Abstract
Background and purpose:
The ionic mechanisms underlying nitrergic inhibitory junction potentials (IJPs) in gut smooth muscle remain a matter of debate. Recently, it has been reported that opening of TWIK-related K+ channel 1 (TREK-1) K+ channels contributes to the nitrergic IJP in colonic smooth muscle. We investigated the effects of TREK-1 channel blockers on nitrergic neurotransmission in mouse and opossum lower oesophageal sphincter (LOS) circular smooth muscle (CSM).
Experimental approach:
The effects of TREK-1 channel blockers were characterized pharmacologically in murine and opossum gut smooth muscle using conventional intracellular and tension recordings.
Key results:
In LOS, L-methionine depolarized the resting membrane potential (RMP) but did not inhibit the nitrergic IJP. Cumulative application of theophylline hyperpolarized the RMP and inhibited the nitrergic IJP concentration dependently. The induced membrane hyperpolarization was prevented by pre-application of caffeine, but not by 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one. 8-Br-cAMP significantly hyperpolarized membrane potential and increased the amplitude of the nitrergic IJP. In opossum LOS muscle strips, L-methionine increased resting tone but had no effect on nerve-mediated LOS relaxation. On the other hand, theophylline markedly inhibited tone. In CSM from mouse proximal colon, L-methionine caused modest inhibition of nitrergic IJPs.
Conclusions and implications:
TREK-1 channels were not involved in the nitrergic IJP in LOS CSM. Not only does L-methionine have no effect on the nitrergic IJP or LOS relaxation, but the effect of theophylline appears to be due to interruption of Ca2+-releasing pathways (i.e. caffeine-like effect) rather than via blockade of TREK-1 channels.
Keywords: ATP, Ca2+-activated Cl- channels, circular smooth muscle, long-lasting slow IJP
Introduction
The lower oesophageal sphincter (LOS) consists of specialized circular smooth muscle (CSM) that maintains myogenic tone at rest, thereby preventing reflux of gastric content into the oesophagus, but relaxes with swallowing to allow ingested food to pass into the stomach (Goyal and Paterson, 1989). The latter is mediated by inhibitory nerves, mainly nitrergic innvervation in opossum LOS, and nitrergic and purinergic innervation in mouse LOS (Zhang and Paterson, 2002; 2003; Zhang et al., 2008).
Nitric oxide (NO) is well established as a mediator of the slow component of the inhibitory junction potential (IJP) in gut CSM; however, the mechanisms underlying this nitrergic IJP remain controversial (Bennett, 1997). It was previously proposed that the opening of K+ channels contributes to the nitrergic IJP; however, none of the established K+ channel antagonists blocked the nitrergic IJP in oesophageal smooth muscle (Crist et al., 1991a; Sanders and Ozaki, 1994). Based on Cl- substitution and pharmacological experiments, we and others have proposed that the nitrergic IJP in oesophageal and LOS smooth muscle is due to closing of Ca2+-activated Cl- channels (ClCa) (Crist et al., 1991a,b; Zhang and Paterson, 2002; 2003; Zhang et al., 2008). Furthermore, we have suggested that spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) primes ClCa via an SR–Ca2+–myosin light chain kinase (MLCK)–ClCa pathway (Zhang and Paterson, 2002; 2003; Zhang et al., 2008). However, recent publications have proposed that TREK-1 K+ channels (nomenclature follows Alexander et al., 2008) are involved in the nitrergic IJP in murine colonic smooth muscle, as the putative TREK-1 K+ channel blockers, L-methionine and theophylline were reported to inhibit the nitrergic IJP (Koh et al., 2006; Hwang et al., 2008).
We have recently characterized the nitrergic innervation of both the clasp and sling muscle fibres of murine LOS CSM (Zhang et al., 2008). In the clasp fibres, transmural nerve stimulation evokes a biphasic IJP, consisting of an initial fast inhibitory junction potential (fIJP) followed by an unusual long-lasting slow inhibitory junction potential (sIJP). The nitrergic innervation is responsible for the entire sIJP and up to half the amplitude of the fIJP, possibly via the closing of Clca, whereas purinergic innervation is responsible for the remainder of the fIJP, via the opening of Ca2+-activated conductance K+ channels. These features constitute a good model in which to study the role of TREK-1 K+ channels in nitrergic neurotransmission, especially the sIJP, in murine LOS clasp muscle fibres.
Methods
All animal care and experimental protocols were approved by the Animal Care Committee of Queen's University. For electrophysiological studies, adult mice (CD1, Charles River Laboratories, Montréal, Canada) of either sex were killed by cervical dislocation after isoflurane anaesthesia. For the muscle strip studies, adult opossums (Didelphis virginiana) of either sex and weighing 2–5 kg were anaesthetized using sodium pentobarbital (40 mg·kg−1) administered by tail vein injection and subsequently killed by intracardiac pentobarbital injection once tissue had been removed.
Mouse studies: tissue preparation and conventional intracellular recordings
The abdominal cavity was exposed via a midline incision, and the stomach, duodenum and part of the attached oesophagus were dissected free and were removed. The LOS was then separated under a dissecting microscope. In mice, the LOS is not an identical ring. Rather, it consists of distinctly thickened ‘clasp’ fibres on the right side and somewhat less distinct ‘sling’ fibres on the left side that resemble gastric muscle (Zhang et al., 2008). Strips (0.5 − 1.0 × 2.0 − 2.5 mm) of the LOS clasp were pinned with the mucosa side facing upward on the bottom of a recording chamber covered by Sylgard (Dow Corning, Midland, MI, USA) and were perfused at 2 mL·min−1 with pre-oxygenated Krebs solution routinely containing nifedipine (1 µM), atropine (3 µM), guanethidine (3 µM) and substance P (SP) (1 µM) at 36°C (Zhang and Paterson, 2001; 2005;) to ensure non-adrenergic, non-cholinergic and non-tachykinergic conditions. CSM from proximal colonic smooth muscle was used as control tissue in some experiments. Nerve stimulation using either 1 or 4 sq wave pulses (20 Hz) with a duration of 0.3 ms and a voltage of 70 V was delivered to the muscle preparations by a pair of silver wires, while electrical activity was recorded using conventional intracellular electrodes as previously described (Zhang and Lang, 1994; Zhang and Paterson, 2002). In general, the tissue was allowed to equilibrate for 1 h prior to the experiment.
Glass microelectrodes were pulled using a vertical microelectrode puller (Sutter Instrument, Novato, CA, USA) and were filled with 3 M KCl. Microelectrode resistance was 70–100 MΩ. The microelectrode was positioned to impale a smooth muscle cell under the guidance of an inverted microscope. The criterion for acceptance of a successful impalement was a sharp voltage drop of approximately −40 mV on penetration that was maintained for at least 2 min. Transmembrane potential was amplified and measured with an intracellular electrometer (Model IE-210, Warner Instrument Corporation, Hamden, CT, USA). Resting membrane potential (RMP) was calibrated upon withdrawal of the microelectrode from the cell. The output of the signal was coupled to the Axon Digidata-1200 acquisition system (Molecular Devices, Sunnyvale, CA, USA). Data were digitized at a frequency of 500 Hz and were stored in a computer for later analysis using Axon Scope 8.0 software (Molecular Devices). The following parameters, which have been described in detail previously (Zhang and Paterson, 2002; 2003;), were used to quantitatively analyse the smooth muscle electrical properties: (i) RMP (mV), (ii) amplitude of IJP (mV) and (iii) half-amplitude duration of IJP (ms).
Opossum studies
The chest and abdominal cavities were exposed via a midline incision, and the lower part of the oesophagus and short segment of the attached stomach were removed and placed in a pre-oxygenated Krebs solution. The distal oesophagus and oesophagogastric junction was opened longitudinally and pinned with the mucosa side up in a dissecting dish. Using a binocular microscope, the mucosa and the connective tissue layers were carefully removed by sharp dissection. The LOS was visible as a distinct thickening of circular muscle in the resultant tissue, located just on the gastric side of the squamocolumnar junction (Sengupta et al., 1987). A strip of LOS (with attached longitudinal muscle) of about 3 × 15 mm was excised and hung in a water-jacketed tissue bath containing 10 mL Krebs solution gassed with 5% CO2+ 95% O2 at 35°C. One end of the strip was fixed to a hook at the base of the tissue bath and the other was tied, using a fine silk thread, to a Grass FT03 isometric force transducer that coupled to a Windaq Data Acquisition system (DATAQ Instruments, Akron, OH, USA). Signals were sampled at 100 Hz and were stored in a computer for subsequent analysis. Strips were initially stretched to 140% of the unloaded length and were equilibrated for at least 1 h. This degree of stretch has been previously shown to result in optimal responses (Uc et al., 1999). The strips were suspended between a pair of platinum electrodes, and nerve-mediated LOS relaxation was assessed by applying electrical field stimulation (0.5 ms pulse duration, 10 Hz, 5 s trains, supramaximal voltage) using a Grass SD9 stimulator (Grass Technologies, West Warwick, RI, USA). In order to quantify drug-induced changes in LOS resting tone and nerve-mediated LOS relaxation, basal tone was defined as the average tension recorded over a 5 min period at the end of the equilibration period and before drug administration, and maximal (i.e. 100%) relaxation was arbitrarily taken as the tone recorded in the strip after it was initially suspended in the organ bath and before stretch was applied.
Statistical analysis
Data are shown as mean ± standard error. n refers to number of animals. For the electrophysiological experiments, only recordings in which a full protocol was completed in the same cell are included in the statistical analysis. Pre- and post-drug comparisons were made using Students t-test, and a P-value of <0.05 was considered statistically significant.
Materials
All drugs were purchased from Sigma (Burlington, Ontario, Canada), except isoflurane (Baxter, Mississauga, Ontario, Canada). The following drugs were used: tetraethylammonium (TEA), nifedipine, atropine, guanethidine, apamin, SP, 8-Br-cAMP, caffeine, 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ), L-methionine and theophylline. Nifedipine and ODQ were dissolved in dimethyl sulphoxide (DMSO), theophylline in 1 N NaOH and others in distilled and deionized water. These were diluted to final concentrations with Krebs solution. The final concentration of DMSO in Krebs solution was no more than 1%, which did not produce any effect on the spontaneous electrical activity of the tissue.
Results
Mouse electrophysiological studies
General electrophysiological properties
Conventional intracellular recordings revealed a RMP of −41.9 ± 1.3 mV (n= 12) in the smooth muscle of LOS clasp in the presence of atropine, guanethidine and SP. Transmural nerve stimulation (four pulses, 20 Hz) induced a biphasic IJP, consisting of an initial fIJP with an amplitude of 29.8 ± 2.1 mV and a half-amplitude duration of 638 ± 27 ms, followed by a long-lasting sIJP with an amplitude of 5.7 ± 0.5 mV and a half-amplitude duration of 12 913 ± 871 ms (n= 12). These data are consistent with our previous reports (Zhang et al., 2008).
Effects of TREK-1 K+ channel blockers L-methionine and theophylline on nitrergic IJPs
Our studies to date have demonstrated that the initial fIJP results from purinergic and nitrergic innervation, while the sIJP is due entirely to nitrergic innervation (Zhang et al., 2008). This provides a good model to test the effects of TREK-1 K+ channel blockers L-methionine and theophylline on the nitrergic IJP. Initially, L-methionine and theophylline at concentrations of 1.0 and 2.5 mM, respectively, were used, which were reported previously to effectively block TREK-1 K+ channels (Park et al., 2005; Koh et al., 2006).
L-methionine depolarized the RMP by about 10 mV with peak effect occurring in 5–10 min but had no significant effect on any component of the biphasic IJP (Table 1 and Figure 1A,B). The effects on the RMP were reversible, with recovery to baseline values occurring within 10–20 min of washout. The time course of the effects of theophylline was rapid and peaked in 2–3 min. Theophylline hyperpolarized the RMP by ∼30 mV over control, significantly attenuated the fIJP and abolished the sIJP (Figure 1A,C). These inhibitory effects completely recovered to baseline values 20–30 min after washing out.
Table 1.
Effect of L-methionine on the RMP and the IJP of murine lower oesophageal sphincter circular smooth muscle
| RMP (mV) | fIJP amplitude (mV) | fIJP duration (ms) | sIJP amplitude (mV) | sIJP duration (ms) | |
|---|---|---|---|---|---|
| Control (n= 4) | 40.4 ± 2.9 | 23.0 ± 1.8 | 611 ± 63 | 4.1 ± 1.2 | 10 522 ± 1101 |
| L-methionine (n= 4) | 30.0 ± 3.0* | 20.0 ± 3.6 | 567 ± 51 | 3.9 ± 1.3 | 10 910 ± 1013 |
P= 0.03 versus control.
IJP, inhibitory junction potential; RMP, resting membrane potential; fIJP, fast inhibitory junction potential; sIJP, slow inhibitory junction potential.
Figure 1.
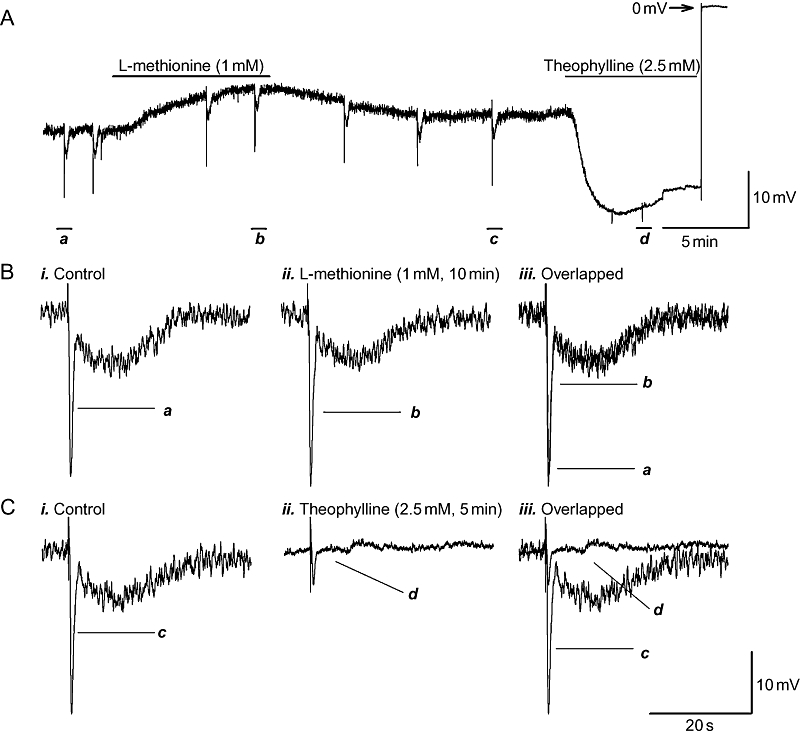
Effects of TREK-1 K+ channel blockers L-methionine and theophylline on the resting membrane potential (RMP) and on the purinergic and nitrergic inhibitory junction potentials (IJPs) in the presence of atropine (3 µM), guanethidine (3 µM) and substance P (1 µM) to ensure non-adrenergic, non-cholinergic, non-tachykinergic conditions. (A) Experimental recording demonstrating that L-methionine (1 mM) depolarized RMP, but theophylline (2.5 mM) hyperpolarized the RMP. (B,C) IJPs depicted in panel A, on an expanded time scale, before and after application of the channel blockers,. L-methionine did not have any significant effects on IJPs. However, theophylline significantly inhibited the fast inhibitory junction potential and abolished the sIJP.
To further characterize the effects of theophylline, cumulative concentration–response experiments were performed. The concentration of theophylline was increased at intervals of 8 min. Figure 2A shows a complete experimental recording of the effects of theophylline on electrical properties before and during application (0.03–3.0 mM), and up to 35 min after washing out. Statistical analysis revealed that theophylline inhibited the amplitude of the fIJP and sIJP with a minimal effective concentration of 0.03 mM and a maximal effective concentration of 1 mM (Figures 2 and 3). The IC50 was 0.19 ± 0.04 mM and 0.24 ± 0.004 with a Hill slope of 2.51 ± 0.48 and 2.59 ± 0.11 (n= 4) respectively. However, the effects of theophylline on the RMP and sIJP were surprising. Theophylline within the range of 0.03–1.0 mM hyperpolarized the RMP with an IC50 of 0.12 ± 0.003 mM and a Hill slope of 2.74 ± 0.22 in a concentration-dependent fashion (Figure 3A), but subsequent application of theophylline (3 mM) significantly depolarized the RMP (Figure 2A). Furthermore, this was not the case when a single 3 mM application of theophylline was applied (Figures 2A and 4A). Moreover, theophylline at a concentration of 0.1 mM actually increased the amplitude of sIJP versus control (Figures 2B and 3B).
Figure 2.
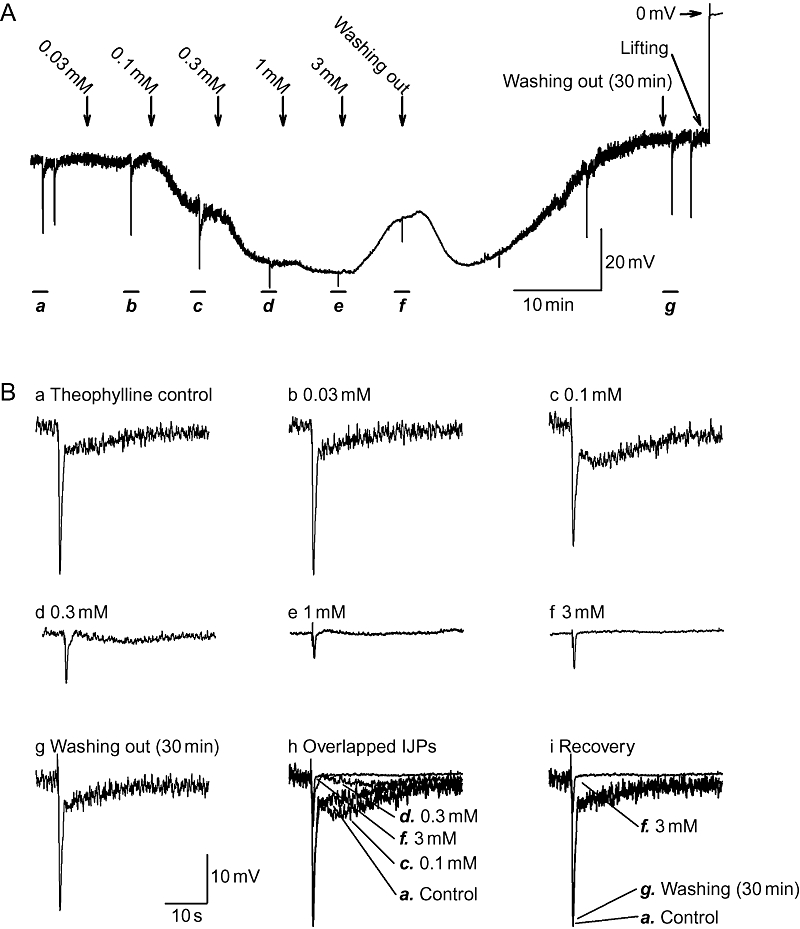
Cumulative concentration–response of theophylline. (A) Time course of effects of cumulative application of theophylline (0.03–3.0 mM) at intervals of 8 min, on electrical properties. Theophylline (0.03–1.0 mM) hyperpolarized resting membrane potential (RMP) in a concentration-dependent fashion, but subsequent bath application of theophylline (3 mM) surprisingly depolarized the RMP. The effects of theophylline were reversible and completely recovered 30 min after washing out. (B) Inhibitory junction potentials (IJPs) depicted in panel A displayed at an expanded time scale. (B, panels h,i) Overlapped IJPs before, during and after the application of theophylline.
Figure 3.
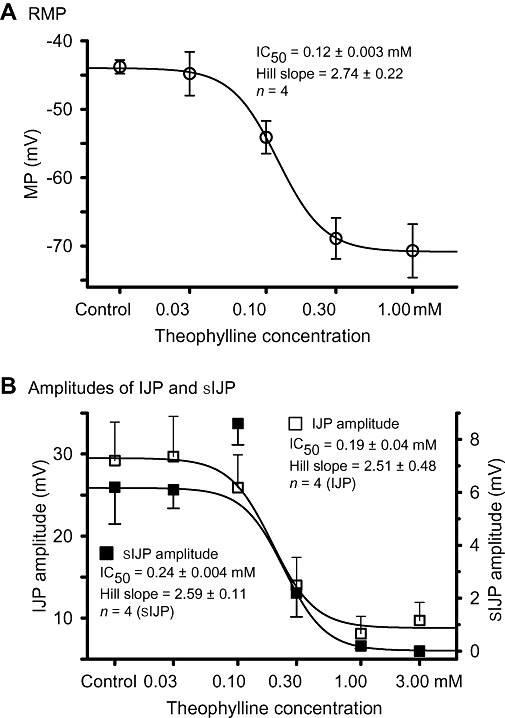
Statistical analysis of concentration–response curves for the theophylline effect on (A) resting membrane potential (RMP), and (B) fast inhibitory junction potential and slow inhibitory junction potential (sIJP). The amplitude of sIJP was significantly increased by theophylline only at the 0.1 mM concentration.
Figure 4.
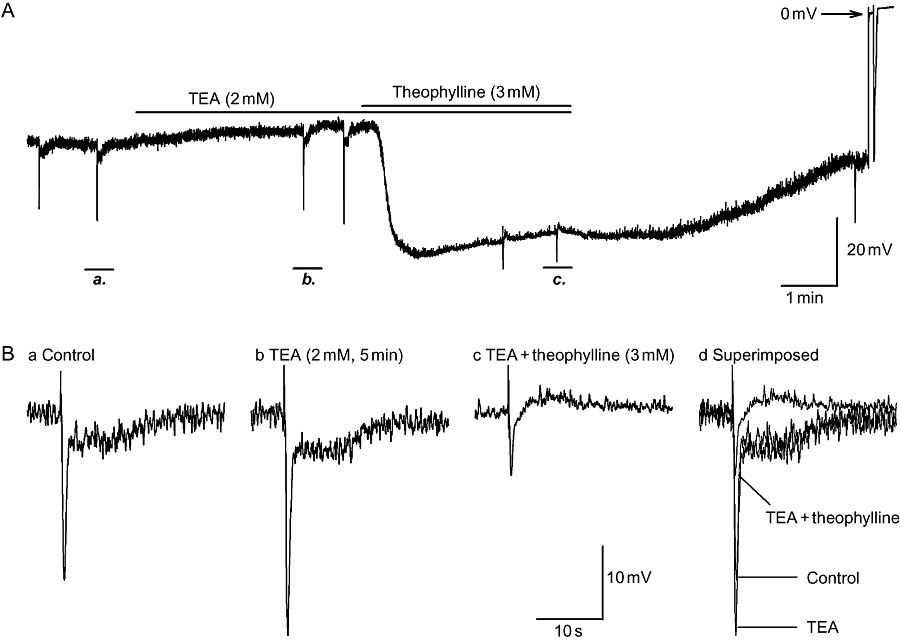
Failure of tetraethylammonium (TEA), a Ca2+-activated large-conductance K+ channels (BK) channel blocker, to prevent the inhibitory effects of theophylline on electrical properties. (A) Continuous recording of the effects of pre-application of TEA (2 mM, 5 min) on the inhibition induced by theophylline (3 mM, 5 min). (B, panels a–c) Inhibitory junction potentials (IJPs) from panel A at an expanded time scale in control, TEA and TEA plus theophylline. TEA failed to prevent the hyperpolarization and abolition of nitrergic IJPs, suggesting that the inhibitory effects of theophylline were not due to the opening of BK channels.
To rule out the possibility that theophylline was inducing membrane hyperpolarization via a presynaptic mechanism, experiments were also performed in the presence of tetrodotoxin (TTX, 1 µM). The degree of theophylline-induced RMP hyperpolarization was not significantly different in the presence or in the absence of TTX (ΔMP 23.0 ± 6.1 mV, with TTX, n= 3; ΔMP 26.9 ± 3.3, without TTX, n= 4; P= 0.576).
Role of phosphodiesterase (PDE) inhibition by theophylline in mediating the RMP hyperpolarization and abolition of the nitrergic IJP
Theophylline has been used therapeutically for a range of diseases as a non-selective PDE inhibitor (Boswell-Smith et al., 2006). Inhibition of PDE results in intracellular accumulation of either cAMP or cGMP. The increase of intracellular cAMP in turn activates Ca2+-activated large-conductance K+ channels (BK), leading to bronchial smooth muscle relaxation (Ise et al., 2003). To determine whether this mechanism mediated the observed inhibitory effects of theophylline, a non-selective K+ channel antagonist, TEA, with known inhibitory effects on BK channels, was used to block BK channels prior to the application of theophylline. Bath application of TEA (2 mM) depolarized RMP, augmented the amplitude of the fIJP and expanded the half-amplitude duration of sIJP. Subsequent application of theophylline still produced marked hyperpolarization, inhibition of the fIJP and abolition of the sIJP to the same extent as evoked by theophylline alone (Table 2 and Figures 1 and 2A).
Table 2.
Role of phosphodiesterase inhibition in theophylline-induced hyperpolarization
| Resting membrane potential (mV) | IJP amplitude (mV) | IJP duration (ms) | sIJP amplitude (mV) | sIJP duration (ms) | |
|---|---|---|---|---|---|
| n= 5 | |||||
| Control | −40.8 ± 2.6 | 27.7 ± 3.2 | 591 ± 11 | 4.9 ± 0.3 | 11 903 ± 1179 |
| +Tetraethylammonium (2 mM) | −35.0 ± 1.7* | 31.6 ± 4.0 | 638 ± 10 | 5.4 ± 0.4 | 13 814 ± 1120* |
| +Theophylline (3 mM) | −67.4 ± 5.3* | 13.0 ± 1.6* | 489 ± 24* | N/M | N/M |
| n= 3 | |||||
| Control | −41.4 ± 2.7 | 33.9 ± 1.7 | 585 ± 47 | 6.3 ± 1.0 | 13 305 ± 898 |
| +Caffeine (5 mM) | −55.6 ± 4.1* | 23.6 ± 2.9* | 371 ± 59* | N/M | N/M |
| +Theophylline (3 mM) | −46.3 ± 0.5* | 17.4 ± 2.3 | 502 ± 29 | N/M | N/M |
| n= 3 | |||||
| 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (20 µM) | −40.0 ± 2.3 | 21.6 ± 2.4 | 437 ± 29 | N/M | N/M |
| +Theophylline (3 mM) | −57.6 ± 6.3* | 14.6 ± 2.5 | 453 ± 41 | N/M | N/M |
| n= 4 | |||||
| Apamin (300 nM) | −33.4 ± 1.5 | 16.3 ± 4.0 | 490 ± 17 | 2.1 ± 0.2 | 12 514 ± 622 |
| +8-Br-cAMP (1 mM) | −40.4 ± 1.8* | 22.3 ± 4.2 | 518 ± 50 | 4.1 ± 0.5* | 17 716 ± 2064* |
P < 0.05.
Duration: half-amplitude duration.
N/M, not measured; IJP, inhibitory junction potential; sIJP, slow inhibitory junction potential.
In addition, to exclude cAMP accumulation as the mechanism underlying the theophylline effect, the membrane-permeable cAMP analogue 8-Br-cAMP was used. In these experiments, apamin (300 nM) was added to the bathing solution to isolate the nitrergic IJP. Administration of 8-Br-cAMP (1 mM) induced RMP hyperpolarization that peaked in 7–10 min (Figure 5A and Table 2). Moreover, 8-Br-cAMP enhanced the amplitude of the biphasic nitrergic IJP (Figure 5B).
Figure 5.
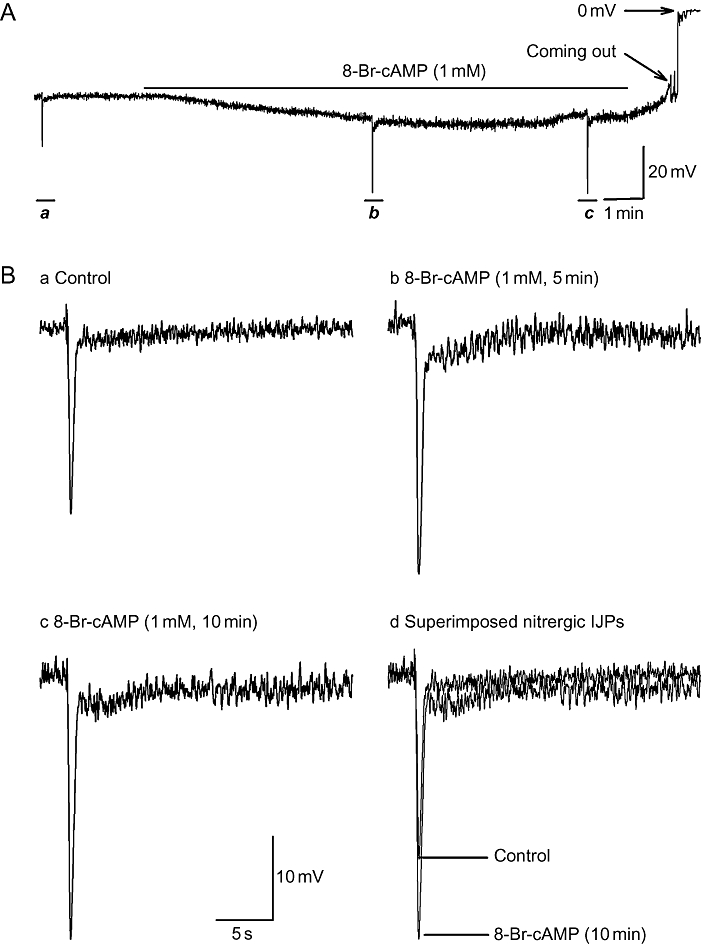
Effects of 8-Br-cAMP on resting membrane potential (RMP), unitary potentials and nitrergic biphasic inhibitory junction potentials (IJPs) in the presence of apamin. Panel A demonstrates that 8-Br-cAMP (1 mM, 10 min) hyperpolarized RMP by about 7 mV over control. (B, panels a–c) Nitrergic IJPs from panel A, on an expanded time scale, in control, 5 min and 10 min after application of 8-Br-cAMP. (B, panel d) Superimposed nitrergic biphasic IJPs in comparison. 8-Br-cAMP increased the amplitude of the biphasic IJPs.
Bath administration of ODQ (20 µM) was employed to interrupt the intracellular cGMP accumulation via the inhibition of guanylate cyclase (Figure 6). ODQ did not affect the RMP, but markedly inhibited the nitrergic IJP. It had no effect on the purinergic IJP (Figure 6B, panel b). The effectiveness of ODQ was validated by the concomitant application of N-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, which had no further inhibitory effect on ODQ-resistant IJPs (Figure 6B, panels c,e). ODQ failed to prevent the RMP hyperpolarization induced by theophylline, excluding a role for intracellularly accumulated cGMP in mediating the inhibitory effects.
Figure 6.
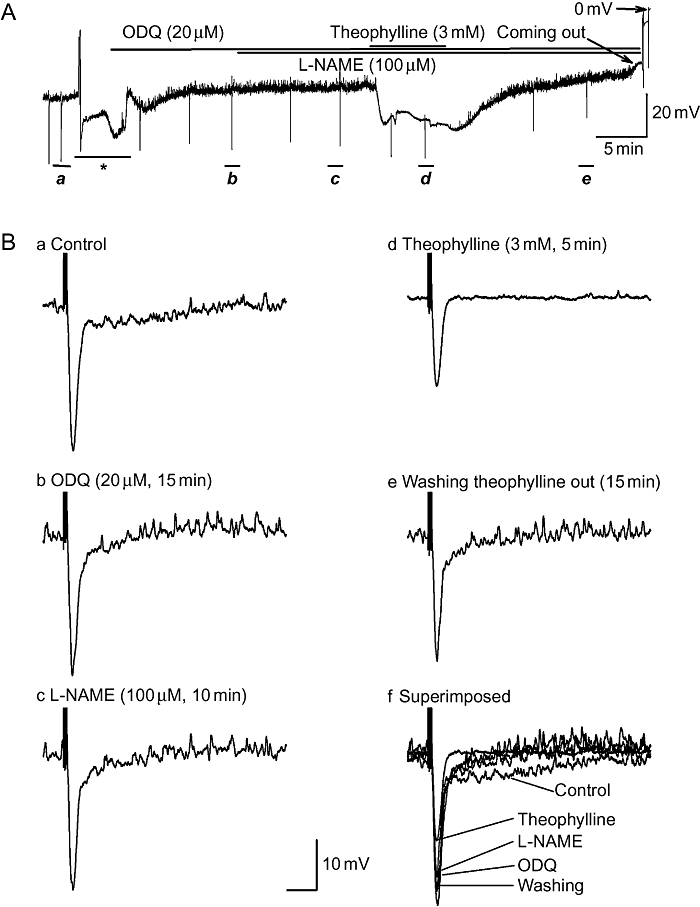
The inhibitory effects of theophylline on the resting membrane potential were not prevented by pre-application of the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ). (A) Full experimental recording of electrical properties before and after bath application of ODQ (20 µM, 15 min) and theophylline (3 mM, 5 min). (B, panels a–e) Inhibitory junction potentials (IJPs) depicted in panel A. displayed at an expanded time scale in control (a), after application of agents (b–d) and recovery (e). Potency of ODQ was validated by further application of N-nitro-L-arginine methyl ester (L-NAME) (100 µM, 10 min), which had no further effect on the IJP. Failure of ODQ to prevent the hyperpolarization excludes the possibility that the hyperpolarization is due to the intracellular accumulation of cGMP resulting from the inhibition of phosphodiesterase by theophylline. The asterisk (*) represents perfusion interference.
Role of SR function in mediating the inhibitory effects of theophylline
The inhibitory effects of theophylline on the RMP andon the biphasic nitrergic IJPs resemble that of caffeine (Zhang and Paterson, 2003), suggesting interruption of Ca2+-dependent signalling primed by spontaneous release of Ca2+ from the SR. This possibility was assessed by experiments in which pre-application of caffeine was used to disable this SR function.
It has been reported that caffeine depletes Ca2+ stores in the SR via massive Ca2+ release (Wang et al., 1992; Large and Wang, 1996). Therefore, it interrupts SR-Ca/CaM kinase II–MLCK–ClCa signalling cascade, leading to RMP hyperpolarization and inhibition of nitrergic IJP in the CSM of opossum LOS (Zhang and Paterson, 2003). In the current studies, caffeine (5 mM) produced maximal RMP hyperpolarization in 1–2 min (Figure 7). Then, the RMP gradually depolarized and reached a steady state in 5 min, at which time the biphasic nitrergic IJPs were abolished. In the presence of caffeine (5 mM), administration of theopylline (3 mM) converted the hyperpolarization to a depolarization (Figure 7A). No furtherstatistically significant effects on purinergic IJPs were observed (Table 2).
Figure 7.
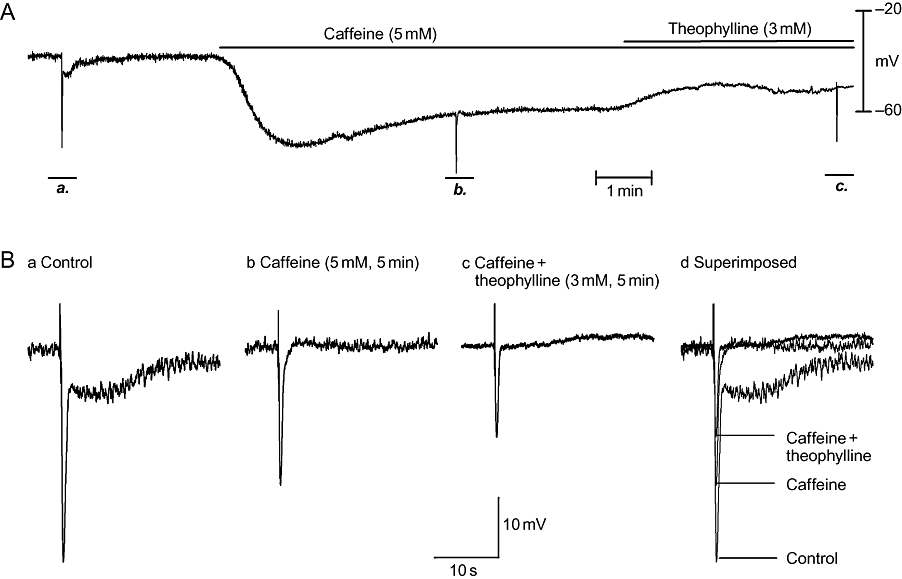
Pre-application of caffeine prevented the resting membrane potential (RMP) hyperpolarization induced by theophylline. In panel A, an experimental recording is shown of the effects of theophylline (3 mM, 5 min) in the presence of caffeine (5 mM, 5 min). Caffeine hyperpolarized the RMP and abolished nitrergic inhibitory junction potentials (IJPs). Further application of theophylline produced RMP depolarization rather than hyperpolarization. (B, panels a–c) IJPs (on an expanded time scale) before and after administration of caffeine and theophylline. (B, panel d) Overlapped IJPs in comparison.
Effects of L-methionine on electrical properties and nitrergic IJPs in colonic smooth muscle
As the effect of L-methionine on the blockade of TREK-1 K+ channels and the inhibition of the nitrergic IJP was initially reported in murine proximal colonic smooth muscle (Koh et al., 2001; 2006; Park et al., 2005), we studied this tissue as well. Experiments were conducted in the presence of atropine (3 µM), guanethidine (3 µM) and SP (1 µM). As shown in Figure 8 and in Table 3, L-NAME (200 µM) depolarized RMP and virtually abolished the long-lasting sIJP, whereas L-methionine (1 mM) depolarized RMP and only partially inhibited the long-lasting sIJP.
Figure 8.
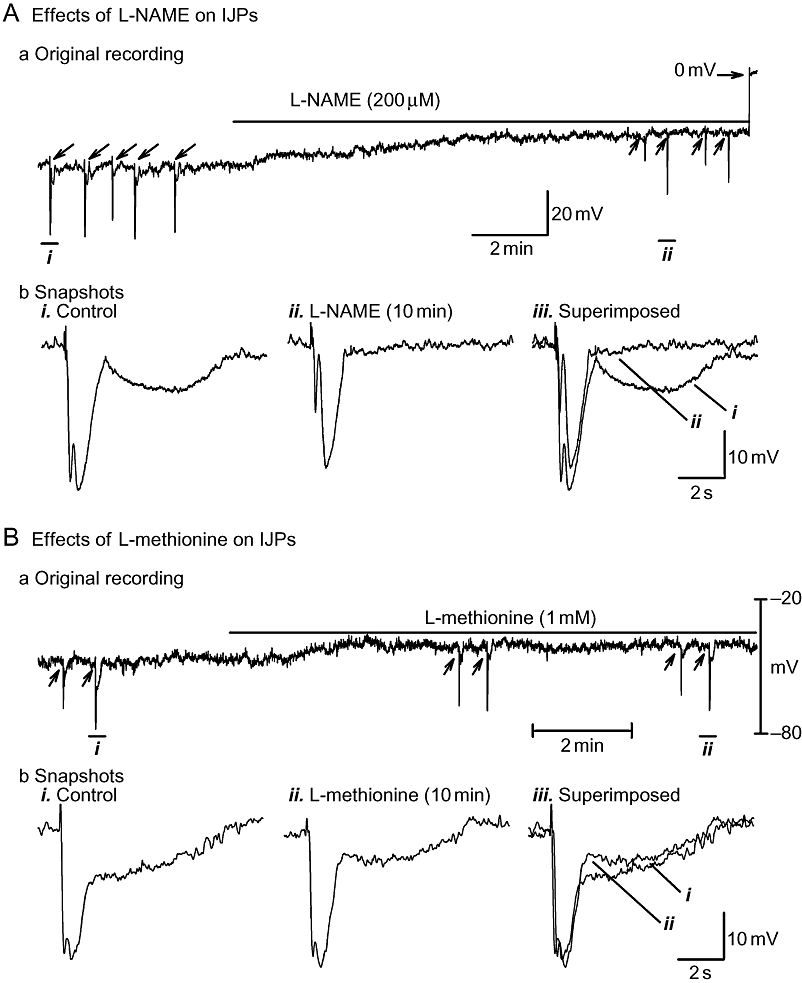
Effects of N-nitro-L-arginine methyl ester (L-NAME) (A) and L-methionine (B) on long-lasting slow inhibitory junction potential (sIJP) in murine proximal colon circular smooth muscle. (A, panel a) and (B, panel a) depict original tracings, demonstrating that both L-NAME and L-methionine depolarize resting membrane potential. (A, panel b) and (B, panel b) show inhibitory junction potentials (IJPs) (on an expanded time scale) induced by four-pulse nerve stimulation before and after drug administration. L-NAME virtually abolishes the long-lasting sIJP, whereas L-methionine causes only partial inhibition.
Table 3.
Effects of L-NAME and L-methionine on IJPs in the murine proximal colon
| MP (mV) | IJP amplitude (mV) | IJP duration (ms) | sIJP amplitude (mV) | sIJP duration (ms) | |
|---|---|---|---|---|---|
| L-NAME | |||||
| Control | −46.0 ± 2.5 | 28.8 ± 1.9 | 1028 ± 41 | 7.7 ± 1.0 | 7007 ± 1188 |
| 200 µM | −34.2 ± 3.0* | 26.7 ± 2.4 | 878 ± 72 | 0.8 ± 0.4* | N/M |
| 10 min | |||||
| L-methionine | |||||
| Control | −48.6 ± 3.3 | 26.8 ± 1.1 | 933 ± 34 | 7.9 ± 0.6 | 6550 ± 549 |
| 1 mM | −40.1 ± 3.0* | 27.9 ± 1.3 | 945 ± 20 | 5.4 ± 0.6* | 7589 ± 1053 |
| 10 min | |||||
L-methionine was tested in eight cells of four mice, and L-NAME was tested in four cells of four mice.
P < 0.05.
Duration: half-amplitude duration.
N/M, not measurable or not measured; IJP, inhibitory junction potential; sIJP, slow inhibitory junction potential.
Opossum muscle strip studies
Effects of L-methionine and theophylline on basal tone and nerve-mediated LOS relaxation
L-methionine (1 mM) increased the basal LOS tone by 135 ± 10% over baseline (n= 6, P= 0.07; measured 10–15 min post-drug application; Figure 9A), whereas theophylline (2.5 mM) decreased the resting tone to 51 ± 4% of baseline (n= 6, P= 0.03). In the presence of L-methionine, LOS relaxation induced by electrical field stimulation was not significantly different from baseline (23 ± 6% vs. 39 ± 10%). Subsequent administration of L-NAME (200 µM) completely antagonized the nerve-mediated relaxation, sometimes unmasking a contraction (Figure 9B). In the presence of theophylline, nerve-mediated LOS relaxation was difficult to discern because the resting tone of the LOS was already low (see above).
Figure 9.
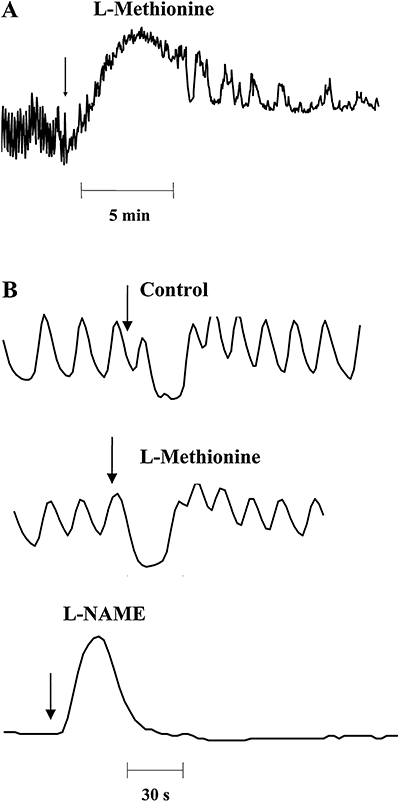
Effects of L-methionine on lower oesophageal sphincter (LOS) basal tone (A) and of nerve-mediated relaxation (B) in opossum LOS. L-methionine significantly increased basal tone but did not inhibit LOS relaxation induced by electrical field stimulation. Subsequent application of N-nitro-L-arginine methyl ester (L-NAME) abolished LOS relaxation and unmasked a contraction. L-NAME also inhibited the spontaneous fluctuations in the basal LOS tone. The arrow in (A) represents the time of application of L-methionine. The arrows in (B) represent the onset of electrical field stimulation. These results are consistent with the electrophysiological data obtained in murine LOS.
Discussion
The current studies found that the putative TREK-1 K+ channel blocker L-methionine depolarized the RMP but failed to affect the nitrergic and purinergic IJPs in the CSM of the murine LOS clasp, and that theophylline, another putative TREK-1 channel blocker, hyperpolarized the RMP and inhibited the nitrergic IJPs in a concentration-dependent fashion. The membrane-permeable cAMP analogue 8-Br-cAMP produced mild hyperpolarization and significantly enhanced the sIJP. Caffeine prevented the hyperpolarization induced by theophylline, but TEA and ODQ failed to do so. Furthermore, tension recording studies using LOS CSM strips from a different species (opossum) showed comparable results.
The TREK-1 K+ channel is a member of the most diverse K+ channel family, which is encoded by more than 80 genes cloned in humans (Patel and Honore, 2002; Honore, 2007). It has been extensively studied in neurons. These channels function as background channels, which are constitutively open at rest and are critical to neural function (Honore, 2007). It has been reported that four transmembrane domains with two pore-forming regions form functional homo- or heterodimeric TREK-1 K+ channels (Doyle et al., 1998). TREK-1 channels are polymodally activated by physical and chemical stimuli, including stretch, heat, intracellular acidosis, lipids and volatile anaesthetics, and are inactivated by actin cytoskeleton and both cAMP/PKA and DG/PKC-dependent phosphorylation (Honore, 2007). Deletion of the TREK-1 gene has provided evidence for the potential role of these channels in pain (Heurteaux et al., 2006), ischaemia, epilepsy (Heurteaux et al., 2004) and depression (Heurteaux et al., 2006).
Recent patch clamp studies have demonstrated that TREK-1 K+ channels are present in gastrointestinal smooth muscle (Koh and Sanders, 2001; Koh et al., 2001; Sanders and Koh, 2006). These channels, with a reported single-channel conductance of 90 pS, were activated by physical stretch, NO and cGMP-dependent protein kinase, and were inhibited by sulphur-containing amino acids and by a wide variety of agents, including theophylline, caffeine, cyclopiazonic acid, thapsigargin, ryanodine and quinidine (Koh and Sanders, 2001; Koh et al., 2001; Park et al., 2005). Furthermore, sulphur-containing amino acids including L-methionine, which block TREK-1 K+ channels, were reported to depolarize RMP and partially inhibited nitrergic IJPs in murine colonic smooth muscle (Park et al., 2005). Therefore, these authors have suggested that the nitrergic IJP is due to the opening of TREK-1 K+ channels.
The current studies also tested the role of TREK-1 K+ channels in the nitrergic neurotransmission to the smooth muscle of murine LOS clasp and colon using two TREK-1 K+ channel blockers, L-methionine and theophylline. As reported by Park et al. (2005), we found that L-methionine depolarizes the RMP. However, we could not demonstrate any inhibitory effect of this agent on the nitrergic IJP in LOS CSM. On the other hand, and in keeping with previous reports (Koh et al., 2001), a modest inhibition (∼35%) of the nitrergic IJP was evident in colonic CSM, suggesting that TREK-1 channel opening may contribute a small component of the nitrergic IJP in this tissue. It is important to note that if opening of TREK-1 K+ channels is responsible for the nitrergic IJP, then TREK-1 K+ channel blockers should depolarize or at least not change the RMP. Surprisingly, theophylline hyperpolarized the RMP and inhibited the nitrergic IJP in a concentration-dependent manner. These inhibitory effects on the nitrergic IJP are unlikely to be related to blockade of the TREK-1 K+ channels. Furthermore, subsequent application of theophylline at the concentration of 3 mM induced RMP depolarization in the presence of cumulatively applied theophylline to a concentration of 1 mM, but theophylline at a single concentration of 3 mM hyperpolarized the RMP. The mechanisms underlying this previously unreported inconsistency were not pursued further in the current studies.
Theophylline was introduced as a bronchodilator nearly 50 years ago (Rabe et al., 1995; Boswell-Smith et al., 2006). Subsequent studies have suggested that theophylline functions as a non-specific PDE inhibitor to increase either intracellular cAMP or cGMP. The increase in intracellular cAMP is linked to activation of BK channels and a resultant decrease in intracellular Ca2+ (Ise et al., 2003). The inhibitory effects of theophylline on the nitrergic IJP in the smooth muscle of murine LOS clasp is unlikely to be attributable to PDE inhibition and consequent accumulation of intracellular cAMP and cGMP based on the following evidence.. Firstly, TEA failed to prevent the theophylline-induced hyperpolarization and inhibition of nitrergic IJPs. Then, application of 8-Br-cAMP, a membrane-permeable analogue of cAMP, hyperpolarized the RMP, but this was much smaller than that evoked by theophylline (∼7 mV vs. ∼27 mV). Furthermore, 8-Br-cAMP significantly augmented, rather than inhibited, the nitrergic IJP amplitude. The mechanism underlying this augmentation was not pursued, but may represent a presynaptic effect. Finally, the blockade of intracellular cGMP generation by ODQ, a guanylate cyclase inhibitor, did not prevent the theophylline-induced RMP hyperpolarization (Figure 6). It is possible that the membrane hyperpolarization of ∼7 mV by cAMP partially contributes to the theophylline-induced hyperpolarization.
The inhibitory effects of theophylline on the electrical properties and on the nitrergic IJP are similar to that of caffeine (Figures 1 and 7), suggesting that theophylline may interrupt the Ca2+-dependent SR-Ca2+/CaM kinase II–MLCK–ClCa signalling cascade (Zhang and Paterson, 2003). Subsequent experiments supported this explanation as interruption of SR function by caffeine prevented the theophylline-induced hyperpolarization. However, the site of action of theophylline on the SR-Ca2+/CaM kinase II–MLCK–ClCa signalling axis remains unknown. In addition, theophylline also partially inhibited the purinergic IJP (Figures 6 and 7). It is possible that this inhibition may be either primary or secondary to the RMP hyperpolarization.
We sought to strengthen these findings by using both a different methodology (muscle tension recordings) and species (opossum). This animal model was chosen because the physiology of its LOS has been extensively studied and it is known that relaxation occurs primarily via nitrergic innervation (Paterson et al., 1992) In keeping with the membrane depolarization induced by L-methionine in murine LOS, this drug increased basal tone in opossum LOS but did not affect the nitrergic LOS relaxation. Furthermore, theophylline induced significant LOS relaxation, as would be expected from the observation that it evoked membrane hyperpolarization in the mouse model.
In summary, the RMP was depolarized, but the nitrergic and purinergic IJPs were not affected by the putative TREK-1 K+ channel blocker L-methionine in the CSM of the murine LOS clasp. In contrast, theophylline, another putative TREK-1 channel blocker, hyperpolarized the RMP and inhibited the nitrergic IJPs. However, the hyperpolarization induced by theophylline was prevented by caffeine but not by TEA and ODQ. It is concluded that that TREK-1 K+ channels are not involved in the nitrergic sIJP in LOS CSM. Not only did L-methionine have no effect on the nitrergic IJP, but the effect of theophylline appears to be due to the interruption of Ca2+-releasing pathways (i.e. caffeine-like effect) rather than via blockade of TREK-1 K+ channels.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research grant # MOP-9978.
Glossary
Abbreviations:
- BK
Ca2+-activated large-conductance K+ channels
- ClCa
Ca2+-activated Cl- channels
- CSM
circular smooth muscle
- fIJP
fast inhibitory junction potential
- L-NAME
N-nitro-L-arginine methyl ester
- LOS
lower oesophageal sphincter
- PDE
phosphodiesterase
- RMP
resting membrane potential
- sIJP
slow inhibitory junction potential
- SP
substance P
- TREK-1
TWIK-related K+ channel 1
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Prog Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl. 1):S252–S257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle. Am J Physiol. 1991a;261:G752–G762. doi: 10.1152/ajpgi.1991.261.5.G752. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated junction potentials in circular muscle of the guinea pig ileum. Am J Physiol. 1991b;261:G742–G751. doi: 10.1152/ajpgi.1991.261.5.G742. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais CJ, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Paterson WG. Esophageal motility. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology. Bethesda, MD: Oxford University Press; 1989. pp. 865–908. [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El YM, Thummler S, Peng XD, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, O'Kane N, Singer C, Ward SM, Sanders KM, Koh SD. Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+ store-active drugs. J Physiol. 2008;586:1169–1184. doi: 10.1113/jphysiol.2007.148718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise S, Nishimura J, Hirano K, Hara N, Kanaide H. Theophylline attenuates Ca2+ sensitivity and modulates BK channels in porcine tracheal smooth muscle. Br. J Pharmacol. 2003;140:939–947. doi: 10.1038/sj.bjp.0705508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001;533:155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM, et al. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- Koh S, O'Kane D, Hwang SJ, Sanders KM, Ward SM. Evidence that Ca2+ release mechanisms are not involved in nitric oxide dependent post-junctional responses in gastrointestinal smooth muscle. Neurogastroenterol Motility. 2006;18:691–692. [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl- conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005;144:1126–1137. doi: 10.1038/sj.bjp.0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Honore E. The TREK two P domain K+ channels. J Physiol. 2002;539:647. doi: 10.1113/jphysiol.2002.014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson WG, Anderson MA, Anand N. Pharmacological characterization of lower esophageal sphincter relaxation induced by swallowing, vagal efferent nerve stimulation, and esophageal distention. Can J Physiol Pharmacol. 1992;70:1011–1015. doi: 10.1139/y92-139. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Magnussen H, Dent G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur Respir J. 1995;8:637–642. [PubMed] [Google Scholar]
- Sanders M, Ozaki H. Excitation-contraction coupling in gastrointestinal smooth muscles. In: Szekeres L, Papp JG, editors. Pharmacology of Smooth Muscle. Berlin: Springer-Verlag; 1994. pp. 331–404. [Google Scholar]
- Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol. 2006;570:37–43. doi: 10.1113/jphysiol.2005.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Paterson WG, Goyal RK. Atypical localization of myenteric neurons in the opossum lower esophageal sphincter. Am J Anat. 1987;180:342–348. doi: 10.1002/aja.1001800404. [DOI] [PubMed] [Google Scholar]
- Uc A, Oh ST, Murray JA, Clark E, Conklin JL. Biphasic relaxation of the opossum lower esophageal sphincter: roles of NO, VIP, and CGRP. Am J Physiol. 1999;277:G548–G554. doi: 10.1152/ajpgi.1999.277.3.G548. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hogg RC, Large WA. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J Physiol. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lang RJ. Effects of intrinsic prostaglandins on the spontaneous contractile and electrical activity of the proximal renal pelvis of the guinea-pig. Br J Pharmacol. 1994;113:431–438. doi: 10.1111/j.1476-5381.1994.tb17007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Nitric oxide contracts longitudinal smooth muscle of opossum oesophagus via excitation-contraction coupling. J Physiol. 2001;536:133–140. doi: 10.1111/j.1469-7793.2001.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Role of Ca2+-activated Cl- channels and MLCK in opossum esophageal smooth muscle. Am J Physiol. 2002;283:G104–G114. doi: 10.1152/ajpgi.00052.2002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol. 2003;140:1097–1107. doi: 10.1038/sj.bjp.0705537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Excitatory purinergic neurotransmission in smooth muscle of guniea-pig taenia caeci. J Physiol. 2005;563:855–865. doi: 10.1113/jphysiol.2004.077636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mashimo H, Paterson WG. Regional differences in nitrergic innervation to smooth muscle of murine lower esophageal sphincter. Br J Pharmacol. 2008;153:517–527. doi: 10.1038/sj.bjp.0707573. [DOI] [PMC free article] [PubMed] [Google Scholar]


