Abstract
We have previously demonstrated that CD4+ Th2 lymphocytes are required to rescue facial motoneuron (FMN) survival after facial nerve axotomy through interaction with peripheral antigen presenting cells, as well as CNS resident microglia. Furthermore, the innate immune molecule, toll-like receptor 2 (TLR2), has been implicated in the development of Th2-type immune responses and can be activated by intracellular components released by dead or dying cells. The role of TLR2 in the FMN response to axotomy was explored in this study, using a model of facial nerve axotomy at the stylomastoid foramen in the mouse, in which blood-brain-barrier (BBB) permeability does not occur. After facial nerve axotomy, TLR2 mRNA was significantly upregulated in the facial motor nucleus and co-immunofluorescence localized TLR2 to CD68+ microglia, but not GFAP+ astrocytes. Using TLR2-deficient (TLR2−/−) mice, it was determined that TLR2 does not affect FMN survival levels after axotomy. These data contribute to understanding the role of innate immunity after FMN death and may be relevant to motoneuron diseases, such as amyotrophic lateral sclerosis (ALS).
Keywords: neuroprotection, PRR, TLR2, facial motoneuron, CNS
INTRODUCTION
Our lab discovered that facial motoneuron (FMN) survival after facial nerve transection outside the skull is decreased in mice that lack functional adaptive immune systems [32]. Furthermore, it has been demonstrated that T cells infiltrate the mouse facial motor nucleus after peripheral facial nerve injury [16,29]. In support of these findings, our laboratory has shown that Th2 cells develop in the draining cervical lymph node after facial nerve axotomy [41] and rescue FMN survival through interaction with microglia [4,11]. Collectively, these data suggest a mechanism for Th2 cell-mediated rescue of FMN after facial nerve axotomy that depends on microglia.
Microglia are thought to be key mediators of CNS inflammation, since they express major histocompatibility complex II [17], are derived from the hematopoietic lineage [12] and are potent cytokine producers [14]. Furthermore, microglia are considered to be the parenchymal macrophages of the CNS, based on expression of F4/80 [28], CD11b [15] and CD68 [18]. However, in contrast to peripheral macrophages, microglia have decreased CD45 expression [31] and increased F4/80 expression [5].
After mouse facial nerve axotomy, microglia encircle and phagocytize damaged or dying FMN [35]. While the specific signals that perpetuate microglial activation are not fully understood, toll-like receptors may be involved through recognition of molecules released by dead/damaged FMN. In agreement, TLR2 has previously been demonstrated to be upregulated on microglia in denervated zones of the hippocampus after transection of axons in the entorhinal cortex [1]. However, it is unknown if TLR2 is involved in FMN-mediated neuroprotection after facial nerve axotomy, whereby the BBB is not permeabilized [29].
It has been previously shown that mouse immunization with the TLR2 agonist, Pam3Cys, induces the Th2 cytokines, interleukin 5 (IL-5) and IL-13 [30]. Furthermore, in a model of chronic asthma, Th2 cytokine levels are significantly decreased in the lungs of TLR2−/− mice [3]. In agreement, staphylococcus aureus, a bacterium that activates TLR2 [34], causes increased expression of Th2 cytokines, IL-4, IL-5 and IL-13, in a mouse model of allergic conjunctivitis [8]. Thus, TLR2 activation appears to be associated with enhanced Th2-type immune responses.
Based on the ability of TLR2 to induce a Th2-type immune response and the requirement of Th2 lymphocytes for rescuing FMN survival after axotomy, we hypothesized that TLR2 plays a role in FMN survival after peripheral facial nerve axotomy. To investigate this hypothesis, we initially used microarray analysis to analyze TLR2 and TLR4 mRNA levels in the facial motor nucleus at a time point consistent with significant T cell infiltration [29]. Based on microarray analysis, we focused our investigation on TLR2. Co-immunofluorescence revealed that TLR2 localizes to microglia, but not astrocytes, after injury. Furthermore, we used the well established facial nerve axotomy paradigm in conjunction with the TLR2−/− mouse model to determine whether TLR2 plays a role in rescuing FMN survival after injury to the facial nerve. Collectively, the results indicate that TLR2 mRNA expression increases in the facial motor nucleus, localizes to microglia, but does not affect FMN survival after injury.
MATERIALS AND METHODS
Animals and surgical procedures
Seven-week old female wild-type (WT; C57BL/6) and TLR2−/− (C57BL/6 background) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and Taconic (Germantown, NY), respectively. Mice were used at 8 weeks of age in all experiments. All surgical procedures were completed in accordance with National Institutes of Health guidelines on the care and use of laboratory animals for research purposes. Mice were anesthetized with 3% isoflurane for all surgical procedures. Using aseptic techniques, the right facial nerve of each animal was exposed and transected at its exit from the stylomastoid foramen [19].
Microarray experiments
At 7 days post-axotomy (DPA), WT mice (n=4) were euthanized with CO2 and the brains rapidly removed. Coronal sections of brain stem including both facial motor nuclei were collected and tissue punches of the left (uninjured control) and right (axotomized) facial motor nuclei obtained from these sections and pooled (right axotomy vs left control). The tissue punches were placed into 0.65 mL microfuge tubes with 20, 1.4 mm Lysing Matrix D beads (Q-Biogene, Morgan Irvine, CA)/tube, and 80 µL working solution D (4M guanidium thiocyanate, 25mM sodium citrate, .5% sarcosyl, 0.1M β-mercaptoethanol in RNAse-free H2O). RNA was isolated from tissue punches and harvested cells by guanidinium-thiocyanate extraction [6]. Samples were processed at Superarray Bioscience Corporation (Frederick, MD) using the Mouse Chemokines and Receptors Microarray, OMM-022. Fold-changes in gene expression (right axotomy/left control) were calculated for pair-wise comparison using the GEArray Expression Analysis suite. Changes in mRNA levels for select inflammatory genes other than TLR2 and TLR4 are demonstrated in supplementary figure 1.
Real-time PCR experiments
WT mouse brains were removed at 1, 4, 7, 14 and 30 DPA (n=3–6/timepoint) and flash frozen [in 62.5% n-Butyl Bromide (Fisher Scientific, Pittsburgh, PA) + 37.5% 2-methylbutane (Fisher Scientific) surrounded by crushed dry ice]. Additional uninjured animals were also included (n=6). Frozen brains were sectioned on the Leica CM3000 cryostat (Leica, Bannockburn, IL) with a temperature of −24°C and immersed into Tissue Teck O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) at 25 µm intervals and thaw-mounted onto membrane-coated glass slides (Leica Microsystems Inc., Bannockburn, IL). Laser microdissection of control and axotomized facial motor nuclei using the Leica AS LMD (Leica Microsystems Inc.) was accomplished, capturing tissue into 0.5 mL microcentrifuge tube caps with 65 uL Extraction Buffer (PicoPure RNA Isolation kit, Arcturus, Mountain View, CA).
A PicoPure RNA Isolation kit (Arcturus) was used to extract RNA and followed the manufacturer’s instructions. Complementary DNA was generated and used in real-time PCR reactions and amplification was detected with SYBR green fluorescent dye (Applied Biosystems, Carlsbad, CA). Primer sequence information was previously described for TLR2 [7,40] and GAPDH [13]. GAPDH served as the reference gene. For each sample, percent change in TLR2 mRNA levels was calculated using the formula (axotomy/control × 100) − 100%.
Immunofluorescence experiments
At 7 DPA, WT mice were euthanized with CO2 followed by flash freezing [in 62.5% n-Butyl Bromide (Fisher Scientific, Pittsburgh, PA) + 37.5% 2-methylbutane (Fisher Scientific) surrounded by crushed dry ice]. Frozen brains were sectioned on the Leica CM3000 cryostat (Leica, Bannockburn, IL) with a temperature of −24°C and immersed into Tissue Teck O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) at 8 µm intervals and thaw-mounted onto pre-cleaned SuperFrost slides (Fisher Scientific). Sections were post-fixed with 4% paraformaldehyde, blocked for endogenous biotin for 5 min. (1% H2O2 in PBS), and blocked for non-specific staining with 10% bovine serum albumin (A4503; Sigma-Aldrich; Saint Louis, MO) in PBS for 1 hr. Sections were incubated with biotinylated anti-TLR2 (6C2; Ebioscience; San Diego, CA) that, has previously been documented with mouse tissue [1,36,37] and was co-incubated with mouse anti-GFAP alexafluor 488 (131-17719; Invitrogen; Carlsbad, CA) or rat anti-CD68 alexafluor 488 (FA-11; AbD Serotec; Raleigh, NC) in PBS at 4°C for 2 days. Sections were washed extensively and incubated with streptavidin-alexafluor 555 for 1 hr. Following extensive washing, sections were covered with ProLong Gold Antifade reagent (Invitrogen). Finally, no signal was detected for sections processed with the isotype control antibody, rat IgG2b [(Ebioscience) (supplementary Fig. 2)].
Images of antibody-stained sections were captured using the IX70 Fluoview (Olympus; Center Valley, PA) microscope attached to a Retiga 2000R (QImaging; Surrey, BC) CCD camera and image capturing system using Image Pro Plus software (v.6.3; Media Cybernetics; Bethesda, MD). Fluorescent images were captured using the 60× objective, for 600× magnification.
All data are presented as means ± SEM. The results from the experiments were analyzed using GB-STAT School Pak (Dynamic Microsystems, Inc.; Silver Spring, MD). Data were analyzed using the analysis of variance (ANOVA) method, followed by post hoc comparisons using the Newman-Keuls test.
RESULTS
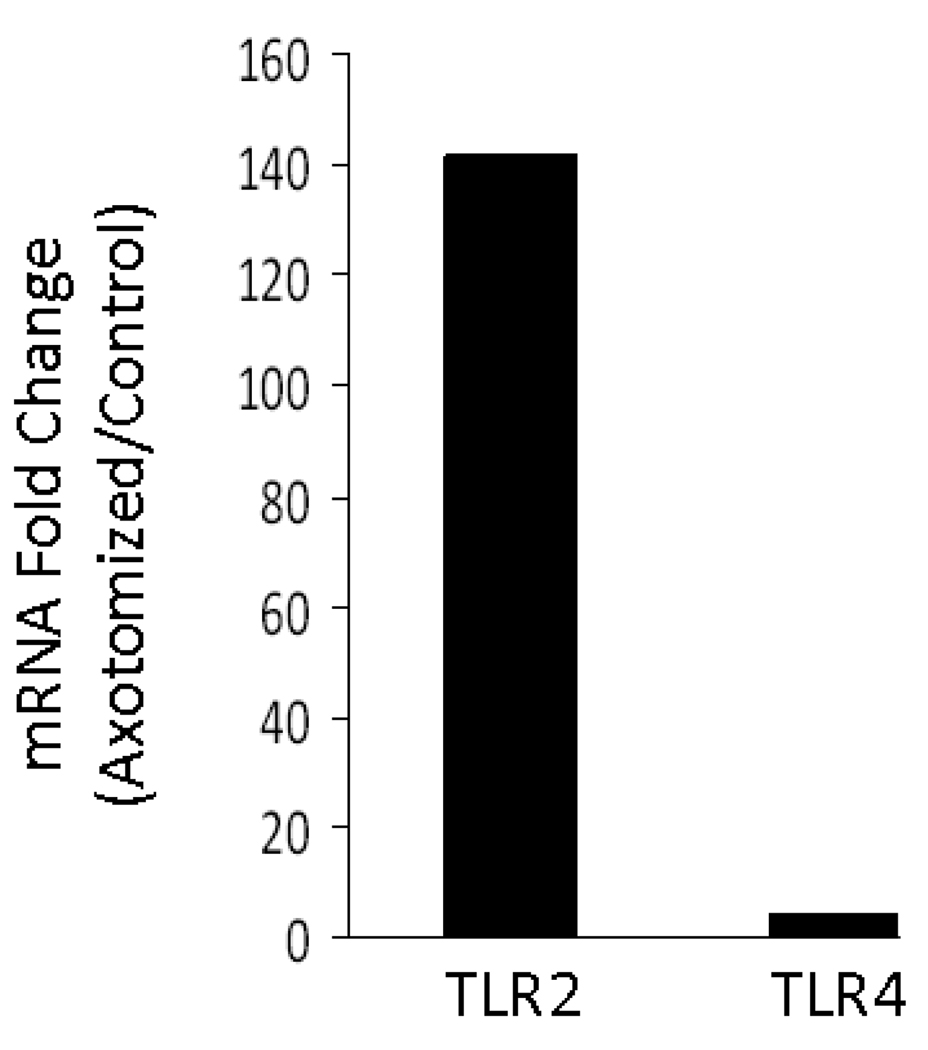
To determine if TLR2 and TLR4 mRNA levels were increased in the facial motor nucleus after facial nerve injury, a preliminary microarray analysis was performed. At 7 DPA, mRNA levels were increased for TLR2 and TLR4, 142 and 4 fold, respectively, in WT mice (Fig. 1). Thus, facial nerve axotomy appears to increase mRNA levels for TLR2 and TLR4 in WT mouse facial motor nucleus.
Figure 1. Toll-like receptor 2 (TLR2) and TLR4 mRNA levels in mouse facial motor nucleus 7 days after facial nerve axotomy.
mRNA levels are displayed as the fold change between the axotomized side and the uninjured (control) side.
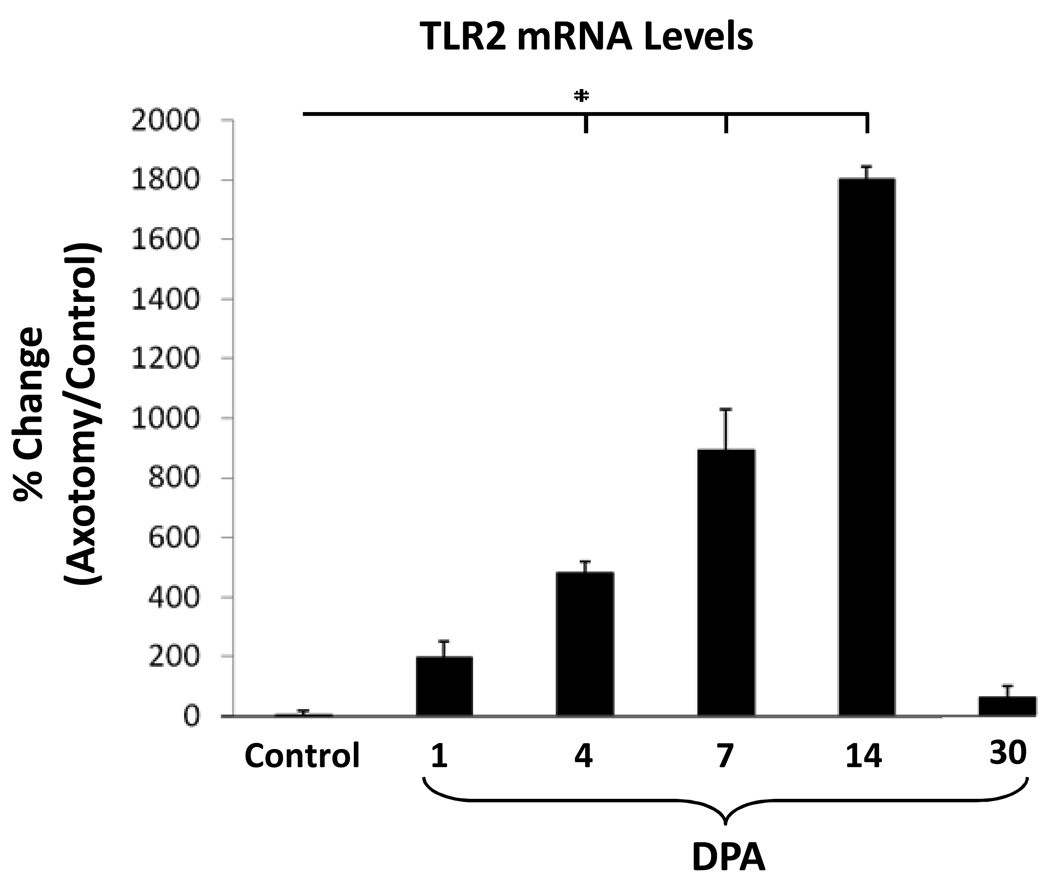
Because TLR2 mRNA changes were so much greater than TLR4, TLR2 mRNA expression was further analyzed in WT mice with real-time PCR (Fig. 2). TLR2 mRNA expression were not significantly different when left and right uninjured facial nuclei were compared. Facial nerve axotomy increased TLR2 mRNA expression at 1, 4, 7, 14 and 30 DPA [194% ± 58%, 481% ± 36%, 893% ± 135%, 1803% ± 38% and 62% ± 38%, (p < 0.01) respectively]. Thus, facial nerve axotomy rapidly induces TLR2 mRNA expression in the WT mouse facial motor nucleus, with a peak at 14 DPA, before significantly decreasing toward baseline levels by 30 DPA.
Figure 2. TLR2 mRNA levels in control or axotomized mouse facial motor nucleus 1, 4, 7, 14 and 30 days post-axotomy (DPA).
Control TLR2 mRNA levels are displayed as the percent change between the right (uninjured) side and the left (uninjured) control side within each animal. At 1, 4, 7, 14 and 30 DPA, TLR2 mRNA levels are displayed as the percent change between the right (axotomized) side and the left (uninjured) control side within each animal. Bar heights represent means (±SEM). * Denotes significant differences at p ≤ 0.01.
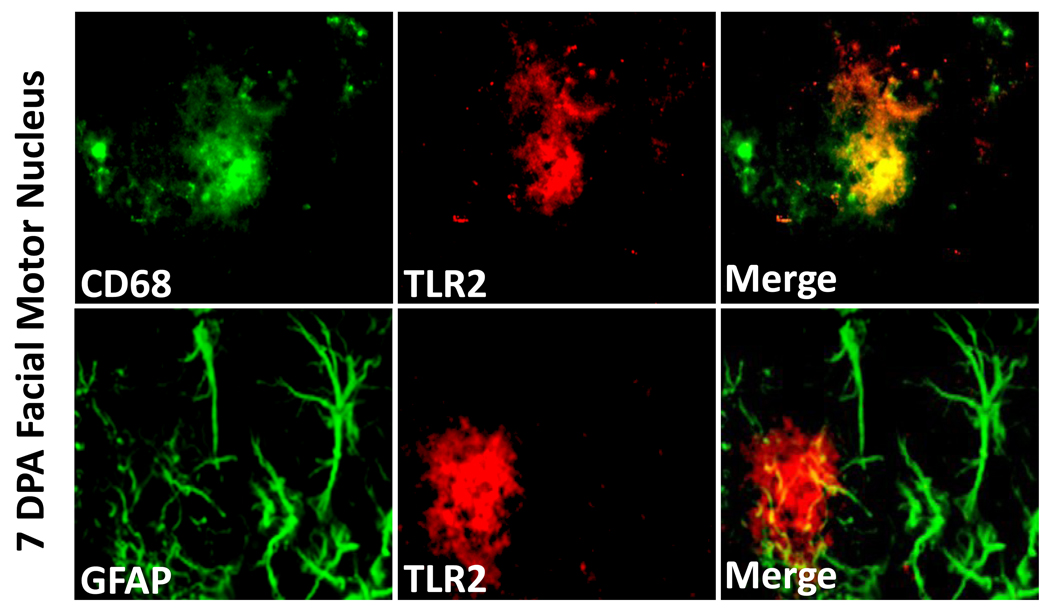
To determine TLR2 cellular localization in the WT injured facial motor nucleus, TLR2 immunofluorescence was performed on brainstem sections from animals 7 DPA (Fig. 3). Co-immunofluorescence for TLR2 with CD68 demonstrated labeling overlap, indicating that TLR2 localizes to microglia in the injured facial motor nucleus. In contrast, co-immunofluorescence for TLR2 with GFAP demonstrated no overlap, showing that TLR2 does not localize to astrocytes in the injured facial motor nucleus. Thus, microglia are reactive for TLR2 in the WT facial motor nucleus after facial nerve axotomy.
Figure 3. TLR2 immunofluorescence in 7 day post-axotomy mouse facial motor nucleus.
High-power (original magnification, 600×) immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for CD68 (green) and TLR2 (red; top row) or GFAP (green) and TLR2 (red; bottom row).
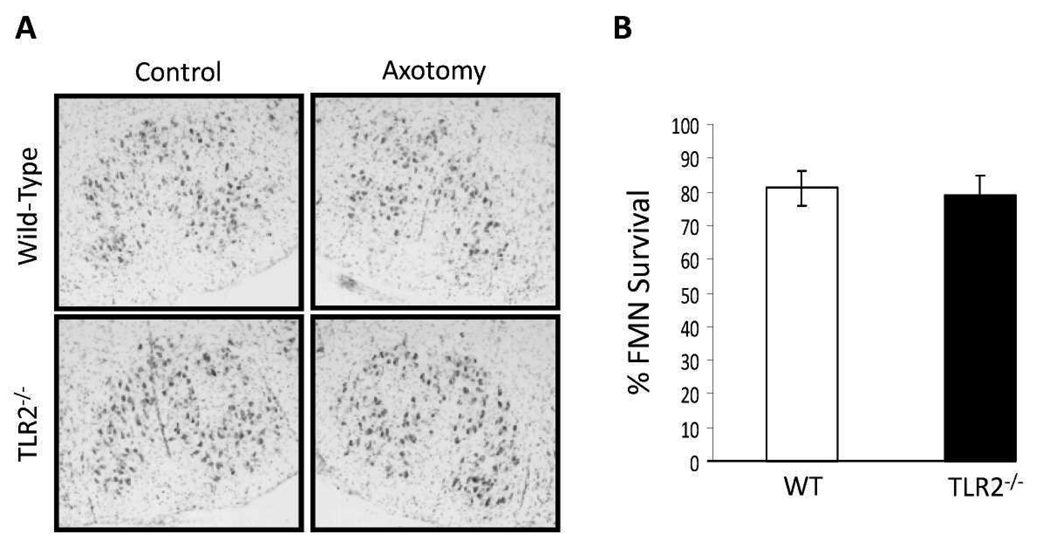
To determine if TLR2 affects FMN survival after injury, a right facial nerve axotomy was performed on WT and TLR2−/− mice. In WT mice, FMN survival levels were 81 ± 5.0%, relative to control values at 4 weeks post-axotomy (Fig. 4). In TLR2−/− mice, FMN survival levels were 79 ± 5.8%, relative to control values at 4 weeks post-axotomy. Thus, TLR2 does not affect FMN survival levels after injury.
Figure 4. Facial motoneuron (FMN) survival levels 4 weeks after facial nerve axotomy in wild-type (WT) and toll-like receptor 2-deficient (TLR2−/−) mice.
(A) Thionin-stained FMN in control and axotomized facial motor nuclei (original magnification, 100×). (B) Average percent FMN survival after facial nerve injury (±SEM).
DISCUSSION
The mechanism underlying axotomy-induced TLR2 mRNA regulation in the facial motor nucleus after facial nerve axotomy is unknown. It has previously been demonstrated that TLR2 is activated by the intracellular proteins, HSP60 [36], HSP70 [39] and HMGB1 [27]. Co-incidentally, HSP70 mRNA expression is induced in the facial motor nucleus of adult hamsters after facial nerve axotomy [25]. Thus, it is possible that axotomy-induced FMN death results in the release of TLR2 agonists, such as HSP70, causing positive feedback for increased TLR2 mRNA expression. Although the effect of TLR2 on cell recruitment was not determined by this study, it is coincidental that maximum TLR2 mRNA expression occurs at 14 DPA, a time point that is consistent with peak T cell infiltration into the facial motor nucleus [29].
In the present study, we hypothesized that TLR2 plays a role in mouse FMN survival after facial nerve axotomy based on previous studies demonstrating TLR2-induced Th2-type immunity [3,8,30] and that Th2 lymphocytes rescue FMN from axotomy induced death [11]. The data indicate an axotomy-regulated increase in TLR2 mRNA levels in mouse facial motor nucleus coincident with T cell infiltration into the facial motor nucleus [29]. It is important to note that TLR4 mRNA levels slightly increased according to microarray analysis, which may be associated with an ability to recognize select intracellular proteins released by dead or dying cells, such as HSP60 [9]. Regarding TLR2 immunoreactivity, localization to microglia demonstrated by this report corroborates previous results from other laboratories [21,23,26]. Interestingly, our data show that TLR2 does not affect FMN survival after facial nerve axotomy, since there was no difference in FMN survival between TLR2−/− and WT mice.
In a mouse model of sciatic nerve lesion, macrophage recruitment to the sciatic nerve distal stump is decreased in TLR2−/− mice [2]. This may be due to the significant decrease in mRNA levels for the macrophage recruiting chemokine, CCL2, which leads to a delay in axonal regeneration and recovery of locomotor function. In contrast, TLR2 regulates T cell-, but not macrophage-recruitment in the hippocampus after transection of axons in the entorhinal cortex [1]. In TLR2−/− mice, CCL3, CCL4, CXCL2 and CXCL10 mRNA is reduced in denervated hippocampus, while CCL2 mRNA expression is unaffected. Thus, the recruitment of specific cells to the sites of neuronal injury likely depends on location of lesion, the type of neuron being injured and the injury-specific chemokine expression.
In conclusion, the literature supports the hypothesis that TLR2 localizes to microglia, has a Th2 response-promoting effect and is upregulated in areas of neuronal damage. Accordingly, this study confirms that TLR2 mRNA expression is increased in the facial motor nucleus after facial nerve axotomy at times when FMN survival is beginning to be affected [33]. Additionally, this investigation confirms that TLR2 localizes to microglia, but a TLR2-deficiency does not affect FMN survival after injury. However, this study cannot rule out the possibility that prolonged periods of TLR2 over-expression affects neuronal survival during CNS infection [22], malignancy [10] or disease, such as ALS [24]. Interestingly, chimeric mSOD1 (ALS) mice with microglia deficient for a commonly-used adaptor molecule for TLR signal transduction, MyD88, exhibit earlier disease onset and a shorter overall lifespan than mSOD1 mice with WT microglia [20]. Therefore, future goals include further elucidating the role of TLR in the facial motor nucleus after facial nerve axotomy using MyD88-deficient mice, to rule out compensatory TLR signaling after facial nerve axotomy.
Supplementary Material
Acknowledgements
This work was supported by NIH grant NS40433 (KJJ and VMS) and the Les Turner ALS Foundation (DAW and KJJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, Owens T. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J. Neurosci. 2006;26:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin A, Pineau I, Barrette B, Filali M, Vallières N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckland KF, O'Connor E, Murray LA, Hogaboam CM. Toll like receptor-2 modulates both innate and adaptive immune responses during chronic fungal asthma in mice. Inflamm. Res. 2008;57:379–387. doi: 10.1007/s00011-008-8004-y. [DOI] [PubMed] [Google Scholar]
- 4.Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J. Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocynate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury P, Sacks SH, Sheerin NS. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin. Exp. Immunol. 2006;145:346–356. doi: 10.1111/j.1365-2249.2006.03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SH, Nam KH, Kweon MN. Staphylococcus aureus accelerates an experimental allergic conjunctivitis by Toll-like receptor 2-dependent manner. Clin. Immunol. 2009;131:170–177. doi: 10.1016/j.clim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J. Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- 10.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS. Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp. Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fargo KN, Alexander TD, Tanzer L, Poletti A, Jones KJ. Androgen regulates neuritin mRNA levels in an in vivo model of steroid-enhanced peripheral nerve regeneration. J Neurotrauma. 2008 May;25(5):561–566. doi: 10.1089/neu.2007.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J. Exp. Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J. Neurosci. Res. 1988;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- 16.Ha GK, Huang Z, Petitto JM. Prior facial motor neuron injury elicits endogenous T cell memory: relation to neuroregeneration. J. Neuroimmunol. 2007;183:111–117. doi: 10.1016/j.jneuroim.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes GM, Woodroofe MN. Cuzner, Microglia are the major cell type expressing MHC class II in human white matter. J. Neurol. Sci. 1987;80:25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- 18.Hutchins KD, Dickson DW, Rashbaum WK, Lyman WD. Localization of microglia in the human fetal cervical spinal cord. Brain. Res. Dev. Brain. Res. 1992;66:270–273. doi: 10.1016/0165-3806(92)90091-a. [DOI] [PubMed] [Google Scholar]
- 19.Jones KJ, LaVelle A. Changes in nuclear envelope invaginations in Axotomized immature and mature hamster facial motoneurons. Brain. Res. 1985;353:241–249. doi: 10.1016/0165-3806(85)90212-3. [DOI] [PubMed] [Google Scholar]
- 20.Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J. Cell. Biol. 2007;179:1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt-Jones EA, Chan M, Zhou S, Wang, J J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laflamme N, Soucy, G G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J. Neurochem. 2001;79:648–657. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Hao W, Dawson A, Liu S, Fassbender K. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J. Biol. Chem. 2009;284:3691–3699. doi: 10.1074/jbc.M804446200. [DOI] [PubMed] [Google Scholar]
- 25.New GA, Hendrickson BR, Jones KJ. Induction of heat shock protein 70 mRNA in adult hamster facial nuclear groups following axotomy of the facial nerve. Metab. Brain. Dis. 1988;4:273–279. doi: 10.1007/BF00999773. [DOI] [PubMed] [Google Scholar]
- 26.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 28.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 29.Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J. Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 31.Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J. Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serpe CJ, Sanders VM, Jones KJ. Kinetics of facial motoneuron loss following facial nerve transection in severe combined immunodeficient mice. J. Neurosci. Res. 2000;62:273–278. doi: 10.1002/1097-4547(20001015)62:2<273::AID-JNR11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Tawaratsumida K, Furuyashiki M, Katsumoto M, Fujimoto Y, Fukase K, Suda Y, Hashimoto M. Characterization of N-terminal structure of TLR2-activating lipoprotein in Staphylococcus aureus. J. Biol. Chem. 2009;284:9174–9152. doi: 10.1074/jbc.M900429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torvik A, Soreide AJ. The perineuronal glial reaction after axotomy. Brain. Res. 1975;95:519–529. doi: 10.1016/0006-8993(75)90125-0. [DOI] [PubMed] [Google Scholar]
- 36.Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, Liew FY, Geller DA, Thomson AW. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ukai T, Yumoto H, Gibson FC, Genco CA. Macrophage-elicited osteoclastogenesis in response to bacterial stimulation requires Toll-like receptor 2-dependent tumor necrosis factor-alpha production. Infect. Immun. 2008;76:812–819. doi: 10.1128/IAI.01241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 39.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 40.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J. Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 41.Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4(+) T cell subsets that develop following mouse facial nerve axotomy. Brain. Behav. Immun. 2008;22:528–537. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.