; O'Rourke et al., 2002). The poor performance of the MLV-based N2A vector virus was not surprising, since oncoretrovirus vectors are known to be incapable of transducing post-mitotic cell types (Lewis and Emerman, 1994). Presumably, the low level of MLV-mediated gene transfer that was detected in the cell cultures was due to the presence of small numbers of mitotically active cells within the cell population, which had not yet undergone terminal maturation.
The FIV-based lentivirus vector was somewhat more efficient in transducing primary mouse monocytes, but was considerably inferior to the HIV-based system. The reasons for this are less obvious, but may relate to the availability of key factors required for vector virus entry, uncoating, integration or expression, etc. Feline immunodeficiency virus has a LTR with weak promoter activity. Price et al. (2002) has revealed transgene expression in human primary hematopoietic cells mediated by second-generation FIV-based vector was almost undetectable and they considered it might be due to weak LTR promoter activity and some unknown elements in the vector backbone they used. FIV-based Felix vector virus was generated based on the second-generation of FIV vector with deletions of all accessory genes and a replacement of the 5′ U3 promoter with the human cytomegavirus enhancer/promoter. This modification allows the vector virus to produce larger quantities of full-length RNA genome following transfection into 293T cells (Curran et al., 2000). It was well accepted that cytomegalovirus promoter/enhancer has stronger regulatory activity in permanent and virus transformed cell lines (Zarrin et al., 1999). In this regard, it may be significant that the green fluorescent protein indicator gene in HIV-based vector construct was expressed under the control of viral LTR, while it was under the transcriptional control of a cytomegalovirus promoter/enhancer in the FIV-based vector virus. This suggests that further studies may be needed to dissect the role and effectiveness of different transcriptional control elements in mediating stable exogenous gene expression in murine monocytes and macrophages. Finally, it is notable that bone marrow-derived monocytes/macrophages were more efficiently transduced by all three of the viral vector systems tested, as compared to blood-derived cells. This is consistent with the notion that there may be important inherent differences between the bone marrow- and blood-derived monocytes/macrophage cell populations (see above).
Overall, the present work establishes methods that permit the isolation and propagation of primary murine monocytes/macrophages, as well as their efficient transduction with HIV-based lentivirus vectors. Present studies also reveal that different approaches can be employed to enhance ex vivo gene transfer efficiency, and show that bone marrow-derived monocytes/macrophages are particularly susceptible to transduction by HIV-based viral vectors. This work thus lays important groundwork for the future development of therapeutic interventions that utilize genetically modified monocytes and macrophages.
Acknowledgements
Authors would like to thank Dr. Stephen Dewhurst for his comments on the manuscript. This study was supported by grants from the National Institutes of Health (S11NS043499 and G12RR003061) and by the Hawaii Community Foundation.
References
- Agger R, Petersen MS, Toldbod HE, Holtz S, Dagnes-Hansen F, Johnsen BW, et al. Characterization of murine dendritic cells derived from adherent blood mononuclear cells in vitro. Scand. J. Immunol. 2000;52:138–147. doi: 10.1046/j.1365-3083.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- Burke B, Summer S, Maitland N, Lewis CE. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J. Leukoc. Biol. 2002;72:417–428. [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman R, Hassen NF, Walker R, Godfrey B, Cutilli J, Hastings JC. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1): monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Kaiser SM, Achcoso PL, Nolan GP. Efficient transduction of nondividing cells by optimized feline immunodeficiency virus vectors. Mol. Ther. 2000;1:31–38. doi: 10.1006/mthe.1999.0007. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovits DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Grace S, Guthrie LA, Johnston RB., Jr. The use of mouse serum and the presence of non-adherent cells for the culture of mouse macrophages. J. Immunol. Methods. 1988;114:21–26. doi: 10.1016/0022-1759(88)90148-2. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Moussavi-Harami F, Sauter SL, Davidson BL. Viral-mediated gene transfer to mouse primary neural progenitor cells. Mol. Ther. 2001;5:16–24. doi: 10.1006/mthe.2001.0512. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Collins MKL, Radcliffe PA, Mitrophanous KA, Takeuchi Y. Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther. 2002;9:932–938. doi: 10.1038/sj.gt.3301708. [DOI] [PubMed] [Google Scholar]
- Kootstra NA, Verma IM. Gene therapy with viral vectors. Annu. Rev. Phamacol. Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J. Immunol. Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kubutsch N, Ogilvie ALJ, Robner S, Koch F, Romani N, et al. An advanced culture for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Mautino MR. Lentiviral vectors for gene therapy of HIV-1 infection. Curr. Gene Ther. 2002;2:23–43. doi: 10.2174/1566523023348165. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Neil S, Martin F, Ikeda Y, Collins M. Postentry restriction to human immunodeficiency virus-based vector transduction human monocytes. J. Virol. 2001;75:5448–5456. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke JP, Newbound GC, Kohn DB, Olsen JC, Bunnell BA. Comparison of gene transfer efficiency and gene expression levels achieved with equine infectious anemia virus- and human immunodeficiency virus type 1-derived lentivirus vectors. J. Virol. 2002;76:1510–1515. doi: 10.1128/JVI.76.3.1510-1515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Case SS, Carbonaro DA, Yu X-J, Pertersen D, Sabo KM. Expression from second-generation feline immunodeficiency virus vectors is impaired in human hematopoietic cells. Mol. Ther. 2002;6:645–652. [PubMed] [Google Scholar]
- Riviere I, Gallardo HF, Hagani AB, Sadelein M. Retroviral-mediated gene transfer in primary murine and human T-lymphocytes. Mol. Biotech. 2000;15:133–142. doi: 10.1385/MB:15:2:133. [DOI] [PubMed] [Google Scholar]
- Rossi GR, Mautino MR, Morgan RA. High-efficiency lentiviral vector-mediated gene transfer into murine macrophages and activated splenic B lymphocytes. Hum. Gene Ther. 2003;14:385–391. doi: 10.1089/104303403321208989. [DOI] [PubMed] [Google Scholar]
- Schreurs MWJ, Eggert AAO, de Boer AJ, Figdor CG, Adema GJ. Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur. J. Immunol. 1999;29:2835–2841. doi: 10.1002/(SICI)1521-4141(199909)29:09<2835::AID-IMMU2835>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Son YI, Egawa SI, Tatsumi T, Redlinger RE, Kalinski P, Kano T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods. 2002;262:145–157. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Verma IM, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- White SM, Renda M, Nam NY, Klimatcheva E, Zhu Y, Fisk J, et al. Lentivirus vectors using human and simian immunodeficiency virus elements. J. Virol. 1999;73:2832–2840. doi: 10.1128/jvi.73.4.2832-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth JJ, Theisen MA, Crowle AJ. Culture conditions required for primary isolation and culture of mouse blood monocytes. J. Reticuloendothel. Soc. 1982;31:325–327. [PubMed] [Google Scholar]
- Young-Ik S, Shin-ichi E, Tomohide T, Richard ER, Jr., Pawel K, Tatsuya K. A novel bulk-culture of method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods. 2002;262:145–157. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Zarrin AA, Malkin L, Fong I, Luk KD, Ghose A, Berinstein NL. Comparison of CMV, RSV, SV40 viral and Vlambdal cellular promoters in B and T lymphoid and non-lymphoid cell lines. Biochem. Biophys. Acta. 1999;1446:135–139. doi: 10.1016/s0167-4781(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Zeng L, Planelles V, Sui Z, Gartner S, Maggirwar SB, Dewhurst S. HIV-1-based defective lentiviral vectors efficiently transducer human monocyte-derived macrophages and suppress replication of wild-type HIV-1. J. Gene Med. 2005;8:18–28. doi: 10.1002/jgm.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T. HIV-1 genotypes in peripheral blood monocytes. J. Leukoc. Biol. 2000;68:338–344. [PubMed] [Google Scholar]