Abstract
Emdogain® (enamel matrix derivative, EMD) is well recognized in periodontology, where it is used as a local adjunct to periodontal surgery to stimulate regeneration of periodontal tissues lost to periodontal disease. The biological effect of EMD is through stimulation of local growth factor secretion and cytokine expression in the treated tissues, inducing a regenerative process that mimics odontogenesis. The major (>95%) component of EMD is Amelogenins (Amel). No other active components have so far been isolated from EMD, and several studies have shown that purified amelogenins can induce the same effect as the complete EMD. Amelogenins comprise a family of highly conserved extracellular matrix proteins derived from one gene. Amelogenin structure and function is evolutionary well conserved, suggesting a profound role in biomineralization and hard tissue formation. A special feature of amelogenins is that under physiological conditions the proteins self-assembles into nanospheres that constitute an extracellular matrix. In the body, this matrix is slowly digested by specific extracellular proteolytic enzymes (matrix metalloproteinase) in a controlled process, releasing bioactive peptides to the surrounding tissues for weeks after application. Based on clinical and experimental observations in periodontology indicating that amelogenins can have a significant positive influence on wound healing, bone formation and root resorption, several new applications for amelogenins have been suggested. New experiments now confirm that amelogenins have potential for being used also in the fields of endodontics, bone regeneration, implantology, traumatology, and wound care.
Keywords: amelogenins, bone formation, endodontics, implantology, wound healing
Introduction
Enamel matrix derivative (EMD) in the form of a purified acid extract of proteins from pig enamel matrix (Emdogain®; Straumann AG, Basel, Switzerland) has been successfully employed to restore functional periodontal ligament, cementum and alveolar bone in patients with severe attachment loss. The first studies on clinical applications with EMD were published in 1997 (1, 2) and since then a wide number of research groups have studied the mechanism of actions of EMD and the clinical potential, as well as worked at further evolving the therapeutic potential of this device. In 2004, a Cochrane review (3) concluded that EMD significantly improve periodontal attachment levels and reduce probing pocket depth when compared with open-flap debridement. A multitude of case reports and clinical studies are now published confirming the clinical effect of EMD when used in periodontal regeneration procedures, among them a follow-up on a series of 107 consecutive EMD cases that show stable periodontal regeneration after 5-years of observation (4).
The role of enamel proteins in periodontal ligament formation is supported by their presence in initial cementum formation during normal development of tooth attachment (5, 6). However, the mechanism(s) by which EMD promotes periodontal regeneration is still somewhat obscure. The major (>95%) constituent of EMD is amelogenins, a family of hydrophobic proteins derived from a single gene by alternative splicing and controlled post-secretory processing. The amelogenins are known to self-assemble into supramolecular aggregates that form an insoluble extracellular matrix (7) with high affinity for hydroxyl apatite and collagens (8). When applied to denuded root surfaces, amelogenins therefore precipitates to form a stable extracellular matrix with a hydrophobic surface with potential for supporting interactions with cells in adjacent tissues.
So far Emdogain® is the only device on the market that has potential for actually triggering clinically significant regenerative responses in periodontal ligament cells. If, as several observations suggest (5, 6), amelogenin deposition precedes cementum formation, then EMD treatment probably mimics odontogenesis and works by restarting dormant developmental programs in cells for (re) generation of the tooth attachment apparatus. Such responses typically involve sequential cascades of growth factors that act on the multitude of cells needed to reconstitute the lost periodontal tissues. It has been assumed that the most important mechanism of action of EMD is to initiate periodontal regeneration through recruitment of cementoblasts to the root-surface and hence to stimulate these to form root-cementum. This novel root-cementum will thereafter secondarily lead to regeneration of periodontal fibers and alveolar bone. However, many recent studies are reporting that amelogenins also can interact directly with cell types other than cementoblasts (9–11), suggesting that these molecules have a more direct role in the regrowth of mesenchymal tissues. This is also supported by clinical observations on effects on tissues outside the oral cavity (12–16), opening possibilities for new applications of amelogenins as discussed below.
Amelogenins in enamel biomineralization
The amelogenins are strongly expressed in the dental enamel organ, and demonstrate a very high overall level of sequence homology among all higher vertebrates examined (> 80%). Both the tyrosine-rich amino terminal and the hydrophilic carboxy-terminal are almost identical between species (17). The high level of sequence conservation suggests that the entire structure of the amelogenin molecule is crucial in enamel formation and biomineralization, and that the amino- and carboxy-terminal motifs are particularly important for their function. Amelogenins are rich in proline residues (∼30%) that are believed to inhibit the formation of classic secondary structures such as β-sheet, α-helix, and random coil, producing an intrinsically disordered protein. However, this disorder also allows amelogenin molecules to self-assemble into hydrophobic supramolecular monodisperse assemblies, so called nanospheres (7). In the mineralizing enamel matrix these amelogenin assemblies bind to hydroxyl apatite crystallites to structure the enamel matrix and to modulate the crystal growth (18). A gradual loss of amelogenins from the matrix occurs within hours after their secretion because of progressive proteolytic processing and translocation of the derived polypeptide fragments from the matrix back into the ameloblast. The hydrophilic carboxy-terminal region is cleaved away from the assembled structures when they bind to apatite. As enamel formation progresses the carboxy-terminal parts of amelogenin assemblies undergo sequential controlled cleavages modulating their apatite binding properties. Amelogenin polypeptides produced by this specific proteolysis become soluble and are absorbed by post-secretory ameloblasts (Fig. 1). The amelogenin nanospheres are ultimately destroyed, and once the full enamel thickness has been deposited, virtually all matrix protein is removed and replaced with tissue fluid from the ameloblast in which the immature enamel crystallites, stretched out during matrix secretion, grow in width and thickness to occlude the space.
Fig. 1.
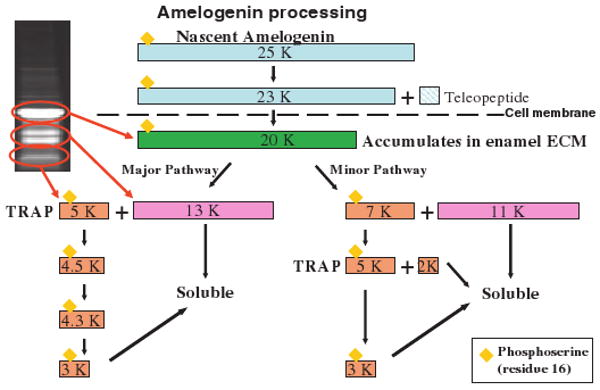
A schematic diagram of extracellular amelogenin proteolytic processing: After secretion from the cell the 20 kDa Amelogenin is processed into smaller peptides by specific proteases. Eventually, the smaller peptides become soluble and are released from the insoluble amelogenin assemblies. TRAP, tyrosine-rich amelogenin peptide, is one candidate for an active peptide that can interact with cellular receptors.
Amelogenin molecular biology
The ability of amelogenins to self-assembly into insoluble nanospheres that are slowly processed by matrix proteases to release active peptides is probably what makes these molecules so applicable in the clinic. The amelogenin self-assembly mechanism is controlled by local changes in temperature, pH, ionic strength, and protein concentration.
While the amelogenins are insoluble at physiological pH, they can be dissolved at either low or high pH. In addition, the solubility is influenced by temperature and as expected for hydrophobic interactions, the best solubility is obtained at low temperature. For clinical use amelogenins are dissolved in an aqueous, acidic solution of propylene glycol alginate (PGA) in a gel formulation suitable for use in a syringe. When applied to a patient, the acidity of the gel is neutralized and the temperature increased, and amelogenins are released, undergo self-assembly and precipitate on the exposed tissue surfaces in the surgical area. Over the course of days and weeks the amelogenin assemblies are processed by matrix proteases, slowly releasing biologically active components into the local environment, promoting regenerative processes and growth.
The activity of EMD has been compared with that of bone morphogenic proteins (BMP) and transforming growth factor (TGF)-β-like molecules (19). Full length amelogenin molecules have been shown to stimulate autocrine production of BMP while the smaller amelogenin fragments of leucine rich amelogenin peptide (LRAP)- and tyrosine-rich amelogenin peptide (TRAP)-related molecules stimulate autocrine production of TGF-β. It has also been shown that EMD increases autocrine synthesis of TGF-β in ligament fibroblasts while TGF-β itself is undetectable in the EMD formulation (9). This study also reported that EMD stimulates the autocrine production of other growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factors (PDGF) and cytokines like interleukin (IL)-6. In addition, other studies have shown that recombinant LRAP, presumably free of any mammalian growth factors, has obvious cell signaling activity (10). Together, these observations suggest that specific amelogenin molecules may trigger an appropriately balanced and sequenced autocrine release of growth factors that orchestrate the regenerative effects of EMD.
A wide range of in vitro and in vivo experimental studies have demonstrated that EMD and amelogenins stimulate growth of multiple mesenchymal cell types including fibroblasts, cementoblasts, osteoblasts, and stem cells (9, 20, 21). These studies also show that EMD and amelogenin enhance the expression of tissue-specific maturation markers, such as alkaline phosphatase (ALP), collagen, and osteocalcin within osseous tissues (12). A secondary stimulation of osteoclast activity is also evident (12, 21) through increased secretion of proteins such as IL-6 and osteoprotegrin. However, a direct cytostatic effect from EMD has been observed in epithelial cells (9, 21, 22) and osteoclasts (21, 23). Osteoclast activity and epithelial stasis are clinically important since one of the prerequisites for a successful regeneration of dental attachment and regrowth bone is the exclusion of epithelial cells, giving the advantage to connective tissue cells and a balance between osteoblast and osteoclast activities.
To be able to understand the effects that amelogenin proteins have on tissues, one has to study how these molecules interact with various cell types. A primary cellular response is initiated by amelogenin binding to receptors on the cell surface, followed by a cyclic adenosine monophosphate (cAMP)-mediated signal that has been observed in several cell types including periodontal ligament cells and blood cells (13). Uptake of EMD nanospheres has been demonstrated in primary human osteoblasts, murine ameloblasts (LS-8), and primary human periodontal ligament cells (12). In human osteoblasts, the EMD assemblies were found to be co-localized with the clathrin adaptor protein complex, AP-2, the major mechanism of cargo sorting into coated pits in mammalian cells (Fig. 2; 12). So far, no receptors specific for amelogenin have been identified, although several putative receptors that bind amelogenin protein isoforms have been identified (24–26). Here, two members of the lysosomal-associated membrane proteins (LAMP) are involved where LAMP-1 interacts with the shorter amelogenin peptide LRAP, whereas LAMP-3/CD63 prefers to bind the longer amelogenin protein isoforms, but none of these two receptors interact with both of these amelogenin molecules (25).
Fig. 2.
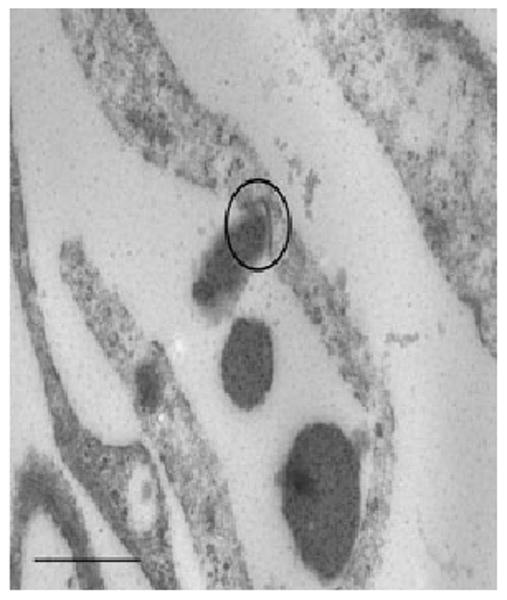
Transmission electron micrographs (TEM) of amelogenin assemblies being transported over the cell membrane in clathrin-coated pits (CCP) in a primary human osteoblast. Osteoblasts were incubated with enamel matrix derivative (EMD; 50 μg/ml) for 3 h. EMD assemblies are stained with a gold-labeled anti-amelogenin antibody (black dots). The CCP is in center of the circle. Further analysis have showed that once in the ccp the EMD assemblies colocalize with the clathrin adaptor–protein complex, AP-2, the major mechanism of cargo sorting into coated pits in mammalian cells, suggesting that uptake of amelogenin is an active process involving specific receptors. Scale bar = 400 nm.
The existence of several isoforms and putative individual receptors indicates that the various isoforms of amelogenin protein may have several different functions. In fact, a gene expression array on the effect of EMD on primary osteoblast showed significant effect on the expression of more than 600 genes (Table 1), and a gene expression profile similar to, but not identical, to that of the bone-promoting hormone parathyroid hormone (PTH) (12). The amelogenins are no longer though to be tissue-specific and exclusively expressed by the ectodermal enamel-producing ameloblast cells. Amelogenin expression has been demonstrated in different long bone cells, in their precursor mesenchymal stem cells, in cartilage cells, and in specific cell layers of the epiphyseal growth plate (27), suggesting that amelogenin has a role not only in enamel biomineralization, but in development, growth and calcification of many skeletal tissues.
Table 1.
Number of genes expressed in osteoblasts regulated by enamel matrix derivative
| Gene functions regulated by amelogenins | No. genes regulated at least threefold | |
|---|---|---|
| Angiogenesis | 17 | |
| Cellular communication | 25 | |
| Related to attachment to other cells | 17 | |
| Cellular defense and repair | 23 | |
| Cellular growth and proliferation | 20 | |
| Related to cell proliferation | 17 | |
| Related to cell growth | 5 | |
| Related to cell maturation | 3 | |
| Molecular transport | 39 | |
| Related to mobilization of Ca2+ | 22 | |
| Cellular signaling | 98 | |
| Cellular migration | 40 | |
| Related to cell homing | 34 | |
| Skeletal growth and remodeling | 19 | |
| Related to skeletal development | 18 | |
Amelogenins in pulpal healing and dentin regeneration
It has been shown that amelogenin participates in the maturation and growth of dental pulp cells during the tooth formation (28). This knowledge is interesting from the perspective of reparative dentin formation following dental insults. It is well known that dental pulp tissue has the potential to form reparative dentine in the adult patient. The mechanism behind this repair process is poorly understood, and as of today the standard treatment of the traumatized dental pulp is application of calcium hydroxide to sterilize the lesion and induce reactive secondary dentin formation. Often, this healing process is not efficient enough to completely resolve the situation, and the potential outcome may be inflammation, internal root resorbtions and/or necrosis of the pulp. A number of scientific publications have evaluated the potential of amelogenin as a pulp-capping material. Experiments in pigs have compared the effect of EMD with calcium hydroxide when used for direct pulp capping (29, 30). The histological evaluation demonstrated a significantly more pronounced formation of secondary dentine in teeth treated with amelogenin (Fig. 3). Other studies in rats (31) and dogs (32) have confirmed these findings and suggest that EMD treatment protects pulp tissue from inflammation and irreversible degenerative changes. Moreover, histopathological findings in these studies also suggest that EMD has a positive effect on endothelial cell growth and capillary sprouting in the pulp, possibly a direct effect of VEGF expression from EMD-stimulated cells.
Fig. 3.
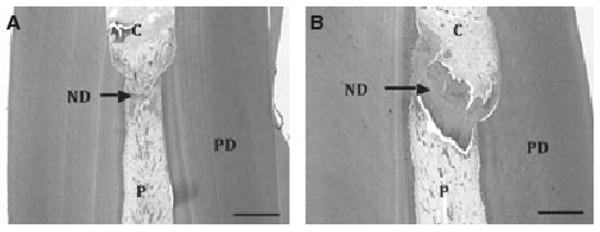
Longitudinal sections through pig incisors 4 weeks after pulpotomy and treatment with (A) calcium hydroxide and (B) enamel matrix derivative (EMD). Formation of new dentin (ND) completely bridging the defect is clearly visible in the EMD-treated tooth, whereas only partial closing of the defect is seen in the calcium hydroxide-treated tooth. C is cavity, PD is primary dentin and P is pulp. Scale bar = 1.0 mm.
A blinded, randomized clinical study, with experimental pulpotomy and pulp capping in healthy premolars scheduled for extraction for orthodontic reasons, has also demonstrated that teeth treated with EMD had significantly less post-operative pain than teeth treated with calcium hydroxide. Again, significantly more formation of pulpal secondary dentine and dentine bridging, and less inflammation was observed (33). However, this study points out that EMD in the present formulation (Emdogain®) is not useful for endodontic purposes since it does not protect against invading microbes, nor does it seal off the cavity or provide support for the restorative material. Based on the above described studies, it seem clear that amelogenins do have the potential for being used as a biological, dentin regenerating capping material, at least in cases where the remaining pulp tissue is healthy and uncontaminated. A new formulation especially developed for an endodontic application must be developed to ensure that a good cavity seal occurs as the seal is essential for a predictable clinical outcome from such procedures.
Amelogenin in bone growth
Micro-array in vitro studies based upon primary human bone cells have demonstrated that amelogenins can induce expression profiles in bone cells similar to that of growing bone (Table 1; 12). Moreover, animal studies in rats have also shown that amelogenins in the form of EMD can be applied to osseous defects in long bones (femur) to induce formation of therapeutically useful trabecular bone (34). Similarly, it has also been shown that amelogenin stimulates bone sialoprotein (BSP) gene transcription in osteoblasts by inducing expression of nuclear proteins that bind to the fibroblast growth factor (FGF)-2 response element and TGF-β1 activation element in the BSP gene promoter (35). BSP is an early phenotypic marker of osteoblast and cementoblast differentiation, and expression of BSP is a prerequisite for osseous calcification. Other studies have also confirmed that amelogenins stimulate growth factor expression and activates intracellular pathways similar to that of BMP and TGF-β (Table 2; Fig. 4; 12, 36, 37).
Table 2.
Cellular pathways activated by enamel matrix derivative
| All cells studied |
| FGF signaling |
| Transforming growth factor-β signaling |
| Wnt/β-catenin signaling |
| PDL fibroblasts |
| vascular endothelial growth factor (VEGF) signaling |
| SHH pathway |
| Pulpal fibroblasts |
| EGF signaling |
| SHH pathway |
| VEGF signaling |
| Interleukin-6 signaling |
| ERK/MAPK signaling |
| Estrogen receptor signaling |
| Bone cells |
| PPAR signaling |
| Nuclear factor-κB signaling |
| Estrogen receptor signaling |
PDL, periodontal ligament; SHH, Sonic Hedgehog; EGF, epidermal growth factor; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferator-activated receptor.
Fig. 4.
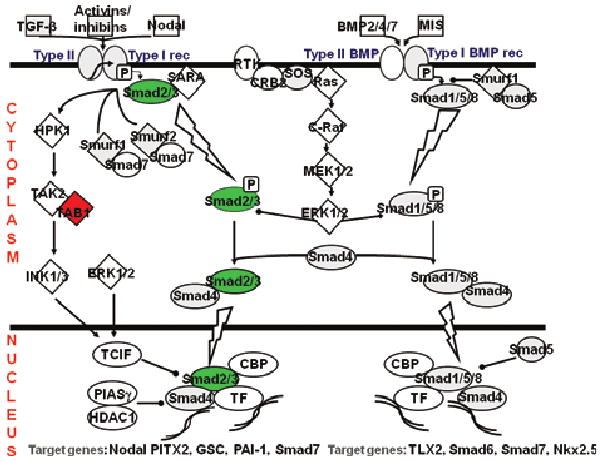
Schematic drawing of the transforming growth factor (TGF)-β pathway: enamel matrix derivative activation of the TGF-β pathway in osteoblasts is clearly visualized by up-regulation of the Smad genes (green) in the gene array studies, generating a nuclear signal for new gene expression. Green symbols indicate a more than fourfold up-regulation of expression of the actual gene. Red indicates a fourfold or more down-regulation. Gray symbols are genes regulated more than twofold (up or down). This drawing is based on data from gene array studies performed on primary human osteoblasts stimulated with amelogenins for 24 h.
Amelogenin has also been tested for its effect on cells seeded into organic scaffolds intended for engineering cruciate ligament tissue (16). This study showed that amelogenin in the form of EMD significantly restricted ligament cell adhesion to the scaffold when used to treat the scaffold before seeding with cells, and had no significant benefit when used in an intra-operative model where EMD and cells were administered concomitantly. However, when EMD was applied after the scaffold had been seeded with the cells the addition of EMD significantly increased proliferation and growth. This finding suggests that EMD may be used to accelerate scaffold colonization, and in turn tissue induction in situ. The observed inhibitory effect on cell attachment implies that EMD may be best suited to postoperative administration.
In a systematic review (SR) on the efficacy of EMD to promote regeneration of osseous tissue in intrabony defects, alone or in combination with membranes, a total of 20 in vivo studies with histomorphometric analysis were evaluated (38). The main results of the SR were that EMD treatment significantly improved bone regeneration when compared with open-flap debridement. However, EMD was not more effective than traditional guided tissue regeneration (GTR) for treatment of intrabony defects of the jaw, nor was there any advantage in combining GTR and EMD as both treatments performed equally well alone. Also, application of EMD was more effective when used in supporting defects, and less effective in non-supporting defects, reflecting the mechanical properties of the gel formulation designed for use in narrow defects, and not for providing primary wound stability during bone healing. This SR also suggests that EMD is not osteoconductive by itself since it is incapable of promoting bone growth into titanium capsules. The various osteoinductive and osteoconductive effects reported for EMD are thus probably mediated by bone cells that are stimulated to produce factors for bone growth and mineralization. This is also supported by one of the studies included in the SR that reported induction of trabecular bone growth around endosseous implants treated with EMD (38, 39).
The potential for using amelogenins for bone regeneration seems undisputable both in the jaws as well as in other bones, but at the same time is restricted by severe formulation issues. Additional research and development is called for before an amelogenin-based product for use in healing critical size bony defects can be introduced to the biomedical community.
Amelogenin in implantology
The successful integration of dental endosseous implants has been thoroughly demonstrated in numerous scientific publications. In recent years, the treatment focus for dental implantology has changed from solely a functional consideration towards achieving shorter healing times and esthetic concerns. Because of the high success rate of dental implants, more compromised and challenging cases are also being treated with dental implants. To ensure a successful treatment outcome in these more difficult cases, novel strategies for improving implant performance are continuously being developed, including shorter time to load and better osseointegration. A plethora of growth factors and other biological mediators have been investigated for stimulation of bone growth and/or mineralization on the implant surface.
Amelogenin is one such candidate that has a potential role in stimulating peri-implant bone growth. However, no studies so far have been able to demonstrate a significant effect from EMD or amelogenins on implant performance when analyzed by biomechanical testing. For example, a study evaluating the effect of the application of EMD onto the surface of dental root-shaped titanium implants was unable to demonstrate improvement in implant stability or osseous growth when tested by removal torque (40). On the other hand, an animal study of rat femurs demonstrated improved osseointegration of implants that were coated with EMD (39). This study showed that when EMD was applied the trabecular bone volume increased significantly at 14 and 30 days post-implantation. The field of implantology is rapidly progressing towards bioactive, osteoinductive surfaces that can be used with superior results in patients with poor bone quality. The role of amelogenins in this picture is uncertain, but intriguing case reports and anecdotal information, especially from treatment of patients with peri-implantitis, justifies further research. As seen for ligament tissue engineering (16) it may be that developing surface coatings based on amelogenins is not the best way forward, since this route of administration seems to hinder cell attachment to the material surface. If alternative methods for amelogenin delivery are developed, the stimulatory effects of amelogenins might be optimized for adjunct use with osseous implants.
Amelogenin in traumatology
Another attractive possibility for application of amelogenins is for their use in replantation procedures with exarticulated teeth. The underpinning mechanism is that root surface conditioning with amelogenins could prevent root resorption and ankylosis, and stimulate periodontal ligament formation after repositioning of the avulsed tooth. Some early case-reports and animal experimental findings suggested that EMD could be used as a bioactive root conditioning for reintegration of avulsed teeth (41), but subsequent studies have struggled with confirming this effect. A study in dogs showed no significant effects from EMD treatment on root resorption and ankylosis after 6 months of healing (42). In this study, the teeth were extracted and reimplanted after killing all cementoblasts on the root surface by drying the teeth in air for 60 min, before repositioning them. Accordingly, a clinical study using EMD to treat both previously ankylosed teeth as well as exarticulated teeth reported that all teeth in the study showed clinical signs of ankylose and concluded that EMD alone was not sufficient to cure or prevent ankylosis (43). Another clinical study, however, assessing the clinical outcome of 22 avulsed permanent incisors replanted with EMD showed significantly less inflammation and root resorption in treated teeth compared with a historical control group from the same region (44). Also, a study using a protocol for replantation of avulsed teeth called anti-resorptive-regenerative therapy (ART) that includes local application of glucocorticoids and EMD prior to the replantation procedure, reported that when ART was used on avulsed teeth that had been stored non-physiologically for longer periods (typically hours), three of eight teeth healed with a functional periodontal ligament (45). It thus seems that when used together with anti-inflammatory drugs, EMD can support functional healing and periodontal regeneration after replantation, even when the avulsed teeth have a severely compromised cementum layer.
In summary, interesting data on the utilization of amelogenins for replantation of avulsed teeth has accumulated. It seems plausible that amelogenins can stimulate regeneration of the tooth attachment apparatus even in cases where the tooth has been stored for significant time outside the oral cavity. However, additional treatment is needed to ensure a stable and predictive treatment outcomes, and new protocols for combination of amelogenin treatment with anti-inflammatory and anti-microbial drugs must be developed and tested before the full potential of amelogenins can be exploited in dental traumatology.
Amelogenins in wound healing
A common clinical observation when using EMD for regenerative periodontal surgery is exceptionally fast wound healing and minimal post-operative symptoms such as pain or swelling. A number of reasons for this observation have been suggested including anti-inflammatory and anti-microbial effects (13, 46). Inspired by clinical observations in oral surgery, several investigators have studied the effect of amelogenins on healing of both acute and chronic skin wounds. The first study to demonstrate that amelogenins stimulate skin wound healing showed that the amount of granulation tissue in an EMD-treated wound was significantly increased, and that wound fill and re-epithelialization of full thickness wounds progressed almost twice as fast in the presence of EMD (47). The mechanisms involved in EMD-assisted skin healing have yet to been completely understood, but it is has been shown that local application of amelogenins stimulates angiogenesis by inducing secretion of VEGF, PDGF, and matrix metalloproteinase-2. Similar mechanisms have previously been reported in a number of in vitro studies (9, 12), and support the idea that amelogenins in general work by stimulating mesenchymal cells to express factors that are important for healing, growth, and regeneration.
The surprisingly rapid healing observed in animal wound models (Fig. 5) led to the development of an amelogenin formulation for skin wounds, based on the original EMD product. Several clinical studies, especially on hard-to-heal venous leg ulcers (VLU), have showed that the observation from oral surgery sites and animal model holds true and that amelogenins work well even in longstanding, hard-to-heal VLU. In the first phase III randomized, clinical multi-center trial on the application of amelogenins in hard-to-heal VLU, the amelogenin-treated wounds showed a threefold reduction in mean ulcer size compared with the control group that was treated with vehicle alone over a 12-week period (14). Statistically significant differences in favour of the Amelogenin-treated group were also found for reduction in ulcer-related pain, reduction in pain at dressing changes and the proportion of patients scored with ‘none’ or ‘low’ levels of exudates.
Fig. 5.
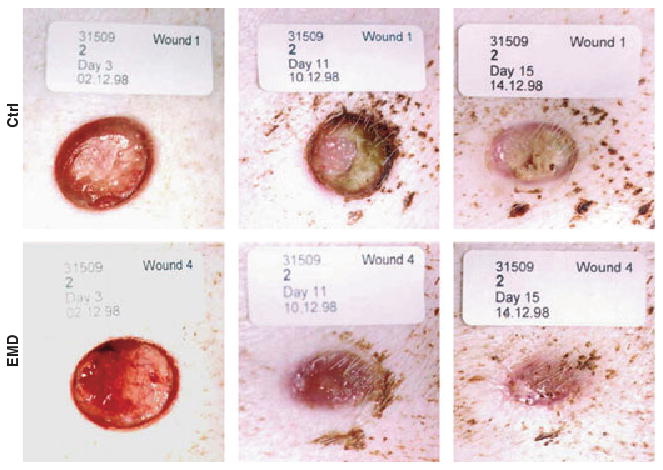
Primary wound healing in full thickness wounds in pigs after treatment with enamel matrix derivative (EMD) or control (propylene glycol alginate vehicle only). After 3 days the EMD-treated wound is significantly better vascularized than the control as visualized here by the vivid red color and presence of blood vessels in the wound surface. After 11 days the EMD-treated wound is almost completely closed and epithelium is covering most of the wound. At this stage the control wound is still covered in granulation tissue and epithelialization has not yet started. After 15 days the EMD-treated wound is completely covered by epithelium, and most of the wound cavity is filled in, while the control wound still show remnants of granulation tissue exposed in the middle of the wound. In this study EMD-treated wounds healed twice as fast as control wounds (the vehicle control used here, polyglycol alginate, is often used in wound care because of its excellent biocompatibility and low pH that restrict bacterial growth and aid healing).
Results of the follow-up from the initial study also showed that the beneficial healing response to amelogenin was maintained even 6 months after the initial treatment started (15). At the follow-up in the amelogenin-treated group, significantly more patients continued to show a reduction in ulcer size from baseline. There were also a significantly higher percentage of patients with diminished wound size, and the overall number of patients with completely healed wounds was three times greater in the amelogenin-treated group than in the control group. Also pain, inflammation and discomfort continued to be significantly reduced in the amelogenin-treated group, indicating that the initial beneficial effects from amelogenins were maintained post-treatment and could be identified by observers at the follow-up.
The experimental and clinical observations on the effect of amelogenins in wound healing are now widely recognized and show that amelogenin therapy as an adjunct to standard wound care is beneficial to patients with hard-to-heal VLU, both in the short and long term. Amelogenins in a formulation named Xelma® (Mölnlycke AB, Gothenburg, Sweden) are now for sale in Europe for this indication, and an application for regulatory approval in the US has been filed to FDA. Moreover, clinical trials investigating the effect of Xelma® in diabetic ulcers, radiation ulcers from cancer therapy, and surgical ulcers have been initiated, and other indications are under development.
Conclusion and clinical perspectives
Over the years, a wide range of regenerative treatment strategies have been suggested for resolving critical loss of tissues like bone, ligaments, and skin. EMD is the first product on the market that is completely based on biomimicry, inducing true periodontal regeneration both in architecture and function. The observed clinical effects of amelogenins have generated much interest in how and why these seemingly pluripotent molecules interact with the intricate cellular and molecular mechanisms that govern healing, regeneration and growth in adults. Findings suggest that amelogenins works on at least two levels: it acts as its own, natural, slow release device that ensures long and stable release of amelogenin peptides even after a single application; and it releases active degradation products that interact directly with cells, inducing a cascade of growth factor expression that secondarily initiates and maintains the anabolic processes. The conserved nature of amelogenins hints at a basic function for these molecules and allows for inter-species use. New data suggest that amelogenins are more widely expressed in the body than previously believed, and that they might be of use in a wide array of applications in regenerative medicine.
Clinical relevance
Application of enamel matrix proteins in the form of Emdogain® has set a modern standard for periodontal regeneration therapy. This clinical application was developed based on the observation that enamel proteins are not exclusive to enamel, but are also deposited on the root surface prior to cementum formation. Emdogain® is an extract of enamel matrix containing almost exclusively amelogenins, the major component of enamel extracellular matrix. Amelogenins are evolutionarily well conserved, hinting at a profound role in biomineralization. New experiments show that amelogenins have potential for clinical use in endodontics, bone regeneration, implantology, traumatology, and wound care.
Contributor Information
S.P. Lyngstadaas, Department of Biomaterials, Faculty of Dentistry, University of Oslo, Oslo, Norway
J.C. Wohlfahrt, Department of Biomaterials, Faculty of Dentistry, University of Oslo, Oslo, Norway
S.J. Brookes, Oral Biology, Leeds Dental, Institute, University of Leeds, Leeds, UK
M.L. Paine, Centre for Craniofacial Molecular Biology, Faculty of Dentistry, University of Southern, Los Angeles, CA, USA
M.L. Snead, Centre for Craniofacial Molecular Biology, Faculty of Dentistry, University of Southern, Los Angeles, CA, USA
J.E. Reseland, Department of Biomaterials, Faculty of Dentistry, University of Oslo, Oslo, Norway
References
- 1.Heijl L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J Clin Periodontol. 1997;24:693–6. doi: 10.1034/j.1600-051x.1997.00693.x. [DOI] [PubMed] [Google Scholar]
- 2.Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705–14. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 3.Esposito M, Coulthard P, Thomsen P, Worthington HV. Enamel matrix derivative for periodontal tissue regeneration in treatment of intrabony defects: a Cochrane systematic review. J Dent Educ. 2004;68:834–44. [PubMed] [Google Scholar]
- 4.Heden G, Wennstrom JL. Five-year follow-up of regenerative periodontal therapy with enamel matrix derivative at sites with angular bone defects. J Periodontol. 2006;77:295–301. doi: 10.1902/jop.2006.050071. [DOI] [PubMed] [Google Scholar]
- 5.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–68. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 6.Spahr A, Hammarström L. Response of dental follicular cells to the exposure of denuded enamel matrix in rat molars. Eur J Oral Sci. 1999;107:360–7. doi: 10.1046/j.0909-8836.1999.eos107507.x. [DOI] [PubMed] [Google Scholar]
- 7.Fincham AG, Moradian-Oldak J, Simmer JP, Sarte P, La EC, Die-kwisch T, et al. Self-assembly of a recombinant amelogenin protein generates supramolecular structures. J Struct Biol. 1994;112:103–9. doi: 10.1006/jsbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- 8.Gestrelius S, Andersson C, Johansson AC, Persson E, Brodin A, Rydhag L, et al. Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J Clin Periodontol. 1997;24:678–84. doi: 10.1111/j.1600-051x.1997.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 9.Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 1991;28:181–8. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 10.Veis A, Tompkins K, Alvares K, Wei K, Wang L, Wang XS, et al. Specific amelogenin gene splice products have signaling effects on cells in culture and in implants in vivo. J Biol Chem. 2000;275:41263–72. doi: 10.1074/jbc.M002308200. [DOI] [PubMed] [Google Scholar]
- 11.Kawase T, Okuda K, Momose M, Kato Y, Yoshie H, Burns DM. Enamel matrix derivative (EMDOGAIN) rapidly stimulates phosphorylation of the MAP kinase family and nuclear accumulation of smad2 in both oral epithelial and fibroblastic human cells. J Periodontal Res. 2001;36:367–76. doi: 10.1034/j.1600-0765.2001.360604.x. [DOI] [PubMed] [Google Scholar]
- 12.Reseland JE, Reppe S, Larsen AM, Berner HS, Reinholt FP, Gautvik KM, et al. The effect of enamel matrix derivative (EMD) on gene expression in osteoblasts. Eur J Oral Sci. 2006;114:205–11. doi: 10.1111/j.1600-0722.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 13.Myhre AE, Lyngstadaas SP, Dahle MK, Stuestøl JF, Foster SJ, Thiemermann C, et al. Anti-inflammatory properties of enamel matrix derivative in human blood. J Periodontal Res. 2006;41:208–13. doi: 10.1111/j.1600-0765.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- 14.Vowden P, Romanelli M, Price P. Effect of amelogenin extracellular matrix protein and compression on hard-to-heal venous leg ulcers. J Wound Care. 2006;16:189–95. doi: 10.12968/jowc.2007.16.5.27043. [DOI] [PubMed] [Google Scholar]
- 15.Romanelli M, Kaha E, Stege H, Wnorowski JW, Vowden P, Majamaa H, et al. Effect of amelogenin extracellular matrix protein and compression on hard-to-heal venous leg ulcers: follow-up data. J Wound Care. 2008;17:20–3. doi: 10.12968/jowc.2008.17.1.27916. [DOI] [PubMed] [Google Scholar]
- 16.Messenger MP, Raif EM, Seedhom BB, Brookes SJ. The potential use of enamel matrix derivative for in situ anterior cruciate ligament. Tissue engineering: a translational in vitro investigation. Tissue Eng. 2007;13:2041–51. doi: 10.1089/ten.2006.0059. [DOI] [PubMed] [Google Scholar]
- 17.Brooks SJ, Bonass WA, Kirkham J, Robinson C. The human amelogenin c-terminal sequence is completely homologous to the C-terminal sequence of amelogenin in all species so far studied. J Dent Res. 1994;73:716–7. doi: 10.1177/00220345940730040401. [DOI] [PubMed] [Google Scholar]
- 18.Lyngstadaas SP, Risnes S, Sproat BS, Thrane PS, Prydz HP. A synthetic, chemically modified ribozyme eliminates amelogenin, the major translation product in developing mouse enamel in vivo. EMBO J. 1995;14:5224–9. doi: 10.1002/j.1460-2075.1995.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S, Nagano T, Yamakoshi Y, Gomi K, Arai T, Fukae M, et al. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-β. J Dent Res. 2005;84:510–4. doi: 10.1177/154405910508400605. [DOI] [PubMed] [Google Scholar]
- 20.Warotayanont R, Zhu D, Snead ML, Zhou Y. Leucine-rich amelogenin peptide induces osteogenesis in mouse embryonic stem cells. Biochem Biophys Res Commun. 2008;367:1–6. doi: 10.1016/j.bbrc.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, Jiang J, Safavi K, Spångberg L, Zhu Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pat Oral Rad Endo. 2004;97:239–45. doi: 10.1016/j.tripleo.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Kawase T, Okuda K, Yoshie H, Burns DM. Cytostatic action of enamel matrix derivative (EMDOGAIN) on human oral squamous cell carcinoma-derived SCC25 epithelial cells. J Periodontal Res. 2000;35:291–300. doi: 10.1034/j.1600-0765.2000.035005291.x. [DOI] [PubMed] [Google Scholar]
- 23.Nishiguchi M, Yuasa K, Saito K, Fukumoto E, Yamada A, Hasegawa T, et al. Amelogenin is a negative regulator of osteoclastogenesis via downregulation of RANKL, M-CSF and fibronectin expression in osteoblasts. Arch Oral Biol. 2007;52:237–43. doi: 10.1016/j.archoralbio.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Thomkins K, George A, Veis A. Charaterization of a mouse amelogenin [A-4] / M59 cell surface protein. Bone. 2006;38:172–80. doi: 10.1016/j.bone.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Harada H, Taniguchi A. The effect of ALMP1 and ALMP3 on M180 amelogenin uptake, localization and amelogenin mRNA induction by amelogenin protein. J Biochem. 2008;144:531–7. doi: 10.1093/jb/mvn096. [DOI] [PubMed] [Google Scholar]
- 26.Iacob S, Veis A. Identification of the functional activity of the [A-4] amelogenin gene splice product in newborn mouse ameloblasts. Bone. 2008;42:1072–9. doi: 10.1016/j.bone.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haze A, Taylor AL, Blumenfeld A, Rosenfeld E, Leiser Y, Dafni L, et al. Amelogenin expression in long bone and cartilage cells and in bone marrow progenitor cells. Anat Rec. 2007;290:455–60. doi: 10.1002/ar.20520. [DOI] [PubMed] [Google Scholar]
- 28.Oida S, Nagano T, Yamakoshi Y, Ando H, Yamada M, Fukae M. Amelogenin gene expression in porcine odontoblasts. J Dent Res. 2002;81:103–8. [PubMed] [Google Scholar]
- 29.Nakamura Y, Hammarstrom L, Lundberg E, Ekdahl H, Matsumoto K, Gestrelius S, et al. Enamel matrix derivative promotes reparative processes in the dental pulp. Adv Dent Res. 2001;15:105–7. doi: 10.1177/08959374010150010201. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Hammarström L, Matsumoto K, Lyngstadaas SP. The induction of reparative dentine by enamel proteins. Int Endod J. 2002;35:407–17. doi: 10.1046/j.1365-2591.2002.00556.x. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi R, Sahara T, Shimizu-Ishiura M, Sasaki T. Porcine enamel matrix derivative enhances the formation of reparative dentine and dentine bridges during wound healing of amputated rat molars. J Electron. 2003;52:227–36. doi: 10.1093/jmicro/52.2.227. [DOI] [PubMed] [Google Scholar]
- 32.Ishizaki NT, Matsumoto K, Kimura Y, Wang X, Yamashita A. Histopathological study of dental pulp tissue capped with enamel matrix derivative. J Endod. 2003;29:176–9. doi: 10.1097/00004770-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Olsson H, Davies JR, Holst KE, Schröder U, Petersson K. Dental pulp capping: effect of emdogain gel on experimentally exposed human pulps. Int Endod J. 2005;38:186–94. doi: 10.1111/j.1365-2591.2004.00932.x. [DOI] [PubMed] [Google Scholar]
- 34.Kawana F, Sawae Y, Sahara T, Tanaka S, Debari K, Shimizu M, et al. Porcine enamel matrix derivative enhances trabecular bone regeneration during wound healing of injured rat femur. Anat Rec. 2001;264:438–46. doi: 10.1002/ar.10016. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu E, Saito R, Nakayama Y, Nakajima Y, Kato N, Takai H, et al. Amelogenin stimulates bone sialoprotein (BSP) expression through fibroblast growth factor 2 response element and transforming growth factor-β1 activation element in the promoter of the BSP Gene. J Periodontol. 2005;76:1482–9. doi: 10.1902/jop.2005.76.9.1482. [DOI] [PubMed] [Google Scholar]
- 36.Heng NHM, N'Guessan PD, Kleber BM, Bernimoulin JP, Pischon N. Enamel matrix derivative induces connective tissue growth factor expression in human osteoblastic cells. J Periodontol. 2007;78:2369–79. doi: 10.1902/jop.2007.070130. [DOI] [PubMed] [Google Scholar]
- 37.Saito K, Konishi I, Nishiguchi M, Hoshino T, Fujiwara T. Amelogenin binds to both heparan sulfate and bone morphogenetic protein 2 and pharmacologically suppresses the effect of noggin. Bone. 2008;43:371–6. doi: 10.1016/j.bone.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Rathe F, Junker R, Chesnutt BM, Jansen JA. The effect of enamel matrix derivative (Emdogain®) on bone formation: a systematic review. Tissue Eng Part B. 2008;14 doi: 10.1089/ten.teb.2008.0065. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu-Ishiura M, Tanaka S, Lee WS, Debari K, Sasaki T. Effects of enamel matrix derivative to titanium implantation in rat femurs. J Biomed Mater Res. 2002;60:269–76. doi: 10.1002/jbm.10064. [DOI] [PubMed] [Google Scholar]
- 40.Franke SV, Johansson CB. Enamel matrix derivative and titanium implants. J Clin Periodontol. 2003;30:359–63. doi: 10.1034/j.1600-051x.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 41.Kenny DJ, Barrett EJ, Johnston DH, Sigal MJ, Tenenbaum HC. Clinical management of avulsed permanent incisors using Emdogain: initial report of an investigation. J Can Dent Assoc. 2000;66:21. [PubMed] [Google Scholar]
- 42.Araujo M, Hayacibara R, Sonohara M, Cardaropoli G, Lindhe J. Effect of enamel matrix proteins (Emdogain') on healing after re-implantation of “periodontally compromised” roots. An experimental study in the dog. J Clin Periodontol. 2003;30:855–61. doi: 10.1034/j.1600-051x.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 43.Schjott M, Andreasen JO. Emdogain does not prevent progressive root resorption after replantation of avulsed teeth: a clinical study. Dent Traumatol. 2005;21:46–50. doi: 10.1111/j.1600-9657.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 44.Barrett EJ, Kenny DJ, Tenenbaum HC, Sigal MJ, Johnston DH. Replantation of permanent incisors in children using Emdogain. Dent Traumatol. 2005;21:269–75. doi: 10.1111/j.1600-9657.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 45.Pohl Y, Filippi A, Kirschner H. Results after replantation of avulsed permanent teeth. II. Periodontal healing and the role of physiologic storage and antiresorptive-regenerative therapy. Dent Traumatol. 2005;21:93–101. doi: 10.1111/j.1600-9657.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 46.Spahr A, Lyngstadaas SP, Boeckh C, Andresson C, Ehrlich M, Podbielski A, et al. Effect of the enamel matrix derivative Emdogain on the growth of periodontal pathogens in vitro. J Clin Periodontol. 2002;29:62–72. doi: 10.1034/j.1600-051x.2002.290110.x. [DOI] [PubMed] [Google Scholar]
- 47.Mirastschijski U, Konrad D, Lundberg E, Lyngstadaas SP, Jorgensen LN, Aagren M. Effects of a topical enamel matrix derivative on skin wound healing. Wound Repair Regen. 2004;12:100–8. doi: 10.1111/j.1067-1927.2004.012117.x. [DOI] [PubMed] [Google Scholar]


