Abstract
Background
White matter integrity has been found to be compromised in adult alcoholics, but it is unclear when in the course of alcohol exposure white matter abnormalities become apparent. This study assessed microstructural white matter integrity among adolescent binge drinkers with no history of an alcohol use disorder.
Methods
We used diffusion tensor imaging to examine fractional anisotropy (FA), a measure of directional coherence of white matter tracts, among teens with (n = 14) and without (n = 14) histories of binge drinking but no history of alcohol use disorder, matched on age, gender, and education.
Results
Binge drinkers had lower FA than controls in 18 white matter areas (clusters ≥27 contiguous voxels, each with p < 0.01) throughout the brain, including the corpus callosum, superior longitudinal fasciculus, corona radiata, internal and external capsules, and commissural, limbic, brainstem, and cortical projection fibers, while exhibiting no areas of higher FA. Among binge drinkers, lower FA in 6 of these regions was linked to significantly greater lifetime hangover symptoms and/or higher estimated peak blood alcohol concentrations.
Conclusions
Binge drinking adolescents demonstrated widespread reductions of FA in major white matter pathways. Although preliminary, these results could indicate that infrequent exposure to large doses of alcohol during youth may compromise white matter fiber coherence.
Keywords: Diffusion Tensor Imaging, Alcohol, Binge Drinking, Adolescence, Brain Imaging, Hangover
Heavy episodic or “binge” drinking is common among adolescents, with 55% of high school seniors reporting having gotten drunk, and 25% reporting consuming 5 or more drinks in a row in the past 2 weeks (Johnston et al., 2007). Despite the prevalence of heavy drinking during adolescence, it remains unclear how binge-pattern alcohol use may affect brain integrity and development.
Magnetic resonance imaging (MRI) studies report white matter degeneration in individuals with alcohol use disorders (AUD). Morphometric studies have demonstrated reductions in overall cerebral (Schottenbauer et al., 2007), gray matter (Chanraud et al., 2007; Makris et al., 2008; Pfefferbaum et al., 1997), white matter (Kril et al., 1997; Pfefferbaum et al., 1997), and corpus callosum (Estruch et al., 1997; Pfefferbaum et al., 1996) volumes among adult alcoholics. Similarly, heavy drinking adolescents with (De Bellis et al., 2005) and without (Medina et al., 2008) comorbid psychiatric disorders exhibit smaller prefrontal white matter volumes. Alcohol-related white matter volume reductions may be due to axonal atrophy, cellular membrane breakdown, or myelin loss (Harper, 1998). While these changes are linked to very heavy drinking, less is known about the impact of monthly to weekly use of 4 to 10 drinks on adolescents' white matter integrity.
Heavy alcohol consumption may exert potent effects on the white matter environment resulting in alterations to fiber structure and orientation, observable with diffusion tensor imaging (DTI). DTI is an MRI technique sensitive to the random movement of water in cells of a target tissue, yielding measures of the magnitude and orientation of this movement (Pierpaoli et al., 1996). Fractional anisotropy (FA), a measure of the directional coherence of brain tissue, provides an estimate of white matter integrity. Higher FA values indicate greater hindrance to water diffusion, which is related to the presence of oriented structures. This index can be used to evaluate the influence of alcohol use on white matter quality (Rosenbloom et al., 2003). FA has been shown to be reduced in the corpus callosum (Pfefferbaum and Sullivan, 2005; Pfefferbaum et al., 2006a; Schulte et al., 2005), right frontal lobe (Harris et al., 2008), and global white matter (Pfefferbaum et al., 2006b) in adult alcoholics, suggesting potential disruption in white matter microstructure. Among adolescents, though, findings are more mixed. In a pilot study, we (Tapert et al., 2003) observed lower FA in the splenium of the corpus callosum among 8 teenagers with AUD and no comorbid Axis I diagnosis, as compared to 8 demographically similar controls. De Bellis and colleagues (2008) reported on 32 adolescents with AUD compared to 28 nondisordered controls, finding no FA decrements among callosal regions, rather increased FA in the rostral portion of the body, and in the isthmus. To date, no known studies have described the effects of binge-level drinking on FA among teenagers who do not meet alcohol abuse or dependence criteria.
Because white matter maturation continues into late adolescence (Hasan et al., 2007; Nagel et al., 2006; Paus et al., 1999; Sowell et al., 2002), understanding the influence of heavy episodic drinking on neurodevelopmental processes is of great relevance. Therefore, the current study serves as an exploratory analysis of white matter integrity in major fiber tracts among typically developing youth (free from any history of psychiatric disorder) with histories of binge drinking, compared to control teens without any past binge episodes. Based on adult and limited adolescent literature, our preliminary hypothesis is that binge drinkers would show compromised white matter fiber coherence (i.e., lower FA). Additionally, greater alcohol involvement was expected to relate to poorer white matter integrity among drinkers.
Materials and Methods
Participants
Subjects were 28 adolescents ages 16 to 19 recruited from a larger study on brain functioning in adolescent substance users (Tapert et al., 2007). Binge drinkers were 14 teens who had consumed at least 5 or 4 alcoholic beverages (for males or females, respectively) in one sitting (NIAAA, 2004; Wechsler et al., 1994) during the 3 months prior to imaging. Controls were 14 teens without a history of a binge drinking episodes matched on age, gender, and level of education, and statistically similar on other demographic measures to the binge drinkers (see Table 1). Exclusionary criteria for this study were: histories of neurological concerns (e.g., learning disorder, head trauma with loss of consciousness >2 minutes, migraine) or psychiatric disorders; history of alcohol or other drug use disorder (abuse or dependence); left-handedness; prenatal exposure to alcohol or drugs; use of psychotropic medication; substance use in the past 72 hours; and MRI contraindications. Written assent and consent were obtained from adolescents and their parent/legal guardians in accordance with the University of California San Diego Human Research Protections Program (18- to 19-year-olds provided their own consent).
Table 1.
Participant Demographic and Substance Use Characteristics
| Binge drinkers (n = 14) M (SD) or % | Controls (n = 14) M (SD) or % | |
|---|---|---|
| Age | 18.09 (0.69) | 17.95 (0.88) |
| % Female | 14.3 | 14.3 |
| % Caucasian | 69.2 | 57.2 |
| % Family history negativea | 92.9 | 92.9 |
| Parent annual salary ($ thousands) | 131.83 (68.27) | 104.92 (61.19) |
| Hollingshead socioeconomic index | 31.14 (17.08) | 33.93 (19.12) |
| Body mass index | 23.98 (4.25) | 22.73 (2.41) |
| Pubertal Development Scale total | 3.53 (0.44) | 3.50 (0.46) |
| Years of education | 11.71 (0.99) | 11.50 (0.76) |
| WASI Vocabulary T-score | 61.43 (9.91) | 57.57 (7.89) |
| WASI Block Design T-score | 56.50 (9.05) | 56.00 (6.69) |
| Beck Depression Inventory total | 2.07 (2.09) | 2.36 (2.92) |
| Spielberger State Anxiety T-score | 35.35 (3.70) | 40.42 (10.19) |
| Child Behavioral Checklist Externalizing T-scoreb | 40.09 (9.35) | 46.57 (10.29) |
| Child Behavioral Checklist Internalizing T-scoreb | 45.36 (9.60) | 45.64 (8.25) |
| Lifetime alcohol use episodes*** | 54.57 (48.80) | 4.57 (6.21) |
| Drinks per month, past 3 months*** | 15.43 (7.82) | 0.21 (0.80) |
| Peak drinks on an occasion, past 3 months*** | 8.21 (3.02) | 0.07 (0.27) |
| Estimated peak BAC, past 3 months*** | 240 (0.070) | 000 (0.010) |
| Number of hangover symptoms, past 3 months** | 1.43 (1.74) | 0.14 (0.53) |
| Days since last alcohol use* | 30.29 (10.53) | 513.29 (417.27) |
| Tobacco cigarettes per day | 0.14 (0.53) | 0.00 (0.00) |
| Lifetime marijuana use episodes | 2.21 (3.09) | 0.64 (1.39) |
| Lifetime other drug use episodes | 0.29 (0.73) | 0.00 (0.00) |
BAC, blood alcohol concentration.
No first or second degree biological relative with alcohol or drug abuse or dependence.
Not available for 3 binge drinkers.
Binge drinkers significantly differ from controls (
p < 0.05,
p < 0.01,
p < 0.001).
Measures
Parents and youth completed screening interviews, including the Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001), to rule out psychiatric disorders and other exclusions listed above. Parents provided socioeconomic status background information which was converted to a Hollingshead Index of Social Position score (Hollingshead, 1965), and completed the Child Behavior Checklist (Achenbach and Rescorla, 2001) yielding continuous indices of internalizing and externalizing psychopathological syndromes normalized by age and gender. Pubertal staging was assessed with the Pubertal Development Scale (Petersen et al., 1988), and calculated separately by gender. The Beck Depression Inventory (Beck, 1978) and Spielberger State Trait Anxiety Inventory (Spielberger et al., 1970) assessed mood state at the time of scanning. Premorbid intellectual functioning was estimated by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) Vocabulary and Block Design subtests. Immediately preceding the scan session, subjects submitted samples for breathalyzer and urine toxicology analyses to ensure against recent intoxication, and were administered a 28-day Timeline Followback (Sobell and Sobell, 1992) to document patterns of recent substance use.
Detailed alcohol involvement history was assessed using the Customary Drinking and Drug Use Record (Brown et al., 1998) interview, administered to teens for lifetime and past 3-month information on alcohol, nicotine, and other drug use, DSM-IV abuse/dependence symptoms (APA, 1994), and hangover/withdrawal experiences. Peak and typical drinking amounts, consumption durations, and body mass index were used to estimate blood alcohol concentrations (BAC) (Fitzgerald, 1995; Widmark, 1922). To differentiate the impact of binge-type drinking versus other alcohol involvement on FA, bivariate analyses were conducted using peak BAC and total withdrawal experiences (indices of binge drinking), as well as years of alcohol use (duration) and total lifetime drinks.
Procedures
Diffusion weighted data were collected on a 3.0-Tesla GE magnetic resonance scanner using whole brain echo planar imaging (repetition time = 12,000 ms; echo time = 93.4 ms; 36 × 3.0 mm thick axial slices; in plane voxel resolution 1.875 × 1.875). Diffusion gradients were applied in 15 directions with 4 averages and a b-value of 2,000 s/mm2. Raw images were uniformly pre-processed by analyzers (TM and SB), blind to participant group status, to correct for: (1) eddy-current distortions using Functional Magnetic Resonance Imaging of the Brain's (FMRIB) Diffusion Toolbox (FDT; 2D with 6 degrees-of-freedom) (Smith et al., 2004); (2) head motion by registering the diffusion images to the b0 image as a reference, and (3) susceptibility distortions by applying field map unwarping (Smith et al., 2006). FA values were computed using a log-linear estimation procedure to fit each data point to a diffusion tensor model via FDT (Basser et al., 1994). FA maps from each participant were submitted to Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006), which involved 3 steps: (1) all datasets were nonlinearly registered and aligned to a study-specific target, which was representative of the sample under investigation, and were resampled to 1 mm3 in MNI-152 space (Evans et al., 2003); (2) a mean aligned FA image was created, from which a thresholded (FA > 0.3) skeletonized mean FA image was derived, representing white matter tracts common to all in the sample; and (3) each subject's FA values were projected onto the skeleton.
Data Analyses
Independent samples t-tests across the skeletonized FA map permitted voxel-wise comparison of mean differences between binge drinkers in controls (Cox, 1996). To control for type I error, a combined t statistic magnitude (p < 0.01) and cluster volume thresholding method was utilized. Monte Carlo simulation revealed that 27 contiguous voxels exceeding the t-threshold were required to protect family-wise error at p = 0.01, assuming 4-mm FWHM intrinsic smoothing (Smith et al., 2006). No additional smoothing kernel was applied to the data. Areas representing group differences of FA on white matter tracts were identified using a DTI atlas (Wakana et al., 2004). To further explore the influence of alcohol use on white matter integrity, follow-up bivariate correlation analyses (using Pearson's r) related binge drinkers' alcohol involvement variables (lifetime hangover symptoms, estimated peak blood alcohol concentration, lifetime drinks, and duration of drinking) to mean FA in clusters that significantly differed between groups.
Results
Independent samples t-tests revealed that teen binge drinkers exhibited lower (p < 0.01) FA in 18 white matter areas (each ≥27 contiguous voxels) throughout the brain, relative to controls. Table 2 itemizes each area by cluster number while Fig. 1 depicts these regions, including the superior (Clusters 6, 15, 16, and 18) and inferior (Cluster 17) longitudinal fasciculi, corona radiata (Clusters 1, 7, and 14), internal (Cluster 8) and external (Clusters 9 and 10) capsules, corpus callosum (Clusters 2, 3, 4, and 5), cerebellum (Clusters 12 and 13), and limbic projection fibers (Cluster 11). No cluster showed higher FA in bingers than controls. All Hedge's g effect sizes exceeded 1.0 (very large range) for each cluster (see Table 2).
Table 2.
Binge Drinkers Show Lower Fractional Anisotropy Than Controls (Clusters ≥27 Contiguous Voxels Each at p < 0.01) in 18 Regions Referred to by Cluster Number
| Cluster number | Anatomic region | Number of voxels | MNI coordinatesa | Effect size Hedge's gb | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal lobe | ||||||
| 1 | Left anterior coronal radiata | 90 | 18 | 12 | 55 | 1.69 |
| 2 | Corpus callosum—body | 44 | −14 | −21 | 21 | 1.58 |
| 3 | Corpus callosum—genu | 29 | −14 | −32 | 8 | 1.47 |
| 4 | Corpus callosum—body & genu | 29 | −1 | −20 | 14 | 1.57 |
| 5 | Corpus callosum—body | 29 | −13 | −15 | 24 | 1.47 |
| 6 | Right superior longitudinal fasciculus | 31 | −49 | 3 | 36 | 1.42 |
| 7 | Right anterior coronal radiata | 27 | −17 | 9 | 60 | 1.31 |
| Subcortical areas | ||||||
| 8 | Left posterior limb of internal capsule | 179 | 25 | 23 | 5 | 1.57 |
| 9 | Left external capsule | 72 | 33 | −9 | −3 | 2.11 |
| 10 | Right external capsule | 40 | −35 | 11 | 5 | 1.42 |
| 11 | Left fornix/stria terminalis | 32 | 28 | 27 | −9 | 1.54 |
| Cerebellum | ||||||
| 12 | Inferior cerebellar peduncle | 42 | 5 | 58 | −32 | 1.58 |
| 13 | Superior cerebellar peduncle | 39 | 9 | 30 | −25 | 1.52 |
| Parietal lobe | ||||||
| 14 | Left posterior coronal radiata | 122 | 24 | 37 | 53 | 1.48 |
| 15 | Right superior longitudinal fasciculus | 33 | −34 | 31 | 39 | 1.32 |
| 16 | Left superior longitudinal fasciculus | 28 | 51 | 18 | 39 | 1.54 |
| Temporal lobe | ||||||
| 17 | Left inferior longitudinal fasciculus | 47 | 36 | 3 | −17 | 1.72 |
| 18 | Left superior longitudinal fasciculus | 29 | 45 | 60 | 0 | 1.56 |
Montreal Neurological Institute (MNI) coordinates point to maximum difference within cluster.
Hedge's g calculated from t-value averaged across all voxels within the cluster.
Fig. 1.
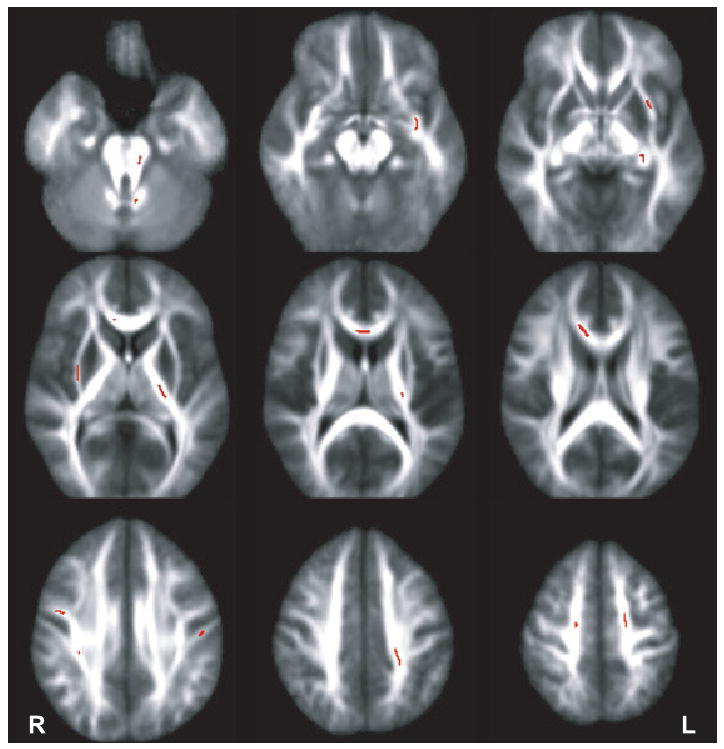
Clusters (darkened areas) overlaid on average fractional anisotropy mask highlight where binge drinking adolescents had lower fractional anisotropy than controls (clusters ≥27 contiguous voxels each at p < 0.01). In no cluster did drinkers have higher fractional anisotropy than controls.
Exploratory bivariate correlations among binge drinkers, uncorrected for multiple comparisons, were run to determine if areas of group differences in FA were associated with alcohol involvement. More lifetime alcohol hangover/withdrawal experiences were related to lower FA in the corpus callosum (Cluster 2: r = −0.65, p < 0.025; Cluster 4: r = −0.71, p < 0.025; and Cluster 5: r = −0.60; p < 0.025; see Fig. 2), and trends were found in the left anterior corona radiata (Cluster 1: r = −0.50, p = 0.06) and left inferior cerebellar peduncle (Cluster 12: r = −0.50; p = 0.06). Peak estimated blood alcohol concentration in the 3 months prior to scanning was also inversely related to FA in the body of the corpus callosum (Cluster 5: r = −0.54, p < 0.05), left internal and right external capsules (Cluster 9: r = −0.53, p < 0.05; and Cluster 10: r = −0.59, p < 0.025), and left posterior corona radiata (Cluster 14: r = −0.62, p < 0.025). No relationships were observed between FA and lifetime drinks, and duration of drinking.
Fig. 2.
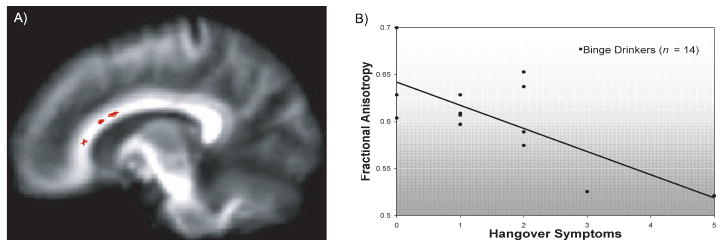
(A) Clusters (darkened area along callosal fibers) and (B) bivariate scatterplot indicate where lower fractional anisotropy was significantly linked to more hangover symptoms in the body and genu of the corpus callosum (r = −0.71, p < 0.005).
Follow-up correlational analyses determined if age might account for FA differences in clusters that differed between groups (n = 28). Age was not linked to FA in any cluster, but advanced pubertal stage was marginally linked to higher FA in the body of the corpus callosum (Cluster 2: r = 0.36, p = 0.06; Cluster 5: r = 0.34, p = 0.07) and fornix/stria terminalis tracts (Cluster 11: r = 0.31, p = 0.10).
Discussion
This study compared white matter integrity between adolescents with binge alcohol consumption relative to controls matched on age, gender, verbal IQ, ethnicity, and socioeconomic status. Despite demographic similarities, widespread reductions in FA were observed in heavy episodic drinkers, suggesting the possibility of compromised white matter integrity in major fiber tract pathways in frontal (including the corpus callosum genu and body), cerebellar, temporal, and parietal regions. These findings extend previous reports of FA diminutions observed in adults both diffusely throughout white matter (Pfefferbaum et al., 2006b), and specifically in the corpus callosum (Harris et al., 2008; Pfefferbaum and Sullivan, 2005; Pfefferbaum et al., 2006a; Schulte et al., 2005) of individuals with alcohol use disorders, yet this is the first report to describe reduced FA in drinkers as early as mid-adolescence and in youth who do not meet criteria for alcohol abuse or dependence.
Although we only observed FA decrements, De Bellis and colleagues (2008) found increased FA in the rostral body and isthmus of the corpus callosum among youth with an alcohol use disorder (AUD) in a region-specific study. Although the current study did not include adolescents with AUD, results here are consistent with our previous study of 8 teenagers with AUD (without concomitant psychiatric issues) compared to 8 control teens (Tapert et al., 2003). This pilot investigation showed reduced FA in the splenium [F(1,16) = 4.39, p = 0.05] and marginally in the body [F(1,16) = 3.46, p = 0.08] of the corpus callosum. Conflicting findings could stem from dissimilarities in samples. While, here, groups were matched by age and free from psychiatric conditions, De Bellis and colleagues studied youths with an AUD who were slightly older than their comparison group (16.9 years vs. 15.9 years, p < 0.003), and had comorbid Axis I disorders (75% cannabis use disorder, 72% lifetime major depressive episode, 69% attention deficit/hyperactivity disorder, and 63% conduct disorder) whereas such diagnoses were exclusionary for controls. These comorbid disorders have previously been linked to white matter abnormalities (Matochik et al., 2005; McAlonan et al., 2007; Medina et al., 2007a; Steingard et al., 2002). Thus, there is much difficulty extricating potential interactive effects of these conditions. However, both the present study and De Bellis and colleagues (2008) offer interesting contributions to understanding the relationships between adolescent alcohol involvement and white matter integrity. The present study examined sub-clinical drinking to better isolate alcohol effects on typically developing teenagers. Binge drinking teens are of particular interest, as it is estimated that more than 30% of minors who begin drinking before age 16 will develop alcohol dependence (Grant and Dawson, 1997). Indeed, studying nondisordered adolescent drinkers is of substantial import due to the increased risk for binge drinking teens to transition into an AUD by adulthood (Hawkins et al., 1992; Viner and Taylor, 2007). The sample in the De Bellis paper is representative of adolescents with an AUD, especially due to high rates of comorbid psychiatric disorders among such youth (Clark et al., 1997; Viner and Taylor, 2007).
White matter aberrancies were related to drinking in a dose-dependent manner. Higher estimations of peak blood alcohol concentrations were linked to poorer fiber tract quality in the corpus callosum, internal/external capsules, and posterior corona radiata. Further, teens reporting multiple hangover symptoms showed more compromised white matter in the body and genu of the corpus callosum, frontal lobe projection fibers (anterior corona radiata), and cerebellar tracts (inferior peduncle). This finding linking postdrinking effects to worse integrity is consistent with white matter deficits observed in adults with histories of alcohol withdrawal seizures (Sullivan et al., 1996). These relationships suggest that high-dose alcohol consumption, to the extent that hangover symptomatology arises, may index adverse influences on white matter caliber, particularly since associations were not observed for drinking frequency or duration. It is also possible that hangover may be more accurately recalled than other aspects of alcohol involvement.
The results of this study align interestingly with prior evidence for alcohol-related interruption of frontocerebellar circuitry (Sullivan and Pfefferbaum, 2005), involving network nodes pons and thalamus, which exhibit volumetric decrements among adult alcoholics (Sullivan et al., 2003). Although we did not examine the integrity of frontocerebellar white matter connectivity specifically, we did observe lower FA in the superior cerebellar peduncle, a fiber tract that connects the thalamus to deep cerebellar nuclei (Wakana et al., 2004). Thalamic projections also emanate from the posterior limb of the internal capsule, where reduced fiber coherence was detected. These observations of altered white matter microstructure, combined with results from other DTI, morphometric, fMRI, and neuropsychological studies, serve as converging lines of evidence pointing towards a deleterious influence of alcohol on frontocerebellar circuitry (Sullivan and Pfefferbaum, 2005). Unlike the aforementioned studies with samples comprised of mature individuals with years of alcoholism, the sample of this exploratory analysis represents teenagers at the beginning stages of risky alcohol use, and related white matter abnormalities in these pathways detectable as early as 16 years of age.
The hippocampus is vulnerable to the effects of ethanol, particularly during adolescence (Medina et al., 2007b; Nagel et al., 2005). Running dorsally to the hippocampus are the fornix and stria terminalis, both compact limbic projections terminating at hippocampal and amygdala regions, respectively (Wakana et al., 2004). Since these fibers are tightly packed together, it is difficult to ascertain whether the decreased FA observed in this area indicates altered white matter integrity to the fibers making up the fornix, terminal stria, or both. As adolescents with alcohol use disorders have demonstrated impairments in learning and memory (Tapert et al., 2002), diminished FA in the fibers extending to the hippocampus may suggest changes in structural connectedness leading to functional aberrations in teenage drinkers. Multimodal neuroimaging studies will help solidify the nature of white matter connectivity between these regions (e.g., frontal lobe, thalamus, pons, cerebellum, insula, hippocampus, and amygdala), and elucidate the influence of heavy drinking on these networks.
Due to binge drinkers' relatively inchoate stages of alcohol use, results could alternatively highlight a premorbid white matter characteristic representing a vulnerability to risky drinking. However, the observed link between alcohol involvement (i.e., hangover) and reduced FA in this study lends support to a possible compromising influence of high-dose alcohol use on white matter quality. Several potential mechanisms could explain how FA might be reduced in heavy drinking teens. Years of alcoholism are linked to decreased N-acetylaspartate in white matter tissue, suggesting compounding axonal insult by chronic alcohol use (Meyerhoff et al., 2004; Schweinsburg et al., 2001). FA decrements could represent such a chemically induced axonal injury at a microstructural level. Animal models provide additional insights to rapid, alcohol-induced neural injury. Adult rats demonstrate neurodegeneration following once-daily ethanol exposure within as little as 5 days (Collins et al., 1998). Additionally, adolescent rats show differential sensitivity to brain damage compared to adults following 4 days of binge alcohol administration (Crews et al., 2000). Thus, human adolescents may be especially vulnerable to the deleterious effects of alcohol on neural tissues, including white matter. Another explanation of poor white matter integrity in binge drinking adolescents could relate to a disruption of neuromaturational processes. Developmental studies of white matter coherence have demonstrated increasing FA across adolescence (Giorgio et al., 2008; Hasan et al., 2007), reflected here by increased FA in the corpus callosum and fornix/stria terminalis with advancing pubertal stage. Low fiber tract coherence among drinkers could be due to an innate delay or an alcohol-related impediment in normal maturational processes. It is also possible that the observed changes may relate to axonal atrophy or membrane breakdown, important in the context of adolescent neuromaturation.
This study is the first we are aware of to characterize white matter integrity in major fiber tracts throughout the cerebrum and cerebellum between subdiagnostic binge drinking teens and nondrinkers. The study is limited by its cross-sectional design and modest sample size, and serves as a preliminary characterization of white matter integrity in adolescent binge drinkers. Due to the exploratory nature of this study, the data require replication. If replicated, these results carry important public health implications as interventions targeting binge drinking behaviors could lessen the risk for developing an alcohol use disorder. Longitudinal investigations, currently underway, will also help ascertain the degree to which heavy drinking and postdrinking effects might influence the developing adolescent brain. Studies with larger sample sizes will examine gender differences, factors predating alcohol use (e.g., family history of alcoholism, personality, conduct disorder), and neuropsychological correlates of white matter abnormalities.
In sum, this study shows differences in white matter quality in adolescents with histories of heavy episodic drinking, revealing widespread areas of compromised white matter in projections to networks underlying complex cognitive abilities of learning, memory, and executive functions. Although preliminary, these results bolster the importance of elucidating the neural sequelae of heavy episodic drinking during adolescence.
Acknowledgments
We would like to acknowledge the support of Dr. Krista Medina, Dr. Sandra A. Brown, Dr. MJ Meloy, Claudia Padula, Jennifer Winward, all staff of the Adolescent Brain Imaging Project, and the participating adolescents and parents. This research was supported by grants R01 DA021182 and R01 AA13419 (Tapert) and R01 MH64729 (Frank).
References
- Achenbach TM, Rescorla LA. Manual for ASEBA School-age Forms & Profiles. University of Vermont Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) Psychological Corporation; San Antonio, TX: 1978. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Clark DB, Pollock N, Bukstein OG, Mezzich AC, Bromberger JT, Donovan JE. Gender and comorbid psychopathology in adolescents with alcohol dependence. J Am Acad Child Adolesc Psychiatry. 1997;36:1195–1203. doi: 10.1097/00004583-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, Urbano-Marquez A. Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3-D statistical neuroanatomical models from 305 MRI volumes. Paper presented at the Proceedings IEEE-Nuclear Science Symposium and Medical Imaging Conference.2003. [Google Scholar]
- Fitzgerald EF. Intoxication Test Evidence. 2nd. Clark Boardman Callaghan; Deerfield, IL: 1995. [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–13. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, Cirino PT, Fletcher JM, Papanicolaou AC, Ewing-Cobbs L. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor Index of Social Position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975-2006: Volume I, Secondary School Students. National Institute on Drug Abuse; Bethesda, MD: 2007. [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Chua SE, Murphy DG, Suckling J, Tai KS, Yip LK, Leung P, Ho TP. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007a;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. NIAAA Newsletter. Vol. 3. NIAAA; Bethesda, MD: 2004. NIAAA Council Approves Definition of Binge Drinking. [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging. 2006a;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006b;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health. 2003;27:146–152. [PMC free article] [PubMed] [Google Scholar]
- Schottenbauer MA, Momenan R, Kerick M, Hommer DW. Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology. 2007;21:337–345. doi: 10.1037/0894-4105.21.3.337. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res. 2001;25:924–934. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene RE. Manual for the State-trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Steingard RJ, Renshaw PF, Hennen J, Lenox M, Cintron CB, Young AD, Connor DF, Au TH, Yurgelun-Todd DA. Smaller frontal lobe white matter volumes in depressed adolescents. Biol Psychiatry. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res. 1996;20:348–354. doi: 10.1111/j.1530-0277.1996.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A. Effects of alcohol dependence comorbidity and antipsychotic medication on volumes of the thalamus and pons in schizophrenia. Am J Psychiatry. 2003;160:1110–1116. doi: 10.1176/appi.ajp.160.6.1110. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Theilmann RJ, Schweinsburg AD, Yafai S, Frank LR. Reduced fractional anisotropy in the splenium of adolescents with alcohol use disorder. Proc Intl Soc Mag Reson Med. 2003;11:2241. [Google Scholar]
- Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. J Epidemiol Community Health. 2007;61:902–907. doi: 10.1136/jech.2005.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA. 1994;272:1672–1677. [PubMed] [Google Scholar]
- Widmark E. A micromethod for the estimation of alcohol in blood. Biochemistry. 1922;131:473–484. [Google Scholar]


