Abstract
Background and purpose:
The intracellular pH (pHi) of neurones is tightly regulated by, for example, membrane-bound acid-exchangers and loaders. Nevertheless, excessive bioelectric activity lowers steady-state pHi. In turn, even a moderate acidification can inhibit neuronal activity, a process believed to be part of a negative feedback loop controlling neuronal excitation. As moclobemide, an antidepressant, and also some antiepileptic drugs can reduce neuronal pHi in hippocampus slices in vitro, we screened a panel of currently used neuropsychopharmaca for comparable effects.
Experimental approach:
BCECF-AM loaded hippocampal slices were superfused with 16 different neuroleptics, antidepressants and antiepileptics under bicarbonate-buffered conditions. Changes in steady-state pHi of CA3 neurones were measured fluorometrically.
Key results:
The antipsychotics haloperidol, clozapine, ziprasidone, and the antidepressants amitriptyline, doxepin, trimipramine, citalopram, mirtazapine, as well as the anticonvulsive drug tiagabine reversibly reduced the steady-state pHi by up to 0.35 pH-units in concentrations of 5–50 µM. In contrast, venlafaxine, the anticonvulsants carbamazepine, clonazepam, gabapentin, lamotrigine, zonisamide, and the mood stabilizer lithium had no effect on neuronal pHi.
Conclusion and implications:
These data substantiate the view that clinically relevant concentrations of neuroleptics and antidepressants can mediate changes in neuronal pHi, which may contribute to their pharmacological mode of action. Effects on pHi should be taken into account when therapeutic or even harmful effects of these drugs are evaluated.
Keywords: antidepressants, antipsychotics, anticonvulsants, lithium, intracellular pH, pH regulation
Introduction
Cellular functions are largely influenced by the concentration of intracellular free H+, which is mainly determined by the interplay of cell metabolism and acid extrusion. In neurones, several lines of evidence point to a pivotal role of intracellular pH (pHi) for inter- and intracellular signalling as well as for cellular and synaptic plasticity (reviewed by Chesler, 2003). It is now widely accepted that neuronal activity modulates pHi and that pHi changes in turn can influence neuronal activity, for example, via altered membrane channel conductivity (Chesler and Kaila, 1992; Takahashi and Copenhagen, 1996; Bonnet et al., 2000a, Xiong et al., 2000; Schuchmann et al., 2006). Membrane-bound antiporters are important as well and, for example, vesicular Ca2+/H+-exchange and Na+/H+-exchange are known to influence neurotransmission (Goncalves et al., 1999; Jang et al., 2006). Catecholamine neurotransmitters can directly activate the neuronal sodium-proton exchange (Smith et al., 1998). Monoamine oxidase (MAO) activity itself, necessary for the degradation of catecholamines and other biogenic amines, is intimately linked to mitochondrial function and ATP production (Cohen and Kesler, 1999) and it has been shown that MAO-A inhibitors themselves, such as moclobemide, clorgyline and pargyline, are able to reduce pHi of CA3 neurones (Bonnet et al., 2000b). With regard to other neuroactive clinical drugs, some anticonvulsants such as levetiracetam (Leniger et al., 2004a), topiramate (Leniger et al., 2004b), valproate (Bonnet et al., 2002) and sulthiame (Leniger et al., 2002) have been demonstrated to influence pHi of hippocampal CA3 neurones. These substances evoked reversible acidifications, which were, at least in part, based on an interference with neuronal pHi-regulation and/or carbonic anhydrase inhibition.
We previously hypothesized that a modestly lowered neuronal pHi might contribute to the therapeutic potential of some neuropsychopharmaca (NPP) (Bonnet and Wiemann, 1999b; Bonnet et al., 2000b), whereas a higher acidification is regarded as being cytotoxic (Siesjöet al., 1993; Ding et al., 2000). In the present investigation, we extended our studies on the pHi modulating effects of clinical drugs to currently used NPP and screened relevant concentrations of antipsychotics, antidepressants and additional anticonvulsants. Neuronal pHi changes were studied in a well-established ex vivo model system, namely the 2′,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein-acetoxymetyl ester (BCECF-AM) loaded hippocampal slice, which allows for the detection of changes in the steady state pHi upon drug treatment in adult hippocampal neuronal somata (Bonnet and Wiemann, 1999a; Hentschke et al., 2006).
Drug concentrations in the extracellular brain fluids of rodents and humans, that are equivalent to therapeutic plasma concentrations, were found to be in the low micromolar range (0.5–10 µM) (Glotzbach and Preskorn, 1982; Walker et al., 2000; Wang et al., 2004; Rambeck et al., 2006). Nevertheless, in the present study, initial experiments were conducted with 50 µM. This concentration was chosen to obtain faster tissue penetration and because BCECF-AM loaded slice preparations bleach over time, restricting the duration of experiments (Bevensee et al., 1995). Moreover, effects on pHi are easier to detect if they occur abruptly. Concentrations more closely resembling the therapeutic range (5–10 µM) were then tested in a second step. With this approach, we show that a considerable number of NPP can modify neuronal pHi, at least in concentrations equivalent to the upper therapeutic range.
Methods
Transverse hippocampal slices (400–500 µm thick) were prepared from brains of adult guinea-pigs (300–400 g) as described previously (Leniger et al., 2004a). Brain slices were pre-incubated for 2 h in 28°C warm saline equilibrated with 5% CO2 in O2. This saline contained in mM: NaCl 124, KCl 3, CaCl2 0.75, MgSO4 1.3, KH2PO4 1.25, NaHCO3 26 and glucose 10. After pre-incubation, slices were transferred to a perspex recording chamber (vol. 4 mL) which was mounted on the stage of an upright microscope (Olympus Bx50Wi, Olympus, Hamburg, Germany) equipped with water immersion objectives. In this chamber, the submerged slices were continuously superfused with 32°C warm saline at a rate of 4.5 mL·min−1. The composition of this control solution was the same as during the pre-incubation period except for the calcium concentration, which was raised to 1.75 mM. The following drugs were added to the superfusate: amitriptyline hydrochloride (pKa: 9.4), doxepin hydrochloride (pKa: 10.4), trimipramine hydrogenmaleonate (pKa: 9.4), citalopram hydrobromide (pKa: 9.5), mirtazapine (pKa: 7,7), venlafaxine hydrochloride (pKa: 9.4), carbamazepine (pKa: 7.0), tiagabine hydrochloride (pKa: 9.4 and 13.3), zonisamide (pKa: 10.2), gabapentin (pKa: 4.5 and 9.5), lamotrigine (pKa: 5.7), clonazepam (pKa: 1.5 and 10.5), ziprasidone hydrochloride (pKa: 6.5), haloperidol (pKa: 8.3), clozapine (pKa: 7.5) and lithium chloride. None of the additives changed the pH of the bicarbonate buffered superfusate, which was continuously controlled to be 7.35–7.4 and kept constant throughout each experiment.
Analysis of pHi changes
Hippocampal slices were stained with 0.5–1 µM BCECF-AM for 3–5 min in the pre-incubation saline. Slices were superfused with saline for at least 30 min without illumination to allow the pHi to stabilize. Then superficially located neurones of the stratum pyramidale of the CA3 region were identified by stained apical dendrites (Bonnet et al., 1998; Bonnet and Wiemann, 1999a) and examined under a 40× or 60× water immersion objective. Up to three neighbouring neurones were recorded simultaneously. In some optical recordings, pHi transients of neuronal somata were also compared with adjacent regions of interest placed, for example, in the stratum radiatum to better reveal specific pHi responses (see Figure 1B). Slices were intermittently excited at 440 and 490 nm (duration of illumination: 0.2 s at each wavelength) using a computer operated filter wheel (Sutter Instruments, Novato, CA, USA) equipped with a 100 W halogen lamp. Fluorescence images were captured (0.05 Hz) by an intensified CCD camera (PTI, Surbiton Surrey, England). Background fluorescence was taken from unstained slices using the same camera and microscope settings. To ratio background subtracted images, a CARAT system (Dr O. Ahrens, Bargteheide, Germany) was used. At the end of an experiment, the ratio 440/490 was calibrated by a standard curve; this was obtained by the in vitro calibration method adapted to an upright microscope (Bonnet and Wiemann, 1999a; Hentschke et al., 2006). Background light was equally included into the standard curve and, thus, eliminated from the measurement.
Figure 1.
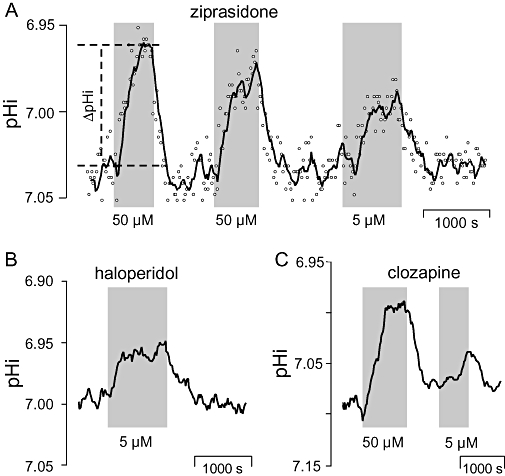
Effects of typical and atypical neuroleptics on the steady-state intracellular pH (pHi) of BCECF-laden hippocampal CA3 neurones. Application periods of drugs at indicated concentrations are shown as shaded areas. (A) Ziprasidone, (B) haloperidol, (C) clozapine. All curves represent sliding averages from eight data points measured in single neuronal somata. Open circles in (A) show original data points measured at 20 s intervals. Definition of ΔpHi (see Tables 1–3 for group data) is demonstrated in (A).
To achieve optical recordings of single CA3 neurones (located in the stratum pyramidale) for up to 3 h, lowest possible intensity of excitation light combined with a near-to-maximum gain of the camera was found to be essential. A fairly stable BCECF fluorescence signal (excitation: 440 nm, emission >520 nm) with a loss of intensity <1% per min was taken as a criterion for neurones to be in good condition (Bevensee et al., 1995). Only neurones fulfilling this criterion were subjected to further drug treatment. None of the drugs changed the background fluorescence parameters when tested at their final concentrations. pHi signals were collected from a region of interest positioned over neuronal somata. Spontaneous baseline ΔpHi-deflections were <0.03 pH-units. Sliding averages of 4–8 values are shown in Figures 1–3 to eliminate noise and demonstrate pHi shifts more clearly.
Figure 3.
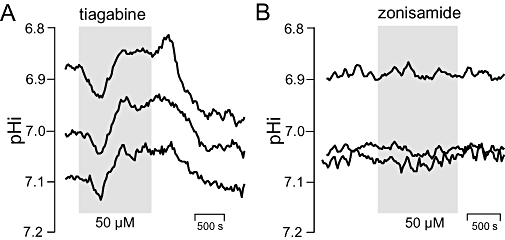
Effects of antiepileptics on steady state intracellular pH of BCECF-laden hippocampal CA3 neurones. Application periods of drugs at indicated concentrations are shown as shaded areas. (A) Tiagabine, (B) zonisamide. Curves represent sliding averages from eight data points measured in three pyramidal somata. Note that neurones respond to tiagabine but not to zonisamide.
Evaluation criteria and definition of ‘pHi activity’
To ensure that the changes in steady-state pHi were mediated by the drug, we used the following three mandatory criteria:
Timing
Changes in pHi must be closely related in time to the onset of drug application.
Reversibility
Changes in pHi must be at least partly reversible upon washout.
Magnitude
Amplitudes of pHi changes must clearly exceed the noise level (ΔpHi > 0.03 pH-units). Calculations of ΔpHi were uniformly carried out on sliding averages of eight values (see Figure 1 for illustration). As drug-mediated pHi deflection may be shaped by ongoing pHi regulation (i.e. by progressive pHi recovery, overshooting pHi deflection upon washout), we calculated ΔpHi between the mean pHi immediately before drug application and the maximum pHi deflection during drug application.
Drugs were designated as being ‘pHi active’ if at least 2/3 of the experiments fulfilled the said criteria. In the case of ‘pHi-activity’ of 50 µM drug concentration (except for lithium), a lower concentration (5 or 10 µM) was additionally tested (except for doxepin). In some experiments, both concentrations were successively applied to the same neurone (see e.g. Figures 1 and 2).
Figure 2.
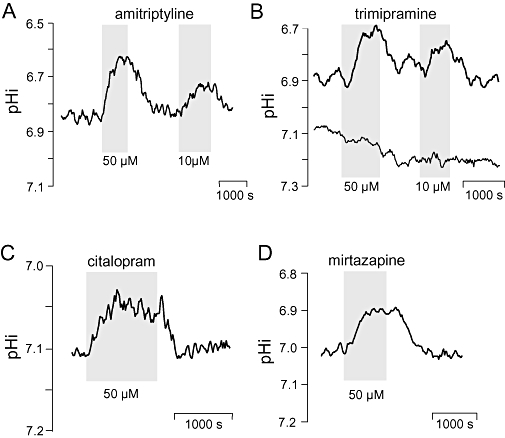
Effects of antidepressants on steady state intracellular pH (pHi) of BCECF-laden hippocampal CA3 neurones. Application periods of drugs at indicated concentrations are shown as shaded areas. (A) Amitriptyline, (B) trimipamine, (C) citalopram, (D) mirtazapine. Curves represent sliding averages from four to eight data points measured in one pyramidal soma. In (B), pHi deflections within near-by regions (150–250 µm2) of the stratum radiatum are additionally shown.
Statistics
Group values are given as mean ± standard deviation (SD). Effects of drug application were tested for significance by Student's t-test for paired samples and P values are indicated in Tables 1–3. Differences between concentration groups (5 or 10 vs. 50 µM) were analysed by independent sample t-tests and confirmed by anova. Significance was assumed for P≤ 0.05.
Table 1.
Effects of antipsychotics on pHi of CA3 neurones
| Drug | Slices | Neurones | Responses | pHi1 | pHi (treatment) | ΔpHi2(range) | ΔpHi2(mean±SD) | pH-active3 |
|---|---|---|---|---|---|---|---|---|
| All | 17 | 22 | 26 of 27 | 7.05 ± 0.07 | 6.98 ± 0.08 | 0.04–0.18 | 0.07 ± 0.03 | |
| Haloperidol | 6 | 8 | 5 µM: 3 of 3 | 7.02 ± 0.08 | 6.95 ± 0.08**4 | 0.06–0.07 | 0.06 ± 0.01 | Y |
| 50 µM: 6 of 6 | 7.03 ± 0.05 | 6.95 ± 0.07** | 0.05–0.15 | 0.09 ± 0.04 | Y | |||
| Clozapine | 5 | 7 | 5 µM: 3 of 3 | 7.07 ± 0.06 | 7.00 ± 0.06* | 0.04–0.10 | 0.06 ± 0.09 | Y |
| 50 µM: 6 of 6 | 7.06 ± 0.08 | 6.98 ± 0.09** | 0.05–0.18 | 0.09 ± 0.05 | Y | |||
| Ziprasidone | 6 | 7 | 5 µM: 5 of 5 | 7.04 ± 0.05 | 6.99 ± 0.05** | 0.04–0.06 | 0.05 ± 0.01 | Y |
| 50 µM: 3 of 4 | 7.07 ± 0.09 | 7.03 ± 0.12* | 0.06–0.07 | 0.06 ± 0.01 | Y |
Steady pHi prior to treatment (control condition).
Responding cells only (ΔpHi > 0.03 pH units).
Y = Yes, N = No.
Asterisks indicate significant differences from control.
P < 0.05;
P < 0.01.
pHi, intracellular pH; SD, standard deviation.
Table 3.
Effects of anticonvulsants on pHi of CA3 neurones
| Drug | Slices | Neurones | Responses | pHi1 | pHi (treatment)ΔpHi2(range) | ΔpHi2 | ΔpHi2(mean±SD) | pH-active3 |
|---|---|---|---|---|---|---|---|---|
| All | 47 | 58 | 18 of 68 | 6.94 ± 0.14 | 6.92 ± 0.14 | 0.04–0.10 | 0.07 ± 0.02 | |
| Carbamazepine | 6 | 6 | 50 µM: 1of 6 | 6.95 ± 0.14 | 6.93 ± 0.15 | 0.10 | 0.10 | N |
| Clonazepam | 6 | 6 | 50 µM: 0 of 6 | 7.01 ± 0.09 | 7.01 ± 0.09 | <0.03 | <0.03 | N |
| Gabapentin | 6 | 6 | 50 µM: 0 of 6 | 6.97 ± 0.10 | 6.97 ± 0.10 | <0.03 | <0.03 | N |
| Lamotrigine | 6 | 6 | 50 µM: 0 of 6 | 6.95 ± 0.08 | 6.95 ± 0.08 | <0.03 | <0.03 | N |
| Tiagabine4 | 8 | 10 | 10 µM: 3 of 3 | 7.03 ± 0.15 | 6.97 ± 0.16*5 | 0.05–0.07 | 0.06 ± 0.01 | Y |
| 50 µM: 11 of 11 | 6.95 ± 0.12 | 6.87 ± 0.12** | 0.04–0.10 | 0.07 ± 0.02 | Y | |||
| zonisamide | 15 | 24 | 50 µM: 3 of 30 | 6.91 ± 0.17 | 6.90 ± 0.16 | 0.04–0.10 | 0.08 ± 0.04 | N |
Steady pHi prior to treatment (control condition).
Responding cells only (ΔpHi > 0.03 pH units).
Y = Yes, N = No.
Only acidic deflections.
Asterisks indicate significant differences from control.
P < 0.05;
P < 0.01.
pHi, intracellular pH; SD, standard deviation.
Drugs
All the drugs were obtained from Sigma (Hamburg, Germany) unless stated otherwise. Mirtazapine was obtained from Organon (Oberschleissheim, Germany); tiagabine hydrochloride from Chemos (Regenstauf, Germany); gabapentin from Parke-Davis (Freiburg, Germany); clozapine from Novartis (Neuss, Germany) and BCECF-AM from Molecular Probes (Leiden, the Netherlands).
Results
This study was carried out on 161 pyramidal neurones of the CA3 region in 121 hippocampal slices. The mean intracellular steady state pHi of the whole collective was 6.98 ± 0.14 (mean ± SD) under control conditions.
Antipsychotics
Effects of the atypical neuroleptics ziprasidone and clozapine and of the typical neuroleptic haloperidol on steady state pHi were evaluated from a total of 27 optical recordings (see Table 1 for details). Mean steady state pHi (±SD) in this series of experiments was 7.05 ± 0.07 prior to drug application. At concentrations of either 5 or 50 µM, all drugs evoked significant decreases of pHi within 5–10 min (Figure 1). The largest pHi deflections were seen for clozapine (0.18 pH units) and haloperidol (0.15 pH units). There were no significant differences between mean values of the concentration groups (5 and 50 µM) for either haloperidol, clozapine or ziprasidone (P values: 0.23, 0.41, and 0.98 respectively). Within the limits of this study, we obtained no evidence that the pHi responses were dependent on the respective starting pHi. All pHi deflections evoked by antipsychotics were reversible. Alkalotic overshoots, possibly pointing to ongoing or disturbed pHi regulation (Figure 1), were not observed in any of the experiments. On the whole, application of 5 or 50 µM of each tested antipsychotic decreased pHi in 26 of the 27 optical recordings (96%).
Antidepressants
Effects of older (amitriptyline, doxepin, trimipramine) and more modern antidepressants (citalopram, mirtazapine, venlafaxine) on pHi were tested in 68 neurones of 46 slices. The mean steady state pH in this series of experiments was 7.01 ± 0.16. The results are summarized in Table 2.
Table 2.
Effects of antidepressants on pHi of CA3 neurones
| Drug | Slices | Neurones | Responses | pHi1 | pHi (treatment) | ΔpHi2(range) | ΔpHi2(mean±SD) | pHi-active3 |
|---|---|---|---|---|---|---|---|---|
| All | 46 | 68 | 55 of 77 | 7.01 ± 0.16 | 6.92 ± 0.16 | 0.05–0.35 | 0.12 ± 0.08 | |
| Amitriptyline | 9 | 10 | 10 µM: 3 of 4 | 6.92 ± 0.09 | 6.80 ± 0.18*4 | 0.07–0.2 | 0.15 ± 0.07 | Y |
| 50 µM: 5 of 7 | 6.94 ± 0.15 | 6.88 ± 0.16* | 0.05–0.2 | 0.07 ± 0.02 | Y | |||
| Doxepin | 4 | 4 | 50 µM: 4 of 4 | 6.96 ± 0.07 | 6.88 ± 0.04* | 0.06–0.12 | 0.09 ± 0.04 | Y |
| Trimipramine | 10 | 20 | 10 µM: 9 of 12 | 6.95 ± 0.07 | 6.86 ± 0.15** | 0.05–0.25 | 0.19 ± 0.10 | Y |
| 50 µM: 7 of 10 | 7.02 ± 0.20 | 6.91 ± 0.19** | 0.06–0.35 | 0.15 ± 0.07 | Y | |||
| Citalopram | 8 | 11 | 10 µM: 3 of 3 | 7.23 ± 0.03 | 7.16 ± 0.02** | 0.06–0.08 | 0.07 ± 0.01 | Y |
| 50 µM: 7 of 10 | 7.04 ± 0.10 | 6.97 ± 0.06** | 0.05–0.13 | 0.08 ± 0.04 | Y | |||
| Mirtazapine | 10 | 16 | 10 µM: 10 of 14 | 7.07 ± 0.15 | 6.96 ± 0.16** | 0.05–0.20 | 0.12 ± 0.05 | Y |
| 50 µM: 5 of 6 | 6.96 ± 0.18 | 6.78 ± 0.18* | 0.05–0.30 | 0.16 ± 0.13 | Y | |||
| Venlafaxine | 5 | 7 | 50 µM: 2 of 7 | 6.99 ± 0.18 | 6.98 ± 0.18 | 0.04–0.05 | 0.05 ± 0.01 | N |
Steady pHi prior to treatment (control condition).
Responding cells only (ΔpHi > 0.03 pH units).
Y = Yes, N = No.
Asterisks indicate significant differences from control.
P < 0.05;
P < 0.01.
pHi, intracellular pH; SD, standard deviation.
The tricyclics amitriptyline, doxepin, trimipramine (applied to 34 neurones) reversibly reduced the steady state pHi of CA3 neurones in the majority of experiments (28 out of 37). There were no significant differences between mean values of the concentration groups (10 and 50 µM) of either amitriptyline and trimipramine (P values: 0.26 and 0.46 respectively).
Citalopram, a selective 5-hydroxytryptamine (5-HT)-reuptake inhibitor, was effective in 10 out of 13 recordings. Mean values of the concentration groups (10 and 50 µM) were also not different from each other (P= 0.33).
In the group in which the effects of combined 5-HT and noradrenaline enhancers were investigated, the results were less uniform. Thus, mirtazapine elicited acidification in 15 out of 20 experiments, whereas venlafaxine was classified as to be not pHi active because small responses were seen in only 2 out of 7 experiments. In the case of mirtazapine, no difference was seen between mean values of the concentration groups (10 and 50 µM, P= 0.43).
Thus, with the exception of venlafaxine, all the antidepressants tested significantly lowered the steady state pHi in a reversible manner (Figure 2)
Anticonvulsants
In the third part of the study, we investigated the older anticonvulsants carbamazepine and clonazepam as well as the newer drugs gabapentin, lamotrigine, tiagabine and zonisamide in 58 neurones from 47 slices. Significant changes in pHi were observed only upon application of the GABA-transport inhibitor tiagabine (Table 3). With 10 and 50 µM tiagabine, an acidification of 0.06 ± 0.01 and 0.07 ± 0.02 pH units occurred, respectively, after at least 10 min (Table 3, Figure 3). The difference between the two concentrations was not significant (P= 0.16). However, in 6 out of 10 neurones, acidification was preceded by a transient alkalosis (ΔpHi 0.053 ± 0.008, P < 0.001), as shown in Figure 3.
Lithium
The mood stabilizer lithium (1.2 mM) failed to evoke pHi changes in 13 CA3-neurones taken from 11 different slices. Mean starting pHi was 7.02 ± 0.14 in these experiments and remained unaltered even when exposure times were extended to more than 40 min.
Discussion
General aspects and limitations of the study
The main finding of this investigation was that 9 out of 16 NPP influenced steady state pHi of hippocampal CA3 neurones in vitro and were, therefore, classified as ‘pHi active’. All the antipsychotics tested (haloperidol, clozapine, ziprasidone) and most of the antidepressants (amitriptyline, doxepin, trimipramine, citalopram, mirtazapine) were among the drugs that lowered pHi by up to 0.35 units. The majority of anticonvulsants, that is, carbamazepine, clonazepam, gabapentin, lamotrigine, zonisamide had no effect and only tiagabine elicited a biphasic response. Lithium which interferes with sodium proton exchange (NHE) and lowers pHi in cultured cells within several days of treatment (Wall et al., 1988; Bitran et al., 1990; Kobaysashi et al., 2000) had no acute effect on steady state pHi of hippocampal CA3 neurones.
When screening NPP, a low (5–10 µM) and a high concentration (50 µM) of each drug evoked largely similar, that is, not significantly different effects. This suggests that drug binding sites responsible for pHi effects may be largely saturated even at low micromolar concentrations. With respect to amplitude and slope of the pHi deflection, a concentration-dependent acidification was observed for single neurones subjected to repetitive treatments with amitriptyline, trimipramine and clozapine (see Figures 1A, C and 2A, B). A more detailed description of dose-response effects awaits further studies on well-defined in vitro systems. Concentrations between 5–10 µM, at which effects on pHi were initially observed, represent the upper range of therapeutic concentrations in the brain (Glotzbach and Preskorn, 1982; Walker et al., 2000; Wang et al., 2004; Rambeck et al., 2006). This and the fact that a lowering of pHi by less than 0.1 pH unit can reduce neuronal excitability or frequency of epileptic discharges (Bonnet et al., 1998; 2000a; Xiong et al., 2000) suggest that our findings may be clinically relevant.
The mechanisms underlying the shift in the steady-state pHi upon application of NPP were beyond the scope of this investigation. Nevertheless, the possibility that drugs directly acidified the cells because their pKa values (see Methods section) were mostly basic or near neutral was excluded. The finding that the basic drugs (pKa >7.0) failed to increased pHi is most likely due to the high buffering capacity of the bicarbonate buffered superfusate and the comparatively low concentration of the drugs. For example, a 200-fold higher concentration of ammonium chloride (pKa: 9.2) is needed to induce a clear alkalinization under equivalent experimental conditions (Bonnet and Wiemann, 1999a). In line with this, 50 µM gabapentin, lamotrigine and clonazepam, all of which showed pKa values below 7.0, elicited no acidification. Therefore, the possibility that all these drugs have a direct effect on the steady-state pHi can be ruled out.
It may appear surprising that nine drugs elicited acidification whereas a sustained increase in the pHi was not observed. In principal, acidification can arise from increased excitatory postsynaptic potentials and action potential firing (Chesler and Kaila, 1992). However, most substances are known to dampen neuronal activity and are, therefore, more likely to diminish neuronal activity, making this possibility less likely. Acidification may also result from increased production of lactic acid, for example, via impaired mitochondrial function, and this mechanism cannot be excluded at present (see examples below). Finally, inhibition of the activity of any acid extrusion system will acidify neurones, because the proton motive force is inwardly directed at a membrane potential of −70 mV. Recent studies on major pHi regulating membrane transporters underline the importance of unimpaired acid extrusion for neuronal excitability. The functional knockout of NHE1, AE3 and NCBE in animal models led to the development of altered neuronal activity and/or changed susceptibility to epileptogenic drugs, even though the steady-state pHi was not significantly changed (Cox et al., 1997; Gu et al., 2001; Hentschke et al., 2006; Jacobs et al., 2008). These studies imply that pharmacological inhibition of a single pHi regulating system may lead to complex neurological changes in the organism, which may, however, only be revealed under particular conditions such as over-excitation. The molecules, membrane transporters and channels currently known to be involved in the regulation of neuronal pHi are summarized in Figure 4 (the abbreviations used conform to Alexander et al., 2008). Many of these components are targeted by neuroactive compounds, and in most cases, these interactions led to neuronal acidification. Whether or not the NPP studied here interfere with these targets should be addressed in future studies.
Figure 4.
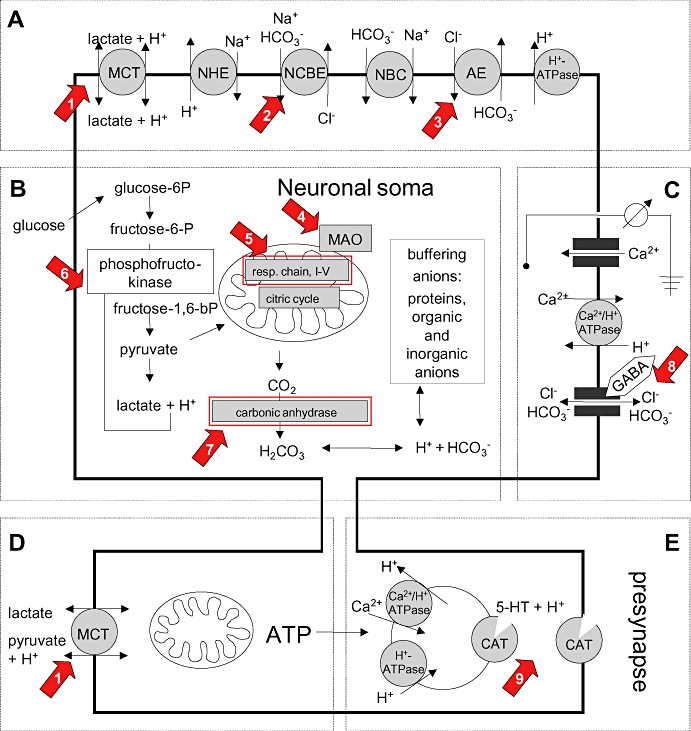
Schematic overview of major components and systems that are relevant to neuronal intracellular pH regulation. (A) Main acid extruders comprise monocarboxylate transporters (MCT), Na+/H+ exchangers (NHE), Na+-dependent Cl-/HCO3- -exchanger (NCBE), Na+/HCO3- -exchanger (NBC), and H+-ATPases. Anion exchangers (AE), e.g. Cl-/HCO3- exchanger, act as acid loaders. (B) Intracellular protons mainly result from lactic acid production (glycolysis) and H2CO3 formation from metabolic CO2 production (citrate cycles) involving the mitochondria. Carbonic anhydrases are important for the level of cellular bicarbonate and, thus, for intracellular buffering capacity. (C) Membrane potential indirectly drives transmembane fluxes of H+ and bicarbonate. (D) Presynaptic mitochondria are preferentially fueled by lactate and pyruvate to provide ATP, for example, for neurotransmitter re-uptake by vesicular and transmembraneous transporters. (E) The pH gradient across the synaptic vesicle membrane is a driving force for transmitter re-uptake mediated by catecholamine-transporters (CAT), which comprise 5-HT transporters (SERT) dopamine transporters, noradrenaline transporters, GABA-transporters and excitatory acid transporters. As an example, the effect of SERT is shown. Arrows numbered 1–9 point to known targets of the following neuropsychopharmaca (see also Discussion): (1) valproate (Rumbach et al., 1986); (2) levetiracetam (Leniger et al., 2004a); (3) topiramate (Leniger et al., 2004b); (4) moclobemide (Bonnet et al., 2000b); (5) fluoxetine, haloperidol, valproate (Rumbach et al., 1986; Wallace and Starkow, 2000); (6) valproate (Benavides et al., 1982); (7) topiramate, sulthiame (Woodbury and Kemp, 1989; Leniger et al., 2002); (8) tiagabine, topiramate, valproate (Kaila, 1994; Lueckermann et al., 1997; Bonnet et al., 2002); (9) 5-HT re-uptake inhibitors, tricyclics, mirtazapine, venlafaxine (Cao et al., 1997).
Sustained alkalinization of neurones was not observed after NPP treatment; for this to occur, an exaggerated extrusion of acid needs to take place, as described in the literature. For example, alkalinization of cultured hippocampal neurones occurs after stimulation of sodium proton transport via β-adrenoceptors (Smith et al., 1998). However, no drug used in the present study binds to or stimulates adrenoceptors. Also, stilbene derivatives can induce an alkalinization in hippocampal neurones, provided that the chloride-bicarbonate-exchange is operating as an acid loader, as has been observed for a subgroup of cultured neurones with a pHi >7.2 (Brett et al., 2002). As the steady-state pHi of neurones in slices was below 7.0, an inhibition of acid loading transporters would have been without effect on pHi. With respect to the drugs tested here, tiagabine was the only substance to induce a transient alkalinization in some neurones and even this was followed by acidification. Tiagabine inhibits GABA re-uptake and increases synaptic GABA, which allows accelerated fluxes of bicarbonate through opened GABA channels (Kaila, 1994; Bonnet and Bingmann, 1995; c.f. Figure 4C). As long as the membrane potential is more negative than the bicarbonate equilibrium potential, an efflux of bicarbonate can take place and this will result in a lowered pHi (Lueckermann et al., 1997). A transient increase in pHi upon addition of tiagabine is therefore unlikely to be due to a direct effect of GABAergic transmission, but may reflect an inhibitory action on neuronal activity, or the involvement of interneurones. Thus, only a few mechanism(s) can lead to the alkalinization of neurones and none of them seems to play role in the effects of NPP.
Effects of antidepressants and antipsychotics on pHi
Synaptic processes are dependent on pH in many ways (see Figure 4B–E). Activation of GABA or glutamate-receptors is followed by a rapid and transient fall in the pHi due to bicarbonate- or proton-fluxes through ion channels or due to stimulation of the re-uptake machinery (Chesler and Kaila, 1992; Hartley and Dubinsky, 1993; Amato et al., 1994). H+-induced fluxes were also observed in several other ion-coupled transporters including the 5-HT and dopamine transporters (Cao et al., 1997; Figure 4E). Much of the signalling involved in the actions of catecholamines is influenced by protons. For example, the release of dopamine from synaptosomes is affected by a drop in pHi (Trudeau et al., 1999; Cannizzaro et al., 2003). Also, the pH gradient spanning the synaptic vesicle membrane provides a major driving force for catecholamine transport (Toll and Howard, 1978; Moriyama and Futai, 1990). Hence, a lowered steady-state pHi secondary to the effects of pH active antidepressants or antipsychotics might be expected to influence the aforementioned synaptic processes with consequent alterations in brain function.
An increased intracellular H+ concentration may also be linked to mitochondrial function and energy metabolism. The mitochondrial membrane contains an NHE subtype (LeBlanc et al., 1988; Orlowski and Grinstein, 1997; Numata et al., 1998), which may help to control the pH of the mitochondrial matrix (Greenbaum and Wilson, 1991; Skulachev, 1999). Presumably by this means, NHE may exert some influence on the permeability of the mitochondrial transition pore (Friberg and Wieloch, 2002). Mitochondrial NHE may also be linked to catecholamine degrading enzymes such as MAO. Interestingly, MAO inhibitors, such as the antidepressant moclobemide, lowered pHi in hippocampal neurones (Bonnet et al., 2000b). Furthermore, antidepressants seem to act as modulators of the membrane permeability transition pore (Marcocci et al., 2002). Also at the level of mitochondria, the antipsychotic haloperidol and the antidepressant fluoxetine were shown to uncouple oxidative phosphorylation (Wallace and Starkow, 2000). This impairment of mitochondrial function may lead to a marked increase in lactic acid formation and, consequently, to metabolic acidification. Clinically, this situation may culminate in a metabolic disaster, known for instance as the malignant neuroleptic syndrome or as the 5-HT syndrome. These examples demonstrate that at least some antidepressants and antipsychotics are able to influence the basic metabolism of cells and outline a field for future research needed to understand the effects of NPP on pHi.
Effects of anticonvulsants on pHi
Epileptiform discharges are sensitive to a moderate drop in pHi (Bonnet et al., 1998; 2000a; Bonnet and Wiemann, 1999a; Xiong et al., 2000). On the other hand, respiratory alkalosis favours febril epileptic seizures (Schuchmann et al., 2006). In the light of these studies, the modest intracellular acidification previously found upon application of valproate, acetazolamide, sulthiame, topiramate or levetiracetam (Bonnet et al., 2002; Leniger et al., 2002; 2004a,b;) probably contributes to the anticonvulsive potency of these drugs. Initial studies on pHi regulation of human cortical brain slices suggest that at least some of these results can be transferred to the human brain (Wiemann et al., 2006). Acidification may also help to limit excitotoxicity and energy expenditure in vulnerable neurones (Tombaugh, 1994). In the present investigation, only tiagabine but neither carbamazepine, clonazepam, gabapentin, lamotrigine nor zonisamide changed pHi in CA3 neurones. With regard to zonisamide, the results from the present study confirm previous findings obtained at the single cell level in neuronal tissue (Thöne et al., 2008). A preliminary conclusion from all these experiments is that about half of the anticonvulsants lower steady state pHi and that this effect probably contributes to the suppression of epileptiform activity both in vitro and in vivo.
pHi and neuroimaging
A few 31P-MRS studies on patients suffering from bipolar or epileptic disorders have provided evidence of dysfunctions in the pHi-regulation of brain cells (Hugg et al., 1992; Garcia et al., 1994; van der Grond et al., 1998; Kato et al., 1998; Hamakawa et al., 2004). These findings support the idea that some antiepileptics and antidepressants may have a therapeutic impact via pHi-changes. A moderate decrease in the pHi could lower aberrant intracellular signalling (Vignes et al., 1996), for example, and this would have a favourable effect in critical regions of the limbic forebrain and temporal lobes, where hyperexcitability is evident in temporal lobe seizures (Koehling et al., 1998) and may occur in affective disorders (Kato et al., 1998). Also, panic disorders may be caused by defects in the regulation of brain pH (Cowley and Arana, 1990; Shioiri et al., 1996; Maddock, 2001). It should be stressed that marked acidification might induce vulnerable neurones to disintegrate or undergo apoptosis (Siesjöet al., 1993; Ding et al., 2000). In some parts of the brain, this might facilitate neurogenesis, which is thought to contribute to the therapeutic effects of some NPP and electroconvulsive therapy (Madsen et al., 2000; Malberg et al., 2000; Duman, 2004). Aberrant pHi regulation in human brain is a developing topic comprising aspects of plasticity, memory and learning (Wemmie et al., 2002). The ability of NPP to acutely influence steady state pHi may, therefore, mean that chronic administration of a drug could initiate slow persistent changes in the brain.
Conclusion
Evidence is provided that many NPP are able to acutely and reversibly lower neuronal steady state pHi in hippocampal CA3 neurones. It appears reasonable to suggest that these moderate acidifications will influence neuronal cell functions, such as excitability and intra- as well as intercellular signalling. Therefore, the pHi activity of NPP should be taken into consideration when therapeutic or even toxic effects of these drugs are evaluated.
Glossary
Abbreviations:
- AE
anion exchanger
- BCECF-AM
2′,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein-acetoxymetyl ester
- CA3
cornu ammonum region 3
- CAT
catecholamine transporter
- MAO
monoamine oxidase
- MCT
monocarboxylate transporter
- NCBE
Na+-dependent Cl-/HCO3- -exchanger
- NBC
Na+/HCO3- exchanger
- NHE
sodium proton exchanger
- NPP
neuropsychopharmaca
- pHi
intracellular pH
- SERT
5-HT transporter
Conflicts of interest
The authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato A, Ballerini C, Attwell D. Intracellular pH changes produced by glutamate uptake in rat hippocampal slices. J Neurophysiol. 1994;72:1686–1696. doi: 10.1152/jn.1994.72.4.1686. [DOI] [PubMed] [Google Scholar]
- Benavides J, Martin A, Ugarte M, Valdivieso F. Inhibition by valproic acid of pyruvate uptake by brain mitochondria. Biochem Pharmacol. 1982;31:1633–1636. doi: 10.1016/0006-2952(82)90392-6. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Schwiening CF, Boron WF. Use of BCECF and propidium iodide to assess membrane integrity of acutely isolated CA1 neurones from rat hippocampus. J Neurosci Methods. 1995;58:61–75. doi: 10.1016/0165-0270(94)00159-e. [DOI] [PubMed] [Google Scholar]
- Bitran JA, Potter WZ, Manji HK, Gusovsky F. Chronic Li+ attenuates agonist- and phorbol ester-mediated Na+/H+ antiporter activity in HL 60 cells. Eur J Pharmacol. 1990;188:193–202. doi: 10.1016/0922-4106(90)90002-f. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D. GABAA-responses of CA3-neurones: contribution of bicarbonate and of Cl--extrusion mechanisms. NeuroReport. 1995;6:700–704. doi: 10.1097/00001756-199503000-00028. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Wiemann M. Ammonium prepulse: effects on intracellular pH and bioelectric activity of CA3-neurones in guinea pig hippocampal slices. Brain Res. 1999a;840:16–22. doi: 10.1016/s0006-8993(99)01687-x. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Wiemann M. Intracellular free protons: relevance for neuropsychopharmacology? Pharmacopsychiatry. 1999b;32:173. [Google Scholar]
- Bonnet U, Wiemann M, Bingmann D. CO2/HCO3--withdrawal from the bath medium of hippocampal slices: biphasic effect on intracellular pH and bioelectric activity of CA3-neurons. Brain Res. 1998;796:161–170. doi: 10.1016/s0006-8993(98)00341-2. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D, Wiemann M. Intracellular pH modulates spontaneous and epileptiform bioelectric activity of hippocampal CA3-neurones. Eur Neuropsychopharmacol. 2000a;10:97–103. doi: 10.1016/s0924-977x(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Leniger T, Wiemann M. Moclobemide reduces intracellular pH and neuronal activity of CA3 neurones in guinea-pig hippocampal slices-implication for its neuroprotective properties. Neuropharmacology. 2000b;39:2067–2074. doi: 10.1016/s0028-3908(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D, Leniger T, Scherbaum N, Widman G, Hufnagel A, et al. Valproate acidifies hippocampal CA3-neurones – a novel mode of action. Eur Neuropsychopharmacol. 2002;12:279–285. doi: 10.1016/s0924-977x(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Brett CL, Kelly T, Sheldon C, Church J. Regulation of Cl- -HCO3- exchangers by cAMP-dependent protein kinase in adult rat hippocampal CA1 neurons. J Physiol. 2002;545:837–853. doi: 10.1113/jphysiol.2002.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Monastero R, Vacca M, Martire M. [3H]-DA release evoked by low pH medium and internal H+ accumulation in rat hypothalamic synaptosomes: involvement of calcium ions. Neurochem Int. 2003;43:9–17. doi: 10.1016/s0197-0186(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Cao Y, Mager S, Lester HA. H+ permeation and pH regulation at a mammalian serotonin transporter. J Neurosci. 1997;17:2257–2266. doi: 10.1523/JNEUROSCI.17-07-02257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Cohen G, Kesler N. Monoamine oxidase and mitochondrial repiration. J Neurochemistry. 1999;73:2310–2315. doi: 10.1046/j.1471-4159.1999.0732310.x. [DOI] [PubMed] [Google Scholar]
- Cowley DS, Arana GW. The diagnostic utility of lactate sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47:277–284. doi: 10.1001/archpsyc.1990.01810150077012. [DOI] [PubMed] [Google Scholar]
- Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson PT, Fu A, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–148. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- Ding D, Moskowitz SI, Li R, Lee SB, Esteban M, Tomaselli K, et al. Acidosis induces necrosis and apoptosis of cultured hippocampal neurones. Exp Neurol. 2000;162:1–12. doi: 10.1006/exnr.2000.7226. [DOI] [PubMed] [Google Scholar]
- Duman RS. Neuronal plasticity: consequences of stress and actions of antidepressant treatment. Dialogues Clin Neurosci. 2004;6:157–169. doi: 10.31887/DCNS.2004.6.2/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H, Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- Garcia PA, Laxer KD, van der Grond J, Hugg JW, Matson GB, Weiner MW. Phosphorus magnetic resonance speectroscopy imaging in patients with frontal lobe epilepsy. Ann Neurol. 1994;35:217–221. doi: 10.1002/ana.410350214. [DOI] [PubMed] [Google Scholar]
- Glotzbach RK, Preskorn SH. Brain concentrations of tricyclic antidepressants: single-dose kinetics and relationship to plasma concentrations in chronically dosed rats. Psychopharmacology. 1982;78:25–27. doi: 10.1007/BF00470582. [DOI] [PubMed] [Google Scholar]
- Goncalves PP, Meireles SM, Neves P, Vale MG. Synaptic vesicle Ca2+/H+ antiport: dependence on the proton electrochemical gradient. Brain Res Mol Brain Res. 1999;71:178–184. doi: 10.1016/s0169-328x(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Greenbaum N, Wilson DF. Role of intramitochondrial pH in the energetics and regulation of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 1991;1058:113–120. doi: 10.1016/s0005-2728(05)80227-0. [DOI] [PubMed] [Google Scholar]
- van der Grond J, Gerson JR, Latxer KD, Hugg JW, Matson GB, Weiner MW. Regional distribution of interictal 31P metabolic changes in patients with temporal lobe epilepsy. Epilepsia. 1998;39:527–536. doi: 10.1111/j.1528-1157.1998.tb01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XG, Yao H, Haddad GG. Increased neuronal excitability and seizures in the Na+/H+ exchanger null mutant mouse. Am J Physiol. 2001;281:C496–C503. doi: 10.1152/ajpcell.2001.281.2.C496. [DOI] [PubMed] [Google Scholar]
- Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci. 2004;58:82–88. doi: 10.1111/j.1440-1819.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate excitotoxicity. J Neurosci. 1993;13:4690–4699. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, et al. Mice with a targeted disruption of the Cl-/HCO3- exchanger display a reduced seizure threshold. Mol Cell Biol. 2006;26:182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugg JW, Laxer KD, Matson GB, Maudsley AA, Husted CA, Weiner MW. Lateralization of human focal epilepsy by 31P magnetic resonance spectroscopic imaging. Neurology. 1992;42:2011–2018. doi: 10.1212/wnl.42.10.2011. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Ruusuvuori E, Sipilä ST, Haapanen A, Damkier HH, Kurth I, et al. Mice with targeted Sic4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci USA. 2008;105:311–316. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I-S, Brodwick MS, Wang Z-M, Jeong H-J, Choi B-J, Akaike N. The Na+/H+ exchanger is a major regulator in GABAergic presynaptic nerve terminals synapsing onto rat CA3 pyramidal neurons. J Neurochemistry. 2006;99:1224–1236. doi: 10.1111/j.1471-4159.2006.04168.x. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kato T, Inubushi T, Kato N. Magnetic resonance spectroscopy in affective disorders. J Neuropsychiatry Clin Neurosci. 1998;10:133–147. doi: 10.1176/jnp.10.2.133. [DOI] [PubMed] [Google Scholar]
- Kobaysashi Y, Pang T, Iwamoto T, Wakabayashi S, Shigekawa M. Lithium activates mammalian Na+/H+-exchangers: isoform specificity and inhibition by genistein. Eur J Physiol. 2000;439:455–462. doi: 10.1007/s004249900195. [DOI] [PubMed] [Google Scholar]
- Koehling R, Luecke A, Straub H, Speckmann E-J, Tuxhorn I, Wolf P, et al. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121:1073–1087. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- LeBlanc G, Bassilana M, Damiano-Forano E. Na+/H+ exchange in bacteria and organelles. In: Grinstein S, editor. Na+/H+ Exchange. Boca Raton, FL: CRC Press; 1988. pp. 103–117. [Google Scholar]
- Leniger T, Wiemann M, Bingmann D, Widman G, Hufnagel A, Bonnet U. Carbonic anhydrase inhibitor sulthiame reduces intracellular epileptiform activity of hippocampal CA3 neurones. Epilepsia. 2002;43:469–474. doi: 10.1046/j.1528-1157.2002.32601.x. [DOI] [PubMed] [Google Scholar]
- Leniger T, Thöne J, Bonnet U, Hufnagel A, Bingmann D, Wiemann M. Levetiracetam inhibits Na+-dependent Cl-/HCO3--exchange of adult hippocampal CA3 neurones from guinea-pigs. Br J Pharmacol. 2004a;142:1073–1080. doi: 10.1038/sj.bjp.0705836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leniger T, Thöne J, Wiemann M. Topiramate modulates pH of hippocampal CA3 neurons by combined effects on carbonic anhydrase and Cl-/HCO3- -exchange. Br J Pharmacol. 2004b;142:831–842. doi: 10.1038/sj.bjp.0705850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueckermann M, Trapp S, Ballanyi K. GABA- and glycine-mediated fall of intracellular pH in rat medullary neurons in situ. J Neurophysiol. 1997;77:1844–1852. doi: 10.1152/jn.1997.77.4.1844. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The lactic acid response to alkalosis in panic disorder: an integrative review. J Neuropsychiatry Clin Neurosci. 2001;13:22–34. doi: 10.1176/jnp.13.1.22. [DOI] [PubMed] [Google Scholar]
- Madsen T, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Malberg J, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci L, De Marchi U, Salvi M, Milella ZG, Nocera S, Agostinelli E, et al. Tyramine and monoamine oxidase inhibitors as modulators of the mitochondrial membrane permeability transition. J Membr Biol. 2002;188:23–31. doi: 10.1007/s00232-001-0169-z. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Futai M. H+-ATPase, a primary pump for accumulation of neurotransmitters, is a major constituent of brain synaptic vesicles. Biochem Biophys Res Commun. 1990;173:443–448. doi: 10.1016/s0006-291x(05)81078-2. [DOI] [PubMed] [Google Scholar]
- Numata M, Petrecca K, Lake N, Orlowski J. Identification of a mitochondrial Na+/H+ exchanger. J Biol Chem. 1998;273:6951–6959. doi: 10.1074/jbc.273.12.6951. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- Rambeck B, Jürgens UH, May TW, Pannek HW, Behne F, Ebner A, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47:681–694. doi: 10.1111/j.1528-1167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Rumbach L, Mutet C, Cremel G, Marescaux CA, Micheletti G, Warter JM, et al. Effects of sodium valproate on mitochondrial membranes: electron paramagnetic resonance and transmembrane protein movement studies. Mol Pharmacol. 1986;30:270–273. [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, et al. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö BK, Katsura K-I, Mellergard P, Ekholm A, Lundgren J, Smith M-J. Acidosis-related brain damage. In: Kogure K, Hossman K-A, Siesjö BK, Welsh FA, editors. Progress in Brain Research. Amsterdam: Elsevier; 1993. pp. 23–48. Vol. 96. [PubMed] [Google Scholar]
- Shioiri T, Kato T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. High-energy phosphate metabolism in the frontal lobes of patients with panic disorder detected by phase-encoded 31P-MRS. Biol Psychiatry. 1996;40:785–793. doi: 10.1016/0006-3223(95)00487-4. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells and organisms. Mol Aspects Med. 1999;20:139–184. doi: 10.1016/s0098-2997(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Smith GAM, Brett CL, Church J. Effects of noradrenaline on intracellular pH in acutely dissociated adult rat hippocampal neurones. J Physiol. 1998;512:487–505. doi: 10.1111/j.1469-7793.1998.487be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K-I, Copenhagen DR. Modulation of neuronal function by intracellular pH. Neurosci Res. 1996;24:109–116. doi: 10.1016/0168-0102(95)00989-2. [DOI] [PubMed] [Google Scholar]
- Thöne J, Leniger T, Splettstoesser F, Wiemann M. Antiepileptic activity of zonisamide on hippocampal CA3 neurons does not depend on carbonic anhydrase inhibition. Epilepsy Res. 2008;79:105–111. doi: 10.1016/j.eplepsyres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Toll L, Howard BD. Role of Mg2+-ATPase and pH gradient in the storage of catecholamines in synaptic vesicles. Biochemistry. 1978;17:2517–2523. doi: 10.1021/bi00606a010. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC. Mild acidosis delays hypoxic spreading depression and improves neuronal recovery in hippocampal slices. J Neurosci. 1994;14:5635–5643. doi: 10.1523/JNEUROSCI.14-09-05635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau L-E, Parpura V, Haydon PG. Activation of neurotransmitter release in hippocampal nerve terminals during recovery from intracellular acidification. J Neurophysiol. 1999;81:2627–2635. doi: 10.1152/jn.1999.81.6.2627. [DOI] [PubMed] [Google Scholar]
- Vignes M, Blanc E, Guiramand J, Gonzalez E, Sassetti I, Rècasens M. A modulation of glutamate-induced phosphoinositide breakdown by intracellular pH changes. Neuropharmacology. 1996;35:1595–1604. doi: 10.1016/s0028-3908(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Walker MC, Tong X, Perry H, Alavijeh MS, Patsalos PN. Comparison of serum, cerebrospinal fluid and brain extracellular fluid pharmacokinetics of lamotrigine. Br J Pharmacol. 2000;130:242–248. doi: 10.1038/sj.bjp.0703337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SM, Kraut JA, Muallem S. Modulation of Na+-H+ exchange activity by intracellular Na+, H+, and Li+ in IMCD cells. Am J Physiol. 1988;255:F331–F339. doi: 10.1152/ajprenal.1988.255.2.F331. [DOI] [PubMed] [Google Scholar]
- Wallace KB, Starkow AA. Mitochondrial targets of drug toxicity. Annu Rev Pharmacol Toxicol. 2000;40:353–388. doi: 10.1146/annurev.pharmtox.40.1.353. [DOI] [PubMed] [Google Scholar]
- Wang X, Ratnaraj N, Patsalos PN. The pharmacokinetic inter-relationship of tiagabine in blood, cerebrospinal fluid and brain extracellular fluid (frontal cortex and hippocampus) Seizure. 2004;2004:574–581. doi: 10.1016/j.seizure.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hagemann AM, Price MP, Nolan BC, et al. The acid-activated ion channel ASIC contibutes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wiemann M, Splettstoesser F, Pannek HW, Behne F, Speckmann EJ, Bingmann D. Effects of levetiracetam and topiramate on pHi regulation of human neocortical brain slices. Acta Physiol. 2006;186(Suppl. 1):126. [Google Scholar]
- Woodbury DM, Kemp JW. Other antiepileptic drugs. Sulfonamides and derivates: acetazolamide. In: Levy R, Mattson R, Meldrum B, Penry JK, Dreyfuss DE, editors. Antiepileptic Drugs. New York: Raven Press; 1989. pp. 855–875. [Google Scholar]
- Xiong ZQ, Saggau P, Stringer JL. Activity-dependent intracellular acidification correlates with the duration of seizure activity. J Neurosci. 2000;20:1290–1296. doi: 10.1523/JNEUROSCI.20-04-01290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


