Abstract
Background and purpose:
We determined if chronic sympatho-inhibition with rilmenidine has functional significance for the kidney by altering responses of renal blood flow (RBF) and plasma renin activity (PRA) to stress and acute hypotension in rabbits with renovascular hypertension.
Experimental approach:
RBF to each kidney and renal sympathetic nerve activity (RSNA) to the left kidney were measured in rabbits in which a renal artery clip induced hypertension (2K1C) and in sham-operated rabbits. After 2 weeks, a subcutaneous minipump was implanted to deliver rilmenidine (2.5 mg·kg−1·day−1) to 2K1C rabbits for 3 weeks.
Key results:
After 5 weeks of renal artery stenosis, mean arterial pressure (MAP) was 23% higher and PRA 3-fold greater than in sham-operated rabbits. Blood flow and renal vascular conductance in the stenosed kidney were lower (−75% and −80%) compared with sham, and higher in the non-clipped kidney (68% and 39%). Responses of RBF and PRA to hypotension were similar in 2K1C and sham rabbits. Airjet stress evoked a greater increase in MAP in 2K1C rabbits than sham controls. Chronic rilmenidine normalized MAP, reduced RSNA and PRA, and did not reduce RBF in the stenosed kidney. Responses of RBF (clipped and non-clipped kidney), RSNA and PRA to hypotension and airjet were little affected by rilmenidine.
Conclusions and implications:
Our observations suggest that chronic sympatho-inhibition is an effective antihypertensive therapy in renovascular hypertension. It normalizes MAP and reduces basal PRA without compromising blood flow in the stenosed kidney or altering responses of MAP, haemodynamics and PRA to acute hypotension and stress.
Keywords: renovascular hypertension, renal sympathetic nerve activity, sympathetic nervous system, renin, rabbits, blood pressure, rilmenidine, stress
Introduction
Overactivity of the sympathetic nervous system (SNS) has been implicated in the development and maintenance of essential and renovascular hypertension in humans (Esler, 1995; 2000; Johansson et al., 1999). Not only can the basal level of SNS activity be increased, but also the sympatho-excitation produced by exposure to stressors is exaggerated in hypertensive patients, those with a genetic propensity to hypertension and in animal models of hypertension (Hollenberg et al., 1981; Noll et al., 1996). Our own studies in both 2K1C hypertension and angiotensin II (Ang II) infusion hypertension using conscious rabbits showed that basal renal sympathetic nerve activity (RSNA) was well maintained (Head and Burke, 2003; 2004; Burke et al., 2008; Burke and Head, 2009), and that the sympathetic responses to acute airjet stress were exaggerated in the hypertensive animals (Head and Burke, 2004). More recently, we have shown that sympathetic baroreflex mechanisms are also affected differently by stress in rabbits with renovascular hypertension, with a markedly greater maximum RSNA response to baroreceptor unloading observed in hypertensive animals compared with normotensive rabbits (Burke and Head, 2009). Inhibition of the SNS by ganglionic blockade produces a much greater reduction in blood pressure in rabbits with renovascular or Ang II-induced hypertension than in normal animals (Burke et al., 2008). Our recent findings indicate that this is due to an enhanced sympathetic vasoconstrictor action as a result of hypertrophied vasculature (Moretti et al., 2009). Collectively, these findings support the view that inhibition of the SNS is an appropriate and effective treatment for renovascular hypertension (Head and Burke, 2003).
Current approaches to pharmacological treatment of renovascular hypertension are problematic (Mancia et al., 2007). European recommendations include calcium antagonists and thiazide diuretics with the possible addition of a renin-angiotensin blocker (Mancia et al., 2007). The latter drugs which include angiotensin converting enzyme inhibitors, AT1 receptor antagonists and more recently renin inhibitors, are most effective in renovascular hypertension but there is a significant risk of acute renal failure which limits their applicability (Spence, 2002). Thus, there is a need to explore alternative therapies such as chronic inhibition of the SNS. Centrally acting antihypertensive agents such as clonidine and α-methyldopa have been available since the 1960s and early studies with clonidine in humans suggest that it is very effective in subjects with renal artery stenosis (Kooner et al., 1991). However, they were shown to have significant side-effects and are now rarely used (Amery et al., 1972). Second generation agents such as moxonidine and rilmenidine have overcome the sedative and other side effects as well as the rebound phenomenon associated with clonidine, and are safe and effective treatments (Head, 1995). Both agents are used clinically, their major action being to stimulate imidazoline and α2-adrenoceptors within the rostral ventrolateral medulla to reduce sympathetic tone and hence arterial pressure (Tibirica et al., 1991; Head et al., 1998). Our studies of acute and chronic treatment with rilmenidine in renovascular hypertensive conscious rabbits show that blood pressure is rapidly reduced to normotensive levels with evidence of marked inhibition of RSNA both at rest and during periods of acute stress (Head and Burke, 2003; 2004; Burke and Head, 2009). Furthermore, RSNA baroreflex function curves are shifted to the left with a marked reduction in the maximum RSNA produced by acute hypotension. However, Li and colleagues found rilmenidine to be less effective on renal function in anaesthetized 1K1C hypertensive rats (Li et al., 1994).
The question addressed by the current study is whether these effects of rilmenidine on RSNA have any functional significance for the kidney in renovascular hypertension. As one of the main roles of RSNA is to facilitate the release of renin during hypotensive periods (DiBona and Kopp, 1997), we sought to determine whether chronic inhibition of the SNS with rilmenidine changes the capacity of the kidney to release renin in response to hypotension. Renal nerves are also known to produce renal vasoconstriction, albeit at higher levels of RSNA than are required for the release of renin (DiBona and Kopp, 1995). Thus, the second aim of the current study was to determine whether the renal vasoconstrictor response to airjet stress is altered by renovascular hypertension and whether chronic inhibition of the SNS is able to reverse the effects of renovascular hypertension. For both aspects of the study, we have included a control group of normotensive animals as a benchmark, in order to determine effects of hypertension and also to determine whether the rilmenidine treatment returned responses to or towards ‘normal’.
Methods
Animals
Experiments were conducted in 27 cross-bred rabbits of either sex, weight ranged from 2.4 to 3.4 kg. Observations from some rabbits from this cohort have previously been published (Burke and Head, 2009) but the present data have not been published previously. All procedures were performed in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (1997) and were approved by the Animal Ethics Committee of the Alfred Hospital/BakerIDI Heart and Diabetes Institute. The rabbits were given water ad libitum and had a controlled diet of pellets and vegetables.
Preliminary operations
All surgical operations were performed under halothane anaesthesia (Fluothane) after induction with intravenous administration of propofol (Diprivan, 10 mg·kg−1). Rabbits were treated with carprofen (Rimadyl, 3 mg·kg−1) intravenously 30 min before surgery and subcutaneously 24 h later. At least 2 weeks were allowed between operations.
A radiotelemetry transmitter (model TA11PA-D70, Data Sciences International, St. Paul, MN, USA) and catheter (150 mm long with 0.7 mm diameter tip), was implanted in the aorta via a small branch of the left iliac artery. In the second operation, an inflatable cuff was fitted around the inferior vena cava (IVC) via a right thoracotomy (Korner et al., 1972). During the third operation, a renal artery clip (with gap averaging 0.5 mm) and transit-time ultrasound flow probe (Model 2SB, Transonic Systems, Ithica, NY, USA) were fitted to the right renal artery via a retroperitoneal incision. Another group of animals underwent a similar procedure but the clip was removed immediately (sham-operated group). Rabbits in the sham-operated group were not equipped with a telemetry transmitter. The cable of the flow probe was tunnelled under the skin and the plug positioned so that it could be readily retrieved for an experiment. Arterial pressure was recorded by telemetry and mean arterial pressure (MAP) was measured between 00 h and 08 h daily so the development of hypertension could be monitored. Of the clipped rabbits, 30% initially became hypertensive, which we defined as a stable increase in MAP of at least 15%. If after 1 week, MAP had not increased significantly, the clip was replaced with a smaller one. With this addition, the success rate of achieving hypertension increased to 85% and 19 clipped (2K1C) rabbits progressed to the next stage.
Two weeks after implantation of the renal clip and rabbits were deemed hypertensive, an osmotic minipump was implanted under the skin between the shoulder blades under local anaesthesia (lidocaine HCl 1%). The pump was filled with either normal saline (vehicle) in sham-clipped (n= 8) and 2K1C (n= 9) rabbits or rilmenidine (41.7 mg−1·mL−1·kg−1 dissolved in 2.2 mL saline) in 2K1C rabbits only (n= 10). Minipumps were initially primed and dispensed the drug at 60 µL day−1. Thus, rabbits received rilmenidine, 2.5 mg·kg−1·day−1, or the same volume of saline. Four weeks after clip implantation, a recording electrode was placed on the left renal nerve via a retroperitoneal incision, positioned as far from the kidney as possible and embedded in a silicone elastomer (Kwik-Sil, World Precision Instruments, FL, USA) (Dorward et al., 1985). At the same time, another flow probe (2SB, Transonic Systems) was fitted around the left renal artery between the electrode and the kidney. The cables of both the electrode and flow probe were tunnelled under the skin of the rabbit's back for later retrieval.
Experimental procedures
The telemetric arterial blood pressure signal was obtained via a receiver placed on the door of the animal's cage. The analogue voltage signal was digitized by an acquisition card (PC Plus, National Instruments, Austin, TX, USA) using software written in the LabVIEW programming language (National Instruments). MAP was calculated on a beat to beat basis and instantaneous heart rate (HR) was calculated from the pulse interval. For experiments in the laboratory, pulsatile arterial pressure was measured via a catheter in the ear artery with a Statham P23ID strain gauge pressure transducer (Statham, Hato Rey, Puerto Rico). In the first experiment, MAP measured by telemetry was matched to MAP measured from the ear artery and any adjustment was applied to all further telemetry recordings. Renal blood flow (RBF) was determined with a flow meter (T206, Transonic Systems). Post-ganglionic RSNA was processed through a low-noise differential preamplifier and amplifier combination (Baker IDI Heart and Diabetes Institute Models 187b and 190) with bandwidth 50 Hz to 2 kHz. Amplified potentials were rectified and integrated using an integrator filter with a 20 ms time constant. MAP, HR, RBF and integrated RSNA were digitized and averaged over 2-s periods. The program also detected synchronized bursts of neural activity, allowing us to subtract background electrode noise. Phenylephrine was injected intravenously to abolish neural bursts. The level of RSNA when burst frequency was zero was taken to equal the intrinsic noise level of the recording system and was subtracted from all RSNA for that experiment. RSNA was normalized in each rabbit relative to the maximum 2 s of RSNA evoked by 50 mL smoke directed at the rabbit's nose at the start of each experiment (Burke and Head, 2003). Maximum RSNA was taken to equal 100 normalized units.
On the day of the experiment, the animal was placed in a single rabbit holding box. Under local anaesthesia (lidocaine HCl 1%), catheters were inserted in the central ear artery and marginal ear vein and the ends of the IVC cuff tubing and plugs of the electrode and flow probes were retrieved. A 1-hour recovery period was allowed before the experiment was commenced. Airjet stress was evoked by a fine stream of compressed air at 15 L·min−1 directed at the rabbit's face. Acute hypotension was evoked by inflating the balloon cuff to lower MAP by 5–10 mm Hg. The inflation was continuously adjusted by hand to maintain this level of MAP for 10 min. The cuff was then further inflated until MAP was close to 15 mm Hg lower than control. MAP was maintained at this level for a further 10 min. Thus, two levels of hypotensive stimulus gave a graded stimulus-response relationship. Blood samples were taken to determine plasma renin activity (PRA) before and after 10 min at each of the lower levels of MAP and the rate at which angiotensin I was generated per 60 min incubation (ng·mL−1) was measured via radioimmunoassay (Oliver et al., 1990).
Experimental design and protocols
One minute averages of MAP were continuously measured by radiotelemetry (only in 2K1C rabbits) from before implantation of the clip until the last experiment. These were averaged each day in 15 rabbits over the 8-h period from midnight, to give daily MAP and HR values. Before implantation of the renal clip, each rabbit underwent an experiment in the laboratory in which baseline ear artery MAP and HR were determined. The main experiment was conducted 6 days after implantation of the renal electrode. After an initial control period of 30 min, each animal was subjected to airjet stress lasting 10 min. At least 15 min after cessation of the stress, when parameters had recovered, there was another 15-min control period and blood samples were taken before and during two periods of acute hypotension.
Data analysis
Haemodynamic data collected in the laboratory were averaged every 2 s and displayed on the computer so that movement artefacts could be excluded. For determination of control values, data were averaged over three periods of 5–15 min each. For analysis of the response to airjet stress, the data were binned into 1-min intervals. Responses to acute hypotension were averaged over 10 min. Renal vascular conductance (RVC) was calculated as the ratio of RBF and MAP and is expressed as µL·min−1·mm Hg−1. In two sham-clipped rabbits, the blood flow probe signal from one kidney was noisy and without clear pulsatility, presumably due to misalignment. In these cases, the signal from the other kidney was substituted.
Statistical analysis
Values are expressed as mean ± SEM or mean difference ± SE. Baseline values and treatment effects of airjet stress and acute hypotension were analysed by split plot repeated measures analysis of variance that allowed for within animal and between animal (group) contrasts (Head and Burke, 2001). The contrasts were adjusted for non-orthogonal comparisons by the Bonferroni method and the repeated nature of the data was adjusted for by the Greenhouse–Geisser method.
Drugs
Fluothane was obtained from AstraZeneca (North Ryde, NSW, Australia); propofol (Diprivan) from Zeneca (Cheshire, UK); carprofen (Rimadyl) from Pfizer (West Ryde, NSW, Australia) and lidocaine HCl 1% from Delta West (WA, Australia). Rilmenidine dihydrogeno-phosphate was obtained from I.R.I. Servier (Courbevoie, France) and phenylephrine hydrochloride from Sigma Chemical Co. (St Louis, MO, USA); both these drugs were dissolved in normal saline (0.9% NaCl) obtained from Baxter Healthcare (Old Toongabbie, NSW, Australia). Receptor nomenclature conforms to British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Development of 2K1C hypertension and effects of rilmenidine
The average MAP measured by telemetry in the rabbits' home cages prior to renal artery clipping or sham clipping was 71 ± 2 mm Hg. One week after renal artery clipping, MAP increased by 26% and after 2 weeks by 33% (to 95 ± 3 mm Hg, P < 0.001, Figure 1). Rilmenidine treatment lowered MAP abruptly over the next day to 78 ± 3 mmHg (n= 8) and MAP remained at this level for 7 days. Over the remaining 2 weeks, MAP further declined (Flinear trend over time= 17, P < 0.001) to be only 5 mm Hg above pre-clip values (Figure 1). In vehicle-treated 2K1C rabbits, MAP remained 36% (102 ± 1 mm Hg, n= 7) above basal levels throughout the 3-week treatment period (Figure 1). Renal artery stenosis initially induced a small tachycardia (+29 ± 9 b·min−1 from 179 ± 8 b·min−1) but HR returned to close to basal levels after 1 week and remained at this level (Figure 1). Rilmenidine treatment initially lowered HR by 20 ± 9 b·min−1 and over the next 2 weeks, HR was 13 ± 3 b·min−1 below basal levels (P < 0.001, Figure 1). The non-clipped normotensive control animals were not studied in this part of the experiment.
Figure 1.
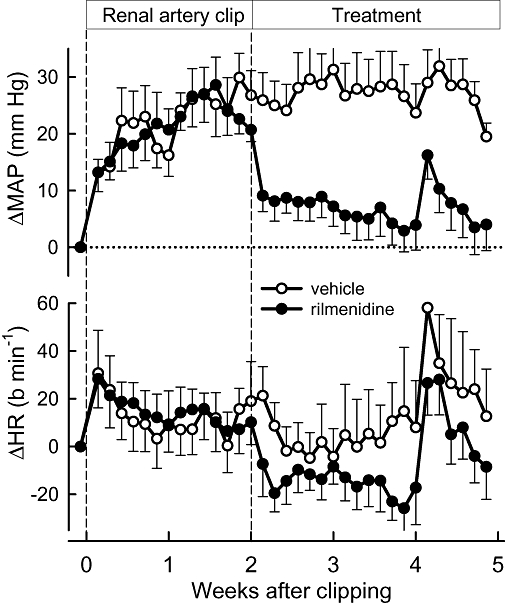
Changes from basal in mean arterial pressure (ΔMAP, upper panel) and heart rate (ΔHR, lower panel) measured daily by telemetry in the rabbits' home cages before and after renal clipping. Data are from vehicle- and rilmenidine-treated 2K1C rabbits during the 5-week protocol from before renal clipping (vertical dashed line at Week 0), the start of treatment (vertical dashed line at Week 2) and the main experiment at Week 5. The abrupt increase in MAP and HR in Week 4 was due to surgery to implant the renal electrode and flow probe. Error bars are SEM indicating variance between animals.
Effects of renal clipping and rilmenidine on haemodynamic variables, PRA and RSNA
Resting values were recorded in the laboratory 5 weeks after renal clipping or sham-clipping (i.e. 3 weeks after beginning treatment with rilmenidine or its vehicle). At this time in 2K1C rabbits, MAP was 23% higher, RSNA was 40% greater and PRA was threefold greater than in sham-clipped rabbits (Table 1). However HR was similar in the two groups (Table 1). After rilmenidine treatment of 2K1C rabbits, MAP and RSNA were markedly lower than in 2K1C vehicle-treated rabbits (−16% and −52%, respectively) such that the levels were similar to those in sham-clipped rabbits (Table 1). Rilmenidine treatment reduced HR to levels that were 18–20% less than in both sham and 2K1C rabbits. Rilmenidine treatment significantly reduced PRA in 2K1C rabbits (Table 1).
Table 1.
Basal haemodynamic values, renal sympathetic nerve activity and plasma renin activity levels in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits
| Sham+vehicle | 2K1C+vehicle | PNT:HT | 2K1C+rilmen | PHT:Ril | PRil:NT | |
|---|---|---|---|---|---|---|
| MAP (mmHg) | 82.5 ± 1.7 | 101.6 ± 4.0 | *** | 85.7 ± 3.1 | *** | NS |
| HR (b·min−1) | 239 ± 17 | 233 ± 6 | NS | 190 ± 7 | *** | *** |
| RSNA (nu) | 8.3 ± 1.6 | 11.7 ± 2.6 | * | 5.6 ± 1.4 | *** | NS |
| PRA (ng·mL−1) | 3.4 ± 0.6 | 12.7 ± 2.1 | *** | 8.0 ± 2.3 | * | NS |
| RBF (non-clipped kidney) (mL·min−1) | 38.9 ± 4.5 | 65.1 ± 5.7 | *** | 48.7 ± 4.8 | *** | * |
| RBF (clipped kidney) (mL·min−1) | 49.5 ± 2.6 | 12.4 ± 2.9 | *** | 18.5 ± 6.2 | NS | *** |
| Total RBF (mL·min−1) | 88.4 ± 5.4 | 81.5 ± 5.3 | NS | 66.5 ± 5.4 | ** | *** |
| RVC (non-clipped kidney) (µL·min−1·mm Hg−1) | 464.8 ± 51.0 | 647.2 ± 57.5 | *** | 587.8 ± 67.6 | NS | * |
| RVC (clipped kidney) (µL·min−1·mm Hg−1) | 599.5 ± 36.5 | 118.4 ± 26.6 | *** | 211.1 ± 64.8 | * | *** |
| Total RVC (µL·min−1·mm Hg−1) | 1064.3 ± 60.0 | 783.7 ± 56.7 | *** | 772.3 ± 75.8 | NS | *** |
| Kidney weight (non-clipped) (g) | 9.7 ± 0.4 | 13.3 ± 0.4 | *** | 12.3 ± 0.5 | NS | ** |
| Kidney weight (clipped) (g) | 10.3 ± 0.5 | 6.2 ± 0.8 | ** | 6.5 ± 1.0 | NS | ** |
| RBF/g kidney (non-clipped) (mL·min−1·g−1) | 4.3 ± 0.3 | 4.9 ± 0.5 | NS | 4.0 ± 0.4 | NS | NS |
| RBF/g kidney (clipped) (mL·min−1·g−1) | 4.8 ± 0.3 | 2.0 ± 0.4 | ** | 2.4 ± 0.6 | NS | ** |
| Body weight (kg) | 3.2 ± 0.1 | 3.0 ± 0.1 | NS | 2.9 ± 0.1 | NS | * |
| n | 8 | 9 | 10 |
P < 0.05,
P < 0.01 and
P < 0.001, NS non-significant for (i) PNT:HT comparison of data from 2K1C rabbits treated with vehicle with sham-operated rabbits; (ii) PHT:Ril comparison of data from 2K1C rabbits treated with vehicle with 2K1C rabbits treated with rilmenidine; (iii) PRil:NT comparison of data from 2K1C rabbits treated with rilmenidine with sham-operated rabbits.
Values are mean ± SEM indicating variance between animals.
MAP, mean arterial pressure; HR, heart rate; RSNA, renal sympathetic nerve activity; PRA, plasma renin activity; RBF, renal blood flow; RVC, renal vascular conductance; rilmen, rilmenidine.
Effects of renal clipping and rilmenidine treatment on RBF and kidney size
Renal blood flow to the stenosed kidney was severely reduced (75% less than sham-clipped animals) and there was a corresponding reduction in RVC in that kidney (80% less than sham-clipped rabbits, Table 1). The clipped kidneys were on average 40% lighter than those of sham-operated rabbits (P < 0.01). Thus, blood flow per gram of tissue was 58% less in the clipped kidney than in the corresponding kidney of sham-clipped animals. These values are closely similar to those obtained in rats with moderate stenosis where RBF was reduced to 2.5 mL·min−1·g−1 in the stenosed kidney (Sigmon and Beierwaltes, 1994). Conversely, blood flow to the non-clipped kidney was 68% greater than to the corresponding kidney in sham-clipped rabbits and RVC in that kidney was 39% greater than in the sham-operated group (Table 1). The non-clipped kidney was 38% heavier than the corresponding kidney of sham-clipped rabbits, so that blood flow per gram of tissue was similar for the left kidneys of the two groups. In 2K1C rabbits, total RBF was similar to that in sham-operated rabbits but total RVC was 26% less, compared with values in sham-operated animals. RBF in the non-clipped kidney was 25% less in rilmenidine-treated rabbits than in vehicle-treated rabbits. RBF in the clipped kidney tended to be greater in rilmenidine-treated than vehicle-treated rabbits (by 49%), but this apparent effect did not reach statistical significance (P= 0.1). Total RBF was 18% less in rilmenidine-treated than vehicle-treated 2K1C rabbits. The mass of the clipped and non-clipped kidneys in 2K1C rabbits was little affected by rilmenidine, so the profile of effects of rilmenidine on RBF was similar, regardless of whether RBF was corrected for kidney weight (Table 1).
Effects of renal clipping and rilmenidine treatment on left ventricular weight
Five weeks after the renal artery had been clipped, left ventricular weight was 15% greater than in sham-clipped rabbits (1.56 ± 0.05 g·kg−1 vs. 1.36 ± 0.04 g·kg−1, P < 0.01). After rilmenidine treatment, the average weight of the left ventricle was 8% less than that of vehicle-treated 2K1C rabbits (1.43 ± 0.04 g·kg−1, P < 0.05) and was not significantly different from that of sham-operated rabbits.
Effects of renal clipping and rilmenidine treatment on responses to hypotension
Acute hypotension was evoked by inflation of the balloon cuff for two periods lasting 10 min each. The average falls in MAP during each period were −7.7 ± 0.6 mm Hg and −14.5 ± 0.9 mm Hg from control (n= 26) and were similar in all groups. The hypotension produced similar marked increases in PRA and RSNA in all rabbits but the increase in HR was 60% greater in rilmenidine-treated 2K1C rabbits than in vehicle-treated 2K1C rabbits (Figure 2, Table 2). Acute hypotension also produced reductions in RBF to both the clipped and non-clipped kidneys. RBF changes in the latter were indistinguishable between the three groups but blood flow reductions were less in the clipped kidney of both vehicle- and rilmenidine-treated rabbits compared with the equivalent but unclipped kidney of the sham-operated rabbits (Figure 3, Table 2). Hypotension evoked a stepwise reduction in RVC (constriction) in both kidneys of sham-operated rabbits, which averaged 14% (Figure 4, Table 2). In 2K1C rabbits, total RVC was not significantly reduced by hypotension due to a lack of response in both kidneys. By contrast, the constriction responses (reduction in conductance) in rilmenidine-treated rabbits from both clipped and non-clipped kidneys were similar to those observed in sham-clipped rabbits (Figure 4, Table 2).
Figure 2.
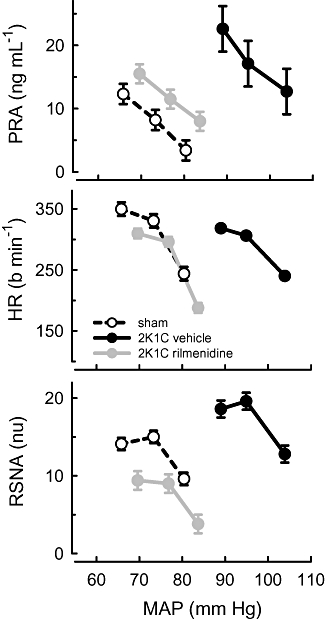
Average values for plasma renin activity (PRA), heart rate (HR) and renal sympathetic nerve activity (RSNA, normalized units) in response to three levels of mean arterial pressure (MAP). MAP was lowered from control in 2 steps by inflation of a cuff on the inferior vena cava in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits. For each line, the control MAP is the highest MAP value. Error bars are SEM indicating within animal variance.
Table 2.
Averaged changes from control in response to two levels of hypotension
| Sham+vehicle | 2K1C+vehicle | PNT:HT | 2K1C+rilmen | PHT:Ril | PRil:NT | |
|---|---|---|---|---|---|---|
| MAP (mmHg) | −10.8 ± 1.1 | −11.9 ± 1.5 | NS | −10.5 ± 1.1 | NS | NS |
| HR (b·min−1) | 96 ± 16 | 72 ± 9 | NS | 115 ± 12 | ** | NS |
| RSNA (nu) | 5.0 ± 1.0 | 6.3 ± 1.7 | NS | 7.2 ± 2.1 | NS | NS |
| PRA (ng·mL−1) | 6.8 ± 1.8 | 7.2 ± 3.5 | NS | 5.5 ± 1.4 | NS | NS |
| RBF (non-clipped kidney) (mL·min−1) | −12.5 ± 2.1 | −8.8 ± 3.0 | NS | −9.8 ± 3.4 | NS | NS |
| RBF (clipped kidney) (mL·min−1) | −11.3 ± 2.9 | −5.8 ± 1.5 | * | −6.3 ± 1.8 | NS | * |
| Total RBF (mL·min−1) | −23.8 ± 4.7 | −13.7 ± 4.4 | * | −13.3 ± 3.8 | NS | * |
| RVC (non-clipped kidney) (µL·min−1·mmHg−1) | −96 ± 23 | −15 ± 32 | NS | −61 ± 50 | NS | NS |
| RVC (clipped kidney) (µL·min−1·mmHg−1) | −74 ± 41 | −43 ± 13 | NS | −68 ± 24 | NS | NS |
| Total RVC (µL·min−1·mmHg−1) | −171 ± 59 | −35 ± 45 | * | −88 ± 56 | NS | NS |
P < 0.05,
P < 0.01, NS non-significant for (i) PNT:HT comparison of data from 2K1C rabbits treated with vehicle with sham-operated rabbits; (ii) PHT:Ril comparison of data from 2K1C rabbits treated with vehicle with 2K1C rabbits treated with rilmenidine; (iii) PRil:NT comparison of data from 2K1C rabbits treated with rilmenidine with sham-operated rabbits.
Values are changes from control during the two periods of hypotension, which have been averaged to give mean difference ± SE indicating between animal variance.
MAP, mean arterial pressure; HR, heart rate; RSNA, renal sympathetic nerve activity; PRA, plasma renin activity; RBF, renal blood flow; RVC, renal vascular conductance; rilmen, rilmenidine.
Figure 3.
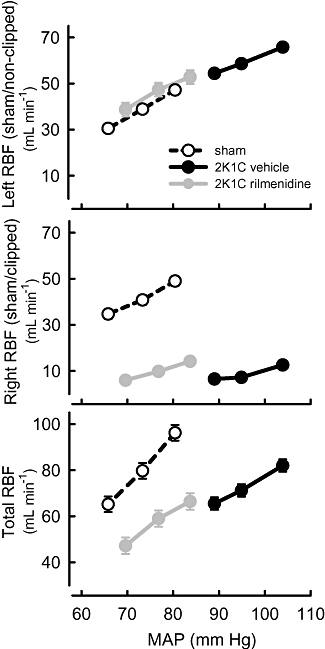
Average values for renal blood flow (RBF) in left (sham or non-clipped), right (sham or clipped) and both (total) kidneys in response to three levels of mean arterial pressure (MAP) in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits. For each line, the control MAP is the highest MAP value. Error bars are SEM indicating within animal variance.
Figure 4.
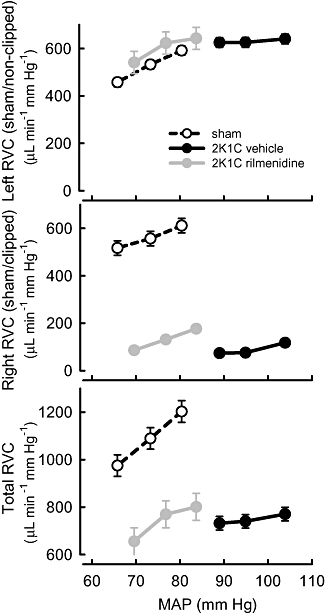
Average values for renal vascular conductance (RVC) in left (sham or non-clipped), right (sham or clipped) and both (total) kidneys in response to three levels of mean arterial pressure (MAP) in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits. For each line, the control MAP is the highest MAP value. Error bars are SEM indicating within animal variance.
Relationship between PRA and RSNA before and during hypotension
In order to examine whether 2K1C hypertension and/or rilmenidine treatment altered the relationship between PRA and the levels of sympathetic activity, these values were correlated using individual values from before, and after 10 and 20 min of hypotension. In normotensive rabbits, there was a positive linear correlation between absolute normalized RSNA levels and the log PRA (r= 0.47, d.f. = 20, P < 0.05, Figure 5). However, no such relationship existed in 2K1C animals or those 2K1C rabbits treated with rilmenidine (r < 0.1, Figure 5).
Figure 5.
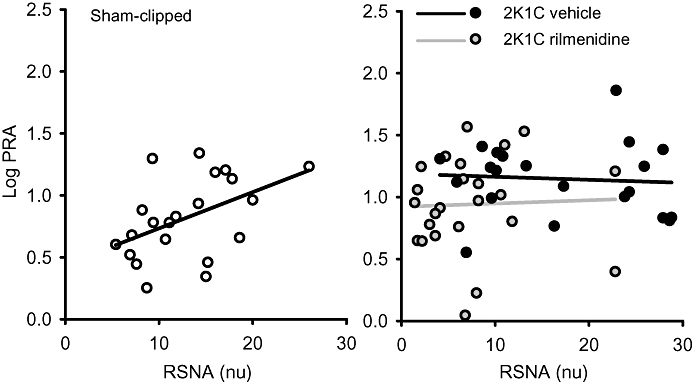
Correlation between renal sympathetic nerve activity (RSNA, normalized units) and log plasma renin activity (PRA) recorded in individual sham-clipped and 2K1C vehicle- and 2K1C rilmenidine-treated rabbits. Solid line represents regression lines for each group.
Effects of renal clipping and rilmenidine treatment on responses to airjet stress
In all rabbits, airjet stress evoked marked increases in RSNA, HR and MAP. RBF was well maintained during airjet stress so calculated RVC fell (Figures 6–8). In 2K1C vehicle-treated rabbits, the effects of airjet stress on RSNA and HR were similar to those in sham-operated rabbits, but the increase in MAP was more than double (P < 0.001, Figure 6). RBF was not altered in any group (Figure 7) but airjet stress markedly reduced RVC in sham-operated rabbits equally in both kidneys. However, in 2K1C vehicle-treated rabbits, airjet stress produced a 66% greater reduction in RVC in the non-clipped kidney (P < 0.05, Figure 8) but a diminished reduction in RVC in the stenosed kidney compared with the corresponding kidneys of sham-operated rabbits (P < 0.001, Figure 8). Rilmenidine treatment in 2K1C animals did not alter the enhanced MAP response to airjet stress nor did it affect RSNA and HR responses (Figure 6). However, in 2K1C rilmenidine-treated rabbits, the RVC response in the clipped kidney was threefold greater than that in 2K1C vehicle-treated rabbits (P < 0.01), thus partially restoring responses towards those observed in the sham-operated rabbits (Figure 8). Rilmenidine treatment tended to further augment the RVC response in the non-clipped kidney (P= 0.07, Figure 8). Thus, total RVC responses in 2K1C rilmenidine-treated rabbits were similar to those of sham animals but markedly enhanced compared with 2K1C vehicle-treated animals (P < 0.05, Figure 8).
Figure 6.
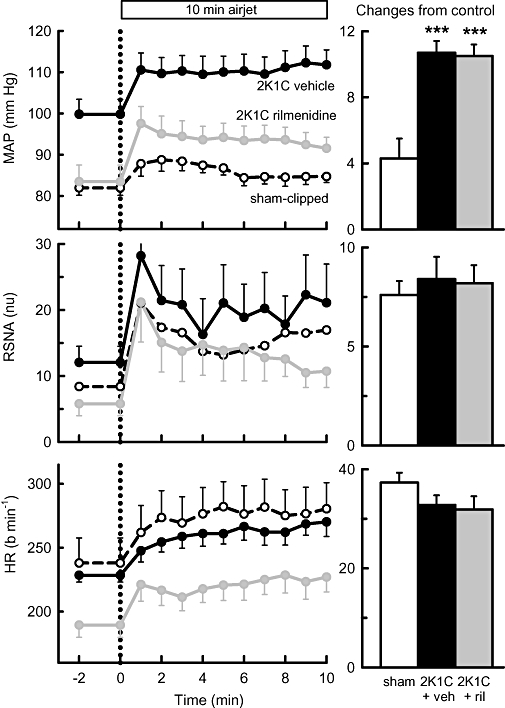
Left panels: average mean arterial pressure (MAP), renal sympathetic nerve activity (RSNA, normalized units) and heart rate (HR, beats·min−1) in response to 10 min exposure to airjet in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits. Right panels: changes from control during airjet stress averaged over 10 min. Errors are SEM indicating between animal variance. ***P < 0.001 for 2K1C vehicle-treated and rilmenidine-treated 2K1C rabbits compared with sham-clipped rabbits. ril, rilmenidine; veh, vehicle.
Figure 8.
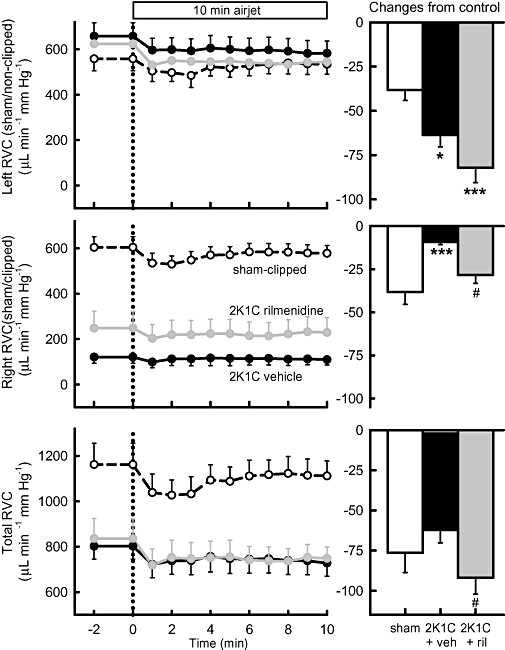
Left panels: average renal vascular conductance (RVC) in left (sham or non-clipped), right (sham or clipped) and both (total) kidneys in response to 10 min exposure to airjet in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits. Right panels: changes from control during airjet stress averaged over 10 min. Errors are SEM indicating between animal variance. *P < 0.05, ***P < 0.001 for vehicle-treated and rilmenidine-treated 2K1C rabbits compared with sham-clipped rabbits and #P < 0.05 for 2K1C vehicle-treated compared with 2K1C rilmenidine-treated rabbits. Veh, vehicle; ril, rilmenidine.
Figure 7.
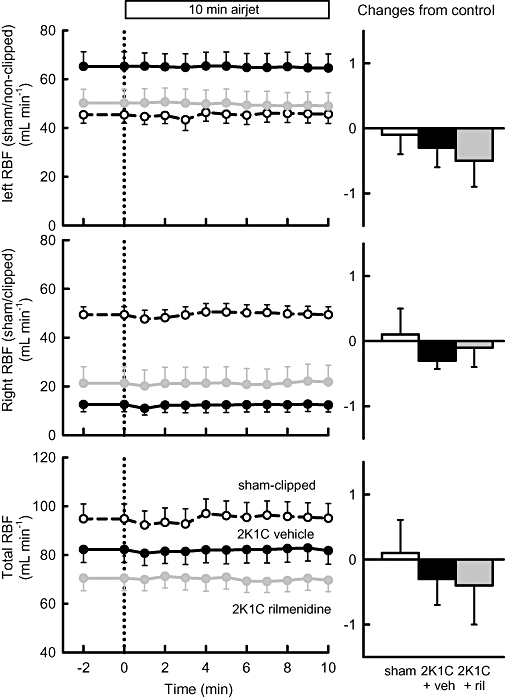
Left panels: average renal blood flow (RBF) in left (sham or non-clip), right (sham or clip) or both (total) kidneys in response to 10 min exposure to airjet in sham-clipped, 2K1C vehicle-treated and 2K1C rilmenidine-treated rabbits. Right panels: changes from control during airjet stress averaged over 10 min. Errors are SEM indicating between animal variance. Veh, vehicle; ril, rilmenidine.
Discussion and conclusions
Our present study provided three important new findings that are relevant to consideration of the use of centrally acting sympatholytic agents for pharmacotherapy in renovascular hypertension. Firstly, we found that chronic rilmenidine treatment not only normalized arterial pressure and reduced RSNA, but that it also markedly reduced basal PRA. This suggests that activation of the renin-angiotensin system in 2K1C hypertension in rabbits is at least partly mediated by renal nerves. Secondly, we found that the lowering of blood pressure with chronic rilmenidine treatment did not further compromise blood flow to the stenotic kidney, suggesting that rilmenidine is unlikely to induce renal failure in renovascular hypertension, as is sometimes seen with agents that directly block the renin-angiotensin system. Thirdly, we found that rilmenidine treatment had no effect on RBF or PRA responses to acute hypotension and airjet stress. Thus, reflex control of the kidney appears little affected by rilmenidine. Taken together, these observations provide further support for the use of centrally acting antihypertensive agents for treatment of renovascular hypertension.
Overall, it appears that chronic rilmenidine treatment is a very effective way of normalizing blood pressure in rabbits with renovascular hypertension. While the initial decrease in MAP was similar to that which we had previously observed during acute treatment with rilmenidine (Head and Burke, 2004), the hypotensive effect increased with time in the 2K1C hypertensive rabbits, such that the final blood pressure was only slightly above the values observed prior to clipping or those observed in sham-operated rabbits. Importantly, we found that rilmenidine markedly reduced RSNA and lowered PRA to a level approximately halfway between the sham and hypertensive groups. Because basal RSNA decreased by one-half, it is likely that this accounted for the reduction in PRA, as the degree of stenosis (assessed by reduction in RBF per gram of stenosed kidney) was similar in both groups. Rilmenidine has also been shown to lower PRA in spontaneously hypertensive rats (SHR) and in dogs (Laubie et al., 1985; Ghaemmaghami et al., 1990) but there have been no other reports of the effect of rilmenidine on PRA in renovascular hypertension. The positive impact of a lower RSNA and PRA after rilmenidine treatment was associated with a tendency for greater blood flow and improved vascular conductance in the stenotic kidney. These findings are more surprising, considering that the blood pressure had been effectively normalized by the treatment, thus reducing the pressure head seen by the stenotic kidney. Thus, chronic rilmenidine treatment appears to allow maintenance, if not improvement, of RBF in the stenosed kidney in the face of the complete abolition of hypertension (Spence, 2002). Indeed, glomerular filtration rate (GFR) is maintained in mild hypertensive patients treated with rilmenidine (Licata et al., 1993) and even subjects with renal failure maintain GFR when treated with moxonidine (Vonend et al., 2003).
A major finding was that the release of renin to a hypotensive challenge was similar in 2K1C hypertensive, 2K1C rilmenidine-treated and sham normotensive rabbits. During acute hypotension, hydrostatic pressure in the afferent arteriole is modulated by RSNA as well as by non-neural mechanisms acting on the macula densa (Kopp and DiBona, 1993; Wagner et al., 1999). Yet despite differences in systemic blood pressure and presumably renal perfusion pressure, the overall PRA response to acute hypotension was relatively normal. This is in accord with findings of Wagner and colleagues using clipped and denervated rat kidneys who suggested that renal nerve activity was an important determinant of the gain of renin stimulation during reduced renal arterial pressure (Wagner et al., 1999). We also observed that the positive correlation between absolute levels of PRA and RSNA, which exists in normal rabbits, was not present in the vehicle- or rilmenidine-treated 2K1C rabbits. Presumably, the correlation is reflective of the importance of RSNA in normal control of renin release. The loss of the dependence of PRA on RSNA in renal artery stenosis may reflect the fact that reduced perfusion pressure in the clipped kidney provides a further stimulus for renin release, which is at least partly independent of the renal nerves.
Rilmenidine is known to improve cardiac baroreflex sensitivity in hypertensive subjects (Finta et al., 2006) and hypertensive animals (Burke and Head, 2009) but decrease the sympathetic baroreflex gain (Burke and Head, 2009). The latter effect of acute or chronic rilmenidine is due to a marked inhibition of the upper plateau of the RSNA baroreflex which we expected to translate into a lesser increase in renin release (Burke and Head, 2009). However, in the current study, the rise in RSNA and the subsequent renin release were equivalent in all three groups. One explanation for this is that the slowness of renin release permits a large degree of baroreceptor resetting, which eliminates the differences in RSNA response (observed during rapid pressure changes) in the different groups. The mechanism of rapid baroreceptor resetting thus ensures that the responsiveness of hypotension-induced renin release is well maintained during 2K1C hypertension as well as during chronic sympathetic inhibition.
Our study also examined the cardiovascular responsiveness to an acute emotional stress in hypertensive, normotensive and rilmenidine-treated rabbits. In 2K1C rabbits, the pressor responses to airjet stress were markedly enhanced but the tachycardia and increase in RSNA were similar to those observed in sham-operated rabbits. While rilmenidine did not attenuate the pressor response to airjet stress at all, the level of blood pressure reached was less than the basal level in untreated 2K1C hypertensive rabbits due to the drug's overall antihypertensive action. Importantly, rilmenidine restored renal vasoconstrictor responses to airjet stress in the clipped kidney and increased slightly the response in the non-clipped kidney. Overall, the reduction in conductance (vasoconstriction) of both kidneys was greater than in the untreated 2K1C rabbits. While increases in RSNA, which were similar in all groups, would be expected to contribute to the vasoconstriction, the uniform maintenance of RBF during stress may be in part also due to renal autoregulation (Guild et al., 2004). We have previously found that the RSNA response to airjet stress was greater after 6 weeks of 2K1C hypertension and not reduced by acute rilmenidine (Head and Burke, 2004). We have now shown that chronic administration of rilmenidine in 5-week hypertensive rabbits also had little effect on the responses to airjet stress. Thus, both acute and chronic rilmenidine treatment preserved the sympathetic response to stress, but in both cases the lowering of MAP and basal RSNA levels before and during stress meant that the overall cardiovascular impact was less. Acute and chronic rilmenidine were equally effective in reducing blood pressure, heart rate and RSNA prior to the airjet stimulus. Thus, it would appear that the central pathways involved in stress do not adapt with chronic treatment with rilmenidine, at least over the several weeks studied here. To our knowledge, there have been few other reports on stress responses during chronic rilmenidine treatment. Two studies using short-term treatment have shown no effect on the responses to mental stress in humans (Panfilov et al., 1995; Fauvel et al., 1999). Esler et al. (2004) examined the effects of mental stress in a cross-over designed study using a 2-week treatment with rilmenidine or placebo. They found that rilmenidine lowered blood pressure and total noradrenaline spillover but the acute response (increase) to mental stress was preserved. These findings closely agree with our studies in conscious rabbits.
Our current findings may have important implications for pharmacotherapy of renovascular hypertension. They indicate that overall, chronic rilmenidine will lessen the negative impact of the stenosis by reducing renal perfusion pressure and circulating levels of Ang II, but will not compromise perfusion of the stenosed kidney. The importance of the current study was that we needed to use chronic rilmenidine to see this benefit. The experiments also showed that chronic rilmenidine restored the ability of the right (clipped) kidney to be able to constrict in the face of a hypotensive stimulus and during airjet stress. Restoration of renal vasoconstriction may at first glance appear to be non-beneficial. However, the normal responsiveness of the kidney to altered RSNA and perfusion pressure allows for fine adjustments in renal function that are essential for long-term maintenance of extracellular fluid volume (DiBona, 2002). Another important benefit of chronic rilmenidine treatment in renovascular hypertension is reflected in the observation of diminished left ventricular hypertrophy. Cardiac hypertrophy is a major contributor to adverse outcomes in various forms of hypertension, including renovascular hypertension, and a reduction in the degree of hypertrophy is seen as crucial in its reversal (Tsilakis et al., 2008). A similar reversal has been demonstrated in SHR and in human hypertensives chronically treated with rilmenidine (Bobik et al., 1998; Farsang et al., 2003).
We conclude that chronic sympatho-inhibition appears to be a beneficial therapy for renovascular hypertension. It normalizes many of the adverse cardiovascular changes that occur without blunting the acute cardiovascular, renal vasculature and renin release responses to acute hypotension and airjet stress. Importantly, RBF in the clipped kidney was maintained or even improved by rilmenidine treatment, despite the fact that arterial pressure was reduced to normotensive levels. Furthermore, rilmenidine appears to restore to normality responses of the renal circulation to altered RSNA and/or arterial pressure induced by acute hypotension and airjet stress. This latter effect may provide more capacity for the renal circulation to respond to physiological challenges, and so for the kidney to maintain homeostasis of extracellular fluid volume.
Acknowledgments
The study was supported by grants from Institut de Recherches Internationales Servier & Compagnie-Developpement (Courbevoie, France) and the National Health and Medical Research Council of Australia.
Glossary
Abbreviations:
- 2K1C
two kidneys, one clip
- Ang II
angiotensin II
- GFR
glomerular filtration rate
- HR
heart rate
- IVC
inferior vena cava
- MAP
mean arterial pressure
- nu
normalized units
- PRA
plasma rennin activity
- RBF
renal blood flow
- RSNA
renal sympathetic nerve activity
- RVC
renal vascular conductance
- SHR
spontaneously hypertensive rats
- SNS
sympathetic nervous system
Conflicts of interest
This study was supported by a grant from Institut de Recherches Internationales Servier & Compagnie-Développement (Courbevoie, France).
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amery AK, Bossaert H, Fagard RH, Verstraete M. Clonidine versus methyldopa. A double blind cross-over study comparing side effects of clonidine and methyldopa administered together with chlorthalidone at a dosage producing the same blood pressure lowering effect. Acta Cardiol. 1972;21:82–99. [PubMed] [Google Scholar]
- Australian Government Publishing Service. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. 6th. Canberra: Australian Government Publishing Service; 1997. [Google Scholar]
- Bobik A, Dilley R, Kanellakis P. Sympatho-adrenal mechanisms regulating cardiovascular hypertrophy in primary hypertension: a role for rilmenidine? J Hypertens. 1998;16:S51–S54. [PubMed] [Google Scholar]
- Burke SL, Evans RG, Moretti J-L, Head GA. Levels of renal and extrarenal sympathetic drive in Ang II-induced hypertension. Hypertension. 2008;51:878–883. doi: 10.1161/HYPERTENSIONAHA.107.100800. [DOI] [PubMed] [Google Scholar]
- Burke SL, Head GA. Method for in-vivo calibration of renal sympathetic nerve activity in rabbits. J Neurosci Methods. 2003;127:63–74. doi: 10.1016/s0165-0270(03)00121-3. [DOI] [PubMed] [Google Scholar]
- Burke SL, Head GA. Cardiac and renal baroreflex control during stress in conscious renovascular hypertensive rabbits: effect of rilmenidine. J Hypertens. 2009;27:132–141. doi: 10.1097/hjh.0b013e328317a7a7. [DOI] [PubMed] [Google Scholar]
- DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Kopp UC. Neural Control of Renal Function – Role in Human Hypertension. New York: Raven; 1995. [Google Scholar]
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- Dorward PK, Riedel W, Burke SL, Gipps J, Korner PI. The renal sympathetic baroreflex in the rabbit. Arterial and cardiac baroreceptor influences, resetting, and effect of anesthesia. Circ Res. 1985;57:618–633. doi: 10.1161/01.res.57.4.618. [DOI] [PubMed] [Google Scholar]
- Esler M. Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol. 1995;26:S24–S28. [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Esler M, Lux A, Jennings G, Hastings J, Socratous F, Lambert G. Rilmenidine sympatholytic activity preserves mental stress, orthostatic sympathetic responses and adrenaline secretion. J Hypertens. 2004;22:1529–1534. doi: 10.1097/01.hjh.0000125453.28861.b8. [DOI] [PubMed] [Google Scholar]
- Farsang C, Lengyel M, Borbas S, Zorandi A, Dienes BS. Value of rilmenidine therapy and its combination with perindopril on blood pressure and left ventricular hypertrophy in patients with essential hypertension (VERITAS) Curr Med Res Opin. 2003;19:205–217. doi: 10.1185/030079903125001659. [DOI] [PubMed] [Google Scholar]
- Fauvel JP, Najem R, Ryon B, Ducher M, Laville M. Effects of rilmenidine on stress-induced peak blood pressure and renal function. J Cardiovasc Pharmacol. 1999;34:41–45. doi: 10.1097/00005344-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Finta E, Laude D, Alfoldi S, Farsang C, Elghozi JL. Effects of rilmenidine on 24-h rhythmicity of blood pressure and spontaneous baroreflex sensitivity in essential hypertensive subjects. J Hypertens. 2006;24:1619–1625. doi: 10.1097/01.hjh.0000239298.63377.db. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami F, Ibanez J, Geelen G, Vincent M, Frutoso J, Gharib C. Effects of swim training alone and in combination with clonidine and rilmenidine on blood pressure, plasma electrolytes, vasopressin, and renin activity in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1990;15:68–74. doi: 10.1097/00005344-199001000-00011. [DOI] [PubMed] [Google Scholar]
- Guild SJ, Malpas SC, Eppel GA, Nguang SK, Evans RG. Effect of renal perfusion pressure on responses of intrarenal blood flow to renal nerve stimulation in rabbits. Clin Exp Pharmacol Physiol. 2004;31:35–45. doi: 10.1111/j.1440-1681.2004.03947.x. [DOI] [PubMed] [Google Scholar]
- Head GA. Importance of imidazoline receptors in the cardiovascular actions of centrally acting antihypertensive agents. Ann N Y Acad Sci. 1995;763:531–540. doi: 10.1111/j.1749-6632.1995.tb32447.x. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL. Renal and cardiac sympathetic baroreflexes in hypertensive rabbits. Clin Exp Pharmacol Physiol. 2001;28:972–975. doi: 10.1046/j.1440-1681.2001.03567.x. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL. Are centrally acting imidazoline agents appropriate therapy for renovascular hypertension? In: Piletz JE, Regunathan S, Ernsberger P, editors. Agmatine and Imidazolines: Their Novel Receptors and Enzymes. New York: Annals of the New York Academy of Sciences; 2003. pp. 234–243. vol. 1009. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL. Sympathetic responses to stress and rilmenidine in 2K1C hypertensive rabbits: evidence of enhanced non-vascular neuroeffector mechanism. Hypertension. 2004;43:636–642. doi: 10.1161/01.HYP.0000116301.02975.aa. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL, Chan CK. Site and receptors involved in the sympathoinhibitory actions of rilmenidine. J Hypertens Suppl. 1998;16:S7–S12. [PubMed] [Google Scholar]
- Hollenberg NK, Williams GH, Adams DF. Essential hypertension: abnormal renal vascular and endocrine responses to a mild psychological stimulus. Hypertension. 1981;3:11–17. doi: 10.1161/01.hyp.3.1.11. [DOI] [PubMed] [Google Scholar]
- Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, et al. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- Kooner JS, Peart WS, Mathias CJ. The sympathetic nervous system in hypertension due to unilateral renal artery stenosis in man. Clin Auton Res. 1991;1:195–204. doi: 10.1007/BF01824987. [DOI] [PubMed] [Google Scholar]
- Kopp UC, DiBona GF. Neural regulation of renin secretion. Semin Nephrol. 1993;13:543–551. [PubMed] [Google Scholar]
- Korner PI, Shaw J, West MJ, Oliver JR. Central nervous system control of baroreceptor reflexes in the rabbit. Circ Res. 1972;31:637–652. doi: 10.1161/01.res.31.5.637. [DOI] [PubMed] [Google Scholar]
- Laubie M, Poignant JC, Scuvee-Moreau J, Dabire H, Dresse A, Schmitt H. Pharmacological properties of (N- dicyclopropylmethyl)-amino-2-oxazoline (S3341), an α2 adrenoceptor agonist. J Pharmacol. 1985;16:259–278. [PubMed] [Google Scholar]
- Li P, Penner SB, Smyth DD. Attenuated renal response to moxonidine and rilmenidine in one kidney one clip hypertensive rats. Br J Pharmacol. 1994;112:200–206. doi: 10.1111/j.1476-5381.1994.tb13052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata G, Scaglione R, Guillet C, Capuana G, Parrinello G, Indovina A, et al. Double-blind controlled study of rilmenidine versus hydrochlorothiazide in mild hypertension: clinical and renal haemodynamic evaluation. J Hum Hypertens. 1993;7:153–157. [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- Moretti J-L, Burke SL, Evans RG, Lambert GW, Head GA. Enhanced responses to ganglion blockade do not reflect sympathetic nervous system contribution to Angiotensin II-induced hypertension. J Hypertens. 2009;29:1838–1848. doi: 10.1097/HJH.0b013e32832dd0d8. [DOI] [PubMed] [Google Scholar]
- Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, et al. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation. 1996;93:866–869. doi: 10.1161/01.cir.93.5.866. [DOI] [PubMed] [Google Scholar]
- Oliver JR, Korner PI, Woods RL, Zhu JL. Reflex release of vasopressin and renin in haemorrhage is enhanced by autonomic blockade. Am J Physiol Heart Circ Physiol. 1990;258:H221–H228. doi: 10.1152/ajpheart.1990.258.1.H221. [DOI] [PubMed] [Google Scholar]
- Panfilov V, Morris AD, Donnelly R, Scemama M, Reid JL. The effects of rilmenidine and atenolol on mental stress, dynamic exercise and autonomic function in mild to moderate hypertension. Br J Clin Pharmacol. 1995;40:563–569. doi: 10.1111/j.1365-2125.1995.tb05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon DH, Beierwaltes WH. Degree of renal artery stenosis alters nitric oxide regulation of renal hemodynamics. J Am Soc Nephrol. 1994;5:1369–1377. doi: 10.1681/ASN.V561369. [DOI] [PubMed] [Google Scholar]
- Spence JD. Treatment options for renovascular hypertension. Expert Opin Pharmacother. 2002;3:411–416. doi: 10.1517/14656566.3.4.411. [DOI] [PubMed] [Google Scholar]
- Tibirica E, Feldman J, Mermet C, Monassier L, Gonon F, Bousquet P. Selectivity of rilmenidine for the nucleus reticularis lateralis, a ventrolateral medullary structure containing imidazoline-preferring receptors. Eur J Pharmacol. 1991;209:213–221. doi: 10.1016/0014-2999(91)90172-m. [DOI] [PubMed] [Google Scholar]
- Tsilakis D, Parzakonis N, Andrikopoulos G, Fouskarinis I, Koulouris S, Manolis AS. The impact of reducing hypertensive left ventricular hypertrophy on sudden cardiac death. Hospital Chronicles. 2008;(Supplement):210, 214. [Google Scholar]
- Vonend O, Marsalek P, Russ H, Wulkow R, Oberhauser V, Rump LC. Moxonidine treatment of hypertensive patients with advanced renal failure. J Hypertens. 2003;21:1709–1717. doi: 10.1097/00004872-200309000-00021. [DOI] [PubMed] [Google Scholar]
- Wagner C, Hinder M, Kramer BK, Kurtz A. Role of renal nerves in the stimulation of the renin system by reduced renal arterial pressure. Hypertension. 1999;34:1101–1105. doi: 10.1161/01.hyp.34.5.1101. [DOI] [PubMed] [Google Scholar]


