Abstract
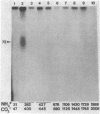
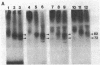
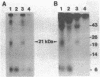
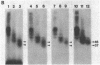
Purified and reconstituted sodium channels from rat brain have been photoaffinity labeled with a photoactivable derivative of the alpha-scorpion toxin V from Leiurus quinquestriatus (LqTx). A battery of sequence-specific antibodies has been used to determine which of the peptides produced by chemical and enzymatic cleavage of the photolabeled sodium-channel alpha subunit contain covalently attached LqTx. Nearly all of the covalently attached LqTx is found within homologous domain I. Two site-directed antisera, which recognize residues 317 to 335 and residues 382 to 400, respectively, specifically immunoprecipitate a 14-kDa peptide produced by CNBr digestion to which LqTx is covalently attached. It is proposed that a portion of the receptor site for alpha-scorpion toxins is formed by peptide segment(s) between amino acid residues 335 and 378 which is located in an extracellular loop between transmembrane helices S5 and S6 of homologous domain I of the sodium channel alpha subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S. Voltage-regulated sodium channel molecules. Annu Rev Physiol. 1984;46:517–530. doi: 10.1146/annurev.ph.46.030184.002505. [DOI] [PubMed] [Google Scholar]
- Barchi R. L. Probing the molecular structure of the voltage-dependent sodium channel. Annu Rev Neurosci. 1988;11:455–495. doi: 10.1146/annurev.ne.11.030188.002323. [DOI] [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore by neurotoxins. An allosteric model. J Biol Chem. 1977 Dec 10;252(23):8669–8676. [PubMed] [Google Scholar]
- Catterall W. A. Binding of scorpion toxin to receptor sites associated with sodium channels in frog muscle. Correlation of voltage-dependent binding with activation. J Gen Physiol. 1979 Sep;74(3):375–391. doi: 10.1085/jgp.74.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Risk M. Toxin T4(6) from Ptychodiscus brevis (formerly Gymnodinium breve) enhances activation of voltage-sensitive sodium channels by veratridine. Mol Pharmacol. 1981 Mar;19(2):345–348. [PubMed] [Google Scholar]
- Catterall W. A. The molecular basis of neuronal excitability. Science. 1984 Feb 17;223(4637):653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- Couraud F., Jover E., Dubois J. M., Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20(1):9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Darbon H., Jover E., Couraud F., Rochat H. Alpha-scorpion neurotoxin derivatives suitable as potential markers of sodium channels. Preparation and characterization. Int J Pept Protein Res. 1983 Aug;22(2):179–186. doi: 10.1111/j.1399-3011.1983.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Darbon H., Jover E., Couraud F., Rochat H. Photoaffinity labeling of alpha- and beta- scorpion toxin receptors associated with rat brain sodium channel. Biochem Biophys Res Commun. 1983 Sep 15;115(2):415–422. doi: 10.1016/s0006-291x(83)80160-0. [DOI] [PubMed] [Google Scholar]
- Elmer L. W., O'Brien B. J., Nutter T. J., Angelides K. J. Physicochemical characterization of the alpha-peptide of the sodium channel from rat brain. Biochemistry. 1985 Dec 31;24(27):8128–8137. doi: 10.1021/bi00348a044. [DOI] [PubMed] [Google Scholar]
- Feller D. J., Talvenheimo J. A., Catterall W. A. The sodium channel from rat brain. Reconstitution of voltage-dependent scorpion toxin binding in vesicles of defined lipid composition. J Biol Chem. 1985 Sep 25;260(21):11542–11547. [PubMed] [Google Scholar]
- Fontecilla-Camps J. C., Almassy R. J., Suddath F. L., Bugg C. E. The three-dimensional structure of scorpion neurotoxins. Toxicon. 1982;20(1):1–7. doi: 10.1016/0041-0101(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Gordon D., Merrick D., Auld V., Dunn R., Goldin A. L., Davidson N., Catterall W. A. Tissue-specific expression of the RI and RII sodium channel subtypes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8682–8686. doi: 10.1073/pnas.84.23.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Merrick D., Wollner D. A., Catterall W. A. Biochemical properties of sodium channels in a wide range of excitable tissues studied with site-directed antibodies. Biochemistry. 1988 Sep 6;27(18):7032–7038. doi: 10.1021/bi00418a054. [DOI] [PubMed] [Google Scholar]
- Greenblatt R. E., Blatt Y., Montal M. The structure of the voltage-sensitive sodium channel. Inferences derived from computer-aided analysis of the Electrophorus electricus channel primary structure. FEBS Lett. 1985 Dec 2;193(2):125–134. doi: 10.1016/0014-5793(85)80136-8. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci U S A. 1986 Jan;83(2):508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Jover E., Massacrier A., Cau P., Martin M. F., Couraud F. The correlation between Na+ channel subunits and scorpion toxin-binding sites. A study in rat brain synaptosomes and in brain neurons developing in vitro. J Biol Chem. 1988 Jan 25;263(3):1542–1548. [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Rodriguez H. A discontinuous electrophoretic system for separating peptides on polyacrylamide gels. Anal Biochem. 1983 Sep;133(2):515–522. doi: 10.1016/0003-2697(83)90118-5. [DOI] [PubMed] [Google Scholar]
- Messner D. J., Feller D. J., Scheuer T., Catterall W. A. Functional properties of rat brain sodium channels lacking the beta 1 or beta 2 subunit. J Biol Chem. 1986 Nov 15;261(32):14882–14890. [PubMed] [Google Scholar]
- Meves H., Simard J. M., Watt D. D. Interactions of scorpion toxins with the sodium channel. Ann N Y Acad Sci. 1986;479:113–132. doi: 10.1111/j.1749-6632.1986.tb15565.x. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Poli M. A., Mende T. J., Baden D. G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol Pharmacol. 1986 Aug;30(2):129–135. [PubMed] [Google Scholar]
- Ray R., Morrow C. S., Catterall W. A. Binding of scorpion toxin to receptor sites associated with voltage-sensitive sodium channels in synaptic nerve ending particles. J Biol Chem. 1978 Oct 25;253(20):7307–7313. [PubMed] [Google Scholar]
- Rochat H., Bernard P., Couraud F. Scorpion toxins: chemistry and mode of action. Adv Cytopharmacol. 1979;3:325–334. [PubMed] [Google Scholar]
- Rossie S., Gordon D., Catterall W. A. Identification of an intracellular domain of the sodium channel having multiple cAMP-dependent phosphorylation sites. J Biol Chem. 1987 Dec 25;262(36):17530–17535. [PubMed] [Google Scholar]
- Schmidt J. W., Catterall W. A. Palmitylation, sulfation, and glycosylation of the alpha subunit of the sodium channel. Role of post-translational modifications in channel assembly. J Biol Chem. 1987 Oct 5;262(28):13713–13723. [PubMed] [Google Scholar]
- Sharkey R. G., Beneski D. A., Catterall W. A. Differential labeling of the alpha and beta 1 subunits of the sodium channel by photoreactive derivatives of scorpion toxin. Biochemistry. 1984 Dec 4;23(25):6078–6086. doi: 10.1021/bi00320a027. [DOI] [PubMed] [Google Scholar]
- Strichartz G., Rando T., Wang G. K. An integrated view of the molecular toxinology of sodium channel gating in excitable cells. Annu Rev Neurosci. 1987;10:237–267. doi: 10.1146/annurev.ne.10.030187.001321. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Talvenheimo J. A., Catterall W. A. The sodium channel from rat brain. Reconstitution of neurotoxin-activated ion flux and scorpion toxin binding from purified components. J Biol Chem. 1984 Feb 10;259(3):1676–1688. [PubMed] [Google Scholar]
- Wollner D. A., Catterall W. A. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Ayeb M., Bahraoui E. M., Granier C., Rochat H. Use of antibodies specific to defined regions of scorpion alpha-toxin to study its interaction with its receptor site on the sodium channel. Biochemistry. 1986 Oct 21;25(21):6671–6678. doi: 10.1021/bi00369a052. [DOI] [PubMed] [Google Scholar]