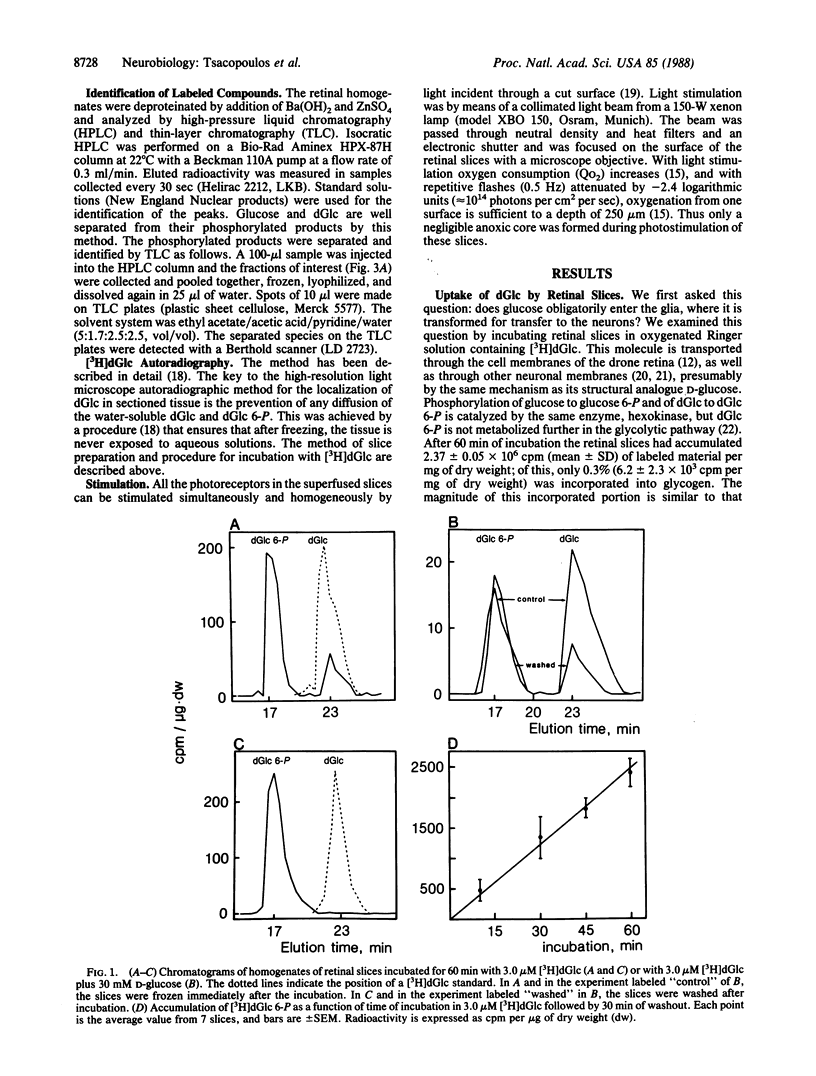
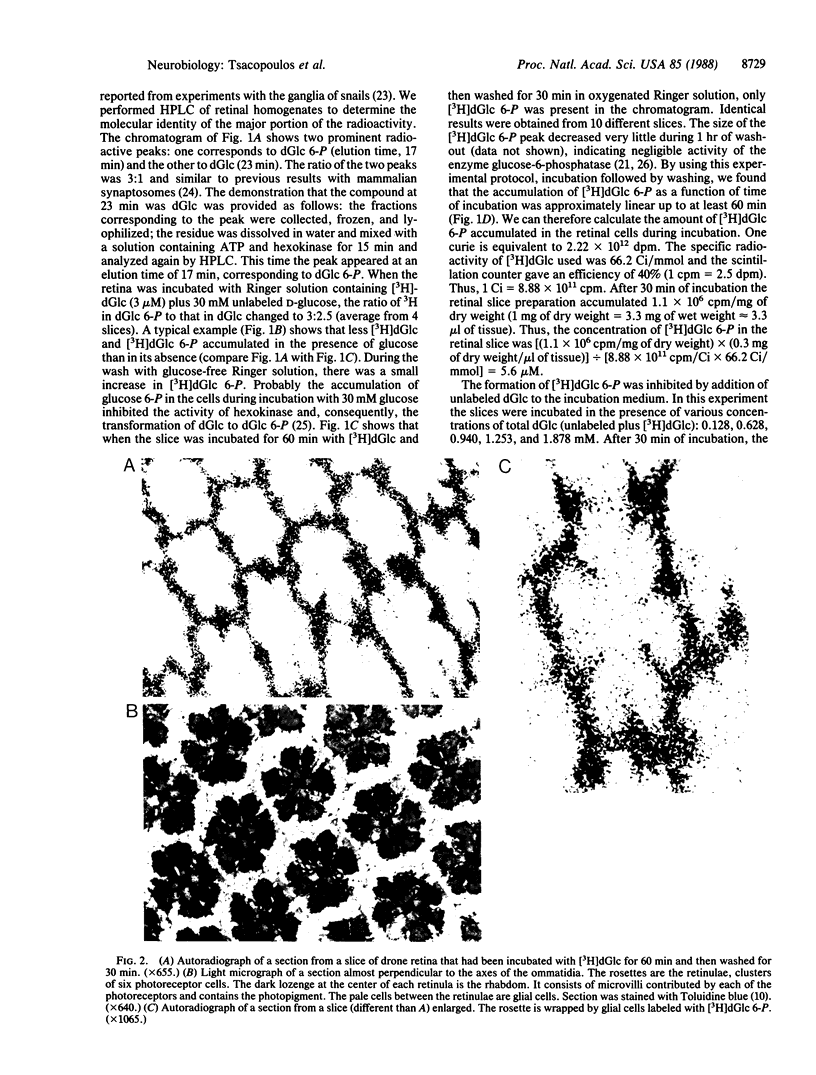
Abstract
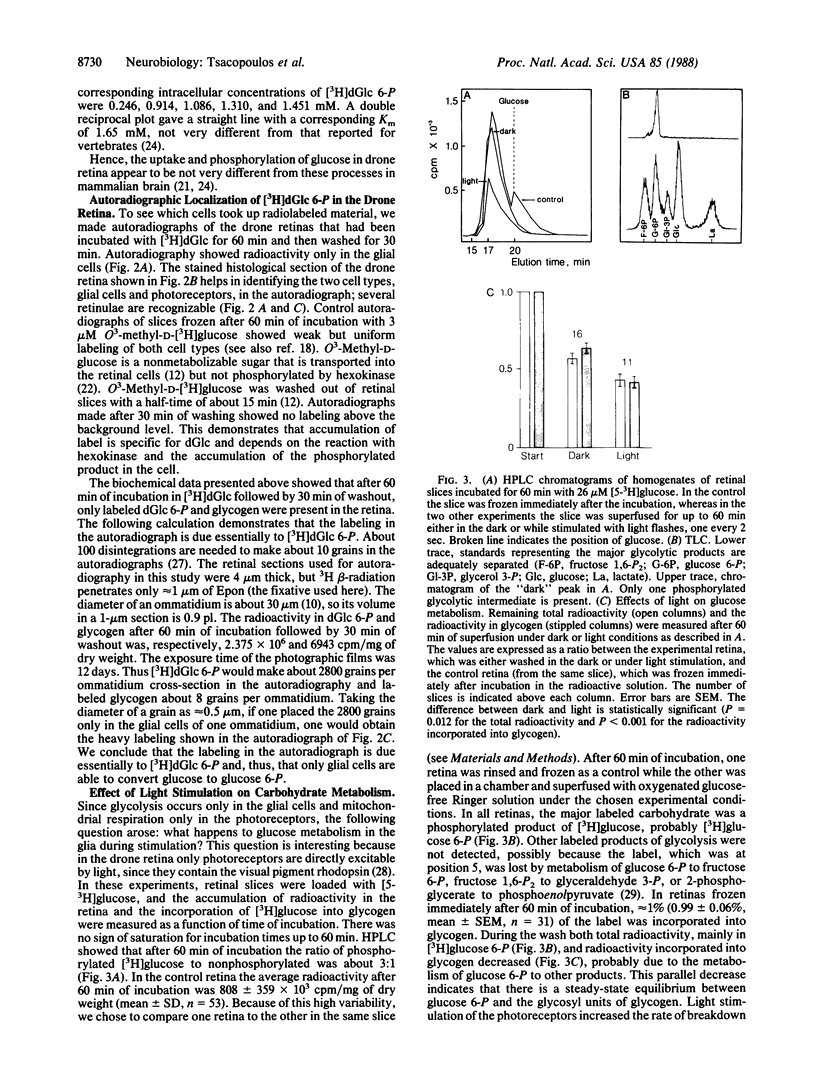
The retina of the honeybee drone is a nervous tissue in which glial cells and photoreceptor cells (sensory neurons) constitute two distinct metabolic compartments. Retinal slices incubated with 2-deoxy[3H]glucose convert this glucose analogue to 2-deoxy[3H]glucose 6-phosphate, but this conversion is made only in the glial cells. Hence, glycolysis occurs only in glial cells. In contrast, the neurons consume O2 and this consumption is sustained by the hydrolysis of glycogen, which is contained in large amounts in the glia. During photostimulation the increased oxidative metabolism of the neurons is sustained by a higher supply of carbohydrates from the glia. This clear case of metabolic interaction between neurons and glial cells supports Golgi's original hypothesis, proposed nearly 100 years ago, about the nutritive function of glial cells in the nervous system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann F., Mauro A. Effect of hypoxia on the change in membrane conductance evoked by illumination in arthropod photoreceptors. Nat New Biol. 1973 Aug 1;244(135):146–148. doi: 10.1038/newbio244146b0. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Coles J. A., Tsacopoulos M. Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol. 1979 May;290(2):525–549. doi: 10.1113/jphysiol.1979.sp012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond E., Fishman R. A. High affinity transport of 2-deoxyglucose in isolated synaptic nerve endings. Nature. 1973 Mar 9;242(5393):122–123. doi: 10.1038/242122a0. [DOI] [PubMed] [Google Scholar]
- Duffy T. E., Nelson S. R., Lowry O. H. Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem. 1972 Apr;19(4):959–977. doi: 10.1111/j.1471-4159.1972.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Guth L., Watson P. K. A correlated histochemical and quantitative study on cerebral glycogen after brain injury in the rat. Exp Neurol. 1968 Dec;22(4):590–602. doi: 10.1016/0014-4886(68)90151-9. [DOI] [PubMed] [Google Scholar]
- Jones G. J., Tsacopoulos M. The response to monochromatic light flashes of the oxygen consumption of honeybee drone photoreceptors. J Gen Physiol. 1987 May;89(5):791–813. doi: 10.1085/jgp.89.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Retinal glycogen. Arch Ophthalmol. 1961 Nov;66:680–688. doi: 10.1001/archopht.1961.00960010682013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Muri R. B., Jones G. J. Microspectrophotometry of single rhabdoms in the retina of the honeybee drone (Apis mellifera male). J Gen Physiol. 1983 Oct;82(4):469–496. doi: 10.1085/jgp.82.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Lucignani G., Atlas S., Crane A. M., Dienel G. A., Sokoloff L. Reexamination of glucose-6-phosphatase activity in the brain in vivo: no evidence for a futile cycle. Science. 1985 Jul 5;229(4708):60–62. doi: 10.1126/science.2990038. [DOI] [PubMed] [Google Scholar]
- Pentreath V. W., Kai-Kai M. A. Significance of the potassium signal from neurones to glial cells. Nature. 1982 Jan 7;295(5844):59–61. doi: 10.1038/295059a0. [DOI] [PubMed] [Google Scholar]
- Perrelet A. The fine structure of the retina of the honey bee drone. An electron microscopical study. Z Zellforsch Mikrosk Anat. 1970;108(4):530–562. doi: 10.1007/BF00339658. [DOI] [PubMed] [Google Scholar]
- Phelps C. H. Barbiturate-induced glycogen accumulation in brain. An electron microscopic study. Brain Res. 1972 Apr 14;39(1):225–234. doi: 10.1016/0006-8993(72)90797-4. [DOI] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M., Coles J. A., Van de Werve G. The supply of metabolic substrate from glia to photoreceptors in the retina of the honeybee drone. J Physiol (Paris) 1987;82(4):279–287. [PubMed] [Google Scholar]
- Tsacopoulos M., Poitry S., Borsellino A. Diffusion and consumption of oxygen in the superfused retina of the drone (Apis mellifera) in darkness. J Gen Physiol. 1981 Jun;77(6):601–628. doi: 10.1085/jgp.77.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M., Poitry S. Kinetics of oxygen consumption after a single flash of light in photoreceptors of the drone (Apis mellifera). J Gen Physiol. 1982 Jul;80(1):19–55. doi: 10.1085/jgp.80.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]