Abstract
The increasing use of single cell gel electrophoresis (the comet assay) highlights its popularity as a method for detecting DNA damage, including the use of enzymes for assessment of oxidatively damaged DNA. However, comparison of DNA damage levels between laboratories can be difficult due to differences in assay protocols (e.g. lysis conditions, enzyme treatment, the duration of the alkaline treatment and electrophoresis) and in the end points used for reporting results (e.g. %DNA in tail, arbitrary units, tail moment and tail length). One way to facilitate comparisons is to convert primary comet assay end points to number of lesions/106 bp by calibration with ionizing radiation. The aim of this study was to investigate the inter-laboratory variation in assessment of oxidatively damaged DNA by the comet assay in terms of oxidized purines converted to strand breaks with formamidopyrimidine DNA glycosylase (FPG). Coded samples with DNA oxidation damage induced by treatment with different concentrations of photosensitizer (Ro 19-8022) plus light and calibration samples irradiated with ionizing radiation were distributed to the 10 participating laboratories to measure DNA damage using their own comet assay protocols. Nine of 10 laboratories reported the same ranking of the level of damage in the coded samples. The variation in assessment of oxidatively damaged DNA was largely due to differences in protocols. After conversion of the data to lesions/106 bp using laboratory-specific calibration curves, the variation between the laboratories was reduced. The contribution of the concentration of photosensitizer to the variation in net FPG-sensitive sites increased from 49 to 73%, whereas the inter-laboratory variation decreased. The participating laboratories were successful in finding a dose–response of oxidatively damaged DNA in coded samples, but there remains a need to standardize the protocols to enable direct comparisons between laboratories.
Introduction
Alkaline single cell gel electrophoresis (the comet assay) is a method used to measure single strand breaks (SSB) and alkali-labile sites (ALS). One reason for the increasing interest in using the method is the low number of cells required to measure DNA lesions. A range of different types of DNA lesions can be measured by adding lesion-specific enzymes (1). A common modification of the assay is to measure the level of 8-oxoguanine as well as other altered purines by incorporating a digestion with the bacterial DNA repair enzyme formamidopyrimidine DNA glycosylase (FPG) (1). In addition, the comet assay can be modified to measure DNA incision activity reflecting base excision repair (2) and nucleotide excision repair (3). Several guidelines for the comet assay have been published (4–7), but there are still considerable differences in protocols used by different research groups. These differences affect inter-laboratory comparisons of results and there is no general agreement about the normal background level of DNA lesions measured by the comet assay. Important steps in the comet assay procedure that may affect the variability are: (i) cell treatment/dilution in agarose, (ii) duration of enzyme treatment, (iii) duration and pH of alkaline treatment, (iv) electrophoresis conditions and (v) slide scoring. In addition, the fact that different laboratories use different end points (i.e. %DNA in tail, tail moment, tail length and arbitrary units as well as various descriptions of the distribution of images) when reporting their results further complicates the comparison of data between different laboratories. Møller et al. (8) have previously shown that there is substantial variation when different investigators score the same slides by visual classification of comets. Forchhammer et al. (9) recently reported that inter-individual differences in visual scoring could be reduced to a large extent by using investigator- and protocol-specific calibration curves.
The aim of this study was to assess variation in estimates of oxidatively damaged DNA, in terms of FPG-sensitive sites, measured with the comet assay by 10 different European Comet assay Validation Group (ECVAG) partners using their own protocols when analyzing coded samples.
Materials and methods
Study design
In order to investigate the inter-laboratory variation in the analysis of oxidation damage to DNA, 10 laboratories measured the level of DNA damage in four γ-ray irradiated calibration samples and three coded samples of human cells using their own protocols. The three coded samples contained cells with different amounts of 8-oxoguanine in their DNA. Cryopreserved calibration curve samples, coded samples and aliquots of FPG from the same batch were distributed on dry ice to the participating laboratories. Laboratories were instructed to analyse the calibration samples together with the coded samples, in order to create laboratory-specific calibration curves. Each laboratory completed a questionnaire on their comet assay protocol (Table I).
Table I.
Comet assay conditions used by the 10 different laboratories
| Laboratory | LMP agarose (% in PBS) | Enzyme incubation (min) | Electrophoresis |
Alkali (min) | Stain | Software | |||
| Time (min) | Voltage (V) | V/cma | Current (mA) | ||||||
| 1 | 0.75 | 45 | 20 | 30 | 0.9 | 300 | 40 | YOYO-1 | Comet IV (Perceptive Instruments) |
| 2 | 0.75 | 30 | 30 | 24 | 1.5 | 300 | 40 | EtBr | Komet 4.0 (Kinetic Imaging Ltd) |
| 3 | 0.65 | 10 | 20 | 25 | 0.9 | 300 | 20 | EtBr | Comet III (Perceptive Instruments) |
| 4 | 0.65 | 25 | 24 | 22 | 1.5 | 300 | 20 | EtBr | Comet IV (Perceptive Instruments) |
| 5 | 0.75 | 45 | 20 | 25 | 1.1 | 300 | 40 | YOYO-1 | — |
| 6 | 0.6 | 30 | 20 | 30 | 0.9 | 300 | 20 | PI | Komet 5.5 (Kinetic Imaging Ltd) |
| 7 | 1 | 30 | 30 | 25 | 1.6 | 260–300 | 40 | DAPI | Comet IV (Perceptive Instruments) |
| 8 | 1b | 45 | 20 | 25 | 1.1 | 300 | 40 | EtBr | Cometa 1.5 (Immagini e Computer, Italy) |
| 9 | 1 | 30 | 30 | 25 | 0.8 | 300 | 20 | DAPI | LUCIA 4.61 (Precoptic C, Czech Republic) |
| 10 | 0.8 | 30 | 20 | 23 | 1.2 | 300 | 40 | EtBr | Komet 5.5 (Kinetic Imaging Ltd) |
The dyes used are 4,6′-diamidino-2-phenylindole dihydrochloride (DAPI), ethidium bromide (EtBr), propidium iodide (PI) and YOYO®-1 iodide (YOYO-1). All laboratories used low-melting point (LMP) agarose.
V/cm was calculated using a spreadsheet published on COMICS’ homepage (http://comics.vitamib.com/electrophoresis-physics). COMICS is an European Union funded project which focuses on refinement of the comet and DNA repair assays.
The agarose was diluted in TAE (buffer solution containing a mixture of Tris base, acetic acid and EDTA) instead of PBS.
Reagents and enzymes
Trypsin–ethylenediaminetetraacetic acid (EDTA), Dulbecco's Minimal Essential Medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 medium, foetal bovine serum (FBS), penicillin–streptomycin and sodium pyruvate were purchased from Invitrogen Corporation, Karlsruhe, Germany. FPG was supplied by one of the participating laboratories (Department of Nutrition, University of Oslo, Norway).
Preparation of calibration curve samples
Samples of human acute monocytic leukaemia THP1 cells in phosphate-buffered saline (PBS) were irradiated with γ-rays at 0, 2.5, 5 and 10 Gy as described previously (9). Aliquots of cells were frozen slowly to −80°C in 50% FBS, 40% RPMI 1640 medium and 10% dimethyl sulfoxide (DMSO).
Preparation of coded samples with 8-oxoguanine
A549 human type II alveolar epithelial cells were cultured and harvested as described previously (10). The cells were centrifuged at 400 × g for 5 min at 4°C; the cell pellet was suspended in PBS and centrifuged again. Cells were divided into three portions and suspended in PBS or in PBS containing 0.2 or 0.8 μM of the photosensitizer Ro 19-8022. Treatment with photosensitizer Ro 19-8022 and visible light predominantly generates 8-oxoguanine (11). All three cell suspensions were irradiated on ice for 5 min from a distance of 33 cm with a 500-W tungsten halogen lamp. Following irradiation, cells were immediately centrifuged (500 × g) for 5 min at 4°C. The cell pellets of the coded samples were suspended in freezing medium consisting of 20% FBS, 70% DMEM and 10% DMSO, aliquoted and frozen slowly to −80°C.
The comet assay
The 10 participating laboratories used their own comet assay protocols to measure the level of FPG-sensitive sites. The basic procedure was common for all laboratories. Cells were embedded in low-melting point agarose (for concentrations see Table I) and then lysed (1% Triton X-100, 2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, pH 10) for a minimum of 1 h at 4°C. In addition, the lysis solution of Laboratory 3 contained 1% DMSO and the lysis solution of Laboratory 8 contained both 1% DMSO and 1% N-lauroylsarcosine sodium salt. For the coded samples, an additional step of digestion with FPG was added in order specifically to measure oxidative DNA lesions. FPG recognizes and removes specific oxidatively damaged purines creating an apurinic site, leading to a strand break either by the enzyme's associated lyase activity or by the subsequent alkaline treatment. After lysis, the gels were washed 3× in buffer (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA, 0.2 mg/ml bovine serum albumin, pH 7.8–8) and then incubated with buffer containing FPG. Enzyme incubation was performed at 37°C for between 10 and 45 min, depending on the laboratory (Table I). Eight laboratories (1–3 and 5–9) incubated parallel gels in enzyme buffer with and without FPG, thereby enabling estimation of background DNA breaks and the calculation of net FPG-sensitive sites, whereas two laboratories (4 and 10) only incubated gels with FPG. Slides were placed in an alkaline solution (0.3 M NaOH, 1 mM EDTA, pH > 13) for 20–40 min (Table I), followed by electrophoresis in the same solution for 20–30 min (Table I). By their ability to relax DNA supercoiling, strand breaks allow DNA to migrate out from the nucleoids under the influence of the electric field. The DNA was stained (Table I) and the comets, with tails of relaxed and mainly single-stranded DNA, were analysed either by visual scoring or by computerized image analysis.
Different comet assay end points
The magnitude of the comet tail can be reported in different units e.g. %DNA in tail, tail moment and tail length when using computerized scoring or arbitrary units when using visual scoring. Some laboratories reported several end points.
Arbitrary units (from visual scoring) are normally reported in the range 0–400 (1). A direct comparison of the data obtained by computerized and visual scoring can be obtained by dividing the visual score by a factor of 4, so that the visual score has the same range as %DNA in tail (0–100), though this is only an approximate equivalence. Since Laboratory 5 only reported results in arbitrary units, its results have been transformed in this way and are referred to as %DNA in tail.
Calculations of the amount of lesions/106 bp
One gray of γ-rays induces 0.29-strand breaks/109 Da DNA [based on a mean of the levels reported by Ahnström et al. (12) and Kohn et al. (13)]. Based on this dose equivalence and the assumption that 1 bp has a mass of 650 Da, the slopes of the calibration curves (%DNA in tail/Gy) were used to calculate how many lesions/106 bp a particular percentage of DNA in the tail corresponded to:
In order to get the number of lesions/106 Guanines (Gua), the number of lesions/106 bp is multiplied by 0.44. The slope of the calibration curve refers to the slope of each laboratory-specific calibration curve. THP1 cells contain 6 × 1012 Da DNA, whereas A549 cells contain 12 × 1012 Da DNA. However, we assume that the %DNA in tail in the standard curve samples would not have been affected if another cell line with a different amount of DNA had been used.
Statistics
Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The statistical analyses were carried out by parametric tests using general linear models (GLM), correlation analyses, z-tests (Fisher's z transformation) and Levene's tests. In the GLM analyses, the level of DNA lesions was the dependent variable, the laboratory was a random variable and the rest of the variables, i.e. concentration of photosensitizer, duration of alkaline treatment, duration of electrophoresis, duration of enzyme treatment and agarose density, were covariate (continuous) variables. Assessment of the contribution of each variable to the overall variation was calculated from the sum of squares in the statistical analysis. In Levene's test, the homogeneities of variances were tested between data transformed with the laboratory-specific calibration curves and data transformed by a mutual standard curve (the mean of the laboratory-specific calibration curves). Throughout this study, P values < 0.05 were considered as indicating statistical significance. The statistical analyses are based on data both from %DNA in tail and by visual score.
Results
First, we describe the measurement of calibration samples, i.e. cells γ-ray irradiated with different doses, in the different laboratories; the results give a picture of inter-laboratory variation in assaying simple DNA breaks. ‘DNA breaks’ includes DNA SSB and ALS. Next, we give the results obtained with coded samples, first looking at simple DNA breaks. The coded samples contained different amounts of 8-oxoguanine, which were converted to strand breaks with FPG; thus, the breaks measured include DNA breaks and 8-oxoguanine. By subtracting the level of DNA breaks, we obtain net FPG-sensitive sites, i.e. 8-oxoguanine; this is obviously possible only for the eight laboratories that measured DNA breaks. The data are analysed as ‘raw’ values (%DNA in tail) and then converted to lesions/106 bp using the calibration curve for each laboratory.
DNA breaks in calibration curve samples
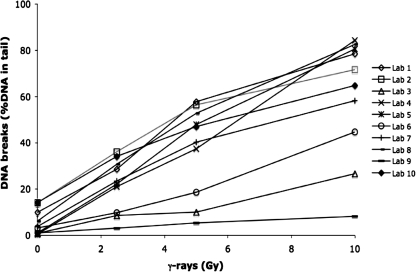
The mean level of DNA breaks (i.e. SSB and ALS) in the calibration curve samples measured as (or converted to) %DNA in tail by 10 different laboratories using their own comet assay protocols are presented in Figure 1. The level of DNA breaks measured as both %DNA in tail (n = 10) and tail moment (n = 7) are presented as mean ± SD in Table II. There were very large variations in levels of DNA breaks reported by the different laboratories, which is probably explained by differences in the comet assay protocols. The dose of ionizing radiation, inter-laboratory variation and unexplained variation contributed to 59, 28 and 13%, respectively, of the total variation (GLM analysis). Both the dose of ionizing radiation and the inter-laboratory variation contributed significantly (P < 0.001) to the level of DNA breaks expressed as %DNA in tail. All laboratories reported significant linear dose–response relationships (R2mean = 0.970, range: 0.931–0.995).
Fig. 1.
The levels of DNA breaks in calibration curve samples of THP1 cells exposed to 0, 2.5, 5 or 10 Gy. Ten laboratories used their own comet assay protocols to measure the levels of DNA breaks. The levels of DNA breaks are presented as mean values of % DNA in tail (n = 10, R2 = 0.594, P < 0.001).
Table II.
DNA breaksa in calibration curve samples consisting of THP1 cells exposed to 0, 2.5, 5 and 10 Gy, respectively, measured with the comet assay by 10 different laboratories using their own protocols
| Dose (Gy) | %DNA in tail, n = 10 |
Tail moment, n = 7 |
||
| Mean (SD) | CV (%) | Mean (SD) | CV (%) | |
| 0 | 5.5 (5.4) | 99 | 1.3 (2.0) | 147 |
| 2.5 | 21.8 (11.3) | 52 | 6.2 (4.8) | 78 |
| 5 | 37.3 (19.3) | 52 | 13.4 (10.3) | 76 |
| 10 | 60.0 (25.9) | 43 | 29.9 (20.7) | 69 |
Some laboratories have reported data with more than one end point.
i.e. SSB and ALS.
DNA breaks in the coded samples
The level of DNA breaks in the coded samples measured as %DNA in tail (n = 8), tail moment (n = 6) and lesions/106 bp (n = 8) are presented as mean ± SD in Table III. As can be seen, there is a dose-dependent increase for both the %DNA in tail as well as the number of lesions/106 bp. In fact, seven of eight laboratories reported a positive ranking of DNA breaks with concentration of photosensitizer in the three coded samples. The concentration of photosensitizer, inter-laboratory variation and unexplained variation contributed 7, 87 and 6%, respectively, to the total variation of DNA breaks expressed as %DNA in tail (GLM analysis). Such a low contribution of the concentration was expected since the photosensitizer induces mainly oxidative damage to DNA and not DNA breaks. Nonetheless, both the contribution of the concentration of photosensitizer and the inter-laboratory variation to the total variation of %DNA in tail was statistically significant (P < 0.001). The mean of the linear dose–response relationships reported by the laboratories was R2mean = 0.83 (range: 0.09–1.00). When transforming the %DNA in tail to lesions/106 bp using the laboratory-specific calibration curves, the Pearson correlation coefficient r increased from 0.27 to 0.36 (correlation between the level of DNA breaks and concentration of photosensitizer). The concentration of photosensitizer, inter-laboratory variation and unexplained variation contributed 13, 79 and 9%, respectively, of the total variation of DNA breaks expressed as lesions/106 bp (GLM analysis).
Table III.
DNA lesions in coded samples consisting of A549 cells exposed to 0, 0.2 and 0.8 μM photosensitizer Ro 19-8022 and light, respectively, measured with the comet assay
| Photosensitizer (μM) | %DNA in tail |
Tail moment |
Lesions/106 bp |
||||
| Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) | ||
| DNA breaksa,b | |||||||
| 0 | 7.1 (5.6) | 78 | 2.1 (2.3) | 108 | 0.27 (0.16) | 59 | |
| 0.2 | 8.4 (6.1) | 73 | 2.6 (2.9) | 111 | 0.33 (0.19) | 59 | |
| 0.8 | 11.2 (8.1) | 72 | 3.6 (3.3) | 90 | 0.46 (0.29) | 62 | |
| DNA breaksa and FPG-sensitive sitesc | |||||||
| 0 | 14.3 (7.8) | 54 | 4.4 (3.4) | 78 | 0.49 (0.19) | 38 | |
| 0.2 | 27.2 (15.8) | 58 | 9.7 (7.3) | 75 | 0.96 (0.36) | 38 | |
| 0.8 | 50.7 (27.9) | 55 | 27.2 (17.7) | 65 | 1.75 (0.55) | 32 | |
| Net FPG-sensitive sitesb | |||||||
| 0 | 6.1 (4.2) | 68 | 2.1 (2.2) | 101 | 0.20 (0.13) | 64 | |
| 0.2 | 19.2 (13.3) | 69 | 6.8 (6.0) | 87 | 0.69 (0.28) | 40 | |
| 0.8 | 40.5 (22.6) | 56 | 22.9 (16.5) | 72 | 1.41 (0.43) | 31 | |
Lesions/106 bp have been calculated by using the laboratory-specific calibration curves. Some laboratories have reported data with more than one end point.
i.e. SSB and ALS.
End points presented are %DNA in tail (n = 8), tail moment (n = 6) and lesions/106 bp (n = 8).
End points presented are %DNA in tail (n = 10), tail moment (n = 7) and lesions/106 bp (n = 10).
DNA breaks and FPG-sensitive sites in the coded samples
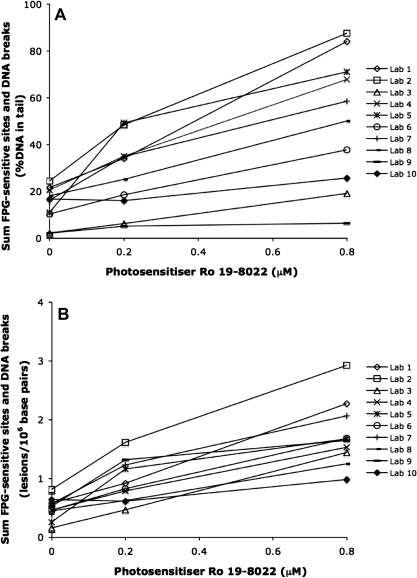
The results from FPG treatment (i.e. the sum of DNA breaks and FPG-sensitive sites) expressed as %DNA in tail and lesions/106 bp (n = 10) in the three coded samples are shown in Figure 2A and B, respectively. Nine of 10 laboratories reported a positive ranking of the FPG-treated samples with concentration of photosensitizer in the coded samples (Figure 2). The mean of the linear dose–response relationships for the sum of DNA breaks and FPG-sensitive sites was R2mean = 0.94 (range: 0.76–1.00). The dose–response was highly significant both for data reported as %DNA in tail and after conversion to lesions/106 bp using the laboratory-specific calibration curves (P < 0.001). The Pearson correlation coefficient was increased from r = 0.64 to r = 0.80 after conversion to lesions/106 bp. However, this increase of the Pearson correlation coefficient was not statistically significant (z-test, P > 0.05). The sum of DNA breaks and FPG-sensitive sites measured as %DNA in tail (n = 10), tail moment (n = 7) and lesions/106 bp (n = 10) are presented as mean ± SD in Table III. The coefficient of variation in the sum of DNA breaks and FPG-sensitive sites between the participating laboratories was lower for all concentrations after converting the end point %DNA in tail to lesions/106 bp using the laboratory-specific calibration curves (Table III). However, this decreased variation between laboratories was only significant for 0.8-μM photosensitizer Ro 19-8022 (P < 0.05, Levene's test).
Fig. 2.
The sum of DNA breaks and FPG-sensitive sites in coded samples with A549 cells exposed to light and 0, 0.2 or 0.8 μM of photosensitizer Ro 19-8022 in PBS. Ten laboratories used their own comet assay protocols to measure the sum of DNA breaks and FPG-sensitive sites. Data are presented as (A) %DNA in tail and as (B) lesions/106 bp. The amount of lesions/106 bp has been calculated based on the assumption that 1 Gy induces 0.29 strand breaks/109 Da DNA and using laboratory-specific calibration curves.
Both the concentration of photosensitizer (P < 0.001, positive association) and the inter-laboratory variation (P < 0.001) contributed significantly to the total variation of the sum of DNA breaks and FPG-sensitive sites expressed as %DNA in tail (GLM analysis, Table IV). In a multiple regression analysis of the concentration of photosensitizer and the different protocol steps, the contribution to the variation of the concentration of photosensitizer (P < 0.001, positive association) and the density of agarose gels (P < 0.001, negative association) were highly significant and the contribution of the duration of alkaline treatment (P < 0.01, positive association), duration of electrophoresis (P < 0.01, positive association) and duration of enzyme treatment (P < 0.05, positive association) were significant (GLM analysis, Table IV). By converting the %DNA in tail to lesions/106 bp, the contribution of the concentration of photosensitizer was increased from 41 to 64% (GLM analysis, Tables IV and V). In a multiple regression analysis of the concentration of photosensitizer and the different protocol steps, the contribution of the concentration of photosensitizer remained highly significant (P < 0.001, positive association). The duration of electrophoresis (P < 0.001, positive association) and agarose density (P < 0.01, negative association) also contributed significantly to the variation in the sum of DNA breaks and FPG-sensitive sites expressed as lesions/106 bp (Table V).
Table IV.
Contribution of the concentration of photosensitizer Ro 19-8022, analyzing laboratory and protocol steps to the variation of the sum of DNA breaks and FPG-sensitive sites expressed as %DNA in tail
| Variable | R2model | % of total variation |
SDres | ||
| Concentration | Variable | Residual | |||
| Concentration | 0.406 | 41*** | — | 59 | 18.5 |
| Concentration + laboratorya | 0.845 | 41*** | 44*** | 16 | 9.4 |
| Concentrationb | 0.761 | 38*** | 22 | 11.7 | |
| Alkaline treatment (min)b | 8** | ||||
| Electrophoresis (min)b | 9** | ||||
| Enzyme treatment (min)b | 6* | ||||
| Agarose densityb | 17*** | ||||
| Concentration + alkaline treatment (min)c | 0.566 | 41*** | 16** | 43 | 15.8 |
| Concentration + electrophoresis (min)c | 0.410 | 41*** | 0ns | 59 | 18.4 |
| Concentration + enzyme treatment (min)c | 0.520 | 41*** | 11* | 48 | 16.6 |
| Concentration + agarose densityc | 0.415 | 41*** | 1ns | 59 | 18.3 |
*P < 0.05, **P < 0.01 and ***P < 0.001.
Concentration and laboratory in one model.
Concentration and different protocol steps together in one model.
Concentration and one protocol step at a time in a model.
Table V.
Contribution of the concentration of photosensitizer Ro 19-8022, analyzing laboratory and protocol steps to the variation of the sum of DNA breaks and FPG-sensitive sites after conversion to lesions/106 bp using the laboratory-specific calibration curves (n = 10)
| Variable | R2model | % of total variation |
SDres | ||
| Concentration | Variable | Residual | |||
| Concentration | 0.643 | 64*** | — | 36 | 0.39 |
| Concentration + laboratorya | 0.878 | 64*** | 24** | 12 | 0.23 |
| Concentrationb | 0.856 | 60*** | 13 | 0.25 | |
| Alkaline treatment (min)b | 1ns | ||||
| Electrophoresis (min)b | 18*** | ||||
| Enzyme treatment (min)b | 2ns | ||||
| Agarose densityb | 6** | ||||
| Concentration + alkaline treatment (min)c | 0.667 | 64*** | 2ns | 33 | 0.38 |
| Concentration + electrophoresis (min)c | 0.761 | 64*** | 12** | 24 | 0.32 |
| Concentration + enzyme treatment (min)c | 0.652 | 64*** | 1ns | 35 | 0.38 |
| Concentration + agarose densityc | 0.646 | 64*** | 0ns | 35 | 0.39 |
*P < 0.05, **P < 0.01 and ***P < 0.001.
Concentration and laboratory in one model.
Concentration and different protocol steps together in one model.
Concentration and one protocol step at a time in a model.
It should be noted that the residual variation (the unexplained fraction of the variation) was higher in models with DNA lesions as dependent variable and only the concentration of photosensitizer as independent variable than in multiple regression models that also contained either laboratory or different protocol steps as independent variables (Tables IV and V). The model with the concentration of photosensitizer and laboratory as independent variables had a lower unexplained fraction of the variation compared with models with specific protocol differences (Tables IV and V).
Net FPG-sensitive sites in the coded samples
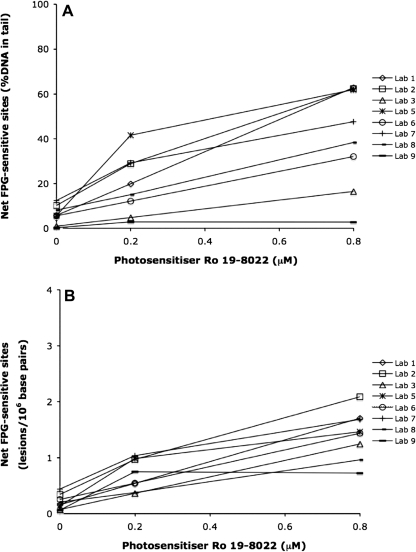
The levels of net FPG-sensitive sites expressed as %DNA in tail and lesions/106 bp (n = 8) in the three coded samples are shown in Figure 3A and B, respectively. Seven of eight laboratories reported a positive ranking of net FPG-sensitive sites with concentration of photosensitizer in the coded samples. The mean of the linear dose–response relationships was R2mean = 0.90 (range: 0.45–1.00). The dose–response relationship was highly significant both for data reported as %DNA in tail and after conversion to lesions/106 bp using the laboratory-specific calibration curves (P < 0.001). The Pearson correlation coefficient was increased from r = 0.70 to r = 0.85 after conversion to lesions/106 bp. This increase of the Pearson correlation coefficient was not statistically significant (z-test, P > 0.05). The amount of net FPG-sensitive sites in the coded samples measured as %DNA in tail (n = 8), tail moment (n = 6) and the amounts of lesions/106 bp (n = 8) are presented as mean ± SD in Table III. These values correspond to 0.09, 0.30 and 0.62 net FPG-sensitive sites/106 Gua for the concentrations 0, 0.2 and 0.8 μM photosensitizer, respectively. The variation in the measured net FPG-sensitive sites between the participating laboratories was lower for all concentrations of the photosensitizer after converting the end point %DNA in tail to lesions/106 bp (Table III). However, this decrease was not statistically significant (Levene's test).
Fig. 3.
The level of net FPG-sensitive sites (the sum of DNA breaks and FPG-sensitive sites minus the level of DNA breaks) in coded samples with A549 cells exposed to light and 0, 0.2 or 0.8 μM of photosensitizer Ro 19-8022 in PBS. Eight laboratories used their own comet assay protocols to measure the net FPG-sensitive sites. Data are presented as (A) %DNA in tail and as (B) lesions/106 bp. The fraction of explained variation strongly increased when using lesions/106 bp as end point. The amount of lesions/106 bp was calculated based on the assumption that 1 Gy induces 0.29 strand breaks/109 Da DNA and using laboratory-specific calibration curves.
Both the concentration of photosensitizer (P < 0.001, positive association) and the inter-laboratory variation (P < 0.01) contributed significantly to the total variation of net FPG-sensitive sites expressed as %DNA in tail (GLM analysis, Table VI). In a multiple regression analysis of the concentration of photosensitizer and the different protocol steps, it was found that the concentration of the photosensitizer explained 54% (P < 0.001, positive association) of the variation when using the end point %DNA in tail. In addition, the contributions of the duration of alkaline treatment (P < 0.01, positive association) and agarose density (P < 0.05, negative association) were significant (Table VI). After conversion to the end point lesions/106 bp, the contribution of the concentration of photosensitizer was improved from 49 to 73% (GLM analysis of concentration of photosensitizer and laboratory, Tables VI and VII). In a multiple regression analysis of the concentration of the photosensitizer and the protocol steps, the contributions of the concentration of photosensitizer (P < 0.001, positive association), duration of alkaline treatment (P < 0.05, positive association), duration of electrophoresis (P < 0.05, positive association) and agarose density (P < 0.05, negative association) were significant (Table VII).
Table VI.
Contribution of the concentration of photosensitizer Ro 19-8022, analyzing laboratory and protocol steps to the variation of the net FPG-sensitive sites expressed as %DNA in tail
| Variable | R2model | % of total variation |
SDres | ||
| Concentration | Variable | Residual | |||
| Concentration | 0.485 | 49*** | — | 51 | 14.8 |
| Concentration + laboratorya | 0.826 | 49*** | 34** | 17 | 8.6 |
| Concentrationb | 0.808 | 54*** | 22 | 9.0 | |
| Alkaline treatment (min)b | 16** | ||||
| Electrophoresis (min)b | 1ns | ||||
| Enzyme treatment (min)b | 0ns | ||||
| Agarose densityb | 6* | ||||
| Concentration + alkaline treatment (min)c | 0.747 | 49*** | 26*** | 25 | 10.4 |
| Concentration + electrophoresis (min)c | 0.485 | 49*** | 0ns | 51 | 14.8 |
| Concentration + enzyme treatment (min)c | 0.595 | 49*** | 11* | 40 | 13.1 |
| Concentration + agarose densityc | 0.487 | 49*** | 0ns | 51 | 14.8 |
*P < 0.05, **P < 0.01 and ***P < 0.001.
Concentration and laboratory in one model.
Concentration and different protocol steps together in one model.
Concentration and one protocol step at a time in a model.
Table VII.
Contribution of the concentration of photosensitizer Ro 19-8022, analyzing laboratory and protocol steps to the variation of the net FPG-sensitive sites after conversion to lesions/106 bp using the laboratory-specific standard curves (n = 8)
| Variable | R2model | % of total variation |
SDres | ||
| Concentration | Variable | Residual | |||
| Concentration | 0.727 | 73*** | — | 27 | 0.31 |
| Concentration + laboratorya | 0.878 | 73*** | 15ns,b | 12 | 0.20 |
| Concentrationc | 0.864 | 72*** | 13 | 0.22 | |
| Alkaline treatment (min)c | 4* | ||||
| Electrophoresis (min)c | 5* | ||||
| Enzyme treatment (min)c | 0ns | ||||
| Agarose densityc | 5* | ||||
| Concentration + alkaline treatment (min)d | 0.778 | 73*** | 5* | 22 | 0.28 |
| Concentration + electrophoresis (min)d | 0.756 | 73*** | 3ns | 24 | 0.29 |
| Concentration + enzyme treatment (min)d | 0.729 | 73*** | 0ns | 27 | 0.30 |
| Concentration + agarose densityd | 0.729 | 73*** | 0ns | 27 | 0.30 |
*P < 0.05, **P < 0.01 and ***P < 0.001.
Concentration and laboratory in one model.
Borderline significance (P = 0.052).
Concentration and different protocol steps together in one model.
Concentration and one protocol step at a time in a model.
As in the case of the sum of FPG-sensitive sites and DNA breaks, the residual variation was higher in models with net FPG-sensitive sites as dependent variable and only the concentration of photosensitizer as independent variable than in multiple regression models that also contained either laboratory or different protocol steps as independent variables (Tables VI and VII). The model with the concentration of photosensitizer and laboratory as independent variables had a lower unexplained fraction of the variation compared to models with specific protocol differences (Tables VI and VII).
Discussion
The laboratories in this ECVAG study were successful in finding a dose–response relationship of FPG-sensitive sites, where nine of 10 participating laboratories identified the dose–response for the sum of DNA breaks and FPG-sensitive sites. Regarding net FPG-sensitive sites, seven of eight laboratories identified this dose–response. In a similar trial conducted by European Standards Committee on Oxidative DNA damage (ESCODD) published in 2003, only three of eight laboratories could detect the dose–response relationship in net FPG-sensitive sites using the comet assay (14). In the ESCODD trial, human transformed epithelial (HeLa) cells were exposed to 0, 0.2 or 0.4 μM photosensitizer Ro 19-8022 and light for 2 min, whereas in the present study, A549 cells were exposed to 0, 0.2 or 0.8 μM Ro 19-8022 and light for 5 min. The narrower concentration range used in the ESCODD trial might in part explain why the present study was more successful at detecting the dose–response.
Any comet assay end point can be expressed as lesions/106 bp, as lesions/109 Da DNA, as lesions/cell or as FPG-sensitive sites/106 Gua by calibration with ionizing radiation. In the present study, the slopes of the laboratory-specific calibration curves were used to calculate how many lesions/106 bp particular amounts of DNA lesions expressed as %DNA in tail corresponded to. The difference between the 10 laboratories was reduced (Figures 2 and 3), after the level of DNA lesions expressed as %DNA in tail had been converted to lesions/106 bp. By converting %DNA in tail to lesions/106 bp, the coefficient of variation was decreased for all doses, although statistical significance was not reached for all tests (Table III). The increased values of Pearson's correlation coefficients after conversion to lesions/106 bp for both sum of DNA breaks and FPG-sensitive sites (from r = 0.64 to r = 0.80) and net FPG-sensitive sites (from r = 0.70 to r = 0.85) underscore the ability of laboratory-specific calibration curves to reduce the inter-laboratory variation although these increases were not statistically significant. It should be kept in mind that transformation of primary comet assay end points using calibration curves or reference standards is associated with a variation that is equal to the sum of the variations in the sample and calibration sample. Therefore, even though the reduction of variation was not significant, it should be underlined that such a reduction is relevant since it managed to cope with the variation introduced by the transformation and reduce the overall variation. It is reasonable to assume that inter-laboratory differences in protocols were responsible for a large part of the variation. When analyzing how much of the variation in net FPG-sensitive sites was caused by the concentration of the photosensitizer and the analyzing laboratory, it was found that the contribution of the concentration of the photosensitizer was increased from 49 to 73% by converting %DNA in tail to lesions/106 bp (Tables VI and VII). Similar analyses for DNA breaks were not made since the Ro 19-8022 plus light mainly induces oxidatively damaged DNA and not DNA breaks. The decrease of the inter-laboratory variation after the conversion to lesions/106 bp observed in the present study indicates that such a conversion would facilitate comparisons between data from different research groups by decreasing the influence of the differences in used protocols. Forchhammer et al. (9) have shown previously that it is possible to reduce the variation in DNA lesions caused by using different comet assay protocols by using protocol-specific calibration curves. Use of lesions/106 bp, lesions/109 Da DNA, lesions/cell or FPG-sensitive sites/106 Gua as end points instead of other end points (e.g. %DNA in tail and arbitrary units from visual scoring) also has other advantages. The amount of lesions/106 bp would be easier to understand for individuals who are not familiar with the comet assay compared to end points such as %DNA in tail, tail moment, arbitrary units, tail length and different types of comet distribution. Furthermore, if scientists could agree on one end point it would facilitate comparisons between different studies and laboratories. The amount of lesions/106 bp can be calculated regardless of whether visual or computerized comet scoring is used. Conversion to lesions/106 bp does, however, require calibration with ionizing radiation. A second option would be to introduce an authentic internal standard. An authentic internal standard consisting of ‘reference’ cells (which generate reference-comets that can be readily distinguished from test comets present in the same gel) can be used to reduce intra- and inter-experiment variability in measures of DNA damage formation and repair (15).
The 10 participating laboratories in this validation trial used different protocols, but the solutions used were either very similar (lysis solution) or identical (enzyme and alkaline/electrophoresis solutions). The agarose densities used varied between 0.65 and 2% agarose, the duration of the alkaline treatment varied between 20 and 40 min, the duration of the electrophoresis varied between 20 and 30 min and the length of the FPG treatment varied between 10 and 45 min (see Materials and Methods and Table I). Forchhammer et al. (9) previously showed that the duration of both alkaline treatment (20 and 30 min) and electrophoresis (20 and 40 min) significantly affected the level of oxidatively damaged DNA detected by visual scoring. It should be emphasized that the present study was not designed to investigate the potential influence of differences in protocols, and the degrees of freedom and number of participating laboratories are not optimal for such analyses. However, in the present study, statistical models with laboratory as categorical variable explained more of the variation than models with specific steps in the comet assay protocols (Tables IV–VII). In statistical models with only the concentration of the photosensitizer as independent variable, the residual variation (the unexplained fraction of the variation) was larger than in all models with either laboratory or different protocol steps as independent variables (Tables IV–VII). This indicates that the inter-laboratory variation in DNA damage to a large extent is explained by the variation in protocols but that it is difficult to point out any critical step in the protocol. It is possible that differences in protocols not included in the statistical analyses such as the use of different software systems also might contribute to the inter-laboratory variation.
In conclusion, the participating laboratories were successful in finding a dose–response relationship of oxidatively damaged DNA where nine of 10 laboratories reported the same ranking of the FPG treatment and seven of eight laboratories reported the same ranking of net FPG-sensitive sites in the coded samples. The inter-laboratory variation in assessment of oxidatively damaged DNA was largely due to differences in the protocols. The statistical analyses imply that it is assay protocols rather than single steps in the procedure that give rise to the inter-laboratory variation. After conversion of the data to lesions/106 bp using laboratory-specific calibration curves, the variation between the laboratories was reduced for all concentrations of photosensitizer Ro 19-8022. In addition, the contribution of the concentration of Ro 19-8022 to the variation in net FPG-sensitive sites was increased from 49 to 73% by conversion from %DNA in tail to lesions/106 bp using the laboratory-specific calibration curves. We plan to repeat the present study with standardized protocols to enable comparisons between laboratories without the influence of protocol differences.
Funding
Environmental Cancer Risk, Nutrition and Individual Susceptibility, a network of excellence operating within the European Union 6th Framework Program, Priority 5: ‘Food Quality and Safety’ (Contract No 513943); the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS); the European Union 6th Framework Program strategic targeted research project ‘COMICS’ (Contract No 037575); Swedish Research Council (Vetenskapsrådet).
Acknowledgments
The photosensitizer Ro 19-8022 was a generous gift from F. Hoffmann-La Roche, Basel, Switzerland.
Conflict of interest statement: None declared.
References
- 1.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 2.Collins AR, Dusinska M, Horvathova E, Munro E, Savio M, Stetina R. Inter-individual differences in repair of DNA base oxidation, measured in vitro with the comet assay. Mutagenesis. 2001;16:297–301. doi: 10.1093/mutage/16.4.297. [DOI] [PubMed] [Google Scholar]
- 3.Langie SA, Knaapen AM, Brauers KJ, van Berlo D, van Schooten FJ, Godschalk RW. Development and validation of a modified comet assay to phenotypically assess nucleotide excision repair. Mutagenesis. 2006;21:153–158. doi: 10.1093/mutage/gel013. [DOI] [PubMed] [Google Scholar]
- 4.Albertini RJ, Anderson D, Douglas GR, et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat. Res. 2000;463:111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 5.Burlinson B, Tice RR, Speit G, et al. Fourth International Workgroup on Genotoxicity testing: results of the in vivo Comet assay workgroup. Mutat. Res. 2007;627:31–35. doi: 10.1016/j.mrgentox.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann A, Agurell E, Beevers C, et al. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Møller P. The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin. Pharmacol. Toxicol. 2006;98:336–345. doi: 10.1111/j.1742-7843.2006.pto_167.x. [DOI] [PubMed] [Google Scholar]
- 9.Forchhammer L, Brauner EV, Folkmann JK, Danielsen PH, Nielsen C, Jensen A, Loft S, Friis G, Møller P. Variation in assessment of oxidatively damaged DNA in mononuclear blood cells by the comet assay with visual scoring. Mutagenesis. 2008;23:223–231. doi: 10.1093/mutage/gen006. [DOI] [PubMed] [Google Scholar]
- 10.Johansson C, Rytter E, Nygren J, Vessby B, Basu S, Möller L. Down-regulation of oxidative DNA lesions in human mononuclear cells after antioxidant supplementation correlates to increase of gamma-tocopherol. Int. J. Vitam. Nutr. Res. 2009;78:183–194. doi: 10.1024/0300-9831.78.45.183. [DOI] [PubMed] [Google Scholar]
- 11.Will O, Gocke E, Eckert I, Schulz I, Pflum M, Mahler HC, Epe B. Oxidative DNA damage and mutations induced by a polar photosensitizer, Ro19-8022. Mutat. Res. 1999;435:89–101. doi: 10.1016/s0921-8777(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 12.Ahnström G, Erixon K. In: DNA Repair: A Laboratory Manual of Research Procedures. Friedberg EC, Hanawalt PC, editors. New York, NY, USA: Marcel Dekker; 1981. pp. 403–418. [Google Scholar]
- 13.Kohn KW, Erickson LC, Ewig RA, Friedman CA. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976;15:4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- 14.ESCODD. Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic. Biol. Med. 2003;34:1089–1099. doi: 10.1016/s0891-5849(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 15.Zainol M, Stout J, Bowman K J, Almeida G M, Jones G D D, ECVAG Introducing a true internal standard for the Comet assay to minimize intra- and inter-experiment variability in measures of DNA damage and repair. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp826. 10.1093/nar/gkp826, October 14, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]