Abstract
Background and purpose:
Certain saponins synergize with antitumour drugs to enhance their efficacy, but the mechanisms underlying this synergy in vivo are not well studied. Here, we describe the distribution of Saponinum album (Spn) from Gypsophila paniculata L. in mice after subcutaneous injection.
Experimental approach:
The [3H]-labelled Spn used for in vivo experiments was biologically active, as it still increased the cytotoxicity of a chimeric toxin in vitro. Distribution of [3H]-Spn was measured in BALB/c mice, with or without subcutaneous tumours in the flank. Labelled Spn was subcutaneously injected in the neck, and samples of organs, blood, urine and tumour tissue were analysed for radioactivity, 5–240 min after the injection.
Key results:
The majority of [3H]-Spn distributed within 10 min throughout the entire animal, with high levels of radioactivity in the urine by 30 min. No preferential accumulation in tumour tissue or other organs was observed. In tumour-bearing mice, using a sequential combination of Spn (given first) and a chimeric toxin against the epidermal growth factor receptor, ErbB1, we tested two different pretreatment times for Spn. There was high antitumour efficacy (66% inhibition of tumour growth) after 60 min pre treatment with Spn, but no significant inhibition after 10 min pre treatment with Spn.
Conclusions and implications:
[3H]-Spn was rapidly cleared from the mice after s.c. injection, and antitumour synergy with chimeric toxins was correlated with the removal of excess Spn from tissues. Disposition of Spn in vivo may critically determine antitumour synergy with chimeric toxins.
Keywords: immunotoxin, chimeric toxin, saporin, epidermal growth factor, cancer therapy, Saponinum album
Introduction
Saponins are plant glycosides that contain a hydrophobic core structure, the aglycone, which is either a steroid or a triterpenoid (see Güçlü-Ustündağ and Mazza, 2007), and, usually, one or more sugar chains are covalently linked to the core structure. The saponins have several properties. Among them are permeabilization (Baumann et al., 2000), haemolysis (Chwalek et al., 2006), anticancer (Wang et al., 2006b; Zheng et al., 2006) and antimicrobial effects (Wina et al., 2005). In addition, saponins possess many inhibitory effects on tumour cells (see Bachran et al., 2008a). Besides growth inhibitory effects (Haridas et al., 2001; Liu et al., 2005; Wang et al., 2006a), induction of apoptosis (Haridas et al., 2001; Kim et al., 2008; Niu et al., 2008) and reduction of invasiveness and multidrug resistance (Yanamandra et al., 2003; Kwon et al., 2008), the enhancement of inhibitory effects of other cytostatic drugs by some saponins seems to be a promising option for tumour therapy. Several chemotherapeutic agents have been combined with saponins, and synergistic effects on tumour growth inhibition were observed both in vitro and in vivo (see Fuchs et al., 2009).
Immunotoxins (ITs) and chimeric toxins (CTs) are targeted drugs designed for more efficient tumour treatment (see Fuchs and Bachran, 2009). They consist of at least two functional parts, a cell-targeting moiety and a cytotoxic compound. The target structures for CTs and ITs include growth factor receptors, such as the epidermal growth factor (EGF) receptor, ErbB1 (receptor nomenclature follows Alexander et al., 2008), vascular endothelial growth factor receptor, interleukin (IL) receptors, such as IL-2 receptors and leucocyte antigens (CD22, CD30). In 1999, the American Food and Drug Administration approved the first CT named Ontak (a fusion of IL-2 and parts of diphtheria toxin) for the treatment of cutaneous T-cell lymphoma (Talpur et al., 2002; Frankel et al., 2003). In addition, two further chemically coupled ITs, Zevalin (Chinn et al., 1999; Witzig et al., 2002) and Mylotarg (Hamann et al., 2002), have been approved and several ITs are under investigation in clinical trials (Fuchs and Bachran, 2009). In spite of the progress with ITs for the successful treatment of cancer, several problems persist, among them (i) side effects of the toxic compound due to non-specific toxicity, which often results in diffuse endothelial damage and concomitant vascular leak syndrome (Soler-Rodriguez et al., 1993); and (ii) ineffective uptake of the drug into target cells. A few amino acids in the toxic moieties of ITs have been modified to reduce non-specific toxicity and vascular leak syndrome (Smallshaw et al., 2003; Onda et al., 2008). The insertion of a cleavable adapter was another approach to reduce side effects of ITs by separating the toxic moiety from the targeting moiety (Goyal and Batra, 2000; Fuchs et al., 2007). In our earlier work, we reported a synergistic mechanism between CT and Saponinum album (Spn), a saponin composite from Gypsophila paniculata L. We showed that a non-toxic, non-permeabilizing concentration of Spn synergistically enhanced the cytotoxicity of CTs up to more than 100 000-fold in cell culture experiments (Heisler et al., 2005; Bachran et al., 2006). Thus, saponins provide a hopeful option for improving CT uptake and tumour therapy.
We have here described the distribution of Spn in mice in order to make informed choices in the optimization of the combination of Spn and CT for targeted tumour therapy. The main obstacle to effective studies of the biodistribution of saponins is the generation of a labelled form that also retains biological activity. We applied a method based on modification of the sugar side chains with [3H]-labelled acetyl residues. Our results revealed that the majority of [3H]-Spn distributed within minutes throughout the body of the mouse and accumulated in the urine. However, a synergistic antitumour effect of a sequential combination of Spn and CT was observed 60 min after Spn injection but not after 10 min.
Methods
Expression and purification of the CTs
The CTs used in this study [SA2E and chimeric toxins containing saporin and EGF (SE)] consist of an N-terminal 6× His-tag, saporin-3 and human EGF as ligand. In SA2E, the toxin and EGF are linked by the molecular adapter (Keller et al., 2001). CTs were expressed for 3 h at 37°C in an isopropyl β-D-thiogalactopyranoside-inducible Escherichia coli-expression system, as described earlier (Bachran et al., 2008b). Cells were harvested by centrifugation (10 min, 4°C, 5000×g). Culture pellets resuspended in phosphate buffer solution (PBS) (150 mM NaCl, 8.3 mM Na2HPO4, 1.7 mM KH2PO4, pH 7.4) were supplemented with 20 mM imidazole and stored at −20°C.
The CTs were purified under native conditions by nickel-nitrilotriacetic acid agarose affinity chromatography, as described by Bachran et al. (2008b). The eluted CTs were concentrated in Amicon centrifugal devices (Millipore, Eschborn, Germany) and desalted against PBS by using PD10 columns (GE Healthcare, Munich, Germany). The CTs were estimated to be >90% pure, as evaluated by Coomassie staining after sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The concentration of purified proteins was estimated by using Advanced Protein Assay Kit (Cytoskeleton Inc., Denver, CO, USA). The quantity of full-length toxins was corrected after analyses by SDS-PAGE [12% (w/v) gel] under reducing conditions and comparison to protein standards.
Spn isolation and radio labelling with 3H-acetic anhydride
Spn for cytotoxicity assays and in vivo studies was obtained from Merck, Darmstadt, Germany, and was further characterized (Weng et al., 2008; 2009a;). The labelling with 3H-acetic anhydride (25 mCi) (Amersham, Munich, Germany) was performed as described elsewhere (Weng et al., 2009b). Specific, vehicle-free activity was determined by liquid scintillation and was calculated to be approximately 0.6 MBq·mg−13H-Spn (Weng et al., 2009b).
Cell culture experiments
Cell culture experiments were performed with modified murine NIH-3T3 Swiss mouse embryo fibroblasts (HER14 cells), stably transfected with human ErbB1. The model of tumour growth in mice used murine mammary adenocarcinoma TSA cells, stably transfected with human ErbB1 (TSA-ErbB1 cells). HER14 and TSA-ErbB1 cells were maintained in Dulbecco's Modified Eagle's Medium with Glutamax™-1 (PAA, Pasching, Austria) supplemented with 100 mL·L−1 fetal calf serum (Biochrom, Berlin, Germany), 100 U·mL−1 penicillin and 100 µg·mL−1 streptomycin and were cultivated at 37°C, 5% CO2 and 95% humidity.
The dose–response curves to [3H]-Spn alone and the combinations Spn/SE and [3H]-Spn/SE were obtained as described (Bachran et al., 2006). The effects of [3H]-Spn given alone were assessed over the concentration range of 1–30 µg·mL−1. The combined application of Spn/SE was performed with SE in the range of 0.03 to 10 nM while for [3H]-Spn/SE, SE was used in concentrations between 0.1 and 30 nM. The concentration of Spn and 3H-Spn was 1.5 µg·mL−1 each. The cells were cultivated for 48 h, as described above and subsequently analysed in a cytotoxicity assay, which is based on the cleavage of fluorescein diacetate by living cells (Bachran et al., 2006). The relative cell survival was calculated after blank subtraction (wells without cells) as the percentage of living cells in treated wells in relation to untreated cells (cells without toxin).
Animal experiments
All animal care and experimental procedures were carried out in compliance with German law under licence number G 0274/03 of the Regional Authorities for Health and Social Affairs LAGeSo of Berlin. We used 6- to 8-week-old (18–20 g) female BALB/c mice (Bundesinstitut für Risikobewertung, Berlin, Germany). After purchase, the mice were acclimatized for 1 week before the experiments were started. The mice were housed in groups of five in Plexiglas cages under standard conditions of temperature (21–24°C), humidity (40–60%) and 12-h, light–dark cycles, with light period starting at 08:00 h. Food and water were supplied ad libitum.
Distribution of 3H-Spn in vivo
For distribution studies of 3H-Spn, mice were injected s.c. in the right flank with 2.5 × 105 TSA-ErbB1 cells in 100 µL Dulbecco's PBS on day 1. After 2 weeks, tumours had a diameter of about 5 mm, and biodistribution studies were initiated. A further group of mice without tumours was used for comparison. Radioactivity of 40 kBq 3H-Spn (22.5 µg) were injected s.c. in 100 µL Dulbecco's PBS into the neck. After different time intervals (5, 10, 30, 60 and 240 min), the mice were killed by a 2-min exposure to isoflurane, and blood and urine were collected. A range of tissues (skin from the abdomen, skin from the injection site, kidney, spleen, liver, lung, heart, ovary/oviduct, muscle, brain) and the tumour were dissected and homogenized (3 × 10 s at 6800 rpm with 30 s pause) with 700 µL PBS in a Precellys homogenizer (Peqlab, Erlangen, Germany) with CK14 beads and CK28 beads. Aliquots (500 µL) of the homogenate were analysed by liquid scintillation counting in 15 mL Ultima Gold AB (Perkin-Elmer, Überlingen, Germany) liquid scintillation solution and measured for 10 min.
Analysis of 3H-Spn by high performance thin layer chromatography
Mouse urine was analysed by high-performance, thin-layer chromatography (HPTLC) with silica gel 60 glass plates and chloroform/methanol/water (70:50:10) as eluent. The urine of four mice [5, 10 (two samples) and 60 min] without tumour was used for these analyses. Plates were dried (100°C), and the radioactivity of the application spot and of the region where saponins are being detected was measured by liquid scintillation counting with Optiphase Supermix scintillation cocktail (Perkin-Elmer).
Tumour growth inhibition in vivo
Analyses of tumour inhibition in BALB/c mice were performed with syngeneic TSA-ErbB1 cells. HER14 cells are not of BALB/c origin and, therefore, not suitable for the mouse model. Nevertheless, HER14 cells were used for cell culture experiments here, so we could more readily compare them with our earlier results on the enhancement of cytotoxicity by Spn. For efficacy studies of the combination therapy, groups of 10 BALB/c mice were injected s.c. in the right flank with 1.25 × 105 TSA-ErbB1 cells in 100 µL Dulbecco's PBS on day 1. Treatment with Spn/SA2E started on day 6 to allow adhesion and moderate growth of the tumour cells. Tumour treatment was performed with the adapter-containing SA2E instead of SE because SA2E induces fewer side effects and is more effective in vivo (Fuchs et al., 2007). Two different treatment methods were analysed with six applications on days 6, 9, 13, 16, 20 and 23 with 30 µg (1670 µg·kg−1) Spn in 100 µL Dulbecco's PBS injected s.c. in the neck and 0.1 µg (5.6 µg·kg−1) SA2E in 100 µL Dulbecco's PBS injected (i) after 10 min or (ii) after 60 min s.c. in the right flank in the vicinity of the tumour. The controls were injected twice with 100 µL Dulbecco's PBS in the neck first and in the right flank after 10 min. Therapeutic efficacy was characterized by measuring tumour growth in vivo by using a calliper and by weighing the excised tumour after killing the mice on day 27. Only mice with growing tumours were used for the experiment. The volume of the tumour was calculated assuming a spheroid and calculated with the shape-modified formula (adjusted to the typical shape of the tumours) 3/5 ×π× a × b × c (a, b and c are the diameters of the tumour).
Data analysis
Data are shown as means ± SEM or SD, as shown in the text. The statistical analysis for determining P values was performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) by using the Mann–Whitney U-test. A P value < 0.05 was considered as statistically significant.
Results
Cytotoxicity of 3H-Spn and of the combination 3H-Spn/SE
Purification, testing of the enzymatic activity and determination of dose response curves are well established for the CTs (Heisler et al., 2002; 2003;). For all experiments, only those toxins that exhibited at least 90% purity (as evaluated by Coomassie staining) were used. The cytotoxicity of 3H-labelled Spn alone and of 3H-Spn in combination with SE, a CT against ErbB1, was analysed on HER14 cells (Figure 1). 3H-Spn displayed no cytotoxicity on HER14 cells at concentrations up to 30 µg·mL−1. For the combination 3H-Spn/SE, the amount of 3H-Spn was 1.5 µg·mL−1 in order to achieve comparable results to those obtained with unlabelled Spn that was generally used at this concentration determined to be optimal in previous experiments. The 50% growth inhibitory concentration (GI50) for SE was about 3 pM in the case of Spn/SE but 30 pM when 3H-Spn/SE was used. Although the potency of the combination was reduced by labelling the Spn, relative to the unlabelled form, the potency of SE was still considerably enhanced over that of SE alone (GI50 about 3000 pM; Figure 1B).
Figure 1.
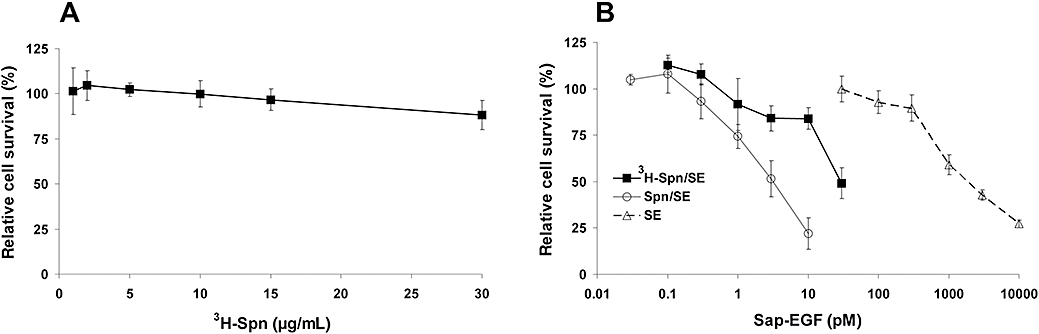
Cytotoxic effects of 3H-Spn alone and combined with SE on HER14 cells. The cells were incubated for 48 h (A) in the presence of varying concentrations of 3H-Spn and (B) with either 1.5 µg/mL 3H-Spn or unlabelled Spn, and varying concentrations of SE applied 5 min after Spn, or with SE alone. Living cells were quantified by their ability to cleave fluorescein diacetate. Relative cell survival was calculated as the number of living cells after treatment in relation to (A) untreated cells and (B) cells treated only with 3H-Spn, Spn without SE or untreated cells. Error bars indicate the SEM of n independent experiments performed in triplicate (n= 4 for 3H-Spn toxicity, n= 3 for 3H-Spn/SE and Spn/SE, and n= 5 for SE). SE, chimeric toxins containing saporin and epidermal growth factor; Spn, Saponinum album from Gypsophila paniculata L.
Biodistribution of 3H-Spn
For any effect of the presence of tumours on 3H-Spn distribution in vivo to be assessed, the investigations were performed in tumour-bearing mice and tumour-free mice (Figure 2). In both groups, the majority of the labelled Spn was found in the urine as soon as 30 min after injection (Figure 2A,C). After 5 min, the highest levels of radioactivity were detected at the injection site in the skin of the neck. However, 3H-Spn disappeared very quickly from the injection site, dispersed through the whole body and reached the urine. At 30 min after injection, radioactivity at the injection site was drastically reduced. The activity (per g tissue) was slightly increased in the kidneys and the liver in comparison with the other organs (Figure 2B,D). Radioactivity was highest in the kidneys after 10 min while at every time point only marginal, low levels of 3H were detected in the blood. This indicates a rapid transfer from the blood to the urine via the kidneys. Only small amounts of 3H-Spn remained in the different organs. While the activity of 3H-Spn in the urine reached peak values of about 150–200 kBq·g−1 by 30 min, the highest amounts in the organs were in a much lower range, 1–2 kBq·g−1 tissue. No preferential accumulation of 3H-Spn in the tumours was observed.
Figure 2.
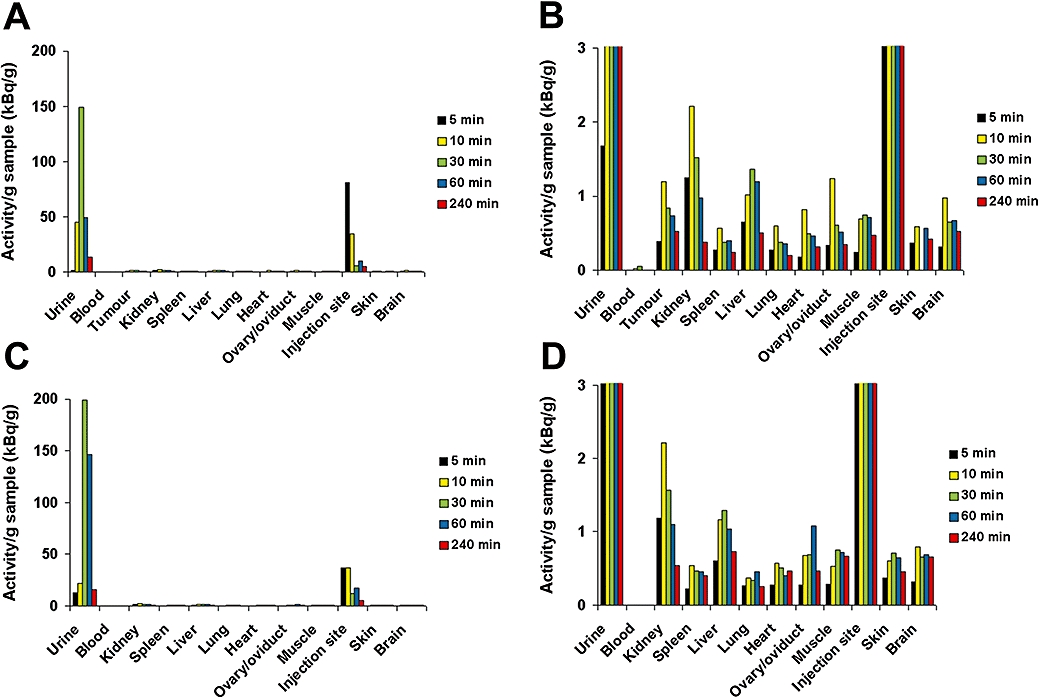
Biodistribution of 3H-Spn in mice. 3H-Spn was injected s.c. in the neck of BALB/c mice with tumours at the flank (A and B) and mice without tumours (C and D). Values were determined after several time points (5–240 min) in the blood, urine and tissues, as shown. The organs were homogenized, and the radioactivity in the samples was measured by liquid scintillation counting. Panels (B) and (D) show the same organ-specific radioactivity as (A) and (C) but on an expanded scale. Values shown are means from two mice at each sampling time. Spn, Saponinum album from Gypsophila paniculata L.
Analysis of 3H-Spn in urine
The radioactivity in the urine was further analysed by HPTLC in order to identify whether unchanged 3H-Spn was present in the urine or whether the radioactivity was derived from 3H-acetate, cleaved from 3H-Spn. The analysis of different areas on the chromatogram by liquid scintillation counting revealed that unchanged 3H-Spn was detected in the urine of the mice (Figure 3). The radioactivity found in the Spn area was equivalent to 34.7% of the total 3H in the sample analysed at 5 min after injection, 27.6 and 24.4% at 10 min after injection and 27.3% at 60 min after injection. At the origin of the chromatogram where 3H-acetate is expected, only small amounts of 3H-activity were found (12.7% at 5 min, 5.8 and 7.8% at 10 min and 1.9% at 60 min after injection).
Figure 3.
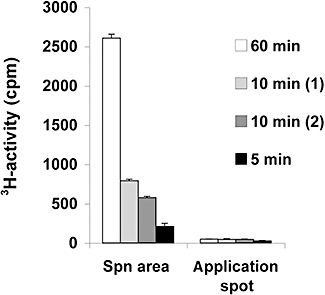
Analysis of [3H]-Spn in the urine of mice by HPTLC. Four urine samples from mice without tumours collected 5 min, 10 min (two samples) and 60 min after 3H-Spn injection were analysed by HPTLC. The radioactivity of the application spot and of the area where Spn migrated on the silica gel glass plate was analysed by liquid scintillation counting. The error bars on the data represent the variation in the HPTLC assay carried out in triplicate. HPTLC, high-performance, thin-layer chromatography; Spn, Saponinum album from Gypsophila paniculata L.
Tumour growth inhibition in vivo
Inhibition of tumour growth by the combination of Spn and SA2E (another CT targeted at ErbB1) was studied with two different treatment schedules: (i) SA2E injected 10 min after Spn; and (ii) SA2E injected 60 min after Spn (Figure 4). We did not treat tumour-bearing mice with SA2E alone, as earlier experiments had shown significantly higher inhibition with Spn/SA2E combinations; 0.1 µg SA2E resulted in a slight tumour growth, while Spn alone did not (Bachran et al., 2009). In our present experiments, both treatment schedules inhibited tumour growth. However, the schedule with 60-min pre treatment was more effective than that with 10 min pre treatment. The comparisons and statistical analyses of tumour growth were performed with the data of day 27 (end of the experiment). Assay of tumour volumes (Figure 4) showed that, compared with sham-treated mice, treatment with the combination given 10 min apart resulted in 46% inhibition, which was not significantly different from the sham-treated mice (P= 0.094). However, treatment with Spn 60 min before adding the SA2E significantly inhibited tumour growth by 66% (P= 0.004). The weights of the tumours after 27 days were as follows: sham-treated mice: 0.800 ± 0.389 g (mean ± SD); mice treated with 10-min interval between Spn and SA2E application: 0.512 ± 0.238 g; mice treated with 60-min interval between Spn and SA2E application: 0.348 ± 0.214 g (P= 0.102 for 10-min interval and P= 0.011 for 60 min; different from sham-treated values; n= 9 for each group; Mann–Whitney U-test).
Figure 4.
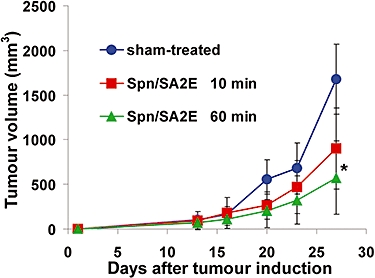
Effects on tumour volume of treatment (s.c.) with Spn/SA2E with 10 min interval between injections (n= 9), Spn/SA2E with 60 min interval between injections (n= 9) and sham-treated BALB/c mice (n= 9). Each group was treated six times with the indicated drugs, beginning on day 6 and continuing twice per week, and the experiment ended on day 27. Only mice with developing tumours were used for the experiment. Tumour volume (shown as means ± SD) was calculated, assuming a spheroid. * indicates significantly (P < 0.05) reduced tumour volume in comparison with the sham-treated mice. Spn, Saponinum album from Gypsophila paniculata L.
The only detectable side effect during the experiment was the reversible and local hardening of the skin at the injection site of Spn in the neck. The effect was more noticeable for the mice that were treated with Spn/SA2E with 10 min incubation between both drugs. During both treatments, no further side effects were observed. This is in accordance with earlier studies where the combination Spn/SA2E induced only reversible moderate side effects, as displayed by kinetic studies on blood plasma parameters (Bachran et al., 2009). One mouse of each group was not included in the evaluation, as the mice had to be removed from the experiment or died early without any obvious signs during the experiment.
Discussion
Tumour therapy with saponins is a well-studied field in biomedicine. Various saponins were tested for their antiproliferative properties, and several saponins induced satisfactory tumour growth inhibition. The field is rapidly growing because several new saponins were isolated and tested for their antitumourigenic potential. In some studies, saponins were combined with conventional chemotherapeutic agents or with radiation to drastically increase their effects on tumour growth (Wang et al., 2008a,b;). Nevertheless, especially for those saponins that are intensively studied in relation to tumour therapy, insights into their mode of action on the cellular and molecular level will lead to a better understanding of saponins in tumour therapy (see Bachran et al., 2008a).
In a recent study, the ginsenoside Rg3 increased the post-operative 3-year survival in non-small cell lung cancer patients with tumours positive for vascular epithelial growth factor, both alone and in combination with a complex chemotherapy regimen, while chemotherapy alone did not (Lu et al., 2008). Another saponin, magnesium isoglycyrrhizinate, is a potential treatment for hepatitis and in HIV therapy. In a phase I study for this saponin (Sun et al., 2007), the compound was well tolerated and induced no side effects in the applied doses of 300 mg in a single dose or 100 mg in multiple doses. Given i.v., the half-life was in the range of 24 h. In earlier experiments in rats, its stereoisomer magnesium glycyrrhizinate was injected i.v. and demonstrated accumulation in the liver (Hu et al., 2003). This result is in contrast to our results obtained in the present study, as Spn showed no accumulation in any organ, and the results demonstrated a rapid distribution of 3H-Spn within minutes throughout the body. Only traces of 3H-Spn were detected in the blood, while the majority was found in the urine. We conclude that Spn was removed immediately from the blood when it passes the kidneys. A weak retention of 3H-Spn was observed in the organs. A further difference between the two saponins is their toxicity. While the median lethal dose (LD50) of magnesium isoglycyrrhizinate in mice after i.v. injection is 685 mg·kg−1 (Sun et al., 2007), the LD50 of Spn is in the range of 11 mg·kg−1 (Bachran et al., 2009). Due to the structural differences between Spn and magnesium isoglycyrrhizinate, the properties of the saponins are drastically different.
The successful labelling of Spn was essential for this study. Labelling was difficult because Spn does not possess many suitable residues for chemical coupling. Furthermore, the aldehyde group of the aglycone has to be preserved to retain the function of Spn (Bachran et al., 2006). Metabolic labelling would guarantee retained function but is too laborious. The use of other labels (e.g. fluorescent dyes) is not advisable, as the bulky dyes would very likely interfere with the binding properties of Spn, while 3H-acetyl labelling of the sugar residues retained the activity of Spn in vitro (Weng et al., 2009b). However, continuous measurements of the distribution in vivo are not possible, as 3H has to be measured by liquid scintillation counting. On the basis of our results of the kinetics of Spn distribution in mice, we expected an increased tumour inhibition by SA2E when it was applied 10 min instead of 60 min, after Spn injection. As we already achieved a 94% reduction in tumour growth when SA2E was applied 60 min after Spn (Bachran et al., 2009), we used here a less-than-optimal regimen (later onset of treatment, extended treatment intervals) to better detect a gain in efficacy when SA2E was applied 10 min after Spn. Surprisingly, in spite of a higher level of 3H-Spn in the tumours and in the tissues at 10 min after injection of Spn, the effects of the subsequent injection of SA2E were reduced; that is, tumours were not significantly inhibited. There was more and significant tumour inhibition with 60-min interval between the injection of Spn in the neck and that of the CT, SA2E in the flank. It would appear that the overall increased saponin concentration in the mice 10 min after Spn injection actually decreased the antitumour effect of the combination. It is known that SA2E is, to a certain degree, internalized into non-target cells and tissues due to non-specific uptake, that is, not mediated by ErbB1 (Heisler et al., 2003). Spn also increased this non-specific uptake but less than the increase in ErbB1-specific uptake, about sevenfold higher (Bachran et al., 2006). Nevertheless, the ErbB1-non-specific uptake of SA2E by non-target tissue is higher when the Spn concentration is higher and, thus, may result in less SA2E in the vicinity of the tumour. This idea is supported by the observation of increased hardening of the skin at the neck in those mice with only 10-min interval between the injection of Spn and SA2E. The high local dose of Spn may result in this increased side effect by the non-specific action of Spn/SA2E. It thus appears that a very low systemic concentration of Spn is better able to show the synergy in antitumour activity. This is in agreement with cell culture experiments, revealing that the Spn concentration required for the synergistic effect is below toxic and permeabilizing concentrations (Hebestreit et al., 2006). Another possible explanation for the reduced efficacy of the treatment with only 10-min interval between Spn and SA2E injections, is that a critical Spn concentration in the target cell membrane is required to exhibit Spn-mediated increased cytotoxicity. Although the amount of saponin in several organs was higher after 10 min than after 60 min, it may take up to 60 min to reach the critical concentration in the membrane. Further experiments are necessary to expose the mechanisms underlying the decreased antitumour effect of the 10 min interval between Spn and SA2E. The reduction in tumour growth with SA2E applied 60 min after Spn was 66% and, thus, less than the 94% described previously (Bachran et al., 2009). This can be attributed to the suboptimal treatment regimen used in the present experiments, for the reasons explained above.
The biodistribution data provide valuable background information for future work on the combination of saponins, from Gypsophila paniculata L. or other sources, with CTs for targeted tumour therapy. Differences in the disposition of Spn could crucially affect the efficacy of Spn–CT combinations in different models and in human patients.
Acknowledgments
We acknowledge the generous financial support of the Deutsche Forschungsgemeinschaft (FU 408/3-1), the Sonnenfeldstiftung via a scholarship for D. B. and the Berliner Krebsgesellschaft (FUFF200801).
Glossary
Abbreviations:
- CT
chimeric toxin
- EGF
epidermal growth factor
- ErbB1
EGF receptor
- GI50
50% growth inhibitory concentration
- HPTLC
high-performance, thin-layer chromatography
- IT
immunotoxin
- SA2E
chimeric toxin containing saporin, a molecular adapter and EGF
- SE
chimeric toxins containing saporin and EGF
- Spn
Saponinum album from Gypsophila paniculata L
Conflicts of interest
None declared.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachran C, Sutherland M, Heisler I, Hebestreit P, Melzig MF, Fuchs H. The saponin-mediated enhanced uptake of targeted saporin-based drugs is strongly dependent on the saponin structure. Exp Biol Med (Maywood) 2006;231:412–420. doi: 10.1177/153537020623100407. [DOI] [PubMed] [Google Scholar]
- Bachran C, Bachran S, Sutherland M, Bachran D, Fuchs H. Saponins in tumor therapy. Mini Rev Med Chem. 2008a;8:575–584. doi: 10.2174/138955708784534445. [DOI] [PubMed] [Google Scholar]
- Bachran C, Heisler I, Bachran D, Dassler K, Ervens J, Melzig MF, et al. Chimeric toxins inhibit growth of primary oral squamous cell carcinoma cells. Cancer Biol Ther. 2008b;7:237–242. doi: 10.4161/cbt.7.2.5264. [DOI] [PubMed] [Google Scholar]
- Bachran C, Durkop H, Sutherland M, Bachran D, Muller C, Weng A, et al. Inhibition of tumor growth by targeted toxins in mice is dramatically improved by saponinum album in a synergistic way. J Immunother. 2009;32:713–725. doi: 10.1097/CJI.0b013e3181ad4052. [DOI] [PubMed] [Google Scholar]
- Baumann E, Stoya G, Volkner A, Richter W, Lemke C, Linss W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000;102:21–35. doi: 10.1078/0065-1281-00534. [DOI] [PubMed] [Google Scholar]
- Chinn PC, Leonard JE, Rosenberg J, Hanna N, Anderson DR. Preclinical evaluation of 90Y-labeled anti-CD20 monoclonal antibody for treatment of non-Hodgkin's lymphoma. Int J Oncol. 1999;15:1017–1025. doi: 10.3892/ijo.15.5.1017. [DOI] [PubMed] [Google Scholar]
- Chwalek M, Lalun N, Bobichon H, Ple K, Voutquenne-Nazabadioko L. Structure-activity relationships of some hederagenin diglycosides: haemolysis, cytotoxicity and apoptosis induction. Biochim Biophys Acta. 2006;1760:1418–1427. doi: 10.1016/j.bbagen.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Frankel AE, Fleming DR, Hall PD, Powell BL, Black JH, Leftwich C, et al. A phase II study of DT fusion protein denileukin diftitox in patients with fludarabine-refractory chronic lymphocytic leukemia. Clin Cancer Res. 2003;9:3555–3561. [PubMed] [Google Scholar]
- Fuchs H, Bachran C. Targeted tumor therapies at a glance. Curr Drug Targets. 2009;10:89–93. doi: 10.2174/138945009787354557. [DOI] [PubMed] [Google Scholar]
- Fuchs H, Bachran C, Li T, Heisler I, Durkop H, Sutherland M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J Control Release. 2007;117:342–350. doi: 10.1016/j.jconrel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Fuchs H, Bachran D, Panjideh H, Schellmann N, Weng A, Melzig MF, et al. Saponins as tool for improved targeted tumor therapies. Curr Drug Targets. 2009;10:140–151. doi: 10.2174/138945009787354584. [DOI] [PubMed] [Google Scholar]
- Goyal A, Batra JK. Inclusion of a furin-sensitive spacer enhances the cytotoxicity of ribotoxin restrictocin containing recombinant single-chain immunotoxins. Biochem J. 2000;345(2):247–254. Pt. [PMC free article] [PubMed] [Google Scholar]
- Güçlü-Ustündağ O, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- Haridas V, Higuchi M, Jayatilake GS, Bailey D, Mujoo K, Blake ME, et al. Avicins: triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci USA. 2001;98:5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebestreit P, Weng A, Bachran C, Fuchs H, Melzig MF. Enhancement of cytotoxicity of lectins by Saponinum album. Toxicon. 2006;47:330–335. doi: 10.1016/j.toxicon.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Heisler I, Keller J, Tauber R, Sutherland M, Fuchs H. A colorimetric assay for the quantitation of free adenine applied to determine the enzymatic activity of ribosome-inactivating proteins. Anal Biochem. 2002;302:114–122. doi: 10.1006/abio.2001.5527. [DOI] [PubMed] [Google Scholar]
- Heisler I, Keller J, Tauber R, Sutherland M, Fuchs H. A cleavable adapter to reduce nonspecific cytotoxicity of recombinant immunotoxins. Int J Cancer. 2003;103:277–282. doi: 10.1002/ijc.10809. [DOI] [PubMed] [Google Scholar]
- Heisler I, Sutherland M, Bachran C, Hebestreit P, Schnitger A, Melzig MF, et al. Combined application of saponin and chimeric toxins drastically enhances the targeted cytotoxicity on tumor cells. J Control Release. 2005;106:123–137. doi: 10.1016/j.jconrel.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Hu Q, Ding J, Liu S, Li P, Hu G. Pharmacokinetics of magnesium glycyrrhizinate following intravenous administration of magnesium glycyrrhizinate in rats. Eur J Drug Metab Pharmacokinet. 2003;28:259–264. doi: 10.1007/BF03220177. [DOI] [PubMed] [Google Scholar]
- Keller J, Heisler I, Tauber R, Fuchs H. Development of a novel molecular adapter for the optimization of immunotoxins. J Control Release. 2001;74:259–261. doi: 10.1016/s0168-3659(01)00329-7. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kwon HC, Ko H, Park JH, Kim HY, Yoo JH, et al. Anti-tumor activity of the ginsenoside Rk1 in human hepatocellular carcinoma cells through inhibition of telomerase activity and induction of apoptosis. Biol Pharm Bull. 2008;31:826–830. doi: 10.1248/bpb.31.826. [DOI] [PubMed] [Google Scholar]
- Kwon HY, Kim EH, Kim SW, Kim SN, Park JD, Rhee DK. Selective toxicity of ginsenoside Rg3 on multidrug resistant cells by membrane fluidity modulation. Arch Pharm Res. 2008;31:171–177. doi: 10.1007/s12272-001-1137-y. [DOI] [PubMed] [Google Scholar]
- Liu MJ, Yue PY, Wang Z, Wong RN. Methyl protodioscin induces G2/M arrest and apoptosis in K562 cells with the hyperpolarization of mitochondria. Cancer Lett. 2005;224:229–241. doi: 10.1016/j.canlet.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Lu P, Su W, Miao ZH, Niu HR, Liu J, Hua QL. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- Niu YP, Li LD, Wu LM. Beta-aescin: a potent natural inhibitor of proliferation and inducer of apoptosis in human chronic myeloid leukemia K562 cells in vitro. Leuk Lymphoma. 2008;49:1384–1391. doi: 10.1080/10428190802090151. [DOI] [PubMed] [Google Scholar]
- Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- Soler-Rodriguez AM, Ghetie MA, Oppenheimer-Marks N, Uhr JW, Vitetta ES. Ricin A-chain and ricin A-chain immunotoxins rapidly damage human endothelial cells: implications for vascular leak syndrome. Exp Cell Res. 1993;206:227–234. doi: 10.1006/excr.1993.1142. [DOI] [PubMed] [Google Scholar]
- Sun L, Shen J, Pang X, Lu L, Mao Y, Zeng M. Phase I safety and pharmacokinetic study of magnesium isoglycyrrhizinate after single and multiple intravenous doses in Chinese healthy volunteers. J Clin Pharmacol. 2007;47:767–773. doi: 10.1177/0091270007299757. [DOI] [PubMed] [Google Scholar]
- Talpur R, Apisarnthanarax N, Ward S, Duvic M. Treatment of refractory peripheral T-cell lymphoma with denileukin diftitox (ONTAK) Leuk Lymphoma. 2002;43:121–126. doi: 10.1080/10428190210183. [DOI] [PubMed] [Google Scholar]
- Wang G, Chen H, Huang M, Wang N, Zhang J, Zhang Y, et al. Methyl protodioscin induces G2/M cell cycle arrest and apoptosis in HepG2 liver cancer cells. Cancer Lett. 2006a;241:102–109. doi: 10.1016/j.canlet.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2006b;59:589–560. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wang W, Rayburn ER, Hao M, Zhao Y, Hill DL, Zhang R, et al. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate. 2008a;68:809–819. doi: 10.1002/pros.20742. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008b;98:792–802. doi: 10.1038/sj.bjc.6604227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng A, Jenett-Siems K, Gorick C, Melzig MF. Enhancement of cytotoxicity of ribosome-inactivating-protein type I by saponinum album is not based on stimulation of phagocytosis. J Pharm Pharmacol. 2008;60:925–930. doi: 10.1211/jpp.60.7.0015. [DOI] [PubMed] [Google Scholar]
- Weng A, Bachran D, Gorick C, Bachran C, Fuchs H, Melzig MF. A simple method for isolation of gypsophila saponins for the combined application of targeted toxins and saponins in tumor therapy. Planta Med. 2009a;75(13):1421–1422. doi: 10.1055/s-0029-1185706. [DOI] [PubMed] [Google Scholar]
- Weng A, Gorick C, Melzig MF. A brief communication: enhancement of toxicity of saporin-based toxins by gypsophila saponins – kinetic of the saponin. Exp Biol Med (Maywood) 2009b;234:961–966. doi: 10.3181/0902-BC-71. [DOI] [PubMed] [Google Scholar]
- Wina E, Muetzel S, Becker K. The impact of saponins or saponin-containing plant materials on ruminant production – a review. J Agric Food Chem. 2005;53:8093–8105. doi: 10.1021/jf048053d. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:3262–3269. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- Yanamandra N, Berhow MA, Konduri S, Dinh DH, Olivero WC, Nicolson GL, et al. Triterpenoids from Glycine max decrease invasiveness and induce caspase-mediated cell death in human SNB19 glioma cells. Clin Exp Metastasis. 2003;20:375–383. doi: 10.1023/a:1024043104803. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zheng J, Zhao Y, Wang B, Wu L, Liang H. Three anti-tumor saponins from Albizia julibrissin. Bioorg Med Chem Lett. 2006;16:2765–2768. doi: 10.1016/j.bmcl.2006.02.009. [DOI] [PubMed] [Google Scholar]


