Abstract
Despite regulatory directives requiring the reduction of animal use in safety testing, recent modifications to genotoxicity testing guidelines now propose the use of two in vivo genotoxicity assays as a follow-up to an in vitro positive (International Conference on Harmonization Consensus Draft Guidance S2[R1] released March, 2008). To address both goals, the in vivo comet and micronucleus (MN) assays can be successfully combined into one informative study. Combining these two assays with such differences in sensitivity, endpoints measured and the type of data generated significantly improves upon the current standard capabilities for detecting genotoxicity without requiring additional animals. But to take full advantage of the benefits of incorporating the comet assay in safety testing, these same differences must be recognized and considered. Developed from over 15 years experience using the in vivo comet and MN assays in genotoxicity testing of chemicals and pharmaceuticals, this paper presents guidelines for the appropriate experimental design, dose selection and data interpretation for combined in vivo comet/MN assay studies. To illustrate the approach, data from combined assay studies are presented and discussed.
Introduction
Since the European Commission Cosmetic Products Directive in 1976, there has been a significant increase in efforts to reduce the use of animals in safety testing. Meanwhile, to improve the safety and protection of humans and the environment, the Registration, Evaluation and Authorization of Chemicals law entered into force in June, 2007 and the recent modification of the International Conference on Harmonization (ICH) Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use S2(R1) now proposes additional in vivo target organ testing of chemicals as a follow-up to positive in vitro findings. In response to the requirements of these conflicting legislative directives, proposals to combine the in vivo comet assay with the in vivo micronucleus (MN) assay and/or to integrate the two assays into repeat-dose toxicity studies are currently under consideration.
As a supplement to the standard test battery for genotoxicity, the in vivo comet assay is used to screen potential drug candidates early in development or it is used as a Tier II or Weight of Evidence study to assess the results of in vitro or in vivo genotoxicity tests. Due to its flexibility, the in vivo comet assay can be combined with or incorporated into most standard testing batteries to provide supplemental target organ data without the additional expenditure of time and resources required by an independent study. To ensure that the appropriate study design is used and an accurate interpretation of the comet data is achieved from such combined studies, the incorporation of the comet assay into standard genotoxicity tests and/or toxicology studies requires the recognition of the significant differences between the comet assay and the MN assay. Based on the Organization for Economic Co-operation and Development (OECD) guidelines for the in vivo MN assay (OECD 474), the proposed ICH S2(R1) guidelines recommend the use of the limit dose (e.g. 2000 mg/kg) or the maximum tolerated dose (MTD) as the high dose in all in vivo genotoxicity tests including the comet assay. It also states that concurrent positive control treatments may be excluded once a laboratory has ‘established competence in the use of the assay’. But these and similar recommendations should be adjusted for the comet assay to take full advantage of the increased sensitivity and flexibility it provides. This paper addresses three of the primary issues that are critical to the successful integration of the combined comet/MN assay and provides recommendations for ensuring the optimization of each:
Experimental design,
Dose selection/cytotoxicity and
Data analysis and interpretation.
To demonstrate the effectiveness of the protocol/recommendations proposed here, data from several studies are presented and discussed focusing on the unique aspects of the comet assay. All studies were blind studies conducted in accordance with the US Food and Drug Administration (FDA) (21 CFR Part 58) and the OECD Principles of Good Laboratory Practice (as revised in 1997), ENV/MC/CHEM (98) 17 and all subsequent consensus documents. Proprietary test article information and data are not disclosed.
Materials and methods
Although no definitive guidelines exist for the comet assay, the test methodology is in accordance with recommendations by Hartmann et al. and Burlinson et al. (1,2).
Test animals
Non-fasted virus antibody-free male or female Sprague Dawley rats or CD-1 mice 8–9 weeks of age with a mean body weight (BW) variation of ≤ ±10% at the start of dosing were used for each study. Uniquely identified animals were single housed in polysulphone cages with absorbent bedding and are maintained at temperatures of 18–23°C with a relative humidity of 40–60% and an air exchange rate of 70 ± 1 exchanges per hour. Lighting was controlled to maintain 12 h of light and 12 h of dark and animals were provided Purina Certified Rodent Chow 5002 (Purina Mills, Raleigh, NC) and water ad libitum. All animal procedures were in compliance with the National Research Council Guide for the Care and Use of Laboratory Animals (1996) and the US Animal welfare Act Regulations (9 CFR 1–4). All the protocols and procedures were reviewed and approved by the Helix3 Institutional Animal Care and Use Committee.
Controls
The concentrations of positive control compounds such as mitomycin C and cyclophosphamide (CP) that induce MNs in the bone marrow and peripheral blood can also induce cytotoxicity in any of the multiple tissues that can be tested in the comet assay. Since cytotoxicity can have confounding effects on the comet assay by increasing and/or decreasing DNA migration (1,3,4), the individual compound concentrations and/or the longer exposures that reliably induce MNs may not be optimal for use with the comet assay. Therefore, recommendations for combining the two assays in vivo have included (i) the use of separate positive control dose groups for the two separate assays with at least four to six animals in each; (ii) the use of one positive control dose group for comet and the use of positive control slides from independent studies for the MN positive control or (iii) the exclusion of concurrent positive controls for one or both endpoints. Either option compromises the benefits of the combined study either by adding more animals or by removing the ability to make concurrent control comparisons. It is of critical importance to note here that not only are concurrent controls necessary for adequate evaluation of comet assay data but also concurrent control data for each tissue sampled must be included as different tissues in the same animal can react differently to exposures depending on the pharmacokinetics of a test compound.
Our protocol for integrating the two assays into one study with either rats or mice avoids using any additional animals or resources by providing from the same group of animals concurrent positive control data for both endpoints and in every tissue sampled. In our protocol, the positive control group of animals received a combination of intraperitoneal (i.p.) injections of CP (CAS no. 6055-19-2; Sigma, St Louis, MO) with an oral (p.o.) administration of ethyl methanesulphonate (EMS; CAS no. 62-50-0; Sigma, St. Louis, MO) to induce in the same group of animals a positive MN response in the bone marrow and/or the peripheral blood as well as a positive comet response in any tissue sampled. In the typical rodent MN assay study, a single administration (either by i.p. injection or oral gavage) of CP 24 h prior to harvest is sufficient for inducing a positive genotoxic response in the bone marrow while an administration of CP 48 h prior to harvest induces a positive response in the peripheral blood. Therefore, for studies in which the induction of MNs in both the bone marrow and the peripheral blood were to be evaluated, the positive control group received an i.p. injection of CP at 25 mg/kg BW once daily on two consecutive days and 24 h apart with the sample harvest at 24 h after the final injection. To avoid interfering with the comet assay in the gastrointestinal tract, i.p. injection was the route of administration for the CP. For studies evaluating just one sample type for MNs, animals received a single i.p. injection of CP either 24 (bone marrow) or 48 h (peripheral blood) prior to sampling. In the typical rodent comet assay study, a single oral administration of EMS 3–4 h prior to harvest is sufficient for inducing a positive genotoxic response in any tissue sampled. A single administration of EMS at 200 mg/kg is typically sufficient for inducing a positive comet assay response in the liver. But in our experience and as reported by Hartmann et al. (6), a 300 mg/kg concentration of EMS provides a more consistent positive response across a wider range of tissues when tissues other than just the liver (e.g. gastrointestinal or urinary tract) are evaluated (data not shown). Twenty (20) hours after the final CP dose administration and 4 h prior to sampling, the same group of animals that were injected with CP also received a single oral administration of EMS at 200 or 300 mg/kg BW. Both the CP and the EMS were prepared fresh in dH2O immediately prior to dosing and all doses were administered in a volume of 10 ml/kg.
Experimental design
Dose selection and sample times.
The greatest strength of the comet assay is its ability to detect in target organs initial and/or acute DNA damage in the absence of any clinical signs of stress or toxicity and after exposures as short as 1–4 h depending on the pharmacokinetics of the test compound. But these initial lesions can be repaired or lead to cell death (cytotoxicity) and/or mutations, thus decreasing the amount of damage detectable by the comet assay at later time points when clastogenic effects are most likely to be detected. This may require comet sample collection at a time point (e.g. 4 h after the second dose administration) that is different from the time point at which the MN samples should be collected (e.g. 24 h after 14 daily dose administrations).
In addition, it is important to note that the doses used in standard toxicology tests or dose range finders are typically based on single daily dose administrations (e.g. 2000 mg/kg) approximately 24 h apart. However in the comet assay, animals can receive 2 dose administrations (e.g. 2 × 2000 mg/kg or 4000 mg/kg) within 24 h of sampling. This can result in higher cytotoxicity in the comet assay than what was detected at the same dose in a standard test.
Therefore, information about the kinetics and cytotoxicity of a test compound should be obtained prior to or concurrently with the combined and/or integrated comet/MN assay. This would enable one to ensure that the dosing and sampling times for the two assays are appropriate for achieving adequate but non-cytotoxic exposure of the target samples. Although the inclusion of the MTD, the dose sufficient to elicit a biological response (e.g. toxicity) in the target tissue or the test system is preferred by regulatory agencies in genotoxicity testing, it has been acknowledged by the European Medicines Agency (EMEA) that the use of such a dose could prove to be unattainable since dose-limiting toxicity can occur in a tissue other than the target tissue of interest (5). In such cases, the minimum dose at which cytotoxicity occurs in any tissue may be considered the MTD. However, as recommended by Hartmann et al. (6) and the EMEA, toxicokinetic and/or autoradiographic data should be used to provide evidence of test compound bioavailability and target tissue exposure at the time of sampling.
Cytotoxicity.
Although histopathology is often referred to as the ‘Gold Standard’ measurement of in vivo cytotoxicity (2), the EMEA and pathologists recognize the insensitivity of this methodology and therefore emphasize that the absence of histological findings does not exclude toxicity (7–9). While the earliest histological evidence of necrosis may not manifest for several hours to days, tissue-specific enzymes and proteins that are released from necrotic cells can be detected in the blood as early as 1–2 h after cell death (8,9). Therefore, detecting tissue-specific and irreversible cell injury and death using plasma enzyme levels (8,9) or the low molecular weight (LMW) DNA diffusion assay (1,2) may be more relevant to the comet assay. To determine the possible effects of cytotoxicity on the comet assay results, we recommend concurrently conducting the LMW DNA diffusion assay. To ensure that the dose range tested includes at least two to three non-cytotoxic doses, an LMW DNA diffusion assay dose range finder using the same dosing schedule as the comet assay and including at least two animals per dose group may be conducted. Alternatively, additional dose groups may be included in the comet assay study to ensure that at least two to three non-cytotoxic doses are evaluated.
For the studies presented in this paper, dosing and the sample times for the animals exposed to the test article (proprietary data, not shown) were based on pharmacokinetic/toxicity data provided by the sponsor and/or on the results of a dose range finder conducted by Helix3 with the LMW DNA diffusion assay. Although dose administration schedules varied depending on the study, all the studies included at least two dose administrations within 24 h of sampling with one dose 20 h prior to sampling and one more 4 h prior to sampling. Every study included the LMW DNA diffusion assay and histopathology evaluation as concurrent measurements of cytotoxicity.
Comet sample collection
Animals were anaesthetized by CO2 before they were euthanized by exsanguination 4 h after the final dose administration. During exsanguination, peripheral blood comet assay samples were collected from each animal and processed directly to slides. Immediately after exsanguination, at least three additional tissue samples per animal were collected. In the studies presented, the additional comet tissue samples analysed included the liver, bone marrow, duodenum and urinary bladder. To determine germ cell genotoxicity and/or genotoxicity related to the reproductive tract (10), multiple gender-specific tissues (e.g. prostate and testis) and hormone-regulating tissues from the neuroendocrine pathway (e.g. hypothalamus, thyroid, pineal and/or pituitary gland) were also analysed.
MN sample collection
During exsanguination, peripheral blood MN samples were also collected from each animal. Peripheral blood samples were smeared onto at least two slides per animal. After exsanguination, bone marrow samples were collected on slides by flushing at least one femur per animal with foetal bovine serum.
Histopathology sample collection
Additional portions of the tissue samples collected for the comet assay from the test compound and vehicle control animals were also retained in fixative (SafeFix II, Fisher Scientific, Suwanee, GA) for histopathology.
Comet assay
The standard procedure for preparing and processing comet assay slides as described by Hartmann et al. (1) was used. However, since background migration levels can vary depending on the tissue type and procedures may need to be adjusted depending on the mechanism (e.g. strand breaks versus cross-links) of genotoxicity (6), the sample and slide processing conditions used (e.g. electrophoresis time) for each tissue and study were adjusted as necessary to ensure that the methodology was appropriate for providing migration levels in the vehicle control samples sufficient for the sensitive detection of differences. Collected tissue samples were flushed with mincing solution (Mg++- and Ca++-free Hanks balanced salt solution, 10% v/v dimethyl sulfoxide (DMSO) and 20 mM ethylenediaminetetraacetic acid, disodium salt (Na2EDTA), pH 7.4–7.7) and maintained cold and moist with mincing solution until they were minced to produce a cell suspension. For each comet slide prepared, an aliquot of minced cell suspension or peripheral blood was mixed with 0.5% low-melting point agarose, layered onto microscope slides pre-coated with 1% normal melting point agarose and covered with an additional layer of 0.5% low-melting point agarose. To ensure adequate gel adhesion and to minimize intra- and inter-study migration variability, slides were prepared in an environment with a constant relative humidity of ≤60%. After slides were prepared, they were placed in cold working lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, pH 10, with 10% v/v DMSO and 1% v/v Triton X-100) and lysed at 4°C. After at least 1 h in lysis, two replicate slides per sample were rinsed with 0.4 M Tris buffer (pH 7.5) before treating with alkaline electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH > 13) for 20 min. Electrophoresis times are optimized for each study/tissue. However, for direct comparisons, all the tissues presented in this paper were electrophoresed for 40 min at 0.7 V/cm, 300 mA and 4°C. After electrophoresis, slides were neutralized with 0.4 M Tris (pH 7.5), dipped in 100% ethanol and allowed to air-dry. Air-dried slides were stained with SYBR© Gold stain (Molecular Probes, Eugene, OR) and 100 cells (50 cells per replicate slide) were scored per sample using the Komet© GLP Image Analysis System (Andor Technology, Belfast, UK). All slides were scored without knowledge of their identity. DNA migration was determined as the olive tail moment (OTM; measured as the distance between the centre of gravity of the DNA distribution in the tail and the centre of gravity of the DNA distribution in the head multiplied by the fraction of DNA in the tail), the density of migrated DNA (% Tail) and the distance that DNA migrated (TL, tail length in microns measured from the estimated trailing edge of the head). However, since the OTM and the % Tail are the measurements most laboratories with image analysis systems use for dose group comparisons, these are the measurements of focus in this paper.
LMW DNA diffusion assay
Depending on the degree and stage of apoptosis/necrosis of the cells at the time the comet slides are prepared, cytotoxicity-related DNA fragmentation can contribute to increased DNA migration levels in the total cell population that can be misinterpreted as a genotoxic effect (i.e. ‘false positive’) or it can contribute to decreasing DNA migration levels (i.e. ‘false negative’) due to the loss of detectable DNA following overnight lysis and electrophoresis (3,4,11,12). Due to this potentially confounding effect of cytotoxicity on the in vivo comet assay, a concurrent detection of tissue-specific necrosis and/or apoptosis is essential to the accurate interpretation of DNA migration data (6). But as stressed by pathologists in Robbins Pathologic Basis of Disease (1999), cytotoxic pharmaceuticals can induce in tissues considerable apoptosis before it becomes apparent in histological sections. And due to the time lag between stress and the morphological manifestations of necrosis, evidence of these changes may not be detectable by means other than electron microscopy for 12 h to days after irreversible injury has been induced even when that injury was induced within 20–60 min of exposure (8). Complicating matters further, dye exclusion viability assays (e.g. Trypan blue exclusion) are inadequate for use with in vivo comet due to the mincing of tissues and the disruption of cell membranes caused by this process.
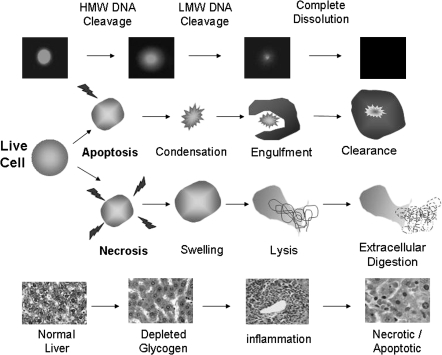
The LMW DNA diffusion assay (also known as the diffusion assay or the neutral diffusion assay) is a sensitive and single-cell detection of apoptosis/necrosis that can easily be conducted concurrently with the comet assay by preparing an extra and identical replicate comet slide that will be removed from lysis after 1 h and fixed without electrophoresis. After only 1 h of lysis and in the absence of electrophoresis, the nuclear DNA of live cells (even those with extensive DNA damage) will appear mostly condensed under microscopic evaluation. But cells with extensive DNA degradation caused by endonuclease activity during apoptosis or necrosis can quickly exhibit a progressively diffuse pattern as increasing amounts of LMW DNA diffuse through the agarose matrix and away from the nucleus. There are overlaps and variations in cellular events that can occur in different tissues and cells. However, the general trend in LMW DNA diffusion in single cells and the corresponding histopathological findings in whole tissues are represented in Figure 1.
Fig. 1.
The progression of cytotoxicity. Before cellular swelling and/or condensation are visible by gross or microscopic examination of a tissue, the earliest detectable measurements of cell death can include an increase in the percentage of individual cells with LMW DNA diffusion and/or evidence of depleted glycogen (e.g. decreased cytoplasmic pallor) in tissue sections. With the lysis of the necrotic cell membranes, more advanced LMW DNA diffusion, increased plasma enzyme levels and/or cytoplasmic eosinophilia (i.e. inflammation) in the tissue may be detected. However, the stage at which tissue necrosis/apoptosis and post-necrotic effects (e.g. compensatory hyperplasia/hypertrophy) can be detected by histopathology is too advanced to be detected by the LMW DNA diffusion assay as complete digestion and clearance of the individual dead cells has occurred.
If cells are processed to comet slides while they are in the initial or early stages of apoptosis/necrosis, the LMW DNA fragments that are larger and therefore closest to the nucleus immediately after lysis can contribute to an increase in DNA migration levels following electrophoresis. But if cells are collected and processed to comet slides while they are in the advanced or final stages of apoptosis/necrosis, DNA migration levels may be decreased as the significantly smaller LMW DNA fragments and even all evidence of a cell’s existence are lost to phagocytosis/extracellular digestion and/or advanced diffusion of the fragments during lysis and electrophoresis. Depending on the dose response curve of the test compound and the range of the doses tested, this decrease in DNA migration at cytotoxic doses (i.e. doses at which histopathological evidence of necrosis is present) as has been experienced by Hartmann et al. (6) and Helix3 can be misinterpreted as an absence of a genotoxic effect (i.e. false negative) when genotoxicity can occur at the lower non-cytotoxic doses as illustrated in Figures 3 and 4.
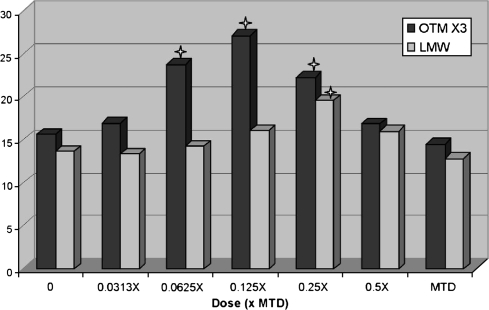
Fig. 3.
Hormetic (∩ shaped) dose response curve of pharmaceutical tested in the comet assay. For viewing the same graph scale with LMW data, OTM values were multiplied by 3. Star indicates statistically significant increase at P < 0.05.
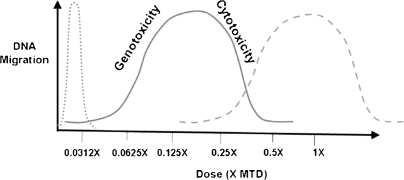
Fig. 4.
Possible dose response curves in the comet assay. While the common assumption is that genotoxicity is most likely to be detected at doses immediately below the MTD (- - -), the genotoxic effect as well as the confounding effects of tissue cytotoxicity (―) and even very steep dose response curves (….) can be detected in the comet assay at doses significantly lower than the MTD.
It is important to note here that while the infamous ‘hedgehog’-shaped comets following electrophoresis can be most likely be attributed to genotoxicity and not cytotoxicity (1,12–14), assessment of diffusion immediately after ≤1 h of lysis and in the absence of electrophoresis is more likely to capture evidence of cytotoxicity due to the speed and nature of the LMW DNA degradation associated with cell death (12,13). However, unlike viability assays such as those that measure ATP levels, membrane permeability or metabolic competency, the LMW DNA diffusion assay does not provide a definitive measurement by which ‘acceptable limits’ (e.g. >70%) may be established. And unlike histopathology evaluation that detects whole tissue necrosis, the LMW DNA diffusion assay also does not provide a definitive marker for cell death by which the exclusion of certain data may be justified. Nor does it distinguish between apoptosis and necrosis. But rather, it provides single-cell data about the pre-processed condition of the nuclear DNA with which detected migration patterns in the comet assay may be interpreted and/or qualified.
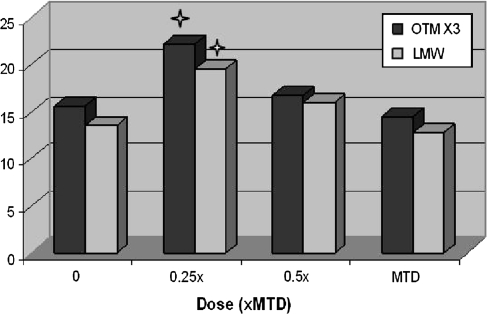
An example of how the LMW DNA diffusion data were used in a study to help interpret the comet assay results is presented in Figures 2 and 3. In the presented study, the non-toxic test compound was initially tested with the MTD as the high dose and 0.5× and 0.25× the MTD as the mid and low doses, respectively (Figure 2). With a statistically significant (P < 0.05) increase in DNA migration present only at the low dose, the conclusion may have been an equivocal or negative classification of genotoxicity. However, a significant increase in LMW DNA diffusion was also detected at the low dose. This combined with the concordant and dose-related decreases in both migration and diffusion at the mid and high doses appeared to indicate that cytotoxicity and the loss of DNA fragments were confounding factors in the determination of genotoxicity at the doses tested. To determine the genotoxicity of the compound in the absence of cytotoxicity, a second experiment was conducted with 0.125× the MTD as the high dose and 0.0625× and 0.0313× as the mid and low doses, respectively. At these doses, significant increases in DNA migration but not in LMW DNA diffusion were detected at 0.125× and 0.0625× the MTD (Figure 3). Therefore, based on a complete set of the data providing a clear hormetic (∩ shaped) dose response curve for the test compound, the compound was classified as positive for genotoxicity with the lowest observable adverse effect level (LOAEL) at 0.0625× the MTD. This study data demonstrate that both genotoxicity and cytotoxicity may be detected at doses well below an MTD that was based on clinical signs and/or histopathology (Figure 4).
Fig. 2.
Dose-related decreases in DNA migration and LMW DNA diffusion in response to cytotoxicity of pharmaceutical tested up to the MTD. For viewing on same graph scale with LMW data, OTM values were multiplied by 3. Star indicates statistically significant increase at P < 0.05.
Although a positive control group was included in the experiments presented in Figures 2 and 3, these data are not included as it is not relevant to the discussion on the LMW DNA diffusion assay. EMS is an established genotoxin that can also induce cytotoxicity in some tissues depending on that tissue's sensitivity. And since the purpose of the positive control is just to verify that the study conditions were sufficient for detecting a positive response, the influence of cytotoxicity on the magnitude of the positive control response is not relevant to the interpretation of comet assay data for test compound exposures.
To assess the percentage of cells with LMW DNA diffusion (% LMW DNA) the methodology described by Vasquez (3) was used. Briefly, one replicate comet assay slide from each sample was removed from lysis after 1 h, neutralized with 0.4 M Tris buffer (pH 7.5), dipped in alcohol and air-dried. Air-dried slides were stained with SYBR© Gold (Molecular Probes) and 100 cells per slide were scored visually for the percentage of diffused versus condensed cells. All slides were scored without knowledge of their identity. It is important to note here that the use of low-signal/high background (e.g. ethidium bromide) or quickly fading (e.g. SYBR© Green) stains and/or a slow deliberate scoring technique can mask or introduce diffusion during scoring, making scores seem inconsistent. In our experience, using the slower-fading, high-signal/background SYBR© Gold stain with an immediate first assessment scoring technique of coded slides are the best methods for ensuring consistent and accurate diffusion scores.
MN assay
MN slides were air-dried before they were fixed in alcohol, air-dried and stained with acridine orange. After staining, 2000 immature erythrocytes (polychromatic erythrocytes, PCEs) per sample (1000 cells per replicate slide) were scored for the incidence of MNs (% MN-PCEs). To assess for a decrease in the ratio of PCEs to total erythrocytes indicative of toxicity or bone marrow suppression, 1000 erythrocytes per sample (500 cells per replicate slide) were scored for the percentage of PCEs (% PCEs). All slides were scored without knowledge of their identity.
Histopathology
To evaluate for the presence of cytotoxicity using histopathology, samples of the comet tissues were embedded in paraffin. In studies where a compound was positive or equivocal for genotoxicity in the comet assay, the tissues in which the effect was detected were prepared to slides, stained with haematoxylin and eosin and evaluated by a veterinary pathologist. Typical morphological changes considered indicative of cell injury and/or death include but are not limited to cellular swelling/shrinking, inflammation, atypical cytoplasm, increased eosinophilia, pyknosis, karyolysis, karyorrhexis and/or compensatory hyperplasia/hypertrophy (8,9). However, as there may be less obvious markers for cell death, the pathologist microscopically evaluated for and reported the presence of any morphological changes compared to normal tissue.
Statistical analysis
Statistical analysis with Analyse-It Software (Leeds, UK) and using individual animal data was conducted on the extent of DNA migration as determined by the OTM and the % Tail, on the % LMW DNA and on the % MN-PCEs. The Shapiro–Wilk test with a confidence level of 95% was conducted on the concurrent vehicle control data to determine the normality of the baseline level distribution. Since test compound exposures can induce either increases or decreases in DNA migration and/or LMW DNA diffusion and studies were conducted blindly without advance provision of mechanistic data, one-tailed tests were used and directional P-values (+ for increases; − for decreases) for significant differences are reported to assist with determining the shape of the dose response curve and interpreting the results. If the data were normally distributed in the concurrent vehicle control (supplementary Figure I, available at Mutagenesis Online), a parametric independent sample t-test with a 95% confidence level and the Welch's approximation for unequal variances (if applicable) was used to compare dose group means to the mean of the concurrent vehicle control. A parametric linear regression test with a 95% confidence level was used to determine the presence of a dose response. If the data were not normally distributed in the concurrent vehicle control (supplementary Figure II, available at Mutagenesis Online), a non-parametric Mann–Whitney test with a 95% confidence level was used to compare dose group medians and a non-parametric Kendall correlation (also known as Jonckheere–Terpstra) test was used to determine the presence of a dose response. Alternatively (and for a direct comparison to the non-parametric statistical analysis), the OTM data that were not normally distributed were normalized by log (natural) transforming the individual cell data after 0.001 was added to each data point to circumvent the potential problem of taking the log of 0. After the natural log transformation (log[n]), the same parametric tests listed above were used to analyse the log transformed data as recommended by Wiklund and Agurell (15) (Table VIII). A one-way analysis of variance (ANOVA) with a Dunnett's post hoc test was also compared to the parametric methods described above (data not shown) with no change in the results between the different statistical analyses.
Table VIII.
Comet assay evaluation of liver in rats exposed to three administrations of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail |
OTM |
OTM log(n) |
% LMW DNA |
||||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Positive | 39.9 ± 4.96 | 17.5 ± 2.25 | < +0.0001* | 4.3 ± 1.00 | +0.0010* | 1.3 ± 0.25 | < +0.0001* | 10.0 ± 5.93 | +0.0436* |
| Vehicle | 21.5 ± 4.94 | 7.8 ± 1.69 | — | 1.2 ± 0.44 | — | −0.8 ± 0.39 | — | 4.8 ± 3.06 | — |
| 25 | 30.7 ± 3.47 | 11.7 ± 1.33 | +0.0006* | 1.9 ± 0.30 | +0.0043* | 0.2 ± 0.18 | < +0.0001* | 3.2 ± 1.94 | 0.1432 |
| 50 | 39.4 ± 5.10 | 16.3 ± 1.95 | < +0.0001* | 3.6 ± 0.78 | +0.0011* | 1.1 ± 0.22 | < +0.0001* | 7.7 ± 3.56 | 0.0851 |
| 100 | 49.1 ± 2.95 | 25.2 ± 1.26 | < +0.0001* | 7.0 ± 0.60 | +0.0011* | 1.8 ± 0.05 | < +0.0001* | 12.7 ± 4.80 | +0.0036* |
| One-tailed trend test P-value | < +0.0001* | < +0.0001* | < +0.0001* | +0.0001* | |||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Although a mean difference in effect of ≥2-fold and/or a mean difference of ≥5% in % Tail DNA has been suggested as possible criteria for a positive response when comparing dose groups to the concurrent vehicle control, these criteria do not address or account for the distribution of the data, inter-animal variability [standard deviation (SD)] or the equality of variances between the compared dose groups and may thus be misleading when attempting to make conclusions about genotoxicity. Therefore, it is more appropriate and objective to use statistical analysis with the appropriate adjustments for distribution and variability and predetermined criteria (e.g. 95% confidence levels) for determining the significance of the genotoxic response.
Determination of a positive response
Use of historical control data.
Significant differences between the MN assay and the comet assay in the way a response is expressed (probability of a change versus magnitude of the change) and the sensitivity with which the response is measured (quantal versus continuous) dictate that the data from these two assays should be evaluated differently. Most genotoxicity endpoints including the MN assay use binomial classification to measure the probability of a response by determining the percentage of damaged cells in a population. However, quantal or discrete data such as these are limited to a finite range of integers and are therefore very insensitive or imprecise due to its susceptibility to counting error and/or inter-animal variability (16–18). For this reason, historical control data are often used with in vivo MN studies to increase the sample size and thereby the sensitivity of dose group comparisons and/or to provide a range of values by which concurrent control data can be validated (19).
Unlike the MN assay, the comet assay scored by image analysis uses an infinite range of possible values to measure the magnitude of the change in response by determining the amount of damage or migration induced in individual cells. This continuous scale method provides more information and uses the data more effectively than the quantal method which takes five times as much data to achieve the same results (20). This sensitivity differential is particularly evident in studies using four to six animals per dose group where the MN assay would require scoring at least 20 000 cells per sample to detect a 2- to 3-fold increase (17) while the comet assay can detect the same 2- to 3-fold increase in damage with up to 80% power by scoring only 100–150 cells per sample (21). With this kind of inherent sensitivity in the comet assay, it is clear that including historical control data to increase the sample size and sensitivity is unnecessary.
Recent modifications to the ICH guidelines recommend using historical control comet data to determine the range of vehicle control values within which small but significant increases in migration are not biologically relevant or by which a threshold effect may be determined. But the almost infinite variety of cell/tissue types, test systems, vehicle controls and experimental conditions with which the comet assay can be used makes it difficult or impossible to generate sufficient and comparable historical control data for every applicable tissue and study design. Even if such a database is developed, the result will be a continuously expanding range of migration levels as even significant differences between the effects of vehicles such as dH2O and methyl cellulose on different tissues can be detected (data not shown), thus making comparisons to the range of vehicle control migrations far less relevant than comparisons to the concurrent controls. Therefore, comparisons between experiments should be limited to comparing the general dose response (e.g. significant and dose-dependent increase in migration) or single change in effect (e.g. significant increase in migration only at cytotoxic dose) in relation to the concurrent controls for the respective experiment.
Historical control comet data should only be used to demonstrate adequate proficiency with the techniques and to justify the methodologies (e.g. electrophoresis conditions) used for processing and analysing a particular sample type under the required experimental conditions.
Dose response curve.
When determining the dose response or change in effect, it is critical to note that due to the sensitivity of the comet assay, the detected low-dose effect may follow a non-linear (e.g. J or U shaped) or even hormetic (∩ shaped) response curve where the significant (and perhaps more biologically relevant) genotoxic effect may be <2-fold greater than the vehicle control and present at doses significantly lower than the MTD (Figures 3 and 4). This is particularly evident in studies in which confounding factors such as toxicity, tissue-specific cytotoxicity, compound bioavailability, cellular repair and division/turnover and/or cell cycle inhibition can influence DNA migration patterns (3,4). Although the probability of a hormetic response in a toxicology study has been debated by many, this phenomenon in genotoxicity has been recognized by the members of the International Workgroup on Genotoxicity Testing in vivo comet group (2) as it has been shown to occur in some in vivo MN studies (22). Further, a 2005 review by Calabrese and Blain (23) of toxicological study data containing ∼5600 dose response relationships across >900 agents from a diverse spectrum of compounds concluded that hormetic dose response relationships occur across numerous animal models and species and across a broad range of susceptibilities to toxic agents.
It was also noted in the Calabrese and Blain (23) review that the quantitative features of the hormetic response revealed that the vast majority (80%) of cases displayed a maximum stimulatory response <2-fold (approximately only 30–60%) greater than the control in endpoints ranging from mutagenesis to immune or metabolic responses to carcinogenesis. Meanwhile, the width of the stimulatory response in nearly 90% of the cases was <100-fold in dose range immediately below the toxicological no observed adverse effect level (NOAEL). This effect has also been frequently evident in some tissues evaluated with the comet assay where the maximum response to a powerful carcinogen such as EMS can be ≤2-fold greater than the minimum response to the vehicle control. Meanwhile, a statistically significant (P < 0.05) and dose-dependent increase in response can reside well within the range between the vehicle and positive control and at doses well below the MTD and even well below the NOAEL. While one cannot help but question the biological relevance of such a small (<2-fold) magnitude of change, it is important to note that one motivation for including the comet assay in safety testing was to take advantage of its increased sensitivity for detecting low levels of damage that might otherwise go undetected by the standard assays from which the ≥2-fold response criteria were originally developed. Further, the Calabrese and Blain (23) review concluded that not only were the majority of stimulatory responses typically of both modest magnitude and width but also these dose response characteristics are biologically significant since they occur independent of biological model, endpoint, chemical class and physical agent.
Data interpretation.
In our view, it is best for the determination of a positive response in the comet assay to limit comparisons of dose groups to the concurrent control groups within the same experiment. Conclusions should be made based on the detected response relative to the range between the concurrently detected minimum response (e.g. vehicle or sham control) and the maximum response detected (e.g. positive control or high dose if the response is greater than the response detected in the positive control) under the experimental conditions of the study as recommended by Murrel et al. (24). Although statistical analysis is recommended for the determination of the significance of a response, it is important to note that it should only be used to aid in the interpretation of the data. But as a basic guide for genotoxicity classifications using the comet assay and in agreement with Hartmann et al. (1), we recommend the following:
A test compound may be positive if both of the following criteria are met:
(a) A significant (P < 0.05) increase (indicative of strand breaks) or decrease (indicative of cross-links) in DNA migration in any tissue evaluated is detected in at least one dose group and
(b) a significant (P < 0.05) dose-dependent increase or decrease in DNA migration in the same tissue is detected.
A test compound may be classified as equivocal for genotoxicity if either (a) or (b) is met, but not both. If an increase in cytotoxicity as measured by LMW DNA diffusion (P < 0.05), plasma enzyme levels and/or histopathological findings is detected in the same tissue and at the same dose concentrations at which DNA migration is significantly increased or decreased, cytotoxicity should be considered a confounding factor in the determination of genotoxicity. In such cases, a repeat test with the same and/or lower doses may be necessary to verify the presence of genotoxicity in the absence of cytotoxicity. If neither (a) nor (b) is met, the test compound may be classified as negative for genotoxicity. To confirm the results of an experiment and/or the biological relevance of the response, the reproducibility of the effect should be tested in an independent experiment.
The final interpretation of the comet assay results and any conclusions made should
(1) consider the appropriateness of the dose selection/sample time,
(2) account for the possibility that a non-linear or non-monotonic dose–response relationship may exist,
(3) describe any confounding factors (e.g. cytotoxicity and bioavailability) and how they may have influenced the migration data and
(4) classify genotoxicity and/or biological relevance based on the reproducibility of a significant response in independent experiments rather than on the absolute magnitude (e.g. x-fold) of the response.
To that effect, it is important for the appropriate and unbiased interpretation of the comet assay dose response to always analyse and report data from every dose group in an experiment regardless of any other effects (e.g. cytotoxicity) that may be present at a particular dose. While many argue that different biological mechanisms such as cytotoxicity play a role at higher doses compared to mechanisms such as genotoxicity at lower doses, this does not imply that the overall dose–response relationship of the mechanisms operate independently of one another. To best understand the dose response of such biological mechanisms and their potential influence on DNA migration, all the data should be presented and addressed for objective review and evaluation. Preemptive exclusion of data provides an incomplete view of the dose response curve and potentially biases the interpretation of results.
Results
The combined in vivo MN and comet assay study proposed in this paper has been in consistent use at Helix3 for over 5 years. To illustrate the effective combination of the assays, a sample of the historical control data from multiple tissues in mice (Table I) and rat studies (Tables II and III) with similar dosing schedules is provided. For studies presented in Tables I and II, both the bone marrow and peripheral blood were evaluated for the presence of MNs. From the same animals, somatic cells from multiple tissues were also evaluated for genotoxicity with the comet assay. In these studies, the positive control animals were dosed by i.p. injection with CP once daily for two consecutive days and 24 h apart. Twenty (20) hours after the second dose administration and 4 h before sampling, the same group of animals received an oral administration of EMS. For studies presented in Table III, the bone marrow was the only sample evaluated for the presence of MNs. But from the same animals, germ cell genotoxicity and genotoxicity related to the reproductive tract was evaluated with the comet assay in multiple gender-specific tissues and in hormone-regulating tissues from the neuroendocrine pathway (10). In these studies, the positive control animals were dosed once with CP 24 h before sampling and once with EMS 4 h before sampling. For all the studies, animals exposed to the test article (proprietary data not shown) or the vehicle were dosed once daily for 3–4 consecutive days and 24 h apart with an additional administration 20 h after the last dose and 4 h prior to sampling. The vehicle control animals received administrations of either dH2O or 0.5% methyl cellulose and each dose group consisted of five to six animals.
Table I.
Combined comet/MN assay in male CD-1 mice
| Study ID | Dose | Bone marrow MN |
Peripheral blood MN |
||
| % PCEs (mean ± SD) | % MN-PCEs (mean ± SD) | % PCEs (mean ± SD) | % MN-PCEs (mean ± SD) | ||
| A | Vehicle | 51.9 ± 10.42 | 0.08 ± 0.094 | 5.5 ± 1.21 | 0.04 ± 0.058 |
| Positivea | 33.2 ± 10.44 | 0.63 ± 0.170 | 3.9 ± 0.88 | 0.23 ± 0.133 | |
| B | Vehicle | 37.7 ± 3.71 | 0.05 ± 0.045 | 5.8 ± 1.99 | 0.07 ± 0.141 |
| Positivea | 25.9 ± 11.47 | 0.80 ± 0.230 | 2.7 ± 1.05 | 0.18 ± 0.097 | |
| Study ID | Dose | Liver comet |
Peripheral blood comet |
Duodenum comet |
||||||
| % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | ||
| A | Vehicle | 6.8 ± 0.49 | 0.8 ± 0.26 | 11.8 ± 15.60 | 6.5 ± 0.97 | 0.5 ± 0.07 | 0.2 ± 0.41 | 15.9 ± 2.80 | 3.5 ± 1.04 | 14.3 ± 8.59 |
| Positivea | 28.9 ± 9.26 | 8.0 ± 3.46 | 27.3 ± 20.27 | 32.2 ± 9.63 | 7.6 ± 3.66 | 13.0 ± 14.30 | 41.6 ± 13.81 | 12.3 ± 5.31 | 18.0 ± 8.39 | |
| B | Vehicle | 11.3 ± 1.37 | 2.3 ± 0.54 | 13.2 ± 6.68 | 10.4 ± 1.12 | 1.3 ± 0.09 | 1.3 ± 1.97 | 24.0 ± 5.17 | 7.4 ± 2.41 | 15.3 ± 7.09 |
| Positivea | 22.4 ± 3.75 | 6.4 ± 1.60 | 33.8 ± 17.10 | 20.7 ± 2.74 | 5.5 ± 1.34 | 9.0 ± 3.85 | 31.4 ± 3.35 | 10.0 ± 1.60 | 20.8 ± 9.85 | |
Two i.p. administrations of 25 mg/kg CP 20 h apart + 300 mg/kg EMS p.o. and 4 h pre-necropsy.
Table II.
Combined comet/MN assay in male Sprague Dawley rats
| Study ID | Dose | Bone marrow MN |
Peripheral blood MN |
||
| % PCEs (mean ± SD) | % MN-PCEs (mean ± SD) | % PCEs (mean ± SD) | % MN-PCEs (mean ± SD) | ||
| C | Vehicle | 39.1 ± 12.60 | 0.07 ± 0.052 | 7.1 ± 0.87 | 0.01 ± 0.020 |
| Positivea | 28.2 ± 8.04 | 2.58 ± 0.392 | 2.8 ± 0.12 | 0.15 ± 0.126 | |
| D | Vehicle | 37.6 ± 18.39 | 0.10 ± 0.040 | 6.7 ± 1.82 | 0.00 ± 0.000 |
| Positiveb | 15.4 ± 3.40 | 1.90 ± 0.450 | 2.2 ± 1.31 | 0.28 ± 0.328 | |
| Study ID | Dose | Liver comet |
Peripheral blood comet |
Duodenum comet |
||||||
| % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | ||
| C | Vehicle | 8.3 ± 1.41 | 1.50 ± 0.64 | 2.20 ± 1.17 | 6.5 ± 0.45 | 0.70 ± 0.19 | 0.70 ± 0.52 | 22.5 ± 2.43 | 6.8 ± 1.56 | 10.8 ± 7.47 |
| Positivea | 28.5 ± 1.76 | 9.00 ± 0.85 | 6.30 ± 3.83 | 32.5 ± 3.36 | 8.80 ± 1.45 | 17.70 ± 4.50 | 46.8 ± 1.66 | 16.7 ± 1.33 | 20.5 ± 5.36 | |
| Bone marrow comet | ||||||||||
| D | Vehicle | 7.8 ± 1.69 | 1.2 ± 0.44 | 4.8 ± 3.06 | 6.2 ± 0.85 | 0.50 ± 0.09 | 1.20 ± 2.40 | 15.6 ± 2.11 | 4.5 ± 0.66 | 4.8 ± 1.47 |
| Positiveb | 17.5 ± 2.25 | 4.3 ± 1.00 | 10.0 ± 5.93 | 19.0 ± 3.71 | 4.00 ± 0.95 | 12.20 ± 5.34 | 26.6 ± 2.55 | 8.1 ± 0.87 | 15.2 ± 7.08 | |
Two i.p. administrations of 25 mg/kg CP 20 h apart + 300 mg/kg EMS p.o. and 4 h pre-necropsy.
Two i.p. administrations of 25 mg/kg CP 20 h apart + 200 mg/kg EMS p.o. and 4 h pre-necropsy.
Table III.
Combined comet/bone marrow MN in male Sprague Dawley rats
| Study ID | Dose | Bone marrow MN |
Hypothalamus comet |
Thyroid comet |
|||||
| % PCEs (mean ± SD) | % MN-PCEs (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | ||
| E | Vehicle | 58.4 ± 7.98 | 0.08 ± 0.052 | 23.7 ± 5.92 | 6.9 ± 3.26 | 11.8 ± 11.55 | 11.0 ± 2.06 | 2.3 ± 0.57 | 5.8 ± 2.71 |
| Positivea | 48.7 ± 11.58 | 1.19 ± 0.501 | 45.4 ± 8.87 | 15.3 ± 3.87 | 21.5 ± 8.83 | 31.7 ± 7.18 | 9.9 ± 3.31 | 9.3 ± 6.31 | |
| F | Vehicle | 43.8 ± 6.12 | 0.13 ± 0.076 | 20.1 ± 3.79 | 4.8 ± 1.35 | 6.7 ± 4.76 | 10.5 ± 2.03 | 1.9 ± 0.85 | 2.8 ± 2.23 |
| Positivea | 44.4 ± 6.71 | 1.47 ± 0.408 | 43.8 ± 5.77 | 13.3 ± 1.81 | 13.2 ± 5.91 | 24.7 ± 3.44 | 6.4 ± 0.87 | 12.9 ± 10.61 | |
| Study ID | Dose | Liver comet |
Duodenum comet |
Pituitary comet |
||||||
| % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | % Tail DNA (mean ± SD) | OTM (mean ± SD) | % LMW (mean ± SD) | ||
| E | Vehicle | 5.0 ± 1.16 | 0.6 ± 0.17 | 4.0 ± 2.53 | 27.1 ± 6.93 | 9.4 ± 3.14 | 18.7 ± 3.01 | 12.1 ± 2.14 | 2.2 ± 0.58 | 3.2 ± 2.04 |
| Positivea | 24.1 ± 6.85 | 6.1 ± 2.16 | 17.8 ± 8.33 | 43.3 ± 9.70 | 14.4 ± 4.21 | 21.2 ± 9.45 | 29.2 ± 7.83 | 8.4 ± 3.02 | 7.2 ± 4.17 | |
| Prostate comet |
Testis comet |
Pineal comet |
||||||||
| F | Vehicle | 11.4 ± 1.93 | 1.9 ± 0.71 | 7.2 ± 3.82 | 9.3 ± 1.59 | 1.5 ± 0.44 | 13.5 ± 6.38 | 11.8 ± 1.55 | 2 ± 0.48 | 5.2 ± 2.14 |
| Positivea | 23.5 ± 1.69 | 5.4 ± 0.79 | 9.0 ± 3.46 | 16.9 ± 2.06 | 3.3 ± 0.35 | 20.5 ± 11.55 | 30.5 ± 7.77 | 7.8 ± 2.63 | 19.7 ± 13.87 | |
One i.p. administration of 25 mg/kg CP 24 h pre-necropsy + 300 mg/kg EMS p.o. and 4 h pre-necropsy.
To provide additional support and examples for the data interpretation and criteria proposed in this paper, the data and results from two acute studies evaluating the dose response in DNA damage induced in rats following oral administrations of a dose range of EMS are also presented and discussed.
Study 1
To compare the sensitivity of the comet and MN assays for detecting DNA damage in comparable tissues of animals exposed to a dose range of EMS, the vehicle or EMS at 25, 50 or 100 mg/kg BW was orally administered to animals once daily on two consecutive days and 24 h apart based on the 48- and 24-h sample time requirements for the peripheral blood and bone marrow MN assay. Twenty (20) hours after the second dose administration and 4 h prior to sampling, the same group of animals received an additional dose administration. This third and final dose administration was included to be consistent with the 2- to 6-h sample time requirement for the comet assay. However, since it was administered only 4 h prior to sampling, it did not contribute to or impact the exposure for the MN assay. Therefore, the response detected in the MN assay was only based on two daily dose administrations while the response detected in the comet assay was based on three dose administrations. But for consistency in reporting, all the data tables for this study refer to the total number (3) of dose administrations (Tables IV–IX). The dose concentrations were selected based on rat toxicity data and bone marrow MN data indicating a NOAEL at 20 mg/kg/day in the rat after 4 weeks of dosing and sampling 24 h after the last dose (E. Glocke, L. Müller, unpublished data). The positive control dose group received a single oral administration of 200 mg/kg EMS 4 h prior to sampling. From each animal, bone marrow and peripheral blood were collected and analysed for both the comet and MN assays. The liver was also collected from each animal and analysed for the comet assay. An additional portion of the same section of the liver analysed with the comet assay was also evaluated by a veterinary pathologist for evidence of cytotoxicity.
Table IV.
Comet assay evaluation of bone marrow in rats exposed to three administrations of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail |
OTM |
% LMW DNA |
|||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Positive | 50.4 ± 3.88 | 26.6 ± 2.55 | < +0.0001* | 8.1 ± 0.87 | < +0.0001* | 15.2 ± 7.08 | +0.008* |
| Vehicle | 39.1 ± 2.93 | 15.6 ± 2.11 | — | 4.5 ± 0.66 | — | 4.8 ± 1.47 | — |
| 25 | 44.5 ± 4.06 | 19.1 ± 3.56 | +0.0323* | 5.2 ± 1.41 | 0.1538 | 11.5 ± 4.04 | +0.004* |
| 50 | 49.7 ± 4.55 | 21.6 ± 2.18 | +0.0004* | 6.4 ± 0.99 | +0.001* | 19.7 ± 8.12 | +0.003* |
| 100 | 50.7 ± 5.51 | 28.7 ± 4.15 | < +0.0001* | 8.9 ± 1.63 | < +0.0001* | 12.2 ± 3.82 | +0.0007* |
| One-tailed trend test P-value | < +0.0001* | < +0.0001* | +0.0375* | ||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Table V.
MN assay evaluation of bone marrow in rats exposed to three administrations of EMS
| Dose (mg/kg) | % PCEs (mean ± SD) | % MN-PCEs (mean ± SD) | P-valuea |
| Positive | 15.4 ± 3.40 | 1.9 ± 0.45 | <0.001* |
| Vehicle | 37.6 ± 18.39 | 0.1 ± 0.04 | — |
| 25 | 29.4 ± 15.16 | 0.0 ± 0.04 | 0.998 |
| 50 | 28.2 ± 7.60 | 0.1 ± 0.10 | 0.226 |
| 100 | 25.9 ± 6.65 | 0.2 ± 0.09 | 0.093 |
| One-tailed trend test P-value | 0.026* | ||
Data based on 1000 cells per animal for % PCEs; 2000 PCEs per animal for % MN-PCEs; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase at P < 0.05.
Table VI.
Comet assay evaluation of peripheral blood in rats exposed to three administrations of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail |
OTM |
% LMW DNA |
|||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Positive | 31.6 ± 3.49 | 19.0 ± 3.71 | +0.0001* | 4.0 ± 0.95 | +0.0002* | 12.2 ± 5.34 | +0.0011* |
| Vehicle | 7.2 ± 1.98 | 6.2 ± 0.85 | — | 0.5 ± 0.09 | — | 1.2 ± 2.40 | — |
| 25 | 19.8 ± 1.88 | 11.3 ± 1.66 | < +0.0001* | 1.5 ± 0.29 | +0.0001* | 2.5 ± 2.07 | 0.1969 |
| 50 | 28.1 ± 1.33 | 15.0 ± 1.62 | < +0.0001* | 2.7 ± 0.38 | < +0.0001* | 9.2 ± 2.93 | +0.0022* |
| 100 | 34.9 ± 2.93 | 20.8 ± 4.03 | +0.0001* | 4.5 ± 1.26 | +0.0003* | 11.7 ± 6.31 | +0.0022* |
| One-tailed trend test P-value | < +0.0001* | < +0.0001* | < +0.001* | ||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Table VII.
MN assay evaluation of peripheral blood in rats exposed to three administrations of EMS
| Dose (mg/kg) | % PCEs (mean ± SD) | % MN-PCEs (mean ± SD | P-valuea |
| Positive | 2.2 ± 1.31 | 0.28 ± 0.328 | 0.001* |
| Vehicle | 6.7 ± 1.82 | 0.00 ± 0.000 | — |
| 25 | 5.0 ± 0.58 | 0.02 ± 0.026 | 0.197 |
| 50 | 6.0 ± 2.63 | 0.03 ± 0.041 | 0.090 |
| 100 | 4.6 ± 1.58 | 0.00 ± 0.000 | 0.531 |
| One-tailed trend test P-value | 0.385 | ||
Data based on 1000 cells per animal for % PCEs; 2000 PCEs per animal for % MN-PCEs; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase at P < 0.05.
Table IX.
Clinical and microscopic evidence of liver toxicity in rats exposed to three administrations of EMS
| Dose (mg/kg) | BW gain (g) (mean ± SD) | Decreased pallora (mean ± SD) | % LMW DNA (mean ± SD) |
| Positive | 9.6 ± 7.75** | 1.5 ± 0.55** | 10.0 ± 5.93** |
| Vehicle | 23.7 ± 5.59 | 0.0 ± 0.00 | 4.8 ± 3.06 |
| 25 | 15.7 ± 8.04 | 0.0 ± 0.00 | 3.2 ± 1.94 |
| 50 | −2.5 ± 14.33** | 0.8 ± 0.75** | 7.7 ± 3.56 |
| 100 | −15.5 ± 7.09** | 2.0 ± 0.00** | 12.7 ± 4.80** |
| One-tailed trend P-value | <0.0001** | <0.0001** | <0.0001** |
Compared to cytoplasm of normal hepatocytes; severity score based on 1 = minimal; 2 = mild.
Statistically significant at P < 0.05.
Bone marrow.
The summary of the bone marrow comet analysis is presented in Table IV and the summary of MN assay analysis is presented in Table V. The bone marrow OTM, % Tail and % LMW DNA data for the concurrent vehicle control were normally distributed (P = 0.165, 0.421 and 0.804, respectively). Therefore, parametric tests were used for the statistical analysis of these parameters. The percentage of micronucleated PCEs (% MN-PCEs) for the concurrent vehicle control was normally distributed (P = 0.87). Therefore, parametric tests were used for statistical analysis of the MN data. After two daily administrations of EMS followed by a third administration 4 h prior to sampling (three total administrations within 48 h), the comet assay mean ± SD minimum (Min) to maximum (Max) dose response range for the OTM was 4.5 ± 0.66 to 8.1 ± 0.87. For the % Tail, the Min to Max dose response range was 15.6 ± 2.11 to 26.6 ± 2.55%. Based on the OTM, a statistically significant and dose-dependent (P < 0.0001) but <2-fold increase in DNA migration was detected in the bone marrow at 50 and 100 mg/kg. Based on the % Tail, a statistically significant and dose-dependent (P < 0.0001) but <2-fold increase in DNA migration was detected in the bone marrow at every dose concentration tested. Based on the % LMW DNA diffusion, a statistically significant and dose-dependent (P < 0.0001) increase in cytotoxicity was also detected at every dose concentration tested. A statistically significant (P < 0.0001) increase in DNA migration and in cytotoxicity was detected in the bone marrow of the positive control group animals.
A significant increase neither in micronucleated PCEs nor in bone marrow suppression was detected by the MN assay at any dose concentration tested. A significant (P < 0.0001) and >10-fold increase in micronucleated PCEs was detected in the bone marrow of the positive control group animals.
Peripheral blood.
The summary of the peripheral blood comet assay analysis is presented in Table VI and the summary of the MN assay analysis is presented in Table VII. The blood OTM and % Tail data for the concurrent vehicle control were normally distributed (P = 0.315 and 0.171, respectively). Therefore, parametric tests were used for the statistical analysis of these parameters. The % LMW DNA and % MN-PCE data were not normally distributed (P = 0). Therefore, non-parametric tests were used for the statistical analysis of these parameters. After three total administrations of EMS within 48 h, the comet assay mean ± SD Min to Max dose response range for the OTM was 0.5 ± 0.09 to 4.0 ± 0.95. For the % Tail, the Min to Max dose response range was 6.2 ± 0.85 to 20.8 ± 4.03%. Based on the OTM and the % Tail, a statistically significant and dose-dependent (P < 0.0001) increase in DNA migration was detected in the blood at every dose concentration tested. Based on the % LMW DNA diffusion, a statistically significant and dose-dependent (P < 0.0001) increase in cytotoxicity was detected at 50 and 100 mg/kg. A statistically significant (P < 0.0001) increase in DNA migration and in cytotoxicity was detected in the peripheral blood of the positive control group animals.
A significant increase neither in micronucleated PCEs nor in bone marrow suppression was detected at any dose concentration tested. A significant (P = 0.001) and >10-fold increase in micronucleated PCEs was detected in the bone marrow of the positive control group animals.
Liver.
The summary of the liver comet assay analysis is presented in Table VIII. The liver OTM data were not normally distributed (P = 0.028). Therefore, non-parametric tests were used for the statistical analysis of this parameter. For comparison of the different statistical approaches, the liver OTM data were also log transformed and analysed as described in the Statistical analysis of this paper. The liver % Tail and % LMW DNA data for the concurrent vehicle control samples were normally distributed (P = 0.145 and 0.846, respectively). Therefore, parametric tests were used for the statistical analysis of these parameters. After three total administrations of EMS within 48 h, the mean ± SD Min to Max dose response range for the OTM was 1.2 ± 0.44 to 7.0 ± 0.60. For the % Tail, the Min to Max dose response range was 7.8 ± 1.69 to 25.2 ± 1.26%. Based on both the OTM and the % Tail, a statistically significant and dose-dependent (P < 0.0001) increase in DNA migration was detected in the liver at every dose concentration tested with a <2-fold increase at 25 mg/kg. This response was unaltered when the OTM data were log transformed and/or when an alternate method of analysis (e.g. ANOVA with Dunnett's test) was conducted. Based on the % LMW DNA diffusion, a statistically significant increase in cytotoxicity was only detected at 100 mg/kg. A statistically significant (P < 0.0001) increase in DNA migration and in cytotoxicity was detected in the liver of the positive control group animals.
Toxicity measurements.
As a measurement of test system toxicity in Study 1, clinical observations and the BWs for each animal were recorded prior to each dose administration. The BW gain of each animal was determined by subtracting the BW at the start of dosing from the BW immediately prior to the final dose administration. Cytotoxicity in the liver was determined by histopathological evaluation and the LMW DNA diffusion assay. The summary of weight gain, histopathology and the % LMW DNA diffusion is presented in Table IX. After three total administrations of EMS within 48 h, the mean ± SD BW gain of the vehicle control animals was 23.7 ± 5.59 with a significant (P < 0.0001) and dose-related decrease in BW gain at doses ≥50 mg/kg including the positive control. Histopathological evaluation of the liver samples from the same animals indicated treatment-induced decreases in glycogen as determined by decreased cytoplasmic pallor at doses ≥50 mg/kg including the positive control (Figure 5). Based on the statistical analysis conducted on the severity scores (1 = minimal; 2 = mild; 3 = moderate; 4 = moderately severe) for the individual animals, this dose-related effect was statistically significant at P < 0.0001. No pathology of inflammation, gross necrosis and/or apoptosis was detected. However, since one of the earliest detectable events in both cell injury and death is the depletion of intracellular glycogen (8,9), and since this effect was also detected in the positive control animals which did not lose body weight the detected decrease in glycogen was considered indicative of cytotoxicity.
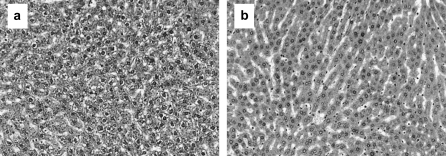
Fig. 5.
Histopathology of glycogen depletion in the liver. Haematoxylin- and eosin-stained normal liver from the vehicle control animals (a) compared to the liver with depleted glycogen from animals dosed with ≥50 mg/kg of EMS (b).
Study 1 conclusion.
As demonstrated by the DNA migration levels in the tissues of animals exposed to cytotoxic concentrations of a potent genotoxic carcinogen such as EMS, the maximum dose response in DNA migration within an experiment can easily be <2-fold greater than the minimum dose response depending on the sensitivity of the specific tissues to a particular compound. But despite this limited range and magnitude of response, the sensitivity of the comet assay and the statistical methods used are more than adequate for detecting small but significant and dose-dependent increases in DNA migration indicative of genotoxicity. While log transformation of non-normally distributed data may provide additional statistical power when the sample size is large (e.g. population studies) and/or when inter- or intra-animal variability is high, log transforming data when the sample size is small (e.g. four to six animals) and/or data variability is stable or low do not appear to enhance the overall specificity or the sensitivity of comet analysis.
Based on the MN assay and after two daily administrations, EMS was neither genotoxic nor cytotoxic in the bone marrow or the peripheral blood. However, based on the comet and LMW DNA diffusion assays in the same animals and after an additional administration of EMS 4 h prior to sampling, EMS was genotoxic in the peripheral blood at 25 mg/kg and in the liver at 25 and 50 mg/kg. In the bone marrow, increased levels of % LMW DNA diffusion that were concordant with the increases in DNA migration detected at every dose concentration tested indicated that cytotoxicity was a confounding factor in the determination of genotoxicity in this tissue and under the experimental conditions of this study. In fact, despite the absence of typical histopathological evidence of gross necrosis/apoptosis or of changes in cell division (e.g. haematopoiesis in the bone marrow), early evidence of dose-related cytotoxicity (e.g. decreases in mean BW and liver glycogen combined with increases in % LMW DNA diffusion in all the tissues sampled) was considered sufficient indication that the multiple administrations of EMS at 25, 50 and 100 mg/kg were too cytotoxic for the accurate assessment of genotoxicity. Therefore, a repeat comet study with lower dose concentrations and/or fewer dose administrations was considered necessary to determine the magnitude of EMS genotoxicity that could be detected in the absence of cytotoxicity.
Study 2
In Study 2, the vehicle or EMS at 25, 50, 100 or 200 mg/kg BW was orally administered to animals once 4 h prior to sampling (Tables X–XIII). Since the 200 mg/kg dose group received the same treatment as the standard positive control dose group, an additional positive control group was not included in the study. Due to the short exposure incompatible with detecting the induction of MNs, the MN component was not included in this study. Therefore, no animals received administrations of CP. From each study animal, bone marrow, peripheral blood, liver and duodenum were collected and analysed for the comet assay.
Table X.
Comet assay evaluation of bone marrow in rats exposed to one administration of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail |
OTM |
% LMW DNA |
|||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Vehicle | 35.9 ± 4.85 | 15.6 ± 2.81 | — | 4.5 ± 1.36 | — | 11.0 ± 3.74 | — |
| 25 | 38.2 ± 9.42 | 15.8 ± 5.32 | 0.4628 | 4.2 ± 2.27 | 0.4045 | 10.0 ± 4.20 | 0.3361 |
| 50 | 42.7 ± 8.45 | 18.0 ± 5.09 | 0.1668 | 4.6 ± 2.11 | 0.4478 | 9.7 ± 4.18 | 0.2867 |
| 100 | 49.3 ± 4.85 | 22.2 ± 3.72 | +0.0030* | 6.2 ± 1.82 | +0.0441* | 11.7 ± 2.50 | 0.3622 |
| 200 | 49.4 ± 5.12 | 23.3 ± 3.82 | +0.0013* | 6.9 ± 1.81 | +0.0127* | 11.8 ± 2.93 | 0.3383 |
| One-tailed trend test P-value | +0.0003* | +0.0027* | 0.1831 | ||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Table XI.
Comet assay evaluation of peripheral blood in rats exposed to one administration of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail |
OTM |
% LMW DNA |
|||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Vehicle | 13.4 ± 4.80 | 8.2 ± 1.28 | — | 0.9 ± 0.21 | — | 0.7 ± 1.21 | — |
| 25 | 14.8 ± 2.88 | 8.9 ± 1.11 | 0.1791 | 0.9 ± 0.19 | 0.2631 | 2.0 ± 2.61 | 0.1970 |
| 50 | 19.7 ± 3.24 | 10.0 ± 0.91 | +0.0085* | 1.3 ± 0.26 | +0.0040* | 5.2 ± 2.56 | +0.0043* |
| 100 | 31.3 ± 2.32 | 13.9 ± 1.32 | < +0.0001* | 2.4 ± 0.22 | < +0.0001* | 8.8 ± 3.31 | +0.0011* |
| 200 | 36.9 ± 4.97 | 20.0 ± 3.63 | < +0.0001* | 4.4 ± 1.17 | +0.0003* | 14.0 ± 4.15 | +0.0010* |
| One-tailed trend test P-value | < +0.0001* | < +0.0001* | < +0.0001* | ||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Table XII.
Comet assay evaluation of liver in rats exposed to one administration of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail |
OTM |
% LMW DNA |
|||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Vehicle | 10.9 ± 2.17 | 5.4 ± 0.72 | 0.7 ± 0.19 | — | 5.2 ± 1.94 | — | |
| 25 | 16.9 ± 2.11 | 7.4 ± 0.90 | +0.0009* | 1.1 ± 0.28 | +0.0101* | 6.7 ± 3.67 | 0.1985 |
| 50 | 24.1 ± 3.14 | 9.5 ± 0.44 | < +0.0001* | 1.3 ± 0.12 | < +0.0001* | 5.5 ± 2.74 | 0.4064 |
| 100 | 33.6 ± 4.08 | 13.1 ± 1.04 | < +0.0001* | 2.5 ± 0.43 | < +0.0001* | 16.2 ± 19.62 | 0.1145 |
| 200 | 45.4 ± 2.24 | 19.7 ± 2.26 | < +0.0001* | 4.7 ± 0.54 | < +0.0001* | 13.8 ± 5.74 | +0.0063* |
| One-tailed trend test P-value | < +0.0001* | < +0.0001* | +0.0221* | ||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Table XIII.
Comet assay evaluation of duodenum in rats exposed to one administration of EMS
| Dose (mg/kg) | TL (mean ± SD) | % Tail DNA |
OTM |
% LMW DNA |
|||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | ||
| Vehicle | 51.6 ± 5.33 | 23.0 ± 2.14 | — | 6.9 ± 0.93 | — | 13.7 ± 5.50 | — |
| 25 | 54.8 ± 5.44 | 26.4 ± 3.50 | +0.0340* | 8.4 ± 1.55 | +0.0325* | 11.3 ± 4.37 | 0.2174 |
| 50 | 54.9 ± 4.76 | 26.6 ± 4.24 | +0.0464* | 8.2 ± 2.09 | 0.2944 | 18.0 ± 14.21 | 0.2510 |
| 100 | 56.6 ± 6.09 | 29.7 ± 5.00 | +0.0063* | 9.4 ± 2.17 | +0.0130* | 16.7 ± 15.07 | 0.3311 |
| 200 | 56.0 ± 8.23 | 32.6 ± 5.84 | +0.0042* | 10.0 ± 2.23 | +0.0043* | 21.8 ± 14.08 | 0.1076 |
| One-tailed trend test P-value | +0.0002* | +0.0038* | 0.0682 | ||||
Data based on 100 cells scored per animal; six animals per dose group.
One-tailed pairwise comparison of dose group to the concurrent vehicle control.
Statistically significant increase (+) or decrease (−) at P < 0.05.
Bone marrow.
The summary of the bone marrow comet assay analysis is presented in Table X. The bone marrow OTM, % Tail DNA and % LMW DNA data for the concurrent vehicle control were normally distributed (P = 0.068, 0.139 and 0.362, respectively). Therefore, parametric tests were used for the statistical analysis of these parameters. After one administration of EMS 4 h before sampling, the mean ± SD Min to Max dose response range for the OTM was 4.5 ± 1.36 to 6.9 ± 1.81. For the % Tail, the Min to Max dose response range was 15.6 ± 2.81 to 23.3 ± 3.82%. Based on the OTM and on the % Tail DNA, a statistically significant and dose-dependent (P ≤ 0.0027) but <2-fold increase in DNA migration was detected in the bone marrow at 100 and in the positive control group at 200 mg/kg. Based on the % LMW DNA diffusion, an increase in cytotoxicity was not detected at any dose concentration tested.
Peripheral blood.
The summary of the peripheral blood comet assay analysis is presented in Table XI. The blood OTM and % Tail DNA data for the concurrent vehicle control were normally distributed (P = 0.971 and 0.207, respectively). Therefore, parametric tests were used for the statistical analysis of these parameters. The % LMW DNA data were not normally distributed (P = 0.003). Therefore, non-parametric tests were used for the statistical analysis of this parameter). After one administration of EMS 4 h before sampling, the mean ± SD background to maximum dose response range was 0.9 ± 0.21 to 4.4 ± 1.17 for the OTM and 8.2 ± 1.28 to 20.0 ± 3.63 for the % Tail. Based on the OTM and the % Tail, a statistically significant and dose-dependent (P < 0.0001) increase in DNA migration was detected in the blood at 50, 100 and 200 mg/kg. Based on the % LMW DNA diffusion, a statistically significant and dose-dependent (P < 0.0001) increase in cytotoxicity was also detected at 50, 100 and 200 mg/kg. Neither an increase in DNA migration nor in cytotoxicity was detected at 25 mg/kg.
Liver.
The summary of the liver comet assay analysis is presented in Table XII. After one administration of EMS, the mean ± SD background to maximum dose response range was 0.7 ± 0.19 to 4.7 ± 0.54 for the OTM and 5.4 ± 0.72 to 19.7 ± 2.26% for the % Tail. Based on both the OTM and the % Tail, a statistically significant and dose-dependent (P < 0.0001) increase in DNA migration was detected in the liver at every dose concentration tested with a <2-fold increase at 25 and 50 mg/kg. Based on the % LMW DNA diffusion, a significant increase in cytotoxicity was only detected at the positive control concentration of 200 mg/kg. Histopathological evaluation of the liver samples found no pathology of inflammation, necrosis and/or apoptosis at any dose concentration tested.
Duodenum.
The summary of the duodenum analysis is presented in Table XIII. The duodenum OTM data for the concurrent vehicle control samples were not normally distributed (P = 0.021). Therefore, non-parametric tests were used for the statistical analysis of this parameter. After one administration of EMS 4 h before sampling, the mean ± SD background to maximum dose response range was 6.9 ± 0.93 to 10.0 ± 2.23 for the OTM and 23 ± 2.14 to 32.6 ± 5.84 for the % Tail. Based on the OTM and the % Tail, a statistically significant and dose-dependent (P > 0.0038) increase in DNA migration was detected in the duodenum at every dose concentration tested with a <2-fold increase at every dose. Based on the % LMW DNA diffusion, no increase in cytotoxicity was detected at any dose concentration tested. Histopathological evaluation of the duodenum samples found no pathology of inflammation, necrosis and/or apoptosis at any dose concentration tested.
Study 2 conclusions.
Four hours after a single oral administration and in the absence of cytotoxicity or clinical signs of stress, EMS was genotoxic in the bone marrow, liver and duodenum with the LOAEL in the liver and duodenum at 25 mg/kg. In the peripheral blood, EMS was cytotoxic at doses ≥50 mg/kg resulting in a statistically significant increase in DNA migration. However, no genotoxic or cytotoxic effects were detected at 25 mg/kg. Based on the results from the two independent studies, the concomitantly conducted comet and LMW DNA diffusion assays could detect at doses as low as 25 mg/kg and after only one to three administrations the genotoxic and cytotoxic effects of EMS that are otherwise undetectable by the MN assay and typical histopathology evaluation. Due to this demonstrated sensitivity of the comet assay, careful consideration of the effects of cytotoxicity when incorporating comet into multi-dose studies should be taken.
Discussion
Using the minimal number of animals and the standard comet sample processing methodology published by Hartmann et al. (1), the combined comet/MN assay protocol presented here has proven to be a sensitive and efficient method for detecting within the same animals multiple classes of genotoxins across a wide range of target organs. By including the LMW DNA diffusion assay in our protocol, one can easily and effectively detect DNA fragmentation that occurs in the earliest stages of cell death and that can by increasing or decreasing DNA migration confound the determination of genotoxicity using comet. However, it is critical to be aware that the DNA migration levels reported in this paper including those that were <2-fold greater than the concurrent vehicle control were induced by both a pharmaceutical (Figure 3) as well as by EMS, a potent genotoxic carcinogen. The exposure effects detected by the comet and MN assays in different or even the same tissues can vary widely depending on the tissue and the test compound. Thus, it is ill-advised to arbitrarily assign to all tissues and test compounds evaluated in the comet assay specific positive response criteria (e.g. 2-fold increase) and/or methodology (e.g. sample times or electrophoresis conditions) based on criteria established from the MN assay and/or the comet response of one tissue to a positive control.
All the methodologies outlined in this paper have been proven to generate reproducible results without the incorporation of extreme technical or statistical measures to control variability and/or sensitivity. For the successful integration of the in vivo comet assay and as with scoring the LMW DNA diffusion assay, experience-driven consistency and speed with sample handling and processing are the most critical factors in managing variability. The specificity and/or sensitivity of the comet assay is most dependent on the appropriateness of the study design and the interpretation of the results. For the appropriate application and objective interpretation of the comet assay in the safety testing of compounds and/or complex compounds (e.g. polymer conjugates) with ambiguous or unknown genotoxicity, we recommend the following:
To ensure that the optimal doses and/or sample times are used, the pharmacokinetics (e.g. Cmax) and the cytotoxicity of a test compound should be determined prior to or concurrently with the combined comet/MN assay and based on the comet/MN dosing regime.
To measure cytotoxicity and/or to determine the MTD relevant to the comet assay, early or sensitive detection methods such as LMW DNA diffusion, plasma enzyme levels and/or histopathological evidence of glycogen depletion should be used.
Since cytotoxicity can confound the ability to determine genotoxicity in comet, include in the dose range tested at least two to three doses that are non-cytotoxic in the sampled tissues to allow for the determination of a dose response.
In cases where dose-limiting cytotoxicity in one tissue conflicts with the target tissue of interest, the minimum dose at which cytotoxicity occurs in any tissue may be considered the MTD and toxicokinetic/autoradiographic data may be used to provide evidence of target tissue exposure.
During slide processing (e.g. electrophoresis), analysis and data interpretation account for and make necessary adjustments for the different tissues and the different forms of genotoxins that can express DNA damage as increases or decreases (e.g. cross-linkers) in DNA migration.
Describe in the results/conclusions any confounding factors (e.g. cytotoxicity and cell division) that may have influenced DNA migration.
Account for the possibility that a non-linear or non-monotonic dose–response relationship may exist.
Present and address data from every dose group for unbiased review and interpretation of the dose response.
Only use historical control comet data to demonstrate proficiency with comet in specific tissues and as justification for the methodology (e.g. electrophoresis conditions) used for a particular tissue type.
Limit comparisons of exposure dose groups to concurrent controls and determine the response relative to the range between the concurrently detected minimum (e.g. vehicle control) and maximum response (e.g. positive control) under the experimental conditions of the study.
Standard statistical analysis methods with pre-determined criteria (e.g. 95% confidence levels) and the appropriate adjustments for data distribution and variability should be used to determine the statistical significance of a response.
Classify genotoxicity and/or biological relevance on the reproducibility of a significant response rather than the absolute magnitude (e.g. x-fold) of the response.
The recommendations presented here provide an effective methodology for combining the in vivo comet and MN assays and for interpreting comet assay data. This approach provides to safety testing critical target organ data increasing sensitivity while minimizing animal usage, exposure times and toxicity.
Supplementary data
Supplementary figures are available at Mutagenesis Online.
Funding
Helix3 funded this research. No grants were used.
Supplementary Material
Acknowledgments
Conflict of interest statement: M.Z.V is the sole stock holder and an employee of Helix3 Inc., a contract research organization providing genotoxicity testing and research services.
References
- 1.Hartmann A, Agurell E, Beevers C, et al. Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Burlinson B, Tice RR, Speit G, et al. Fourth International Workgroup on Genotoxicity testing: results of the in vivo comet assay workgroup. Mutat. Res. 2007;627:31–35. doi: 10.1016/j.mrgentox.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Vasquez MZ. Cytotoxicity and its impact on the in vivo comet assay. Environ. Mol. Mutagen. 2007;48:624. [Google Scholar]
- 4.Dewhurst NE, Vasquez MZ. Interpreting data from in vitro genotoxicity tests using the acellular comet assay. Environ. Mol. Mutagen. 2008;7:576. [Google Scholar]
- 5.EMEA. ICH Topic S2A Genotoxicity: Note for Guidance on Genotoxicity: specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals. 2006. (CPMP/ICH/141/95). EMEA, London, UK. [Google Scholar]
- 6.Hartmann A, Schumacher M, Plappert-Helberg U, Lowe P, Suter W, Mueller L. Use of the alkaline in vivo comet assay for mechanistic genotoxicity investigations. Mutagenesis. 2004;19:51–59. doi: 10.1093/mutage/geg038. [DOI] [PubMed] [Google Scholar]
- 7.EMEA Committee for Medicinal Products for Human Use. Non-Clinical Guideline on Drug-Induced Hepatotoxicity. 2008. , (CHMP/SWP/150115/2006). EMEA, London, UK. [Google Scholar]
- 8.Cotran RS, Vinay K, Trucker C. Cellular pathology chapter I: cellular adaptations, cell injury, and cell death. In: Cotran RS, Vinay K, Trucker C, editors. Robbins Pathologic Basis of Disease. 6th edn. Philadelphia, PA: W.B. Saunders Company; 1999. pp. 1–29. [Google Scholar]
- 9.Jones TC, Hunt R, King NW. Veterinary Pathology. 6th edn. Baltimore, MD: Williams & Wilkins; 1997. [Google Scholar]
- 10.Speit G, Vasquez M, Hartmann A. The comet assay as an indicator test for germ cell genotoxicity. Mutat. Res. 2009;681:3–12. doi: 10.1016/j.mrrev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez M, Tice RR. Detecting genotoxic activity against high molecular weight DNA using the single cell gel (SCG) assay. Environ. Mol. Mutagen. 1997;29:53. [Google Scholar]
- 12.Collins AR, Oscoz A, Brunborg G, Gaivão I, Giovannelli L, Kruszewski M, Smith CC, Stĕtina R. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- 13.Morley N, Rapp A, Dittmar H, Salter L, Gould D, Greulick KO, Curnow A. UVA-induced apoptosis studied by the new apo/necro-comet assay which distinguishes viable, apoptotic and necrotic cells. Mutagenesis. 2006;21:243–247. doi: 10.1093/mutage/gel004. [DOI] [PubMed] [Google Scholar]
- 14.Meintieres S, Nesslany F, Pallardy M, Marzin D. Detection of ghost cells in the standard alkaline comet assay is not a good measure of apoptosis. Environ. Mol. Mutagen. 2003;41:260–269. doi: 10.1002/em.10156. [DOI] [PubMed] [Google Scholar]
- 15.Wiklund SJ, Agurell E. Aspects of design and statistical analysis in the comet assay. Mutagenesis. 2003;18:167–175. doi: 10.1093/mutage/18.2.167. [DOI] [PubMed] [Google Scholar]
- 16.Slob W. Dose-response modeling of continuous endpoints. Toxicol. Sci. 2002;66:298–312. doi: 10.1093/toxsci/66.2.298. [DOI] [PubMed] [Google Scholar]
- 17.Kissling GE, Dertinger S, Hayashi M, MacGregor JT. Sensitivity of the erythrocyte micronucleus assay: dependence on number of cells scored and inter-animal variability. Mutat. Res. 2007;634:235–240. doi: 10.1016/j.mrgentox.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaylor D. Quantalization of continuous data for benchmark dose estimation. Regul. Toxicol. Pharmacol. 1996;24:246–250. doi: 10.1006/rtph.1996.0137. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Hashimoto S, Sakamoto Y, Hamada C, Sofuni T, Yoshimura I. Statistical analysis of data in mutagenicity assays: rodent micronucleus assay. Environ. Health Perspect. 1994;102(Suppl. 1):49–52. doi: 10.1289/ehp.94102s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang LW. Toxicology and Risk Assessment: Principles, Methods, and Applications. London, UK: Informa Healthcare; 1996. [Google Scholar]
- 21.Smith CC, Adkins DJ, Martin EA, O'Donovan R. Recommendations for design of the rat comet assay. Mutagenesis. 2008;23:233–240. doi: 10.1093/mutage/gen008. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi M, MacGregor JT, Gatehouse DG, et al. In vivo erythrocytes micronucleus assay. III. Validation and regulatory acceptance of automated scoring and the use of rat peripheral blood reticulocytes, with discussin of non-hematopoietic target cells and a single dose-level limit test. Mutat. Res. 2007;627:10–30. doi: 10.1016/j.mrgentox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis databases: an overview. Toxicol. Appl. Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Murrel JA, Portier CJ, Morris RW. Characterizing dose-response I: critical assessment of the benchmark dose concept. Risk Anal. 1998;18:13–26. doi: 10.1111/j.1539-6924.1998.tb00911.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.