Abstract
Regulated gene expression assembles an extracellular proteinaceous matrix to control biomineralization and the resultant biomechanical function of tooth enamel. The importance of the dominant enamel matrix protein, amelogenin (Amel); a minor transiently expressed protein, dentin sialoprotein (Dsp); an electrogenic sodium bicarbonate cotransporter (NBCe1); the timely removal of the proteinaceous matrix by a serine protease, Kallikrein-4 (Klk4); and the late-stage expression of Amelotin (Amtn) on enamel biomechanical function were demonstrated and measured using mouse models.
Tooth enamel is a composite bioceramic composed largely of carbonated hydroxyapatite, Hap, and very small amounts of protein remnants. Human enamel rarely undergoes catastrophic mechanical failure despite a lifetime of repeated masticatory parafunctional, and occasional impact loading in a hostile wet environment. In fact, tooth enamel is the most durable of all tissues surviving for millennia as long as it is not exposed to carious acid attack. Mature enamel reflects the unique molecular and cellular activities that are owed to its formation during development. The gene activity and protein expression profiles of ectodermal-derived ameloblasts produce a protective mineralized enamel that protects soft dentin from the ravages of wear and erosion in a functional tooth (Figure 1). The dentinoenamel junction, DEJ, durably unites dissimilar hard brittle enamel and tough flexible dentin. In contrast to artificial bonds between restorations and dentin, the DEJ rarely fails, except when it is affected by inherited disorders.
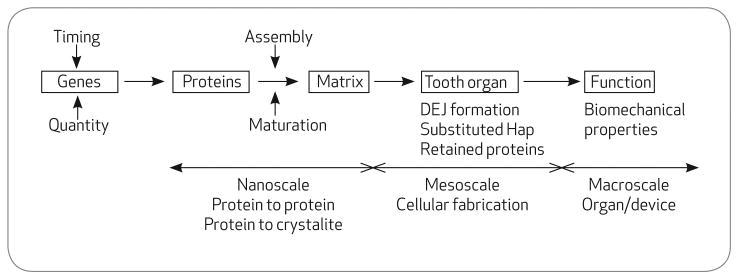
FIGURE 1.
The hierarchy of tooth formation from genes to functioning teeth
Ameloblasts and odontoblasts are lined up face-to-face, or basement membrane-to-basement membrane, in the developing tooth bud. After a series of interactions, the odontoblasts migrate away from the DEJ toward the future pulp, whereas the ameloblasts migrate outward toward eventual tooth surface (Figure 2). Through programmed gene expression, the migratory ameloblasts leave a trail of secreted proteins in their wake. The selection of expressed genes, their timing during development and their relative abundance is under genetic control. This mixture of proteins undergoes self-assembly to form an enamel extracellular organic matrix, or scaffold, that controls the initiation, rate of growth, and habit of the inorganic carbonated hydroxyapatite crystallites that form tooth enamel.1,2 During maturation, almost all of the organic protein matrix is broken down and removed, to be almost completely replaced by inorganic crystallites. Hence, despite an embryonic origin in protein, mature enamel is a hard, wear-resistant, and surprisingly tough composite-ceramic biomaterial.
FIGURE 2.
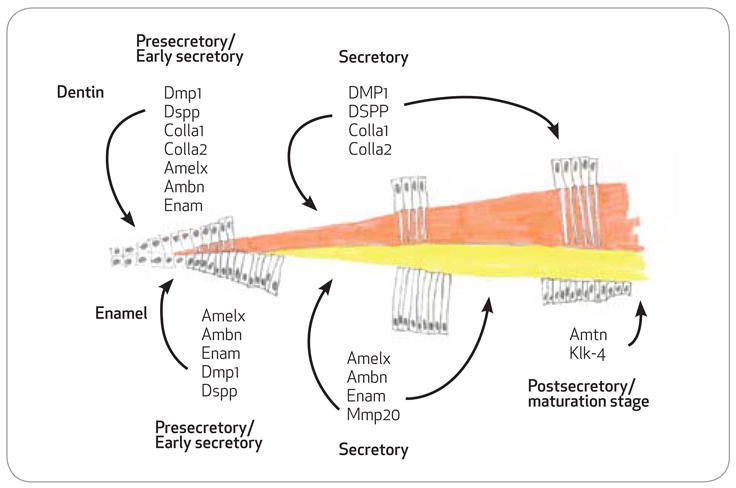
Gene expression through enamel and dentin formation. Schematic of proteins secreted into the dentin matrix by odontoblasts and the enamel matrix by ameloblasts at the various stages of formation. With the exception of collagen, odontoblasts, and ameloblasts, expression profiles for the secreted proteins is very similar in the very early secretory stages. During the secretory stages both odontoblasts and ameloblasts gene expression profiles are entirely distinct as the enamel matures, amelotin, and kallikrein-4 are upregulated.
Enamel has a hierarchical organization (Figure 3). At the nanoscale to macromollecular scale, proteins interact to form a matrix that guide the habit of the individual crystallites to produce long, thin crystallites. Each ameloblast secretes a cylinderlike volume of matrix in which individual crystallites are bundled to form a rod (prism). Organized continuities, and discontinuities, among the matrix cylinders secreted by adjacent ameloblasts create a complex 3-D continuum through interrod crystallites.3 At the microscale or cellular scale, the matrix is defined by the secretory products or zones of influence of individual ameloblasts as they migrate from the DEJ to the outer surface, dance, and weave a complex 3-D web of enamel rods and interrod. At the macroscale or organ level, the tooth is defined by the biomechanical function of its structural components.
FIGURE 3.
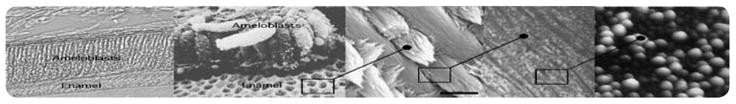
Enamel formation. From left to right: A layer of columnar ameloblasts lay down their protein matrix, which becomes mineralized to form enamel. Each individual ameloblast produces a cylindrical matrix field that becomes mineralized as a rod within a “honeycomb”-like continuum of interrod; the complex migratory vectors of ameloblasts weave rods into a complex fibrous network. Each rod is composed of multitudes of individual crystallites organized by amelogenin nanospheres; amelogenin proteins spontaneously form nanospheres in physologic conditions.
Purpose
The purpose of this article is to review selected advances, highlighting dental student research contributions, in the understanding of the genetic, molecular, and structural aspects of enamel biology. Through examples, a model of enamel formation is presented that relates gene expression to the assembly of an extracellular protein matrix that in turn controls biomineralization, structural hierarchy, and biomechanical function of tooth enamel.
Experimental Strategy
The authors' strategy has been to introduce known mutations to enamel genes, using transgenic (gain of function) and gene knock-in (gene engineering) animal approaches that serve to selectively perturb normal enamel development and structure so that a localizable and quantifiable functional defect can be measured and related to a specific genetic change.
The authors have proposed a long-term paradigm in which these defects will eventually be shown to mirror, or closely resemble, naturally occurring defects in humans that result from genetic defects, such as, amelogenesis imperfecta and acquired human enamel defects, such as fluorosis.1,4,5 Unlike prior studies of enamel defects performed on a few isolated individuals of unknown genetic etiology, or other studies using general toxicity to create defects, the studies described herein are genetic in their origins and thus are repeatable because the single genetic cause is known and consistent. Additionally, the data from the authors' studies will be useful in providing a mechanistic understanding of enamel that will permit the future engineering of replacement enamels.6
Mice and Men
Mouse models have several major advantages. The mouse genome is known and is not unduly dissimilar from our own. Mice also mature quickly, in a matter of weeks. They are small and relatively easy to house and maintain safely. The authors have defined the structural and biomechanical differences between murine and human incisor enamel.7 These differences are a reflection of evolutionary pressures from differences in biomechanical function.
The authors have defined the biomechanical zones of immature, mature, and degrading enamel in the mouse incisor so as to permit reproducible results. Because murine incisors are rather small, the authors used a variety of micro and nanomechanical tests, as well as an array of imaging techniques to measure the effects of known genetic changes on structure and function.3
The Continuously Erupting Mouse Incisor
Because rodent incisors erupt continuously, the whole life cycle of a tooth from stem cells, through maturation, to wear and dissolution can conveniently be found and studied in a single mouse incisor. For enamel and dentin, a midzone was located where data points could be predictably measured. Both dentin and enamel incisal zones had decreased hardness, attributable to oral dissolution. Likewise, apical areas displayed immature and incomplete mineralization, with dentin maturing markedly more slowly than enamel. Knowledge of these zones and the rate of tooth eruption facilitate the use of the continuously erupting mouse incisor as an experimental model to study the impact of both genetic and environmental factors on tooth formation and function.
Amelogenin Self-Assembles Into an Organized Scaffold
Amelogenin (Amel) is the dominant protein in the developing enamel scaffold matrix.8 Amelogenin proteins self-assemble into nanospheres that guide the mineralization of long thin Hap crystallites in an ordered subparallel arrangement, that is the crystallites are almost parallel, but diverging or converging slightly. Two defined domains (A and B) within amelogenin appear essential for this self-assembly according to in vitro model systems. Transgenic animals were used to test the hypothesis that these self-assembly domains operated in vivo.9
Transgenic animals bearing either a domain-A-deleted or domain-B-deleted amelogenin transgene expressed the altered amelogenin exclusively in ameloblasts. This altered amelogenin participated in the formation an organic enamel extracellular matrix and, in turn, in enamel mineralization. At the nanoscale level, the forming matrix adjacent to the secretory face of the ameloblast showed alteration in the size of the amelogenin nanospheres for both transgenic animal lines and the resultant enamel exhibited inferior mechanical properties.10
Amelogenin Isoforms and Function
In mice and humans, alternative splicing creates a dozen or more amelogenin isoforms of different lengths, but their potential functions and/or purpose of this complexity remains unknown. A knock-in genetic approach was used to engineer enamel so that it would be produced from a single amelogenin protein isoform. This knock-in approach reduced amelogenin protein isoform complexity by one order of magnitude, resulting in enamel fabricated with only the most common M180 amelogenin protein. This resulted in an enamel that was significantly harder, or more wear resistant, but also significantly less tough, or less fracture resistant, than wild-type enamel.11
Microstructural organization was indistinguishable between wild-type and transgenic enamel and dentin. Thus, despite a marked reduction in the enamel matrix protein complexity, these substantial design changes produced an engineered enamel with unaltered architecture and acceptable material properties. This finding has profound implications for the future fabrication of replacement enamels. The trade-off, or balance between the opposing mechanical properties of hardness or wear resistance and toughness or fracture resistance provided insights to the importance of the subtle organization in packing of hydroxyapatite crystallites to optimize species-specific biomechanical functions.
Dentin Sialophosphpoprotein, the DEJ, and Enamel Hardness
Rather surprisingly a dentin gene, dentin sialophosphphoprotein (Dspp), is transiently expressed in early-stage, secretory ameloblasts at the time of DEJ formation. This is consistent with Dspp having a role in producing specialized first-formed harder enamel adjacent to the DEJ.12 The expression of “odontoblast” proteins such as DSPP and DMP1, at the time the DEJ is formed, appears to be products of both odontoblasts and ameloblasts. This is well-described in the literature; however, expression of these products (DSPP and DMP1) is short-lived in ameloblasts but continues in odontoblasts until dentine formation is complete.13,14 Crack diffusion by branching and dissipation within this specialized first-formed enamel close to the DEJ prevents catastrophic interfacial damage and gross tooth failure.15 Once Dspp is secreted, it is subjected to proteolytic cleavage that results in three distinct proteins referred to as dentin sialoprotein (Dsp), dentin phosphoprotein (Dpp), and a recently described protein resident between these two termed dentin glycoprotein.16
The authors' purpose was to investigate the contribution of Dsp and Dpp to enamel function. Transgenic mice were engineered to overexpress either Dsp or Dpp throughout the duration enamel formation instead of just very transiently in the first-formed enamel at the DEJ.17 Dsp and Dpp contributions to enamel formation greatly differed. The inclusion of Dsp in bulk enamel significantly and uniformly increased enamel hardness by approximately 20 percent, whereas the inclusion of Dpp softened and weakened bulk enamel.18 This result was consistent with the supposed role of Dsp in the biomechanical function of the DEJ and resulted in an enamel that was biomechanically superior to wild-type enamel being engineered.
Ion Transport: NBCe1 and Enamel Mineralization
After the formation of a competent proteinaceous scaffold to guide mineralization, the ameloblasts secrete the mineral component of enamel in an environment conducive to crystallite precipition. Electrogenic sodium bicarbonate cotransporters (e.g., NBCe1), are found widely in the renal proximal tubule, pancreas, eye, heart, brain, and in developing teeth. Some patients with certain inborn kidney diseases also have enamel abnormalities.
In ameloblasts, NBCe1 helps to maintain the pH buffering system required during mineralization of hydroxyapatite. Protons are released during enamel mineralization when apatite crystals grow from precursor forms of phosphate. The release of protons during hydroxyapatite formation requires a pH buffering system to prevent acidosis and enamel demineralization. By comparing the dentition of NBCe1-null animals to their wild-type littermates, the authors demonstrated that the NBCe1-null mutants produced enamel that was too soft to even measure, and dentin that was significantly softened.19
Dentin may have been less affected than enamel because it is simply less mineralized than enamel or because other ion transport mechanisms may exist. Heterozygous NBCe1 mice produced enamel and dentin of comparable hardness to their wild-type control littermates suggesting that a single copy of NBCe1 may adequately fulfill the biological task or that other ion transporters may be able to compensate in part.
Kallikrein-4 and Removal of the Amelogenin Scaffold
Mature functional enamel contains only very minimal amounts of protein. These minimal remnants may act as a plasticizing agent, allowing some slippage of enamel rods over one another to relieve stresses and provide some toughening.3,20 However, almost all of the enamel scaffold must be removed before mineralization can be completed. Amelogenin, the dominant enamel protein is a known substrate of the Kallikrein-4 (Klk4) proteolytic enzyme in vitro. Kallikrein-4 is a secreted serine protease found primarily in prostate and enamel. Amelogenin is secreted by ameloblasts through early and midenamel formation.
In contrast, Klk4 is normally secreted from these same ameloblasts late in enamel formation. Transgenic mice overexpressing Klk4 in a spatiotemporal pattern simultaneous to endogenous amelogenin expression displayed a soft hypomineralized enamel.21-23 Hence, disruptions to the normal expression pattern of kallikrein-4 in the developing tooth organ clearly impacted the function of the enamel matrix, enamel mineralization, and the integrity of the dentin-enamel junction through premature removal of amelogenin before mineralization has sufficiently matured. Timing is everything.
Ameloblast Maturation, Senescence, and Amelotin
Amelotin (Amtn) is a recently identified enamel matrix protein with expression apparently limited to late-stage enamel formation. The outer layer of last-formed enamel is believed to be softer than bulk enamel and to lack its regular organization, either by design or by default. To date, the role of amelotin in amelogenesis remains unclear. Amelotin-overexpressing animals were generated, in which amelotin gene expression was extended throughout enamel formation.24
The spatiotemporal distribution of the other enamel matrix proteins was not significantly affected, suggesting that the overexpression of amelotin does not exert any inhibitory effect on them. However, the resultant enamel was extremely soft and of a more amorphous structure, consistent with amelotin's likely role in producing the final thin layer of softer outer surface enamel. The mechanism by which amelotin acts remains unknown, but it may play a function in diverse activities such as biomineralization, Ca++ transport, pH balance, and/or cellular differentiation/dedifferentiation.
Clinical Impact
Understanding the genetic and molecular events that regulate the formation of enamel will result in improvements in the prevention, diagnosis, and treatment of heritable and acquired diseases of enamel, including caries, as well as insights that allow the engineering of replacement enamels for therapeutic interventions.
Footnotes
UNIVERSITY OF CALIFORNIA, LOS ANGELES
Successful mentorship creates astute clinicians who are best positioned to advance themselves, their profession, their home institutions, and the wider community through evidence-based practice and therapeutic innovation. Research mentoring should be based upon a foundation of institution values that are shared by students and faculty, supported by appropriate resources, measured through quantitative and qualitative metrics, and rewarded when success is achieved.
Research participation offers an opportunity to work one on one with a professional who is interested in the student and the topic of study. Hands-on research involvement is an experiential, collaborative experience that engages the student and faculty member in an exploration of a subject area, similar to clinical dental education but unlike a traditional didactic learning experience. Research experience is associated with a higher likelihood of acceptance to advanced training programs. Why choose a research experience? It is exciting, fun, rewarding, and informative of a variety of nontraditional dental careers.
Contributor Information
Rick J. Rauth, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Karen S. Potter, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Amanda Y.-W. Ngan, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Deema M. Saad, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Rana Mehr, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Vivian Q. Luong, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Verna L. Schuetter, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry
Vetea G. Miklus, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
PeiPei Chang, Dental students or dental graduates of the University of California, Los Angeles, School of Dentistry.
Michael L. Paine, Faculty members and student research mentors in the Center for Craniofacial Molecular Craniofacial Biology at the University of Southern California, School of Dentistry.
Rodrigo S. Lacruz, Faculty members and student research mentors in the Center for Craniofacial Molecular Craniofacial Biology at the University of Southern California, School of Dentistry.
Malcolm L. Snead, Faculty members and student research mentors in the Center for Craniofacial Molecular Craniofacial Biology at the University of Southern California, School of Dentistry.
Shane N. White, Faculty member and student research mentor at the University of California, Los Angeles, School of Dentistry.
References
- 1.Paine ML, White SN, et al. Regulated gene expression dictates enamel structure and tooth function. Matrix Biology. 2001;20(56):273–92. doi: 10.1016/s0945-053x(01)00153-6. [DOI] [PubMed] [Google Scholar]
- 2.Barlett JD, Ganss B, et al. Protein-protein interactions of the developing enamel matrix. Curr Top Devel Biol. 2006;74:57–115. doi: 10.1016/S0070-2153(06)74003-0. [DOI] [PubMed] [Google Scholar]
- 3.White SN, Luo W, et al. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res. 2001;80(1):321–6. doi: 10.1177/00220345010800010501. [DOI] [PubMed] [Google Scholar]
- 4.Snead ML, Paine ML, et al. Transgene animal model for protein expression and accumulation into forming enamel. Conn Tiss Res. 1998;38(14):279–86. doi: 10.3109/03008209809017048. [DOI] [PubMed] [Google Scholar]
- 5.Kubota K, Lee DH, et al. Fluoride induces ER stress in ameloblasts responsible for dental enamel formation. J Biol Chem. 2005;280(24):23194–202. doi: 10.1074/jbc.M503288200. [DOI] [PubMed] [Google Scholar]
- 6.Snead ML, Zhu DH, et al. Protein self-assembly creates a nanoscale device for biomineralization. Mat Sci Eng C. 2006;26(8):1296–300. [Google Scholar]
- 7.White SN, Chang PP, et al. Murine and human incisal enamel differ in mechanical function. J Dent Res. 2004;83(special issue A) ( iadr.com), Abstract No. 1419.
- 8.Snead ML, Zeichner-David M, et al. Construction and identification of mouse amelogenin cDNA clones. Proc Natl Acad Sci USA. 1983;80(23):7254–8. doi: 10.1073/pnas.80.23.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paine ML, Zhu DH, et al. Enamel biomineralization defects result from alterations to amelogenin self-assembly. J Structural Biol. 2000;132(3):191–200. doi: 10.1006/jsbi.2000.4324. [DOI] [PubMed] [Google Scholar]
- 10.Fong H, White SN, et al. Enamel structure-properties controlled by engineered proteins in transgenic mice. J Bone Min Res. 2003;18(11):2052–9. doi: 10.1359/jbmr.2003.18.11.2052. [DOI] [PubMed] [Google Scholar]
- 11.Rauth RJ, White SN, et al. M180KI Mouse Enamel: Can something so hard be made simple? J Dent Res. 2009;87(special issue A) ( iadr.com), Abstract No. 91.
- 12.White SN, Paine ML, et al. The dentinoenamel junction is a transitional zone uniting dissimilar composite bioceramics. J Am Ceram Soc. 2000;83(1):238–40. [Google Scholar]
- 13.D'Souza RN, Cavender A, et al. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12(12):2040–9. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Yamakoshi Y, et al. Porcine dentin matrix protein 1: gene structure, cDNA sequence, and expression in teeth. Eur J Oral Sci. 2006;114(1):33–41. doi: 10.1111/j.1600-0722.2006.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White SN, Miklus VG, et al. Controlled failure mechanisms toughen the dentinoenamel junction. J Prosthet Dent. 2005;94(4):330–5. doi: 10.1016/j.prosdent.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Yamakoshi Y, Hu JC, et al. Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J Biol Chem. 2005;280(17):17472–9. doi: 10.1074/jbc.M413220200. [DOI] [PubMed] [Google Scholar]
- 17.Paine ML, Luo W, et al. Dentin sialoprotein and dentin phosphoprotein overexpression during amelogenesis. J Biol Chem. 2005;280(36):31991–8. doi: 10.1074/jbc.M502991200. [DOI] [PubMed] [Google Scholar]
- 18.White SN, Paine ML, et al. Ectopic expression of dentin sialoprotein during amelogenesis hardens bulk enamel. J Biol Chem. 2007;282(8):5340–5. doi: 10.1074/jbc.M604814200. [DOI] [PubMed] [Google Scholar]
- 19.Gawenis LR, Bradford EM, et al. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3- cotransporter. J Biol Chem. 2007;282(12):9042–52. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 20.Boyde A. Microstructure of enamel. In: Chadwick DJ, Cardew G, editors. Dental Enamel; Ciba Foundation Symposium 205; New York: Wiley: 1997. pp. 18–31. [DOI] [PubMed] [Google Scholar]
- 21.Paine ML, Luo W, et al. Kallikrein-4 overexpression in the developing enamel organ. J Dent Res. 2006;85(special issue B) ( dentalresearch.org), Abstract No. 797.
- 22.Potter KS, Miklus VG, et al. Kallikrein-4 overexpression in developing murine enamel reduces hardness. J Dent Res. 2006;85(special issue A) ( dentalresearch.org), Abstract No. 1019.
- 23.Potter KS, Miklus VG, et al. Kallikrein-4 overexpression in developing murine enamel profoundly reduces hardness. J Endod. 2007;33(3):364. Abstract No. PR68. [Google Scholar]
- 24.Yazdanshenas D, Lacruz RS, et al. Characterization of transgenic mice overexpressing amelotin in the enamel organ. International Association for Dental Research annual meeting; Miami, Fla.. April 1-4, 2009; Abstract No. 733. [Google Scholar]