Abstract
Background and purpose:
Okadaic acid (OA) and microcystins (MCs) are structurally different toxins with the same mechanism of action, inhibition of serine/threonine protein phosphatases (PPs). Methyl okadaate (MeOk), a methyl ester derivative of OA, was considered almost inactive due to its weak inhibition of PP1 and PP2A. Here, we have investigated the activity and potency of MeOk in hepatic cells in comparison with that of OA and MCs.
Experimental approach:
We tested the effects of MeOK, OA and microcystin-leucine and arginine (MC-LR) on the metabolic rate, the actin cytoskeleton and glucose uptake in a rat hepatocyte cell line (Clone 9) and in primary cultured rat hepatocytes. PP2A was assayed to compare OA and MeOk activity.
Key results:
MeOk disrupted the actin cytoskeleton and depressed the metabolic rate of both types of rat hepatocytes, being six-fold less potent than OA in Clone 9 cells but nearly six-fold more potent in primary cultured hepatocytes. However, unlike OA, MeOk did not change glucose uptake in these cells, suggesting a weak inhibition of PP2A, as confirmed in direct assays of PP2A activity.
Conclusions and implications:
Although MeOk was originally described as a weakly bioactive molecule, it clearly depressed the metabolic rate and disrupted the cytoskeleton in primary and immortalized rat hepatocytes. Furthermore, MeOk affected primary hepatocytes at much lower concentrations than those affecting immortalized cells. These effects were unrelated to PP2A inhibition. Our results suggest the risk to public health from MeOk in foodstuffs should be re-evaluated.
Keywords: actin cytoskeleton, glucose uptake kinetics, metabolic rate, methyl okadaate, microcystin, okadaic acid, phycotoxins, protein phosphatase and rat hepatocytes
Introduction
Many natural toxins such as okadaic acid (OA) potently inhibit the catalytic activity of serine/threonine protein phosphatases (PPs), of which PP1 and PP2A are the most abundant in cells. Both PPs have a wide spectrum of activity being able to dephosphorylate a large number of substrates in vitro (Fernandez et al., 2002). The list of PPs inhibitors includes a structurally diverse group of compounds: polyether fatty acid substances similar to okadaic acid (OA), cyclic peptides like microcystins (MCs) and nodularins, terpenoids such as cantharidin and thyrsiferyl 23-acetate and polyketides, such as tautomycin and calyculin A (Fernandez et al., 2002).
OA is the best representative of the diarrheic shellfish poisoning (DSP) group of toxins. Because OA is a lipophilic compound and inhibits PP, it has been an invaluable tool in studies aimed at elucidating pathways modulating cellular processes, such as glucose uptake (Haystead et al., 1990; Corvera et al., 1991; Tanti et al., 1991; Yoo-Warren et al., 1993; Leira et al., 2002; Louzao et al., 2003; Louzao et al., 2005).
Methyl okadaate (MeOk) is a methyl ester derivative of the OA, artificially produced in the laboratory, but also found in shellfish, and even in naturally collected or cultured dinoflagellates from the genera Prorocentrum and Dinophysis (Hu et al., 1992; Vale and Sampayo, 1999; Suzuki et al., 2004; Suarez-Gomez et al., 2005). Usually, MeOk has been considered as an inactive molecule because of its low activity in inhibiting PPs (Nishiwaki et al., 1990; Takai et al., 1992). However, recent studies indicated that MeOk induced reorganization of the actin cytoskeleton of human neuroblastoma cells (Vilarino et al., 2008), and although the potency of MeOk was lower than that of OA, its mechanism of action was unknown.
There are few toxicity studies of MeOk, as it was considered to be inactive, particularly in gastrointestinal cells. We have used hepatocytes here because the liver is clearly connected to the enteric system (Houten et al., 2006). Studying the effect of this toxin on hepatic cells is thus fundamental to a better understanding of the pharmacokinetics and pharmacodynamics of MeOk (Gomez-Lechon et al., 2004). In addition, hepatic cells have some characteristics very appropriate to our study, such as glucose uptake and the marked actin cytoskeletal structure (Oda et al., 2008).
Hepatic–enteric cell models are particularly useful in studying the effect of hepatotoxins and DSP-related toxins (Louzao et al., 2003; Ares et al., 2005; Espina et al., 2008). At this cellular level, primary cultured cells isolated from rodents' liver are common in vitro models; also immortalized hepatocytes offer a renewable source of hepatocytes. Differences in the potency of toxin effects between immortalized and primary cultured cellular models have been described recently (Stournaras et al., 1996; Jordan and Wilson, 1998; Espina et al., 2008), and this aspect could have consequences for the regulation of toxins in food or for important biomedical applications (McNabb, 2008).
The goal of this work was to investigate the effects triggered by MeOk in immortalized and primary cultured rat hepatocytes, relative to the effects of its parent compound OA. We also tested microcystin-leucine and arginine (MC-LR) as another type of potent PPs inhibitor, very different structurally and in origin, from the group of OA toxins. In our study, we evaluated three cellular parameters: metabolic rate, actin cytoskeleton and glucose uptake kinetics. Additionally, PP2A inhibition assays were carried out to compare directly OA and MeOk activity on this enzyme.
Methods
Cell culture
Clone 9 rat hepatocytes
Rat hepatocytes from the cell line Clone 9 (ECACC N° 88072203) were grown and cultured as previously described (Louzao et al., 2008).
Isolation and culture of rat primary hepatocytes
All animal care and experimental procedures complied with our institutional guidelines for animal care and were approved by the Bioethical Committee of the University of Santiago de Compostela. Rat hepatocytes were obtained from adult male Sprague-Dawley rats. Isolation was performed by a two-step perfusion procedure with collagenase type IV. Viability was tested by Trypan blue exclusion and was always ≥85%. Hepatocytes were seeded on coverslips placed in 8-well sterile plates for attachment as previously described (Barbini et al., 2006).
BE(2)-M17 neuroblastoma cells
Human neuroblastoma cells BE(2)-M17 were routinely grown and cultured as described in Espina et al. (2009).
F and G-actin cytoskeleton staining
After 3 h of incubation with toxins in the culture medium, cells were washed with phosphate-buffered saline and stained for F- and G-actin with Oregon Green 514 phalloidin and Texas Red DNase I, respectively, as described in Espina et al. (2008). Control cells were incubated in the same conditions with the toxin vehicle, dimethylsulphoxide. Percentage of the vehicle added to the cells never exceeded 0.1% (volume/volume) of the incubation media.
Confocal microscopy for visualizing morphology and actin cytoskeleton distribution and measuring
Confocal imaging was carried out with a 40× oil immersion objective of a Nikon Eclipse TE2000-E inverted microscope attached to the C1 laser confocal system (EZC1 V.2.20 software; Nikon Instruments Europe B.V., Amstelveen, the Netherlands). Fluorescent images and measurements were taken as described in Espina et al. (2008).
Results are presented as the percentage of the mean value ± standard error of the mean (SEM) of fluorescence emitted by cells treated with toxins, versus controls, with n≥ 3.
Assays of glucose uptake
Fluorescence of hepatic cells loaded with 500 µM 2-{N-[7-nitrobenz-2-oxa-1, 3-diazol 4-yl] amino}-2-deoxyglucose (2-NBDG) and treated with toxins (1.5 µM OA, 1.5 µM MC-LR, 1.5 µM MeOk, 15 µM MeOk) was measured for up to 30 min. The time course imaging was carried out using the Nikon Eclipse TE2000-E inverted microscope attached to the C1 laser confocal system described above.
Results are presented as the fluorescence mean value ± standard error of the mean (SEM) of hepatic cells treated with toxins versus controls with n≥ 3.
Metabolic rate assay
Cells (40 000 per well) were seeded on to 96-well plates. After 24 h, OA, MeOk, MC-LR or vehicle were added to the cells. Then, a 1:10 dilution of Alamar Blue was added. Fluorescence was measured by using a microplate fluorescence reader FL600 (Bio-Tek/Vermont, USA) after 24 h of incubation with the toxins. Results are presented as percentage of fluorescence versus control; mean values ± SEM, with n≥ 3.
PP2A inhibition assay
PP2A inhibition was assayed by using the Toxiline-DSP Test for detection of diarrheic shellfish toxins (DSP), based in the method described by Vieytes et al. (1997).
Statistical analysis
Results were analysed using the Students's t-test for paired data where appropriate. A probability level of ≤0.05 was set to indicate statistical significance. Drug/molecular target nomenclature follows Alexander et al. (2008).
Materials
OA was from LC Laboratories (Woburn, MA, USA). MeOk was kindly donated by Dr Takeshi Yasumoto and MC-LR was from Sigma (Madrid-Spain). The fluorescent dye Oregon Green 514 phalloidin for F-actin labeling and Texas Red DNase I for G-actin labelling were from Molecular Probes (Leiden, The Netherlands). Alamar Blue was purchased from Biosource (Madrid, Spain). The fluorescent D-glucose derivative, 2-NBDG was from Molecular Probes (Leiden, Netherlands). All other chemicals were reagent grade and purchased from Sigma-Aldrich (Madrid, Spain) or Panreac (Barcelona, Spain).
Results
Effects on cell lines
Our first goal was to study possible toxin-induced changes in the metabolic rate of Clone 9 cells by assays with Alamar Blue (Figure 1). OA and MeOk had a dose-dependent effect with IC50 values as shown in Figure 1A and C respectively. However, no metabolic effects were observed with MC-LR, a PPs inhibitor structurally different from OA and MeOk (Figure 1B). The effect of OA and MeOk was also compared in another cellular model; a human non-epithelial and excitable cell line: BE (2)-M17 neuroblastoma cells. The metabolic rate of neuroblastoma cells after 24 h of incubation with 1.5 µM OA was depressed to the same extent as incubation with 15 µM MeOk, in relation with the controls (Figure 1D).
Figure 1.
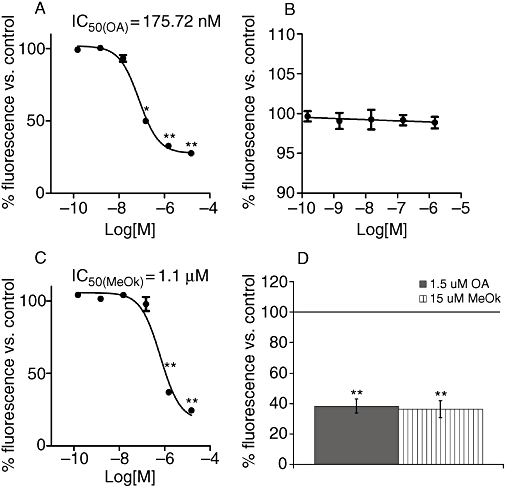
Effects of OA (A), MC-LR (B) and MeOk (C) on metabolic rate in Clone 9 hepatocytes after 24 h incubation. 1D shows the effect of 1.5 µM OA or 15 µM MeOk on the metabolic rate of BE(2)-M17 human neuroblastoma cells after 24 h of treatment. Results are presented as percentage of Alamar Blue fluorescence relative to control cells (100%); mean values ± SEM, with n= 3. *P < 0.05; **P < 0.01, significantly different from control; Student's t-test. MC-LR, microcystin-leucine and arginine; MeOk, methyl okadaate; OA, okadaic acid.
We found that MeOk was an active compound decreasing metabolic rate in Clone 9 cells, but it was almost 10 times less potent than OA. Therefore, we chose 15 µM MeOk and 1.5 µM OA in order to compare their effect on the actin cytoskeleton. Cells incubated with 1.5 µM OA showed a clear modification in cell morphology. They changed from well-extended to rounded shapes with marked blebbing effects, and many of the cells detached from the substrate. F-actin disappeared from the projections and concentrated in the periphery of the cells, showing a different actin cytoskeleton distribution (Figure 2C and D). No effect was found with 1.5 µM MC-LR (Figure 2E and F). Unlike OA, 1.5 µM MeOk was unable to clearly modify the cell shape or actin distribution;; only a slight rounding effect could be observed in few Clone 9 cells (Figure 2G and H). Nevertheless, 15 µM MeOk induced changes in cell morphology and actin cytoskeleton distribution that were very similar to those triggered by 1.5 µM OA (Figure 2I and J).
Figure 2.
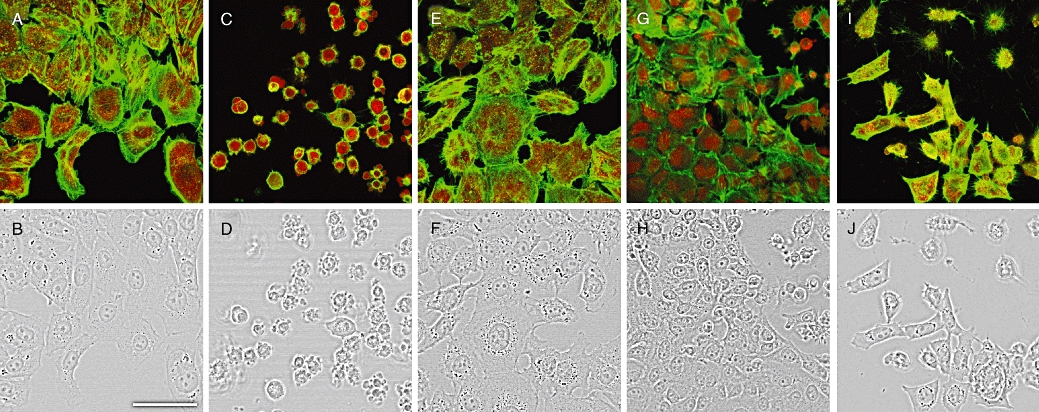
Confocal imaging of F- and G-actin double-staining of Clone 9 rat hepatocytes showing fluorescence (upper row). Transmission pictures of the same cells are presented in the lower row. F and G-actin were labelled with Oregon Green 514 phalloidin and Texas Red DNase I respectively. Figure 2A and B show photographs from control cells, while Figure 2C and D are from cells treated with 1.5 µM OA, Figure 2E and F with 1.5 µM MC-LR, Figure 2G and H with 1.5 µM MeOk and (I) and (J) with 15 µM MeOk. Images are representative of 3 independent experiments. Images are 2× zoom magnifications. Scale bar = 50 µm. MC-LR, microcystin-leucine and arginine; MeOk, methyl okadaate; OA, okadaic acid.
OA and related compounds are known to be involved in the regulation of cellular processes such as glucose transport in hepatic cells. Assays of the glucose uptake kinetics showed an increased 2-NBDG uptake of about 25–30% in cells incubated with OA (Figure 3A). However, neither 1.5 µM nor 15 µM MeOk (Figure 3C and D), nor 1.5 µM MC-LR affected glucose uptake (Figure 3B).
Figure 3.
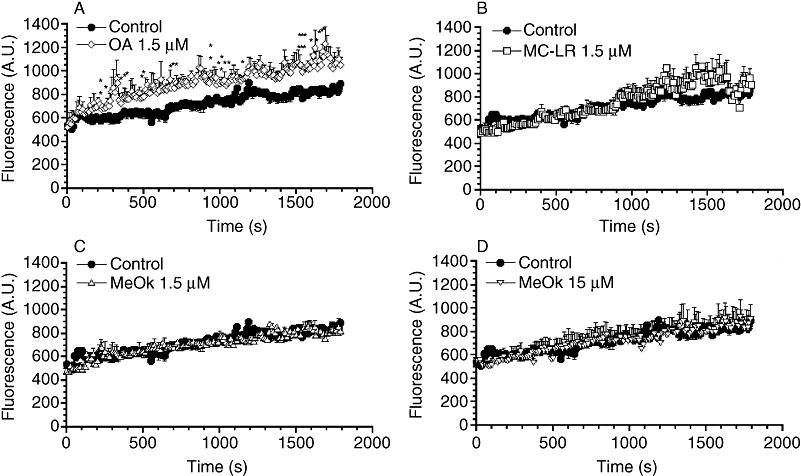
Time course of the effect of OA, MC-LR and MeOk on glucose uptake in Clone 9 hepatocytes. 2-NBDG was added to cells at a final concentration of 500 µM, and fluorescence inside the cells was measured for 30 min, every 5 s. Effect of 1.5 µM OA (A), 1.5 µM MC-LR (B), 1.5 µM MeOk (C), 15 µM MeOk (D) or vehicle (control) was evaluated. Results are presented as mean ± SEM of n= 4 experiments. *P < 0.05; **P < 0.01, significantly different from control; Student's t-test. 2-NBDG, 2-{N-[7-nitrobenz-2-oxa-1, 3-diazol 4-yl] amino}-2-deoxyglucose; A.U., arbitrary units; MC-LR, microcystin-leucine and arginine; MeOk, methyl okadaate; OA, okadaic acid.
Effects on primary cultures of hepatocytes
In order to compare the effects of these drugs between immortalized and primary cultured hepatocytes, the same kind of experiments were carried out with primary cultured rat hepatocytes. Assays revealed a dose-dependent decrease of metabolic rate, induced by all the three toxins tested: OA, MeOk and MC-LR. However, the calculated IC50 with these cells for OA was about six-fold higher than that for MeOk (Figure 4A and C respectively). Moreover, although 150 nM MC-LR did not affect the metabolic rate of primary hepatocytes, higher concentrations (1 µM) were inhibitory (Figure 4B).
Figure 4.
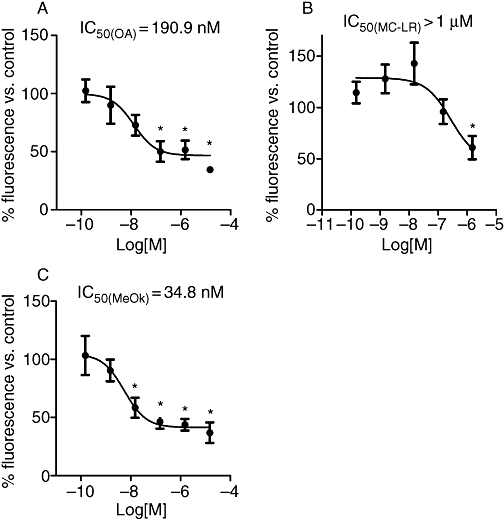
Effects of OA (A), MC-LR (B) and MeOk (C) on metabolic rate in primary cultured rat hepatocytes after 24 h incubation. Results are presented as percentage of Alamar Blue fluorescence relative to control cells (100%); mean values ± SEM, with n= 3. *P < 0.05; **P < 0.01, significantly different from control; Student's t-test. MC-LR, microcystin-leucine and arginine; MeOk, methyl okadaate; OA, okadaic acid.
The morphological effects in primary hepatocytes showed, surprisingly, that 15 µM MeOk was more potent in disrupting the actin cytoskeleton and morphology than 1.5 µM OA (Figure 5C, D, I and J). Many primary hepatocytes detached from substrate when they were treated with 15 µM MeOk. But even more unexpected was the fact that 1.5 µM MeOk triggered the same or even more effects than those induced by the identical concentration of OA (Figure 5C, D, G and H). Most hepatocytes detached from the coverslips, and cells that remained attached were rounded and their prolongations retracted. MC-LR (1.5 µM) also induced detachment and rounding of hepatocytes (Figure 5E and F). F- and G-actin showed a changed distribution in cells treated with all the three toxins. In cells treated with any of the three toxins, the great majority of the F-actin remained in the cell periphery, but the thick F-actin borders of the control hepatocytes (Figure 5A) became thinner. Furthermore, many protrusions rich in G-actin appeared as a part of the blebbing effect (Figure 5C, E, G and I).
Figure 5.
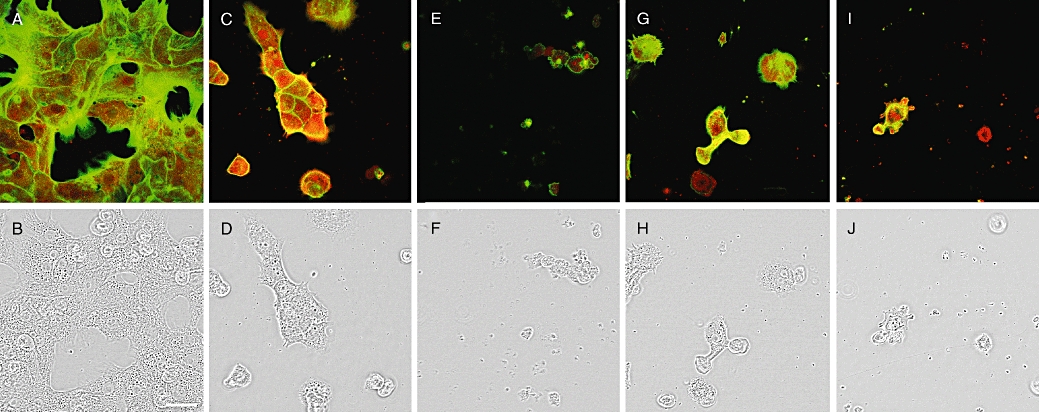
Confocal imaging of F- and G-actin double staining of primary cultured rat hepatocytes showing fluorescence (upper row). Transmission pictures of the same cells are presented in the lower row. F and G-actin were labelled with Oregon Green 514 phalloidin and Texas Red DNase I respectively. Figure 5A and B show photographs from control cells, while Figure 5C and D are from cells treated with 1.5 µM OA, Figure 5E and F with 1.5 µM MC-LR, Figure 5G and H with 1.5 µM MeOk, and Figure 5I and J with 15 µM MeOk. Images are representative of three independent experiments. Scale bar = 50 µm. MC-LR, microcystin-leucine and arginine; MeOk, methyl okadaate; OA, okadaic acid.
Assay of glucose uptake showed that 1.5 µM OA, as well as MC-LR, were able to increase sugar uptake by about 20–30% in primary cultured hepatocytes (Figure 6A and B). However, the pattern of glucose uptake did not change with 1.5 µM MeOk (Figure 6C). Increasing the MeOk concentration up to 15 µM caused the primary hepatocytes to detach from substrate after the first minutes of the experiment, preventing a reliable response (data not shown).
Figure 6.
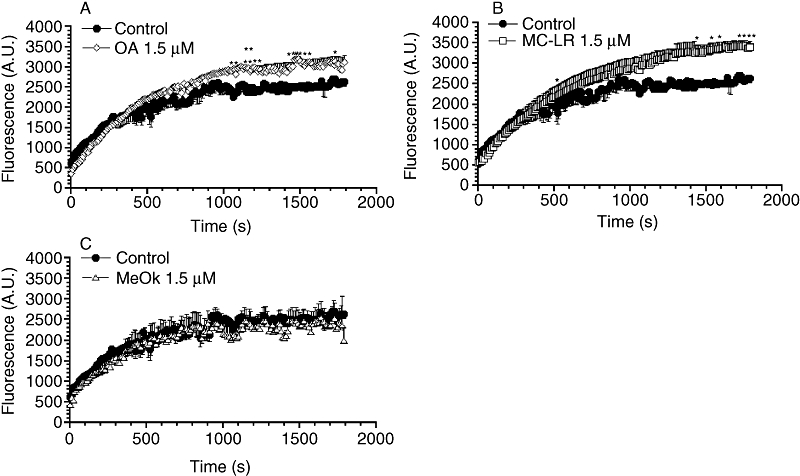
Time course of the effect of OA, MC-LR and MeOk on glucose uptake in primary cultured rat hepatocytes. 2-NBDG was added to cells at a final concentration of 500 µM, and fluorescence in the cells was measured for 30 min every 5 s. Effect of 1.5 µM OA (A), 1.5 µM MC-LR (B), 1.5 µM MeOk (C) or vehicle (control) was evaluated. Results are presented as mean ± SEM of n= 3 experiments. *P < 0.05; **P < 0.01, significantly different from control; Student's t-test. 2-NBDG, 2-{N-[7-nitrobenz-2-oxa-1, 3-diazol 4-yl] amino}-2-deoxyglucose; A.U., arbitrary units; MC-LR, microcystin-leucine and arginine; MeOk, methyl okadaate; OA, okadaic acid.
Finally, PP2A inhibition assays were performed in the presence of increasing concentrations of OA or MeOk. The results showed that MeOk was a poor inhibitor of PP2A, requiring about 500-fold higher concentrations than that of OA to show the same percentage of PP2A inhibition (Figure 7).
Figure 7.

Inhibition of PP2A by OA and MeOk measured using the Toxiline-DSP Test for detection of DSP. Results are expressed as percentage of PP2A inhibition; mean ± SEM of n = 3. DSP, diarrheic shellfish poisoning; MeOk, methyl okadaate; OA, okadaic acid; PP2A, protein phosphatase-2A.
Discussion and conclusions
Micromolar concentrations of OA induce cell retraction and rounding due to the strong reorganization of the F-actin network in IEC-6 cells, a transformed cell line from the small intestine (Fiorentini et al., 1996). Other studies described alterations of cell microfilaments and even depolymerization (Kreienbuhl et al., 1992; Berven et al., 2001; Strnad et al., 2001; Baba et al., 2003; Cabado et al., 2004). In our study, structure and organization of cell morphology and the actin cytoskeleton of Clone 9 cells and primary cultured rat hepatocytes were affected by OA. Downey et al. (1993), who described OA-induced actin assembly in neutrophils, observed similar alterations. In accordance with the previous work of Vilarino et al. (2008) in neuroblastoma cells, an almost 10-fold higher concentration of MeOk than of OA was needed to induce a similar disruption in the actin cytoskeleton and morphology of Clone 9 hepatocytes. However, primary hepatocytes were even more affected by MeOk than by OA. Previously, Arteche et al. (1997) had reported that MeOk presented the same activity as the free acid, OA, in inducing a transient contraction in the oestrogen-primed rat uterus.
In our study, OA decreased the metabolic rate of Clone 9 rat hepatocytes and human neuroblastoma cells. MeOk also depressed the metabolism but with a lesser potency, as the IC50 for OA was more than sixfold lower than that for MeOk in Clone 9 cells. This potency difference is similar to that reported for MeOk-triggered apoptotic death in GH3 pituitary cells and its inhibition of secretion in rat mucosal mast cells, in each case being less potent than OA (Ritz et al., 1997; Ludowyke et al., 1998). On the other hand, we found that MeOk was more potent than OA in decreasing the metabolic activity of primary hepatocytes, which would not support the simple proposition that MeOk could be de-esterified in the cells and thus be converted into OA. Esterification is a common strategy to obtain cell-permeable compounds that are de-esterified intracellularly by esterases. Primary hepatocytes were therefore more sensitive to MeOk itself, and this high potency of MeOk was clearly not due to the weak inhibition of PP2A that we found.
OA and MC-LR are potent inhibitors of Ser/Thr protein phosphatases (PPs). Structural differences in these toxins determine that after oral consumption, MC-LR is specifically hepatotoxic, while OA triggers diarrheic shellfish poisoning (DSP). MC-LR is a peptidic, and thus hydrophilic, molecule requiring an active transport to enter the cell. Previous studies demonstrated that MC-LR enters cells using the bile acid transporters only expressed in a few types of cells, but especially in liver (Runnegar et al., 1991; Dawson, 1998). In our experiments, Clone 9 cells seemed to be insensitive to MC-LR. Clone 9 hepatocytes are an epithelial cell type that grows in monolayer with contact inhibition, as do hepatocytes in liver, and they are undifferentiated and unpolarized (Weinstein et al., 1975). So, Clone 9 hepatocytes may also lack some of the characteristics of mature hepatocytes, such as specialized membrane transporters. This is probably the reason why MC-LR was unable to trigger any effect on this cell type. Furthermore, hepatocytes lose the ability to express bile acid transporters in long-term cultures (Torchia et al., 1996). So we used 24 h primary cultures of rat hepatocytes to avoid the loss of relevant characteristics. In fact, primary hepatocytes appeared rounded and retracted, their F-actin distribution changed, and the metabolic rate fell after the treatment with MC-LR, contrary to what happened with Clone 9 cells.
The PP1 and PP2A are closely involved in D-glucose metabolism (Hubbard and Cohen, 1989; Ugi et al., 2004), and OA and MC-LR have been used to modify the uptake and accumulation of D-glucose inside cells (Corvera et al., 1991; Leira et al., 2002; Louzao et al., 2003; Louzao et al., 2005). Assay of the effects on glucose uptake exerted by OA, MeOk and MC-LR was a measure of their different inhibition of PPs in Clone 9 cells and primary hepatocytes. The lack of effect of MC-LR on glucose uptake in Clone 9 cells could be explained by the fact that this toxin was unable to enter the cells. Unlike OA, MeOk induced no change in glucose uptake, implying a weak inhibition of PP1 or PP2A in both cellular models. However, MeOk was a potent disruptor of the actin cytoskeleton of Clone 9 and primary hepatocytes, indicating that this toxin might inhibit another PP, not involved in glucose intake. This is a serious possibility, because OA was reported not only to inhibit PP1 and PP2A, but also PP4, and less efficiently, PP5 (Honkanen et al., 1994; Hastie and Cohen, 1998; Fernandez et al., 2002; McCluskey et al., 2002). This hypothesis agrees with previous reports that MeOk inhibited PP3 more efficiently than PP1 and PP2A (Honkanen et al., 1994). Another explanation could be that OA and MeOk have targets other than PPs, for their effect on the actin cytoskeleton. In this context, it has been reported that smooth muscle contraction caused by OA is due to a direct effect on the ATP-dependent interaction between actin and myosin, and this latter effect is independent of the inhibition of PPs (Hayakawa et al., 1991).
The effects of OA, MeOk and MC-LR on the actin cytoskeleton of rat hepatocytes are closer than their activities as inhibitors of PPs. These three toxins induced a marked redistribution of the F-actin network concomitant with cell detachment, as described by Kim et al. (2003) in SH-EP human neuroblastoma cells treated with OA. However, the cells did not show the characteristic F-actin punctuated distribution, consequence of the treatment with other natural compounds that disorganize the actin cytoskeleton, for instance, pectenotoxins. These toxins disrupt the actin cytoskeleton of many kinds of cells, depolymerizing F-actin and preventing its polymerization (Ares et al., 2005; Ares et al., 2007; Espina and Rubiolo, 2008;Espina et al., 2008). It is not clear yet if the extensive reorganization of the actin cytoskeleton induced by OA is a direct consequence of its activity or if it is an indirect consequence of the normal apoptotic process. Rounding, detaching and blebbing effects were observed in rat hepatocytes in addition to the actin network reorganization when treated with OA, MeOk or MC-LR. These morphological changes are typical of apoptosis in mammalian cells (Boe et al., 1991).
In summary, this is the first study that provides evidence of MeOk-induced damage in hepatocytes. In addition, the most important and new findings in our work are as follows. Unlike OA, MeOk did not induce any change in glucose uptake kinetics in normal or in immortalized rat hepatocytes, indicating a weak inhibition of PP2A, as later confirmed by direct assay of PP activity. Also, MeOk disrupted the actin cytoskeleton and decreased the metabolic rate of normal and immortalized rat hepatocytes. The potency of MeOk in causing these effects depended on the cell type, with greater potency in normal than in immortalized cells. Lastly, although OA was sixfold more potent than MeOk in immortalized hepatocytes, MeOk was nearly sixfold more potent than OA towards normal hepatocytes.
Therefore, MeOk activity against the actin cytoskeleton and the metabolic rate of rat hepatocytes was similar to that of OA and MC-LR, which are potent PP inhibitors. However, all these effects of MeOk were independent of inhibition of PP1 and PP2A. Interestingly, differences in the sensitivity and potency of various toxins between immortalized and primary cultured cell models have been described recently (Stournaras et al., 1996; Jordan and Wilson, 1998; Espina et al., 2008). This fact could have biomedical applications, for instance in developing new chemotherapeutic drugs, and consequences for the regulation of toxins in foodstuffs.
Acknowledgments
This work was funded with the following grants:
from Ministerio de Ciencia y Tecnología, Spain: AGL2006-08439/ALI, AGL2007-60946/ALI;
from Xunta de Galicia, Spain: GRC 30/2006, and PGIDT07CSA012261PR, PGDIT 07MMA006261PR, 2008/CP389 (EPITOX, Consellería de Innovación e Industria, programa IN.CI.TE.);
from EU VIth Frame Program: IP FOOD-CT-2004-06988 (BIOCOP), STREP FOOD-CT-2004-514055 (DETECTOX) and CRP 030270-2 (SPIES-DETOX); and
from EU VIIth Frame Program: 211326 – CP (CONffIDENCE); STC-CP2008-1-555612 (Atlantox).
Glossary
Abbreviations:
- DSP
diarrheic shellfish poisoning
- MC-LR
microcystin-leucine and arginine
- MeOk
methyl okadaate
- OA
okadaic acid
- PP1
protein phosphatase-1
- PP2A
protein phosphatase-2A
Conflict of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ares IR, Louzao MC, Espina B, Vieytes MR, Miles CO, Yasumoto T, et al. Lactone ring of pectenotoxins: a key factor for their activity on cytoskeletal dynamics. Cell Physiol Biochem. 2007;19:283–292. doi: 10.1159/000100647. [DOI] [PubMed] [Google Scholar]
- Ares IR, Louzao MC, Vieytes MR, Yasumoto T, Botana LM. Actin cytoskeleton of rabbit intestinal cells is a target for potent marine phycotoxins. J Exp Biol. 2005;208:4345–4354. doi: 10.1242/jeb.01897. [DOI] [PubMed] [Google Scholar]
- Arteche E, Strippoli G, Loirand G, Pacaud P, Candenas L, Molto JC, et al. An analysis of the mechanisms involved in the okadaic acid-induced contraction of the estrogen-primed rat uterus. J Pharmacol Exp Ther. 1997;282:201–207. [PubMed] [Google Scholar]
- Baba T, Udaka K, Terada N, Ueda H, Fujii Y, Ohno S, et al. Actin-rich spherical extrusion induced in okadaic acid-treated K562 cells by crosslinking of membrane microdomains. J Histochem Cytochem. 2003;51:245–252. doi: 10.1177/002215540305100213. [DOI] [PubMed] [Google Scholar]
- Barbini L, Gonzalez R, Dominguez F, Vega F. Apoptotic and proliferating hepatocytes differ in prothymosin alpha expression and cell localization. Mol Cell Biochem. 2006;291:83–91. doi: 10.1007/s11010-006-9200-0. [DOI] [PubMed] [Google Scholar]
- Berven G, Saetre F, Halvorsen K, Seglen PO. Effects of the diarrhetic shellfish toxin, okadaic acid, on cytoskeletal elements, viability and functionality of rat liver and intestinal cells. Toxicon. 2001;39:349–362. doi: 10.1016/s0041-0101(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Boe R, Gjertsen BT, Vintermyr OK, Houge G, Lanotte M, Doskeland SO. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991;195:237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- Cabado AG, Leira F, Vieytes MR, Vieites JM, Botana LM. Cytoskeletal disruption is the key factor that triggers apoptosis in okadaic acid-treated neuroblastoma cells. Arch Toxicol. 2004;78:74–85. doi: 10.1007/s00204-003-0505-4. [DOI] [PubMed] [Google Scholar]
- Corvera S, Jaspers S, Pasceri M. Acute inhibition of insulin-stimulated glucose transport by the phosphatase inhibitor, okadaic acid. J Biol Chem. 1991;266:9271–9275. [PubMed] [Google Scholar]
- Dawson RM. The toxicology of microcystins. Toxicon. 1998;36:953–962. doi: 10.1016/s0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Downey GP, Takai A, Zamel R, Grinstein S, Chan CK. Okadaic acid-induced actin assembly in neutrophils: role of protein phosphatases. J Cell Physiol. 1993;155:505–519. doi: 10.1002/jcp.1041550309. [DOI] [PubMed] [Google Scholar]
- Espina B, Rubiolo JA. Marine toxins and the cytoskeleton: pectenotoxins, unusual macrolides that disrupt actin. Febs J. 2008;275:6082–6088. doi: 10.1111/j.1742-4658.2008.06714.x. [DOI] [PubMed] [Google Scholar]
- Espina B, Louzao MC, Ares IR, Cagide E, Vieytes MR, Vega FV, et al. Cytoskeletal toxicity of pectenotoxins in hepatic cells. Br J Pharmacol. 2008;155:934–944. doi: 10.1038/bjp.2008.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espina B, Cagide E, Louzao MC, Fernandez MM, Vieytes MR, Katikou P, et al. Specific and dynamic detection of palytoxins by in vitro microplate assay with human neuroblastoma cells. Biosci Rep. 2009;29:13–23. doi: 10.1042/BSR20080080. [DOI] [PubMed] [Google Scholar]
- Fernandez JJ, Candenas ML, Souto ML, Trujillo MM, Norte M. Okadaic acid, useful tool for studying cellular processes. Curr Med Chem. 2002;9:229–262. doi: 10.2174/0929867023371247. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Matarrese P, Fattorossi A, Donelli G. Okadaic acid induces changes in the organization of F-actin in intestinal cells. Toxicon. 1996;34:937–945. doi: 10.1016/0041-0101(96)00025-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, Donato MT, Castell JV, Jover R. Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab. 2004;5:443–462. doi: 10.2174/1389200043335414. [DOI] [PubMed] [Google Scholar]
- Hastie CJ, Cohen PT. Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters. FEBS Lett. 1998;431:357–361. doi: 10.1016/s0014-5793(98)00775-3. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Okagaki T, Dobashi T, Sakanishi A, Kaneko K, Kohama K. Okadaic acid stimulates the ATP-dependent interaction between actin and myosin of smooth muscle via a direct effect on myosin. Biochem Biophys Res Commun. 1991;177:1155–1160. doi: 10.1016/0006-291x(91)90660-y. [DOI] [PubMed] [Google Scholar]
- Haystead TA, Weiel JE, Litchfield DW, Tsukitani Y, Fischer EH, Krebs EG. Okadaic acid mimics the action of insulin in stimulating protein kinase activity in isolated adipocytes. The role of protein phosphatase 2a in attenuation of the signal. J Biol Chem. 1990;265:16571–16580. [PubMed] [Google Scholar]
- Honkanen RE, Codispoti BA, Tse K, Boynton AL, Honkanan RE. Characterization of natural toxins with inhibitory activity against serine/threonine protein phosphatases. Toxicon. 1994;32:339–350. doi: 10.1016/0041-0101(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. Embo J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Marr J, Defreitas ASW, Quilliam MA, Walter JA, Wright JLC, et al. New diol esters isolated from cultures of the dinoflagellates Prorocentrum lima and Prorocentrum concavum. J natural products. 1992;55:1631–1637. [Google Scholar]
- Hubbard MJ, Cohen P. Regulation of protein phosphatase-1G from rabbit skeletal muscle. 2. Catalytic subunit translocation is a mechanism for reversible inhibition of activity toward glycogen-bound substrates. Eur J Biochem. 1989;186:711–716. doi: 10.1111/j.1432-1033.1989.tb15264.x. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- Kim B, Van Golen CM, Feldman EL. Degradation and dephosphorylation of focal adhesion kinase during okadaic acid-induced apoptosis in human neuroblastoma cells. Neoplasia. 2003;5:405–416. doi: 10.1016/s1476-5586(03)80043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienbuhl P, Keller H, Niggli V. Protein phosphatase inhibitors okadaic acid and calyculin A alter cell shape and F-actin distribution and inhibit stimulus-dependent increases in cytoskeletal actin of human neutrophils. Blood. 1992;80:2911–2919. [PubMed] [Google Scholar]
- Leira F, Louzao MC, Vieites JM, Botana LM, Vieytes MR. Fluorescent microplate cell assay to measure uptake and metabolism of glucose in normal human lung fibroblasts. Toxicol In Vitro. 2002;16:267–273. doi: 10.1016/s0887-2333(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Louzao MC, Vieytes MR, Fontal O, Botana LM. Glucose uptake in enterocytes: a test for molecular targets of okadaic acid. J Recept Signal Transduct Res. 2003;23:211–224. doi: 10.1081/rrs-120025206. [DOI] [PubMed] [Google Scholar]
- Louzao MC, Vieytes MR, Botana LM. Effect of okadaic acid on glucose regulation. Mini Rev Med Chem. 2005;5:207–215. doi: 10.2174/1389557053402747. [DOI] [PubMed] [Google Scholar]
- Louzao MC, Espina B, Vieytes MR, Vega FV, Rubiolo JA, Baba O, et al. Fluorescent glycogen' formation with sensibility for in vivo and in vitro detection. Glycoconj J. 2008;25:503–510. doi: 10.1007/s10719-007-9075-7. [DOI] [PubMed] [Google Scholar]
- Ludowyke RI, Warton K, Scurr LL. Inhibition of antigen and calcium ionophore induced secretion from RBL-2H3 cells by phosphatase inhibitors. Cell Biol Int. 1998;22:855–865. doi: 10.1006/cbir.1998.0332. [DOI] [PubMed] [Google Scholar]
- McCluskey A, Sim AT, Sakoff JA. Serine-threonine protein phosphatase inhibitors: development of potential therapeutic strategies. J Med Chem. 2002;45:1151–1175. doi: 10.1021/jm010066k. [DOI] [PubMed] [Google Scholar]
- McNabb P. Chemistry, metabolism, and chemical analysis of okadaic acid group of toxins. In: Botana LM, editor. Seafood and Freshwater Toxins. Pharmacology Physiology and Detection. Boca Raton, FL: CRC Press; 2008. pp. 209–228. Taylor & Francis Group, LLC. [Google Scholar]
- Nishiwaki S, Fujiki H, Suganuma M, Furuya-Suguri H, Matsushima R, Iida Y, et al. Structure-activity relationship within a series of okadaic acid derivatives. Carcinogenesis. 1990;11:1837–1841. doi: 10.1093/carcin/11.10.1837. [DOI] [PubMed] [Google Scholar]
- Oda H, Yoshida Y, Kawamura A, Kakinuma A. Cell shape, cell-cell contact, cell-extracellular matrix contact and cell polarity are all required for the maximum induction of CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem Pharmacol. 2008;75:1209–1217. doi: 10.1016/j.bcp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Ritz V, Marwitz J, Richter E, Ziemann C, Quentin I, Steinfelder HJ. Characterization of two pituitary GH3 cell sublines partially resistant to apoptosis induction by okadaic acid. Biochem Pharmacol. 1997;54:967–971. doi: 10.1016/s0006-2952(97)00397-3. [DOI] [PubMed] [Google Scholar]
- Runnegar MT, Gerdes RG, Falconer IR. The uptake of the cyanobacterial hepatotoxin microcystin by isolated rat hepatocytes. Toxicon. 1991;29:43–51. doi: 10.1016/0041-0101(91)90038-s. [DOI] [PubMed] [Google Scholar]
- Stournaras C, Stiakaki E, Koukouritaki SB, Theodoropoulos PA, Kalmanti M, Fostinis Y, et al. Altered actin polymerization dynamics in various malignant cell types: evidence for differential sensitivity to cytochalasin B. Biochem Pharmacol. 1996;52:1339–1346. doi: 10.1016/s0006-2952(96)00389-9. [DOI] [PubMed] [Google Scholar]
- Strnad P, Windoffer R, Leube RE. In vivo detection of cytokeratin filament network breakdown in cells treated with the phosphatase inhibitor okadaic acid. Cell Tissue Res. 2001;306:277–293. doi: 10.1007/s004410100455. [DOI] [PubMed] [Google Scholar]
- Suarez-Gomez B, Souto ML, Cruz PG, Fernandez JJ, Norte M. New targets in diarrhetic shellfish poisoning control. J Nat Prod. 2005;68:596–599. doi: 10.1021/np040183t. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Beuzenberg V, Mackenzie L, Quilliam MA. Discovery of okadaic acid esters in the toxic dinoflagellate Dinophysis acuta from New Zealand using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:1131–1138. doi: 10.1002/rcm.1455. [DOI] [PubMed] [Google Scholar]
- Takai A, Murata M, Torigoe K, Isobe M, Mieskes G, Yasumoto T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem J. 1992;284:539–544. doi: 10.1042/bj2840539. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti JF, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y. Effects of okadaic acid, an inhibitor of protein phosphatases-1 and -2A, on glucose transport and metabolism in skeletal muscle. J Biol Chem. 1991;266:2099–2103. [PubMed] [Google Scholar]
- Torchia EC, Shapiro RJ, Agellon LB. Reconstitution of bile acid transport in the rat hepatoma McArdle RH-7777 cell line. Hepatology. 1996;24:206–211. doi: 10.1002/hep.510240133. [DOI] [PubMed] [Google Scholar]
- Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, et al. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004;24:8778–8789. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale P, Sampayo MA. Esters of okadaic acid and dinophysistoxin-2 in Portuguese bivalves related to human poisonings. Toxicon. 1999;37:1109–1121. doi: 10.1016/s0041-0101(98)00247-5. [DOI] [PubMed] [Google Scholar]
- Vieytes MR, Fontal OI, Leira F, Baptista De Sousa JM, Botana LM. A fluorescent microplate assay for diarrheic shellfish toxins. Anal Biochem. 1997;248:258–264. doi: 10.1006/abio.1997.2127. [DOI] [PubMed] [Google Scholar]
- Vilarino N, Ares IR, Cagide E, Louzao MC, Vieytes MR, Yasumoto T, et al. Induction of actin cytoskeleton rearrangement by methyl okadaate – comparison with okadaic acid. Febs J. 2008;275:926–934. doi: 10.1111/j.1742-4658.2008.06256.x. [DOI] [PubMed] [Google Scholar]
- Weinstein IB, Orenstein JM, Gebert R, Kaighn ME, Stadler UC. Growth and structural properties of epithelial cell cultures established from normal rat liver and chemically induced hepatomas. Cancer Res. 1975;35:253–263. [PubMed] [Google Scholar]
- Yoo-Warren H, Kristie JA, Karmann G. Okadaic acid increases glucose uptake in 3T3-L1 adipocytes by stimulating glucose transporter 1 expression. Biochem Biophys Res Commun. 1993;195:310–316. doi: 10.1006/bbrc.1993.2046. [DOI] [PubMed] [Google Scholar]


