Abstract
Background and purpose:
P2Y nucleotide receptors are involved in the regulation of vascular tone, smooth muscle cell (SMC) proliferation and inflammatory responses. The present study investigated whether they are involved in atherosclerosis.
Experimental approach:
mRNA of P2Y receptors was quantified (RT-PCR) in atherosclerotic and plaque-free aorta segments of apolipoprotein E-deficient (apoE–/–) mice. Macrophage activation was assessed in J774 macrophages, and effects of non-selective purinoceptor antagonists on atherosclerosis were evaluated in cholesterol-fed apoE–/– mice.
Key results:
P2Y6 receptor mRNA was consistently elevated in segments with atherosclerosis, whereas P2Y2 receptor expression remained unchanged. Expression of P2Y1 or P2Y4 receptor mRNA was low or undetectable, and not influenced by atherosclerosis. P2Y6 mRNA expression was higher in cultured J774 macrophages than in cultured aortic SMCs. Furthermore, immunohistochemical staining of plaques demonstrated P2Y6-positive macrophages, but few SMCs, suggesting that macrophage recruitment accounted for the increase in P2Y6 receptor mRNA during atherosclerosis. In contrast to ATP, the P2Y6-selective agonist UDP increased mRNA expression and activity of inducible nitric oxide synthase and interleukin-6 in J774 macrophages; this effect was blocked by suramin (100–300 µM) or pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS, 10–30 µM). Finally, 4-week treatment of cholesterol-fed apoE–/– mice with suramin or PPADS (50 and 25 mg·kg−1·day−1 respectively) reduced plaque size, without changing plaque composition (relative SMC and macrophage content) or cell replication.
Conclusions and implications:
These results suggest involvement of nucleotide receptors, particularly P2Y6 receptors, during atherosclerosis, and warrant further research with selective purinoceptor antagonists or P2Y6 receptor-deficient mice.
Keywords: atherosclerosis, apoE, P2Y6, P2Y2, gene expression, macrophage, suramin, PPADS
Introduction
Cardiovascular diseases resulting from atherosclerosis are the leading cause of mortality and morbidity in developing nations. Although not all aspects of their pathogenesis are fully clear, there is consensus that atherosclerosis is a chronic inflammatory disease of the vessel wall. Retention and modification of low density lipoprotein (LDL) in the vessel wall, subsequent recruitment of macrophages in combination with migration of vascular smooth muscle cells (SMCs) are thought to be key events in the pathogenesis of atherosclerosis (Hansson, 2005).
It has been proposed that purinoceptors might be involved in the development of atherosclerotic plaques (Di Virgilio and Solini, 2002). Purinoceptors are activated by extracellularly released nucleotides (like ATP, UTP, ADP and UDP) and are divided into the P2X and P2Y receptor family (Kunapuli and Daniel, 1998). P2X receptors are ion channels activated by ATP, whereas P2Y receptors consist of seven transmembrane domains and are coupled to G-proteins. Until now, eight different P2Y subtypes have been identified (P2Y1, 2, 4, 6, 11, 12, 13, 14) and each subtype has a characteristic agonist profile (Van Der Giet et al., 2002; von Kugelgen, 2006). Stimulation of P2Y1, 2, 4 and 6 receptors causes activation of phospholipase C, whereas P2Y11, 12 and 13 stimulate or inhibit adenylate cyclase.
Previously, it has been shown that activation of P2Y receptors promotes replication of vascular SMCs in vitro (Erlinge et al., 1996; Hou et al., 2002) and in vivo (Seye et al., 1997; Seye et al., 2002), but SMC proliferation is not very prominent in murine or human atherosclerotic plaques (Kockx et al., 1998; Lutgens et al., 1999).
Extracellular nucleotides are currently considered damage-associated molecular-patterns (DAMPs) that contribute to the induction of inflammation by activation of various inflammatory cells (Rubartelli and Lotze, 2007). Indeed, there is increasing evidence that purinoceptors are involved in inflammation. UTP induces up-regulation of vascular cell adhesion molecule-1 on coronary artery endothelial cells (Seye et al., 2003). Moreover, activation of P2X7 receptors by ATP is known to induce cell death of macrophages and trigger the release of interleukin (IL)-1β from these macrophages (Solle et al., 2001). The effects of UTP and UDP (a P2Y6-selective agonist) on macrophage function are less clear, but recent publications point to a pro-inflammatory role of P2Y6 receptors expressed on human monocytes (Warny et al., 2001; Cox et al., 2005) and murine macrophages (Bar et al., 2008).
As purinoceptors are modulators of inflammation, we investigated the potential role of P2Y receptors in murine atherosclerosis. Apolipoprotein E-deficient (apoE–/–) mice are hypercholesterolaemic and spontaneously develop atherosclerotic lesions that resemble the human disease (Meir and Leitersdorf, 2004). Moreover, the lesions arise at predisposed sites along the aorta (i.e. preferentially in the aortic arch, whereas the central thoracic aorta remains virtually plaque-free), which enables comparison of atherosclerotic and non-diseased regions within the same animal (Crauwels et al., 2003).
Methods
Mice
The studies were approved by the Ethical Committee of the University of Antwerp, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Wild-type (WT) and apoE–/– mice, which had been back-crossed in the C57BL/6J background for more than 10 generations, were studied at the age of 4 and 18 months and kept on a regular chow, except for the pharmacological intervention study, when the mice received a Western-type diet to accelerate atherosclerosis.
Pharmacological intervention study
Young apoE–/– mice (age 4–6 weeks) were fed a cholesterol-rich Western-type diet (TD88137, Harlan Teklad, Madison, WI, USA) for 3 months. Two months later, the mice were randomly divided into three groups, and treated with sterile water (control), suramin or PPADS during the final month. The non-selective purinoceptor antagonist suramin (50 mg·kg−1·day−1) was dissolved (200 mg·mL−1) in sterile water and delivered via subcutaneously implanted osmotic minipumps (6 µL·day−1). These pumps provided a continuous release of the drug for 4 weeks. PPADS (25 mg·kg−1·day−1) was dissolved (7.5 mg·mL−1) in sterile water and was injected subcutaneously every 2 days (injection volume 200 µL), as aqueous PPADS solutions showed considerable degradation at 37°C.
Isolation of tissues
Mice were anaesthetized (sodium pentobarbital 75 mg·kg−1, i.p.) and the aorta was carefully dissected. When indicated, endothelial cells were removed by perfusion with Triton X100 as described previously (Guns et al., 2005). A segment of the aortic root (2 mm) was fixed (4% formaldehyde, 24 h) and embedded in paraffin for histology. The aortic arch and the central thoracic aorta were disrupted with a sterile razor blade and transferred to lysis buffer for isolation of total RNA (Microprep-kit) according to the guidelines of the manufacturer.
Real-time RT-PCR
Expression of mRNA was measured by a two-step RT-PCR (RT-PCR core kit). Briefly, RNA was first converted to cDNA with random nonamers and thereafter a quantitative PCR was performed on an ABI 7000 instrument using fluorescent Taqman probes (Guns et al., 2008).
The expression of target genes was normalized to the endogenous reference gene β-actin (i.e. the threshold cycle (CT) of the target gene was subtracted from the CT-value of β-actin resulting in ΔCT-values). From these ΔCT-values, relative expression to the arbitrary chosen calibrator sample was calculated.
In view of changes in cell composition during atherosclerosis, the stability of different potential endogenous reference genes was previously evaluated in aortae with and without plaques (Guns et al., 2008). Geometric averaging of the CT-values with GeNorm (Vandesompele et al., 2002) demonstrated that β-actin and succinate dehydrogenase complex, subunit A (SDHA) were the most stable control genes in aortae with and without atherosclerosis. For practical reasons, β-actin was selected as a reference gene, as it is more frequently used than SDHA. Additionally, the ratio P2Y6/P2Y2 was calculated, which is independent of the expression of β-actin.
Histology
The cross-sectional plaque area was determined on haematoxylin and eosin-stained transverse sections at the level of the aortic root using a computer-assisted image analysis system (Image-Pro Plus, Epix Vision, Buffalo Grove, IL, USA) (Crauwels et al., 2003; Guns et al., 2008). In addition, transverse sections were stained with antibodies against α-smooth muscle actin (mouse monoclonal antibody), mac-3 (rat monoclonal antibody), proliferating cell nuclear antigen (PCNA) and P2Y6 receptors (rabbit polyclonal antibody). Monoclonals were developed by a peroxidase-conjugated rabbit anti-FITC antibody or a biotin-labelled anti-rat IgG (mouse IgG absorbed) respectively, whereas P2Y6 receptors were visualized with anti-rabbit IgG anti-body in combination with avidin-biotin labelling (ABC-kit).
Cell culture
A primary culture of murine aortic SMCs was obtained after limited enzymatic digestion (5 h, collagenase 2) of mouse aortae (Ray et al., 2001).
To examine the effects of nucleotides on macrophage function, J774 macrophage cells were grown in the RPMI 1640 medium supplemented with 10% heat-inactivated foetal bovine serum, 100 U·mL−1 penicillin, 100 U·mL−1 streptomycin, 50 µg·mL−1 gentamycin and 20 U·mL−1 polymyxin B sulphate in a humidified 5% carbon dioxide incubator at 37°C line. Cells were primed with interferon gamma (IFNγ, 100 U·mL−1) and incubated with 0.1–3 mM UDP or ATP.
J774 cell activation was assessed by measuring nitrite – as a proxy for induction of inducible nitric oxide synthase (iNOS) and IL-6 (mouse ELISA) in the supernatants at 42 h. Nitrite was measured by means of the Griess reagent as described previously (Jans et al., 2004). In parallel experiments, the effect of UDP (1000 µM) – with and without PPADS (10 or 30 µM) or suramin (100 or 300 µM) – on iNOS and IL-6 mRNA expression was evaluated after a 6-h incubation.
Statistical analysis
All results are expressed as mean ± SEM; n represents the number of mice or cell cultures. A 5% level of significance was selected.
Materials
The Osmotic minipumps (Alzet®, model 2004) were obtained from Charles River (France); Microprep-kit from Stratagene; RT-PCR core kit from Eurogentec (Seraing, Belgium) and the ABI 7000 instrument from Applied Biosystems (Foster City, CA, USA). The primers and probes for the RT-PCR were purchased from Eurogentec or Applied Biosystems (Assay on Demand; Table 1). The mouse monoclonal antibody, (clone 1A4, FITC-labelled) was obtained from Sigma (Bornem, Belgium); the rat monoclonal antibody (clone M3/84) from Pharmingen (San Diego, CA, USA); the PCNA (MCA1558F, FITC-labelled) from AbD Serotec (Raleigh, NC, USA); the rabbit polyclonal antibody from Alomone Labs (Jerusalem, Israel); peroxidase-conjugated rabbit anti-FITC antibody was from Dako (Glostrup, Denmark) and the biotin-labelled anti-rat IgG (mouse IgG absorbed) from Vector Laboratories (Burlingame, CA, USA). The ABC-kit was obtained from Vector Laboratories. Collagenase 2 was from Worthington. J774 macrophage cells were obtained from the American Type Culture collection (Manassas, VA, USA); the RPMI 1640 medium from Invitrogen (Carlsbad, CA, USA) and mouse ELISA from R&D Systems (Minneapolis, MN, USA).
Table 1.
Sequences of primers and probes for real-time quantitative RT-PCR
| Gene | Sequence (from 5′ to 3′) | Amplicon |
|---|---|---|
| P2Y1 | ||
| Forward | CAAGCAGAATGG AGACACGAGT | |
| Reverse | GCATAGATCATCTCAGGG ATGTCTT | |
| Probe | ACTCAGGAGCTAGGATCTCGTGCC TTCAC | 93 bp |
| P2Y2 | ||
| Forward | ACCTCAAGAGCAGGAGCTGATC | |
| Reverse | GCCATTGATGGTGCTATTCCA | |
| Probe | CTGCTGCCATTGCCCTGGACCT | 75 bp |
| P2Y4 | ||
| Forward | GGCCAGAAGAAGCAGCAGAA | |
| Reverse | GGCTGGGACCTAGTGATGTGA | |
| Probe | CCCAGCTTCCTTAGTCCAGTCCAGGG | 145 bp |
| P2Y6 | ||
| Forward | AGCAAGGCGGCTCGTATG | |
| Reverse | TCTCCAGCACAGGGCAAGA | |
| Probe | CCTACTTGGCTGTGCGCTCCACG | 129 bp |
| β-actin | ||
| Forward | GCTCTGGCTCCTAGCACCAT | |
| Reverse | GCCACCGATCCACACAGAGT | |
| Probe | ATCAAGATCATTGCTCCTCCTGAGCGC | 75 bp |
| CD68 | Mm00839636_g1 | Assay on demand (Applied Biosystems) |
| α-SM-actin | Mm01204962_gh | Assay on demand (Applied Biosystems) |
| iNOS | Mm01309897_m1 | Assay on demand (Applied Biosystems) |
| IL-6 | Mm01210733_m1 | Assay on demand (Applied Biosystems) |
IL-6, interleukin 6; iNOS, inducible nitric oxide synthase.
Results
Expression of P2Y receptors in atherosclerosis
The expression of mRNA for P2Y receptors in atherosclerotic arteries of 18-month-old apoE–/– mice on regular chow was measured and compared with that in plaque-free vessels of age-matched WT mice. P2Y4 receptor mRNA was not detected (CT > 40) in vessels from WT or atherosclerotic apoE–/– mice, whereas the finding that P2Y4 was detected in liver demonstrated the efficacy of the primers and probe. The expression of mRNA for P2Y1 receptors was low (CT > 38) and did not enable accurate quantification. In contrast, P2Y2 and P2Y6 receptor mRNA (CT <34 and <32 respectively) could be measured accurately. The expression of mRNA for P2Y6 receptors was significantly (P= 0.027) increased in atherosclerotic arteries of apoE–/– mice compared with plaque-free vessels from WT mice (Figure 1). In contrast, P2Y2 receptor expression was identical in aortic segments with or without plaques. Calculation of the ratio P2Y6/P2Y2, which is independent of any fluctuation of reference genes, confirmed the increase in P2Y6 (P= 0.001).
Figure 1.
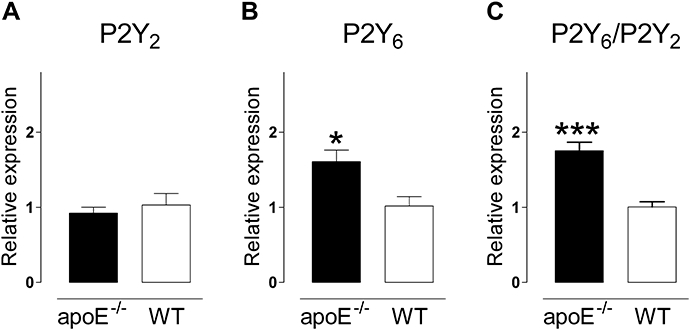
Comparative expression of mRNA for P2Y2 receptors (A), P2Y6 receptors (B) and the ratio P2Y6/P2Y2 (C) in aortic arches of old (18 months) atherosclerotic apoE–/– and plaque-free WT mice (18 months) on regular chow. The expression of the P2Y6 receptor was significantly increased in arteries with atherosclerotic lesions. β-Actin was used as an endogenous reference gene and the mean value of the WT was used as a calibrator and set to one. Results show mean and SEM, n= 6 (apoE–/–) or n= 4 (WT). *P < 0.05, ***P < 0.001. WT, wild-type.
Next, the expression of mRNA for P2Y2 and P2Y6 receptors was evaluated along the aorta within individual apoE–/– mice to exclude possible strain differences. Initial experiments with intact aortic segments indicated an increase in mRNA of P2Y6 receptors, but not of P2Y2 receptors in the atherosclerotic arch (AA) compared with the virtually plaque-free thoracic aorta (data not shown). Thereafter, these results were validated in segments from which the endothelial cells had been removed: 4-month-old apoE–/– mice, which had not yet developed atherosclerotic lesions, displayed no regional differences in the expression of mRNA for P2Y2 and P2Y6 receptors between the atherosclerosis-prone and the atherosclerosis-resistant segment (Figure 2). In contrast, 18-month-old apoE–/– mice, which had developed atherosclerotic plaques in the aortic arch (AA), but not in the central thoracic aorta (TA), showed an elevated expression of P2Y6 receptors in the atherosclerotic region (P= 0.044), whereas the expression of P2Y2 receptors was similar in both regions. The ratio P2Y6/P2Y2 was increased as well (P= 0.012). In WT mice, no regional- or age-related differences in the expression of mRNA for P2Y2 or P2Y6 receptors were observed (data not shown).
Figure 2.
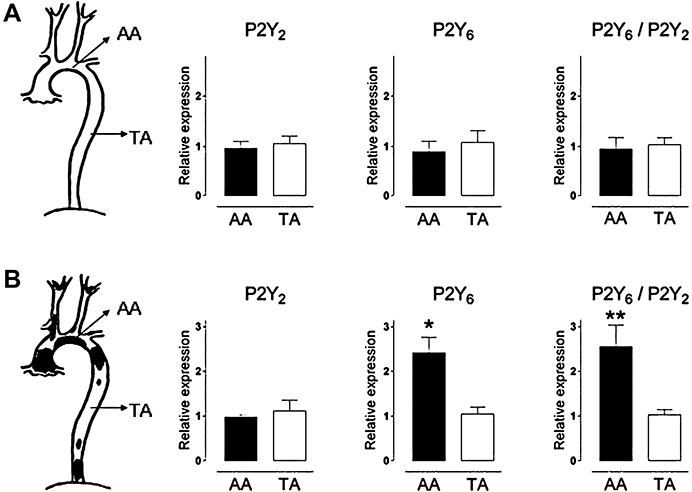
Relative expression of mRNA for P2Y2 receptors, P2Y6 receptors and the ratio P2Y6/P2Y2 in endothelial cell-denuded aortae of young (A) and old (B) apoE–/– mice on regular chow. Young apoE–/– mice (4 months) that had not developed atherosclerotic plaques yet, showed no regional differences in the expression of P2Y2 or P2Y6 receptors between aortic arch (AA) and thoracic aorta (TA). In contrast, old apoE–/– (18 months) with established plaques in the aortic arch had more P2Y6 receptor transcripts in the atherosclerotic region. β-Actin was used as an endogenous reference gene and the mean value of the TA was used as calibrator and set to one. Results show mean and SEM, n= 5. *P < 0.05, **P < 0.01.
In an attempt to pinpoint the cell type causing the increased expression of mRNA in atherosclerotic regions, the mRNA of P2Y2 and P2Y6 receptors was quantified in cell cultures of murine aortic SMCs and J774 macrophages (Figure 3). The P2Y2 receptor subtype was similarly expressed in naïve aortic SMCs, cultivated aorta SMCs and J774 macrophages. The expression of mRNA for P2Y6 receptors, however, decreased significantly upon cultivation of aortic SMCs. In contrast, J774 macrophages showed considerably higher levels of P2Y6 receptor mRNA.
Figure 3.
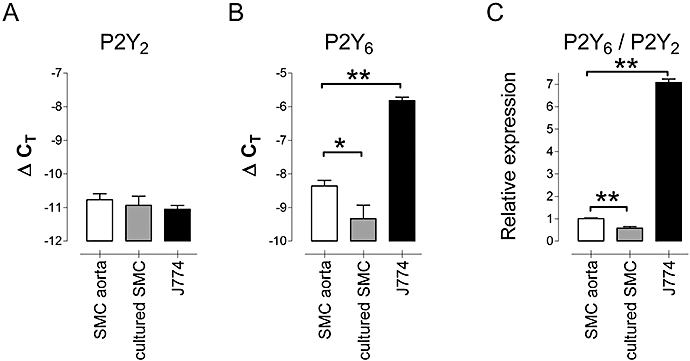
Relative expression of mRNA for P2Y2 receptors (A), P2Y6 receptors (B) and the ratio P2Y6/P2Y2 (C) in naïve murine aortic SMCs (SMC aorta), in a primary culture of aortic SMCs (cultured SMC) and in J774 macrophages (J774). The expression of P2Y6 receptors decreased upon cultivation of SMCs, whereas J774 macrophages displayed a relatively high expression. ΔCT= CTβ-actin – CT P2Y2 or 6. Results show mean and SEM, n= 5; *P < 0.05, **P < 0.01, anova, Bonferroni post hoc test. SMC, smooth muscle cell.
Immunohistochemistry of P2Y6 receptors in atherosclerosis
In transverse sections of WT aortae, and in plaque-free segments of apoE–/–mice, abundant staining for P2Y6 receptors was present in SMCs in the media (Figure 4). In atherosclerotic regions, α-smooth muscle actin staining of the inner SMC layer was often less intense, but P2Y6-positive staining remained prominent in medial SMCs (arrow heads). Cells with a foamy appearance that stained positive for the macrophage marker mac-3 showed the presence of some macrophage-derived foam cells in the inner media as well.
Figure 4.
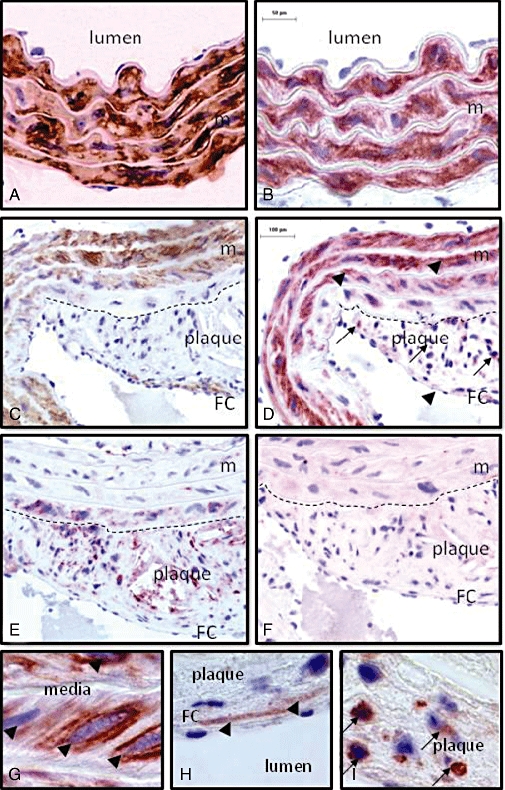
Staining of α-smooth muscle actin (A, C, brown), of P2Y6 receptors (B, D, G, H, I, red) and of the macrophage marker mac-3 (E, red) in consecutive transverse sections of a plaque-free (A, B) and an atherosclerotic (C, D, E, F, G, H, I) segment of the aorta of an apoE–/– mouse (18 months old). α-Smooth muscle actin-positive staining was observed in the media of both segments, but also in SMCs of the fibrous cap. Mac-3-positive staining was found in the plaque, and occasionally in the inner media. P2Y6-positive staining was most abundant in SMCs of the media (arrow heads) in segments with (D, G) or without (B) plaques. However, in the plaque P2Y6 receptors were mostly present in macrophages (D, I, arrows) and in a few SMCs in the fibrous cap (D, H, arrow head). Staining of P2Y6 receptors was completely abolished after incubation of the antibody with the polypeptide that had been used to immunize the rabbits, illustrating the selectivity of the antibody (F). The dashed line shows the border between media and plaque. Magnification: 200× (A, B); 100× (C, D, E, F); 1000× (G, H, I). FC, fibrous cap; m, media; SMC, smooth muscle cell.
Macrophage-derived foam cells that were positive for mac-3 were the most abundant cell type in the plaque. A few α-smooth muscle actin-positive cells, often forming a thin fibrous cap over the necrotic core, were present (Figure 4). In the plaques, most P2Y6 staining occurred in macrophage-derived foam cells. This was evident not only from overlap staining in consecutive sections, but also from their location, their foamy appearance and the nuclear shape of most intra-plaque P2Y6-positive cells. A minority of the P2Y6 positivity was found in SMCs, as shown not only by staining of adjacent sections, but also by their location (in fibrous cap) and characteristic, elongated shape of the nucleus. The absence of P2Y6 receptor staining after incubation of the antibody with the polypeptide that had been used to immunize the rabbits illustrated the selectivity of the antibody.
P2Y6 receptors and macrophage function
Levels of nitrite and IL-6 were monitored after a 42-h incubation of J774 macrophages with UDP (a selective P2Y6 receptor agonist) and ATP (acting via P2X7 and/or P2Y2 receptors). UDP (Figure 5) caused a concentration-dependent increase of nitrite and IL-6 concentrations, whereas ATP was hardly active (Figure 5). Moreover, UDP increased mRNA expression of iNOS, particularly IL-6 in J774 cells after 6 h (Figure 6). The stimulant effect on iNOS was completely blocked by suramin (300 µM) or PPADS (30 µM), whereas the up-regulation of IL-6 was completely inhibited at threefold lower concentrations of the antagonists.
Figure 5.
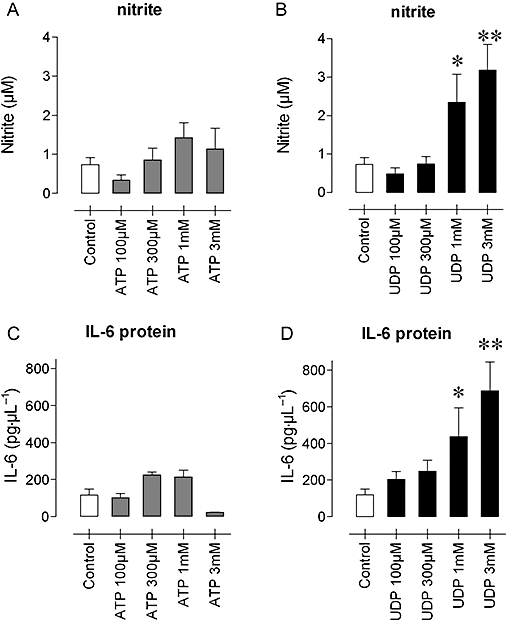
Concentrations of nitrite (A, B) and IL-6 (C, D) in supernatants of J774 macrophages after a 42-h incubation with increasing concentrations of ATP (A, C) or UDP (B, D). Results show mean and SEM of four independent experiments. *P < 0.05, **P < 0.01, anova, Dunnett's post hoc test. ATP, adenosine 5′ triphosphate; IL-6, interleukin 6; UDP, uridine 5′ diphosphate.
Figure 6.
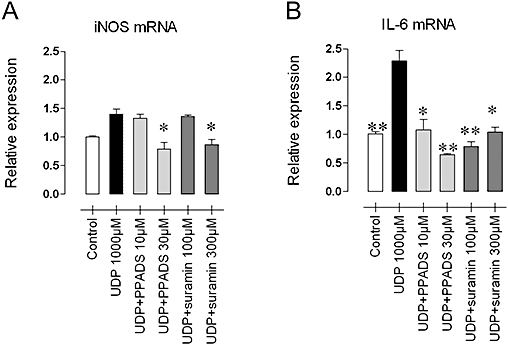
iNOS (A) and IL-6 (B) mRNA expression in J774 macrophages after stimulation (6 h) with 1 mM UDP, with and without suramin (100 and 300 µM) or PPADS (10 and 30 µM). The UDP-induced up-regulation of iNOS and IL-6 was antagonized by both non-selective purinoceptor antagonists. Results show mean and SEM of four independent experiments. *P < 0.05, **P < 0.01 different from 1 mM UDP, anova and Dunnett's test. IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; PPADS, pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid; UDP, uridine 5′ diphosphate.
Purinoceptor blockers and atherosclerosis
To evaluate the role of P2Y receptors during atherosclerosis, the effects of suramin and PPADS (both non-selective purinoceptor antagonists) were studied in cholesterol-fed apoE–/– mice. Body weight and age of the mice were comparable between the three groups [body weight: 27.0 ± 1.6 g (control, n= 6), 28.4 ± 2.4 g (suramin-treated, n= 6) and 27.7 ± 1.4 g (PPADS-treated, n= 6); age: 4.9 ± 0.3, 4.5 ± 0.3 and 4.3 ± 0.3 months respectively]. PPADS treatment did not influence total plasma cholesterol, whereas suramin tended to increase cholesterol levels (control: 6.99 ± 0.56 g·L−1; PPADS-treated: 7.26 ± 0.19 g·L−1 and suramin-treated: 9.43 ± 0.98 g·L−1, P= 0.055; anova).
All mice showed plaques at the aortic root, but the cross-sectional area was significantly smaller in mice treated with suramin or PPADS (Figure 7A). To test whether treatment with PPADS or suramin changed the cell composition of the plaques or cell replication, macrophages and smooth muscle cells and PCNA-positive nuclei were stained by immunohistochemistry. The macrophage marker mac-3 was abundantly present in atherosclerotic plaques, but its relative cross-sectional area was not decreased after suramin or PPADS treatment (Figure 7B). Furthermore, neither suramin nor PPADS had an effect on the relative cross-sectional area of α-SMC actin staining in the plaque (Figure 7C). Also the ratio between α-SMC and mac-3 areas (mean ± SEM: 0.25 ± 0.11 n= 6, 0.33 ± 0.12 n= 6 and 0.32 ± 0.10 n= 6) was not different between control, PPADS and suramin groups respectively. In the control group, 12% of the nuclei in the plaque were PCNA-positive, mainly in macrophage-derived foam cells, but their frequency was not affected by either treatment (Figure 7D).
Figure 7.
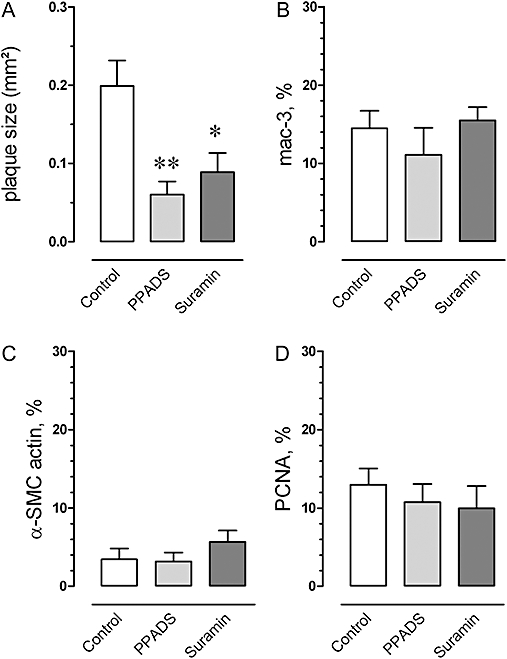
Cross-sectional plaque area at the level of the aortic root in control, suramin (50 mg·kg−1·day−1), and PPADS (25 mg·kg−1·day−1)-treated apoE–/– mice (4 months) on a Western-type diet (A). Plaque size was significantly lower in mice treated with suramin or PPADS. Relative areas of macrophages (mac-3, B) and SMCs (α-SMC actin, C), and cell proliferation (percentage PCNA-positive nuclei, D) were not influenced by suramin or PPADS. Results show mean and SEM, n= 6. *P < 0.05, **P < 0.01, Kruskal Wallis anova. PCNA, proliferating cell nuclear antigen; PPADS, pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid; SMC, smooth muscle cell.
The semi-quantitative immunohistochemical data of the plaque composition were corroborated by mRNA measurements in the atherosclerotic aortic arch (AA) and in plaque-free (TA) segments by means of quantitative, real-time RT-PCR. In the control group, the aortic arch showed about a10-fold higher expression of the mRNA for the macrophage marker CD68 in comparison with the central thoracic aorta. In contrast, mRNA levels of α-smooth muscle actin were about 50% lower. Treatment with suramin or PPADS did not change the expression of marker in segments either with (AA) or without (TA) plaques (Figure 8).
Figure 8.
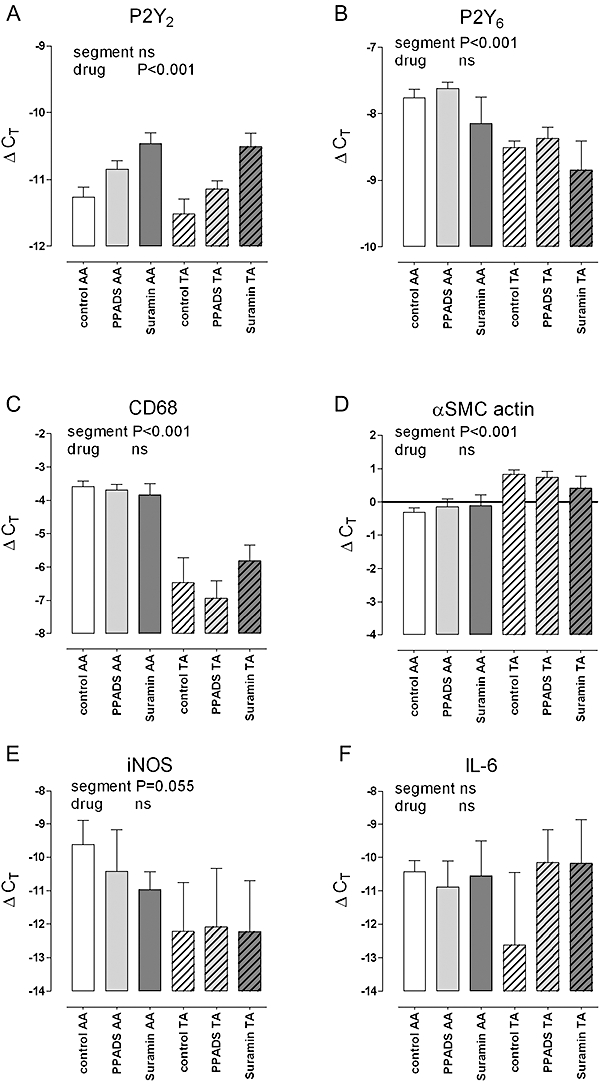
mRNA expression of P2Y2 receptors (A), P2Y6 receptors (B), the macrophage marker CD68 (C), α-SMC actin (D), iNOS (E) and IL-6 (F) in the atherosclerotic aortic arch (AA) and in virtually plaque-free thoracic aorta (TA) segments of control mice and apoE–/– mice (4 months), on a Western-type diet, treated with suramin or PPADS. Atherosclerotic AA segments showed increased mRNA expression of P2Y6 receptors, CD68 and iNOS (tendency), unaltered expression of P2Y2 receptors and IL-6, and slightly decreased α-SMC actin mRNA. Treatment with suramin or PPADS increased mRNA of P2Y2 receptors and showed a tendency to decrease iNOS activation, whereas the expression of P2Y6 receptors, CD68, α-SMC actin and IL-6 were not influenced by the purinoceptor antagonists. Results show mean and SEM, n= 6. Significances of a factorial anova with segment (AA or TA) and drug treatment as factors are shown; the interaction term was never significant. IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; PPADS, pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid; SMC, smooth muscle cell.
Atherosclerotic segments again showed increased mRNA expression of P2Y6 and of the P2Y6/P2Y2 ratio (data not shown), whereas expression of P2Y2 receptors was similar to plaque-free regions (Figure 8). Treatment with PPADS (P= 0.037, Dunnett's post hoc test) or suramin (P < 0.001) increased mRNA of P2Y2 receptors, but this occurred to the same extent in segments with (AA) or without (TA) atherosclerosis.
To investigate the effects of the purinoceptor antagonists on pro-inflammatory mediators, the mRNA of iNOS and IL-6 was measured. The expression of iNOS was low and highly variable, but tended to be higher in the aortic arch, where it seemed to be suppressed by suramin and PPADS. The expression of IL-6 mRNA was low and variable as well, without significant effects of location within the aorta or drug treatment.
Discussion
P2Y receptor expression during atherosclerosis
The present study was the first time mRNA expression of P2Y receptors was investigated in atherosclerotic arteries of apoE–/– mice. Expression of P2Y1 or P2Y4 receptor mRNA was low or undetectable and not influenced by atherosclerosis. Surprisingly, P2Y2 receptor mRNA levels were not affected by atherosclerosis in the apoE–/– model. Previously, P2Y2 receptor up-regulation has been described in models of injury to the media that directly evoke SMC proliferation: balloon denudation in rat aorta (Seye et al., 1997), collar placement in rabbit carotid artery (Seye et al., 2002) and stent placement in pig coronaries (Shen et al., 2004). This discrepancy might be explained by the minor contribution of SMC proliferation to human or murine atherosclerosis: most cell replication (85–95%) in plaques is confined to macrophages (Kockx et al., 1998; Lutgens et al., 1999). It could also be related to species differences, as we also failed to demonstrate up-regulation of P2Y2 receptors upon cultivation of SMCs.
A consistent, although modest (approximately twofold), up-regulation of P2Y6 receptor mRNA was observed in both spontaneous and diet-induced atherosclerosis. Increased mRNA of P2Y6 receptors in atherosclerotic regions was also shown within individual apoE–/– mice, thereby eliminating potential strain differences. As up-regulation of P2Y6 remained after removal of the endothelial cells, that cell type can be excluded as a source of the increased P2Y6 expression. Pronounced staining of P2Y6 receptors was shown by immunohistochemistry in medial SMCs, but this occurred to the same extent in aortae with or without plaques. Their function is not fully clear, as the P2Y6-selective agonist UDP evoked only minor contractile responses (Bar et al., 2008). In addition, cultivation of native SMCs resulted in down-regulation of P2Y6 receptors, and therefore, it is unlikely that SMCs with a proliferative phenotype were responsible for the up-regulation of P2Y6 receptors in atherosclerotic arteries of apoE–/– mice.
After the involvement of endothelial cells and SMCs had been excluded, it appeared that other plaque-associated cell types were involved in the increased expression of P2Y6 receptors. Macrophages, abundantly present in the atherosclerotic lesions, were the most plausible candidates. Indeed, immunohistochemistry of consecutive sections showed the accumulation of P2Y6-positive macrophages in atherosclerotic plaques and mRNA expression of P2Y6 receptors were about 10-fold higher in J774 macrophages than in native or cultured aortic SMCs.
Functional role of P2Y6 receptors on J774 macrophages
The potential (patho)physiological role of P2Y6 receptors on macrophages was characterized in murine J774 macrophages. Application of UDP led to activation of J774 macrophages, as shown by the up-regulation of mRNA of iNOS and IL-6, and the increased concentration of nitrite and IL-6 in supernatants of UDP-stimulated cells. Moreover, the up-regulation and the release of nitrite (results not shown) were fully blocked by suramin or PPADS. In agreement with these results, we recently reported that UDP augmented the release of IL-6 from endotoxin-stimulated murine peritoneal macrophages. This UDP-evoked response was abolished in P2Y6-deficient macrophages (Bar et al., 2008), clearly demonstrating the involvement of P2Y6 receptors in macrophage activation. In agreement with this pro-inflammatory function of P2Y6 receptors in mouse macrophages, in two publications the release of IL-8 from human monocytes after stimulation with the P2Y6 receptor agonist UDP has been described (Warny et al., 2001; Cox et al., 2005). Also ATP has been reported to activate iNOS in RAW 264.7 murine macrophages (Tonetti et al., 1994), but in the present experiments with J774 macrophages, or in peritoneal macrophages (Bar et al., 2008), the ATP responses were markedly smaller than those evoked by UDP.
Purinoceptors and atherosclerosis
The pharmacological intervention study showed that both suramin and PPADS treatment reduced plaque size. Importantly, the decrease was independent of changes in plasma cholesterol levels. Suramin even tended to increase cholesterol levels. A rise in cholesterol levels is expected to stimulate atherosclerosis, whereas suramin treatment had exactly the opposite effect on plaque size.
In this model of diet-induced atherosclerosis, again increased expression of P2Y6 receptors was observed in atherosclerotic regions, which was not influenced by suramin or PPADS. Interestingly, mRNA of P2Y2 receptors was increased in aortae from mice treated with suramin or PPADS. A similar (compensatory) increase of mRNA levels of P2Y2 and P2Y1 receptors was observed in P2Y6-knockout mice (Bar et al., 2008). The (physiological) role of this up-regulation is not clear, but comparable observations are reported with other G-protein coupled receptors (Sharma et al., 2008).
The exact mechanism by which suramin and PPADS reduce plaque size is not yet fully clear. Treatment with suramin or PPADS did not change plaque composition, as indicated by immunohistochemistry (mac-3 and α-SMC actin) and RT-PCR (CD68 and α-SMC actin mRNA), or cell replication-rates, as indicated by PCNA staining. In contrast, both antagonists showed a tendency to normalize iNOS mRNA in atherosclerotic regions towards the levels of non-atherosclerotic vessels. In murine plaques, iNOS is exclusively present in a subset of the macrophages (Crauwels et al., 2003). This reduction of iNOS mRNA by suramin and PPADS points to suppression of macrophage activation and that could be a possible explanation for the reduced plaque formation (Miyoshi et al., 2006). It is also in line with the idea that DAMPs, such as ATP, UDP and UTP contribute to inflammation by activating various inflammatory cells (Rubartelli and Lotze, 2007). In agreement with these in vivo results, both suramin and PPADS inhibited UDP-evoked up-regulation of iNOS in J774 macrophages. Release of IL-6 from macrophages (elicited by UDP), another potential pro-atherogenic mechanism (Huber et al., 1999), was suppressed by suramin and PPADS in vitro. However, neither compound decreased mRNA levels of IL-6 in vivo, presumably because IL-6 is also produced by endothelial cells and SMCs.
Limitations of the study
In view of the potential role of P2Y6 receptors on macrophages, it would have been advantageous to test the effect of a P2Y6-selective antagonist on atherosclerosis. Recently, MRS2578 a promising P2Y6 selective compound was synthesized (Mamedova et al., 2004). However, MRS2578 failed to antagonize UDP-evoked endothelium-dependent relaxations, and also interfered with the phenylephrine-induced contraction of aortic rings in the absence of nucleotides (data not shown). As selectivity and stability of those blockers is not guaranteed in vivo, it was decided to evaluate the effects of non-selective purinoceptor antagonists on plaque progression.
Suramin and PPADS both block multiple P2X and P2Y receptors; therefore, we cannot draw firm conclusions about the exact identity of the nucleotide receptors involved. Antagonism of activating P2Y6 receptors, and of P2X7 receptors, on macrophages might be possible mechanisms by which suramin and PPADS attenuated atherosclerosis. Interference of the blockers with platelet aggregation, mediated by P2Y1, P2Y12 and P2X1 receptors, provides another explanation, although the contribution of platelets to murine atherosclerosis has yet to be proven. Recent reports on the P2Y12 receptor antagonist clopidogrel are controversial. In one study clopidogrel was shown to inhibit atherosclerosis in apoE and low density lipoprotein receptor double-knockout mice (Jawien et al., 2007). However, in another study therapy with acetylsalicylic acid or clopidogrel alone, or in combination, failed to suppress plaque development in apoE–/– mice (Schulz et al., 2008). Finally, reduced atherosclerotic lesions were observed in P2Y1 receptor/apoE double-knockout mice, but decreased plasma cholesterol levels could explain the attenuated plaque growth in that study. Interestingly, bone marrow transplantation experiments pointed to a role of non-haematopoietic-derived P2Y1 receptors, presumably on endothelial cells or smooth muscle cells, rather than mononuclear cells or platelets (Hechler et al., 2008).
In addition, suramin has been reported to bind different growth factors (Middaugh et al., 1992; Kloen et al., 1994) and to inhibit SMC proliferation in vivo (Hu et al., 1999), but PPADS, a chemically unrelated compound, which is not known to bind growth factors, also decreased plaque size.
Both drugs and placebo were administered via different delivery methods, as PPADS could not be infused continuously due to stability issues. Hence, a direct comparison between the treatment with PPADS and suramin is not possible. Even if the drugs had been given via an identical route, it would not be possible to compare both treatments, as virtually nothing is known about the pharmacokinetics in mice and we have no information about their plasma (and more importantly) tissue levels.
Finally, the concentrations of UDP and ATP applied in all studies appear rather high and physiologically questionable. However, the intracellular concentration of nucleotides, especially ATP (3–5 mM), exceeds more than hundred times the extracellular concentration. Hence, lysis of neighbouring cells could cause a marked rise of in the levels of extracellular nucleotides. Therefore, it is likely that in atherosclerotic lesions UDP – leaking from necrotic cells in the core – activates P2Y6 receptors, resulting in iNOS activation and IL-6 release.
Conclusion
To summarize, we showed for the first time an up-regulation of P2Y6 receptors in murine atherosclerotic plaques and documented their involvement in iNOS activation and IL-6 release from murine macrophages. Furthermore, the in vivo suppression of plaque size by suramin is suggestive of the involvement of purinoceptors in atherosclerosis, but could also be explained by the binding of growth factors. However, the results with PPADS, another and chemically unrelated purinoceptor antagonist, also pointed to the involvement of one or more purinoceptors in atherogenesis. Experiments with P2Y6–/–× apoE–/– double-knockout mice could provide more conclusive evidence.
Acknowledgments
P-J G is a Research Assistant of the Fund for Scientific Research Flanders (Belgium-FWO Vlaanderen). The authors wish to thank Rita Van den Bossche and Hermine Fret for technical support.
Glossary
Abbreviations:
- ADP
adenosine 5′ diphosphate
- anova
analysis of variance
- apoE–/–
apolipoprotein E-deficient
- ATP
adenosine 5′ triphosphate
- CT
threshold cycle
- DAMP
damage-associated molecular-pattern
- IFNγ
interferon γ
- IL-6
interleukin 6
- iNOS
inducible nitric oxide synthase
- PCNA
proliferating cell nuclear antigen
- PPADS
pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid
- SMC
smooth muscle cell
- UDP
uridine 5′ diphosphate
- UTP
uridine 5′ triphosphate
- WT
wild-type
Conflicts of interest
The authors state no conflict of interest.
References
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynaems JM, et al. Knock-out mice reveal a role for P2Y6 receptor in macrophages, endothelial cells and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- Cox MA, Gomes B, Palmer K, Du K, Wiekowski M, Wilburn B, et al. The pyrimidinergic P2Y6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem Biophys Res Commun. 2005;330:467–473. doi: 10.1016/j.bbrc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Crauwels HM, Van Hove CE, Holvoet P, Herman AG, Bult H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc Res. 2003;59:189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Heilig M, Edvinsson L. Tyrphostin inhibition of ATP-stimulated DNA synthesis, cell proliferation and fos-protein expression in vascular smooth muscle cells. Br J Pharmacol. 1996;118:1028–1034. doi: 10.1111/j.1476-5381.1996.tb15502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, et al. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Van Assche T, Verreth W, Fransen P, Mackness B, Mackness M, et al. Paraoxonase 1 gene transfer lowers vascular oxidative stress and improves vasomotor function in apolipoprotein E-deficient mice with pre-existing atherosclerosis. Br J Pharmacol. 2008;153:508–516. doi: 10.1038/sj.bjp.0707585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hechler B, Freund M, Ravanat C, Magnenat S, Cazenave JP, Cachet Reduced atherosclerotic lesions in P2YI/apolipoprotein E double-knockout mice: the contribution of non-hematopoietic-derived P2YI receptors. Circulatia. 2008;118:754–763. doi: 10.1161/CIRCULATIONAHA.108.788927. [DOI] [PubMed] [Google Scholar]
- Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, et al. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol Heart Circ Physiol. 2002;282:H784–H792. doi: 10.1152/ajpheart.00997.2000. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999;100:861–868. doi: 10.1161/01.cir.100.8.861. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- Jans DM, Martinet W, Fillet M, Kockx MM, Merville MP, Bult H, et al. Effect of non-steroidal anti-inflammatory drugs on amyloid-beta formation and macrophage activation after platelet phagocytosis. J Cardiovasc Pharmacol. 2004;43:462–470. doi: 10.1097/00005344-200403000-00019. [DOI] [PubMed] [Google Scholar]
- Jawien J, Csanyi G, Gajda M, Mateuszuk L, Lomnicka M, Korbut R, et al. Ticlopidine attenuates progression of atherosclerosis in apolipoprotein E and low density lipoprotein receptor double knockout mice. Eur J Pharmacol. 2007;556:129–135. doi: 10.1016/j.ejphar.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Kloen P, Jennings CL, Gebhardt MC, Springfield DS, Mankin HJ. Suramin inhibits growth and transforming growth factor-beta 1 (TGF-beta 1) binding in osteosarcoma cell lines. Eur J Cancer. 1994;30A:678–682. doi: 10.1016/0959-8049(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens E, Daemen M, Kockx M, Doevendans P, Hofker M, Havekes L, et al. Atherosclerosis in APOE*3-Leiden transgenic mice: from proliferative to atheromatous stage. Circulation. 1999;99:276–283. doi: 10.1161/01.cir.99.2.276. [DOI] [PubMed] [Google Scholar]
- Mamedova LK, Joshi BV, Gao ZG, von Kugelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- Middaugh CR, Mach H, Burke CJ, Volkin DB, Dabora JM, Tsai PK, et al. Nature of the interaction of growth factors with suramin. Biochemistry. 1992;31:9016–9024. doi: 10.1021/bi00152a044. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, et al. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci. 2006;79:525–531. doi: 10.1016/j.lfs.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2001;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Schulz C, Konrad I, Sauer S, Orschiedt L, Koellnberger M, Lorenz R, et al. Effect of chronic treatment with acetylsalicylic acid and clopidogrel on atheroprogression and atherothrombosis in ApoE-deficient mice in vivo. Thromb Haemost. 2008;99:190–195. doi: 10.1160/TH07-03-0235. [DOI] [PubMed] [Google Scholar]
- Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, et al. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, et al. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, et al. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278:24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- Sharma V, Parsons H, Allard MF, McNeill JH. Metoprolol increases the expression of beta(3)-adrenoceptors in the diabetic heart: effects on nitric oxide signaling and forkhead transcription factor-3. Eur J Pharmacol. 2008;595:44–51. doi: 10.1016/j.ejphar.2008.07.042. [DOI] [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M. Cloning, upregulation, and mitogenic pole of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol. 2004;66:1265–1274. doi: 10.1124/mol.104.002642. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Tonetti M, Sturla L, Bistolfi T, Benatti U, De Flora A. Extracellular ATP potentiates nitric oxide synthase expression induced by lipopolysaccharide in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1994;203:430–435. doi: 10.1006/bbrc.1994.2200. [DOI] [PubMed] [Google Scholar]
- Van Der Giet M, Giebing G, Tolle M, Schmidt S. The role of P2Y receptors in the control of blood pressure. Drug News Perspect. 2002;15:640–646. doi: 10.1358/dnp.2002.15.10.740237. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warny M, Aboudola S, Robson SC, Sevigny J, Communi D, Soltoff SP, et al. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]


