Abstract
The inwardly rectifying potassium channel Kir6.2 assembles with sulfonylurea receptor 1 to form the ATP-sensitive potassium (KATP) channels that regulate insulin secretion in pancreatic β-cells. Mutations in KATP channels underlie insulin secretion disease. Here, we report the characterization of a heterozygous missense Kir6.2 mutation, G156R, identified in congenital hyperinsulinism. Homomeric mutant channels reconstituted in COS cells show similar surface expression as wild-type channels but fail to conduct potassium currents. The mutated glycine is in the pore-lining transmembrane helix of Kir6.2; an equivalent glycine in other potassium channels has been proposed to serve as a hinge to allow helix bending during gating. We found that mutation of an adjacent asparagine, Asn-160, to aspartate, which converts the channel from a weak to a strong inward rectifier, on the G156R background restored ion conduction in the mutant channel. Unlike N160D channels, however, G156R/N160D channels are not blocked by intracellular polyamines at positive membrane potential and exhibit wild-type-like nucleotide sensitivities, suggesting the aspartate introduced at position 160 interacts with arginine at 156 to restore ion conduction and gating. Using tandem Kir6.2 tetramers containing G156R and/or N160D in designated positions, we show that one mutant subunit in the tetramer is insufficient to abolish conductance and that G156R and N160D can interact in the same or adjacent subunits to restore conduction. We conclude that the glycine at 156 is not essential for KATP channel gating and that the Kir6.2 gating defect caused by the G156R mutation could be rescued by manipulating chemical interactions between pore residues.
Keywords: ABC Transporter, Ion Channels, Membrane Biophysics, Pancreatic Islet, Potassium Channels, KATP Channel, Kir6.2, Gating, Hyperinsulinism, Inward Rectification
Introduction
Inwardly rectifying potassium (Kir) channels are expressed in a wide variety of cell types where they regulate membrane excitability in response to diverse signals (1). They are characterized by two-transmembrane helices, TM1 and TM2, a re-entrant pore-helix, and cytoplasmic N and C termini (1). The structural mechanism by which Kir channels gate, a process that switches the channel between open and closed conformation, is not clearly understood. Collective structural and functional data from bacterial and mammalian Kir channels have led to a proposed gating model in which ligand binding to the cytoplasmic domains induces movement of an amphipathic helix called the slide helix along the lipid bilayer followed by lateral movement of the outer helix TM1. This then allows rotation and bending of the inner helix TM2 to open or close a lower gate located at the bundle crossing where the inner helices (TM2) of the four Kir subunits converge (2–6). Within TM2, a central glycine that is conserved in most Kir channels was proposed in the bacterial MthK channel to serve as a hinge to allow the helix to bend during gating (7). However, whether this glycine serves a similar role in Kir channels remains unresolved (8–12). Another important gating characteristic of Kir channels is that they conduct ions more readily in the inward rather than the outward direction because of blocking by intracellular Mg2+ and polyamines at positive membrane potentials (13, 14). Although the inward rectification property is conferred by multiple structural features in the pore helix and the extended cytoplasmic pore of Kir channels, a residue in TM2 plays a critical role (14–16). Thus, in weak inward rectifiers, such as in Kir1.1 and Kir6.2, this residue is a neutral amino acid asparagine, whereas in strong inward rectifiers such as Kir2.1 and Kir3.1, the residue is a negatively charged aspartate (14).
Among Kir channels, Kir6.2 is unique in requiring co-assembly with the sulfonylurea receptor (SUR)2 for functional expression (17). The channel complex formed by Kir6.2 and SUR is known as the ATP-sensitive potassium (KATP) channel (17, 18). Kir6.2, being the pore subunit of the KATP channels, integrates signals from the regulatory subunit SUR1 and multiple ligands to control potassium ion conduction (17–20). Membrane phosphoinositides (such as phosphatidylinositol 4,5-bisphosphate) and long chain acyl-CoA (LC-CoA) activate the channel, whereas ATP inhibits the channel by direct interactions with Kir6.2. MgADP on the other hand stimulates channel activity by interacting with SUR1. Sensitivities of KATP channels to intracellular nucleotides ATP and MgADP enable the channels to couple cell metabolism to cell excitability (20, 21).
In pancreatic β-cells, the KATP channel composed of Kir6.2 and sulfonylurea receptor 1 (SUR1) is essential for triggering glucose-stimulated insulin secretion (21–23). Upon glucose stimulation, the relative ATP to ADP concentrations in β-cells increase, driving KATP channel closure, membrane depolarization, Ca2+ influx, and insulin release. Mutations in Kir6.2 and SUR1 that lead to loss or gain of KATP channel function are well recognized as the major cause of congenital hyperinsulinism or neonatal diabetes, respectively (23–27). Functional analysis of mutant KATP channels has offered insight into not only disease mechanisms but also the structure-function relationship of the channel (23–27). Here, we report functional characterization of a congenital hyperinsulinism-associated heterozygous Kir6.2 mutation of glycine 156 (the central glycine in TM2 hypothesized to serve as a gating hinge in potassium channels) to an arginine. Homomeric G156R mutant KATP channels are well expressed at the cell surface but do not give rise to potassium currents (28). When co-expressed with WT Kir6.2 to simulate heterozygous expression, the mutant subunit was incorporated into the channel complex to alter channel function. We show that a second site mutation, N160D, which converts Kir6.2 from a weak inward rectifier to a strong inward rectifier in the WT background, restores ion conduction in the G156R channel. The G156R/N160D channel exhibits no voltage-dependent block by spermine, suggesting that electrostatic interactions of the two substituted amino acids underlie restoration of ion conduction. Interestingly, this interaction can occur both within the same subunit and between two neighboring subunits. Furthermore, G156R/N160D channels have single channel conductance and nucleotide sensitivities comparable to WT channels and are activated by metabolic inhibition in intact cells. These results indicate that the physical chemical property of the glycine residue at position 156 is not an absolute requirement for permeation and gating in Kir6.2; rather, appropriate interactions among the pore residues provide a structural environment conducive to permeation and gating.
MATERIALS AND METHODS
Molecular Biology
Rat Kir6.2 cDNA is in pCDNAI/Amp vector and hamster SUR1 in pECE. Site-directed mutagenesis was carried out using the QuikChange site-directed mutagenesis kit (Stratagene), and the mutations were confirmed by sequencing. Mutant clones from two or more independent PCR reactions were analyzed to avoid false results caused by undesired mutations introduced by PCR.
Kir6.2 Tetramer Construction
The concatenated Kir6.2 tetramer design is shown in Fig. 3A. A modified version of pCDNAI/Amp was generated by introducing a 5′ ClaI site into the multiple cloning sites of WT rat Kir6.2 pCDNA1/Amp using the QuikChange site-directed mutagenesis kit (Stratagene). This modified pCDNA1/Amp-ClaI was used for the final subcloning to create the concatenated Kir6.2 tetramer and for expression in COSm6 cells. To generate individual subunits with appropriate linkers and 5′ and 3′ restriction sites for subcloning as shown in Fig. 3A, the following PCR primer design was employed. For subunit 1, a forward and reverse primer pair was used to generate a ClaI site (underlined) at the 5′ end and a 5×Gln linker (parentheses) followed by a NheI site (underlined) at the 3′ end: forward (5′-TATAATCGATACTCTGCAATGAGGCCCTAGGCCAAGCCAGTGTAGTGCC-3′) and reverse (5′-ATATGCTAGC(CTGCTGCTGCTGCTG)GGACAAGGAATCCGGAGAGATGC 3′). For subunit 2, a forward and reverse primer pair was used to generate an NheI site (underlined) followed by a 5×Gln linker (parentheses) at the 5′ end and a 5×Gln linker (parentheses) followed by a BamHI site (underlined) at the 3′ end: forward (5′-TATAGCTAGC(CAGCAGCAGCAGCAG)ATGCTGTCCCGAAAGGGCATTATCCC-3′) and reverse (5′-ATATGGATCC(CTGCTGCTGCTGCTG)GGACAAGGAATCCGGAGAGATGC-3′). For subunit 3, a forward and reverse primer pair was used to generate a BamHI site (underlined) followed by a 5×Gln linker (parentheses) at the 5′ end, and a 5×Gln linker (parentheses) followed by a NheI site (underlined) at the 3′ end: forward (5′-TATAGGATCC(CAGCAGCAGCAGCAG)ATGCTGTCCCGAAAGGGCATTATCCC-3′ and reverse (5′-ATATGCTAGC(CTGCTGCTGCTGCTG)GGACAAGGAATCCGGAGAGATGC-3′. For subunit 4, a forward and reverse primer pair was used to generate a NheI site (underlined) followed by a 5×Gln linker (parentheses) at the 5′ end and an EcoRI site (underlined) at the 3′ end: forward (5′-TATAGCTAGC(CAGCAGCAGCAGCAG)ATGCTGTCCCGAAAGGGCATTATCCC-3′) and reverse (5′-ATATGAATTCCTGCAGCCCGACAAGTATCTTGTAACACCCCAGGCACACAGCGGC-3′.
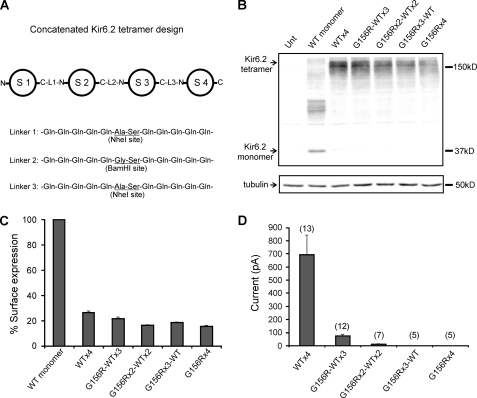
FIGURE 3.
Analysis of Kir6.2 tandem tetramers containing 0–4 G156R mutant subunits. A, shown is a schematic of the concatenated Kir6.2 tetramer design (S1–4, subunit 1–4; L1–3, linker 1–3). The detailed protocol for constructing the tetramer including primer information is provided under “Materials and Methods.” B, shown is a Western blot of Kir6.2 monomer and various tandem tetramers expressed in COS cells. All cells were co-transfected with SUR1. Note in the Kir6.2 monomer lane that higher molecular weight bands likely representing oligomeric forms of Kir6.2 monomer are seen, as reported in previous studies (33). The tubulin blot was included as loading controls. C, chemiluminescence assays are shown comparing surface expression levels of channels formed in cells co-transfected with fSUR1 and Kir6.2 monomer or various Kir6.2 tandem tetramers (cDNA amount was adjusted to give an equal molar ratio for Kir6.2 monomer and tandem tetramers). D, shown is averaged patch current amplitude of the various Kir6.2 tandem tetramer channels expressed in COS cells. Each bar represents the mean ± S.E. of 5–13 patches (as shown above the bar).
PCR fragments were purified by gel extraction, digested with ClaI and NheI for subunit 1, NheI and BamHI for subunit 2, BamHI and NheI for subunit 3, and NheI and EcoRI for subunit 4. Subunits 1 and 2 were ligated into an intermediate vector pJPA5, and subunits 3 and 4 were ligated into pJPA7 to generate dimers. Positive dimers were verified by sequencing and digested with ClaI and BamHI for pJPA5 subunits 1 and 2 or BamHI and EcoRI for pJPA7 subunits 3 and 4. The dimer fragments were then ligated into pCDNAI/Amp-ClaI to generate the final tetramer fusion constructs.
Western Blotting and Chemiluminescence Assay
Cell surface expression levels of mutant channels were assessed by Western blot and by a quantitative chemiluminescence assay using a SUR1 that was tagged with a FLAG epitope (DYKDDDDK) at the N terminus (fSUR1), as described previously (29). COSm6 cells grown in 35-mm dishes were transfected with 0.4 μg of rat Kir6.2 and 0.6 μg of fSUR1 using FuGENE 6® and lysed 48–72 h post-transfection in 20 mm HEPES, pH 7.0, 5 mm EDTA, 150 mm NaCl, 1% Nonidet P-40 with Complete Protease Inhibitors (Roche Applied Science). Proteins in the cell lysate were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, analyzed by incubation with appropriate primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences), and visualized by enhanced chemiluminescence (Super Signal West Femto; Pierce). For chemiluminescence assay, cells were fixed with 2% paraformaldehyde for 30 min at 4 °C. Fixed cells were pre-blocked in phosphate-buffered saline (PBS) plus 0.1% bovine serum albumin (BSA) for 30 min, incubated in M2 mouse monoclonal anti-FLAG antibody for fSUR1 (10 μg/ml, Sigma) for 1 h, washed 4 × 30 min in PBS plus 0.1% BSA, incubated in horseradish peroxidase-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch, Inc., 1:1000 dilution) for 20 min, and washed again 4 × 30 min in PBS plus 0.1% BSA. Chemiluminescence of each dish was quantified in a TD-20/20 luminometer (Turner Designs) after 10 s of incubation in Power Signal enzyme-linked immunosorbent assay luminol solution (Pierce). All steps after fixation were carried out at room temperature.
86Rb+ Efflux Assay
COSm6 cells were plated onto 35-mm culture dishes and transfected with wild-type SUR1 and control or mutant rat Kir6.2 cDNA as described above. Cells were incubated for 24 h in culture medium containing 86RbCl (1 μCi/ml) 2 days after transfection. Before measurement of 86Rb+ efflux, cells were incubated for 30 min at room temperature in Krebs-Ringer solution with metabolic inhibitors (2.5 μg/ml oligomycin and 1 mm 2-deoxy-d-glucose). At selected time points the solution was aspirated from the cells and replaced with fresh solution. At the end of a 40-min period, cells were lysed. The 86Rb+ in the aspirated solution and the cell lysate was counted. The percentage efflux at each time point was calculated as the cumulative counts in the aspirated solution divided by the total counts from the solutions and the cell lysate (25).
Electrophysiology
Patch clamp recordings were performed in the inside-out configuration as previously described (30). Briefly, COSm6 cells were transfected with cDNA encoding WT or mutant channel proteins as well as cDNA for the green fluorescent protein to help identify positively transfected cells. Patch clamp recordings were made 36–72 h post-transfection. Micropipettes were pulled from non-heparinized Kimble glass (Fisher) with resistance typically ∼1–2 megaohms. The bath (intracellular) and pipette (extracellular) solution (K-INT) had the following composition: 140 mm KCl, 10 mm K-HEPES, 1 mm K-EGTA, pH 7.3. ATP and ADP were added as the potassium salt. For measuring ATP sensitivity, 1 mm EDTA was included in K-INT to prevent channel rundown (31). Unless specified, all currents were measured at a membrane potential of −50 mV (pipette voltage = +50 mV), and inward currents are shown as upward deflections. The MgADP or diazoxide response was calculated as the current in a K-INT solution containing either 0.1 mm ATP and 0.5 mm ADP or 0.1 mm ATP and 0.3 mm diazoxide (both with 1 mm free Mg2+) relative to that in plain K-INT solution. When ATP sensitivity was reduced, base-line currents were obtained using 10 mm BaCl2 block at +50 mV.
For spermine block measurements, holding potential was stepped from 0 to −100 mV (+100-mV membrane potential) for 30 ms, then ramped from −100 to +100 mV in 200 ms and stepped back to 0 mV for total recorded time of 1 s. In the tandem tetramer experiments shown in Fig. 7, holding potential was stepped to −150 mV for 50 ms and ramped to 100 mV in 300 ms. Note in these experiments that control K-INT solution did not include EDTA.
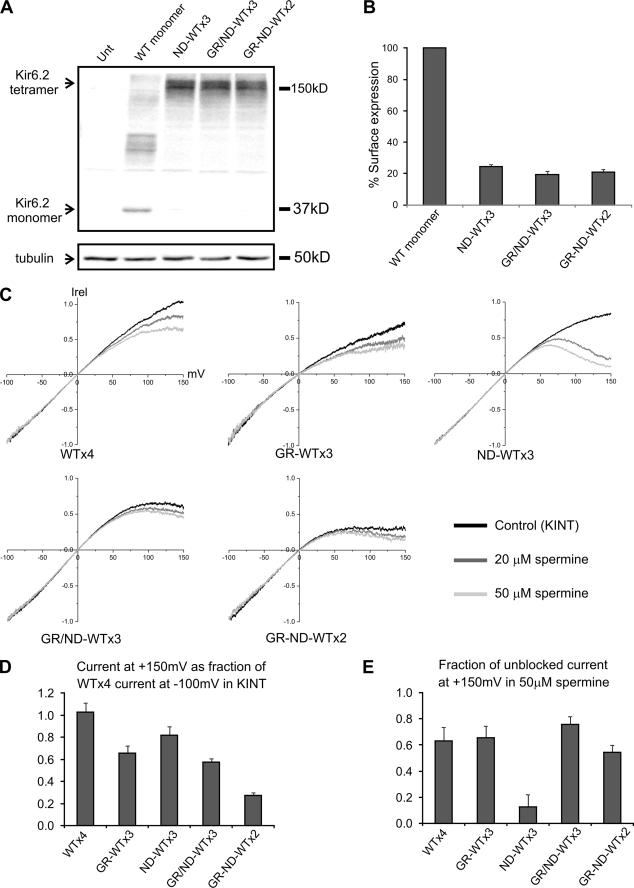
FIGURE 7.
Intra- and intersubunit interactions between G156R and N160D in the Kir6.2 tetramer. A, shown is a Western blot of Kir6.2 monomer and various tandem tetramers expressed in COS cells. All cells were co-transfected with SUR1, and the tubulin blot was shown as the loading controls. B, chemiluminescence assay results show surface expression of the various mutant tandem tetramer channels, although at a much reduced level compared with channels formed by co-expressing SUR1 and Kir6.2 monomer. Note in this figure that GR denotes G156R, and ND denotes N160D. GR/ND refers to G156R and N160D in the same subunit, and GR-ND refers to G156R and N160D in adjacent subunits in the Kir6.2 tandem tetramer. C, inside-out patches containing various tandem tetramer channels were subjected to a voltage-ramp protocol (+150 to −100 mV) in control K-INT solution or K-INT solution containing 20 or 50 μm spermine. Averaged current-voltage profiles of the channels (from four-six patches) are shown. Currents were normalized (Irel) to that seen at −100 mV. D, current of the various mutants at +150 mV in K-INT control solution was normalized to that of WT tandem tetramer (denoted as WT×4) at −100 mV in K-INT to show the spermine-independent difference caused by the mutations. E, Current observed at +150 mV in the presence of 50 μm spermine for each channel was shown as the fraction of current observed at the same membrane potential in K-INT solution for the respective channel.
To measure single channel conductance, currents were recorded using borosilicate glass (Sutter Instruments) electrodes coated with Sylgard and polished with a microforge (Narishige) to produce bath resistances of 8.0–12.0 megaohms. Currents were acquired at 100 kHz with an applied analog 4-pole Bessel filter with a cutoff frequency of 5–10 kHz. Recordings were filtered again offline using a gaussian or 8-pole Bessel filter with a cutoff of 2 kHz.
Data Analysis
Data were analyzed using pCLAMP software (Axon Instrument). Off-line analysis was performed using Microsoft Excel and Origin programs. Currents amplitudes in the voltage ramp experiments were averages of three consecutive voltage ramps and normalized to the most negative current amplitude (at −100 mV membrane potential). Statistical analysis was performed using the following. For current amplitudes, diazoxide and MgADP response, we used independent two-population two-tailed Student's t test; for ATP dose response and two-point estimation shown in Fig. 8, A and B, we used one-way analysis of variance (ANOVA); for single channel conductance, we used both one-way ANOVA and non-parametric Kruskal-Wallis tests (to test accuracy of significance with low data repetition).
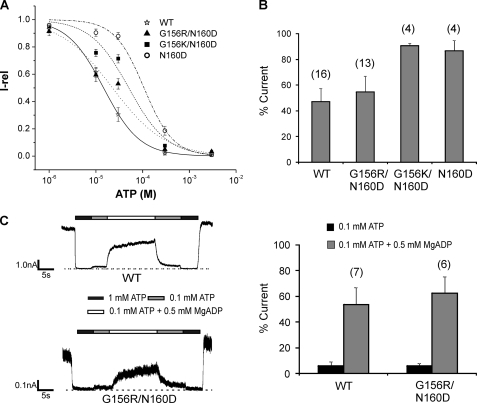
FIGURE 8.
Nucleotide sensitivities of G156R/N160D charge-pair mutant channels. A, ATP dose-response curves obtained by fitting the data points to the modified Hill equation (Irel = 1/(1+ ([ATP]/IC50)H)), where Irel is the current relative to that seen in the absence of ATP, IC50 is [ATP] necessary for half-maximal inhibition of channel activity, and H the Hill coefficient. Recordings were made in K-INT/1 mm EDTA solution to minimize rundown (38). Error bars represent S.E. of 5–6 patches. The IC50 values are as shown in Table 1. The H values are WT = 1.2 ± 0.2, G156R/N160D = 0.8 ± 0.2, G156K/N160D = 1.2 ± 0.5, and N160D = 1.4 ± 0.2. B, ATP sensitivity was also assessed by comparing channel activity at 20 μm ATP. The number of patches for each channel type is shown above the bars. There was no significant difference between WT and G156R/N160D (p > 0.05), but a significant difference was observed between WT and G156K/N160D or N160D (p < 0.01). C, left, representative traces of WT and G156R/N160D channels show channel response to MgADP. Patches were exposed to various concentrations of ATP and ADP as indicated by the bars above the recordings. Free [Mg2+] was 1 mm in all solutions. Right, quantification is shown of channel response to ATP and MgADP. Currents in 0.1 mm ATP or 0.1 mm ATP plus 0.5 mm ADP were normalized to currents in nucleotide-free solution. Again, there is no significant difference (6–7 patches) in MgADP response between WT and G156R/N160D (p > 0.05).
RESULTS
The G156R Mutation Abolishes Channel Activity without Preventing Expression
The G156R mutation in Kir6.2 was originally reported in a large scale study of dominant KATP channel mutation cases of congenital hyperinsulinism (28). The proband and her younger sister heterozygous for the G156R mutation presented with hypoglycemia in the newborn period. The younger sister responded to treatment with diazoxide. The proband received a pancreatectomy after treatment with diazoxide seemed ineffective, but the trial was likely too brief to judge its effectiveness. Initial in vitro reconstitution of the G156R mutant channel in COSm6 cells indicated relatively normal surface expression but no channel activity (28). The biochemical and functional data characterizing this mutant phenotype are presented in Fig. 1. Western blots of lysates from COSm6 cells transfected with G156R or WT channel cDNAs showed comparable SUR1 and Kir6.2 protein levels as well as a complex-glycosylated SUR1 band (upper) corresponding to SUR1 that has exited the endoplasmic reticulum and traversed medial Golgi (Fig. 1A). Because SUR1 trafficking requires co-assembly with Kir6.2 into an octameric structure before endoplasmic reticulum export (44), the results indicate fully assembled G156R KATP channels reaching the cell surface. In agreement with the Western blot results, chemiluminescence measured from antibody-labeled G156R channels at the cell surface was ∼80% of WT (Fig. 1B).
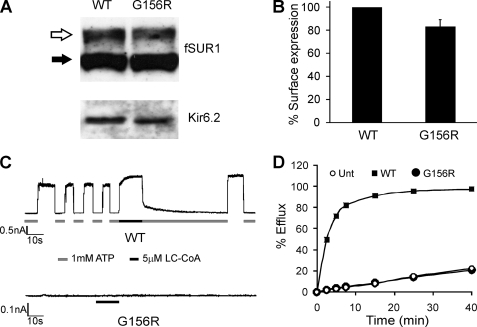
FIGURE 1.
Characterization of G156R mutant channels reconstituted in COS cells. A, shown are Western blots of SUR1 and Kir6.2 in cells transfected with cDNAs of fSUR1 (SUR1 that has been tagged with a FLAG epitope at the extracellular N terminus) and WT- or G156R-Kir6.2. fSUR1 was probed with anti-FLAG antibody, and Kir6.2 was probed with a rabbit antiserum raised against the C-terminal cytoplasmic domain of Kir6.2 (45). SUR1 was detected as a lower band (filled arrow), corresponding to the core-glycosylated form, and an upper band (open arrow), corresponding to the complex-glycosylated form. B, quantification is shown of cell surface expression of channel complexes using the chemiluminescence assay described under “Materials and Methods.” The expression level of the mutant was shown as percent of that of WT channels. The error bar represents S.E. from four independent experiments. C, channel activity was assessed by inside-out patch clamp recordings. Top, the WT current trace shows the characteristic inhibition by ATP and activation by LC-CoA (oleoyl-CoA). Bottom, no currents were detected in cells expressing the G156R mutant. Recordings in this and the subsequent figures were made at −50 mV, and inward currents are shown as upward deflections. D, shown is a representative 86Rb+ efflux experiment assessing KATP channel activity in untransfected cells or cells co-transfected with SUR1 and WT- or G156R-Kir6.2. Cells were treated for 30 min with metabolic inhibitors to activate KATP channels. Although WT channel activity was readily observed as 86Rb+ efflux over time, the G156R mutant channels did not exhibit efflux above the background seen in untransfected cells.
Despite normal surface expression, channel activity was not observed in inside-out excised patches even after exposure to phosphatidylinositol 4,5-bisphosphate or LC-CoA (oleoyl-CoA was used throughout the study) which are both known to increase KATP channel open probability (Fig. 1C). 86Rb+ efflux experiments also showed no detectable mutant channel activity above background upon metabolic inhibition, in contrast to WT channels (Fig. 1D). Moreover, channel current was not observed when other ions were substituted for K+, including Rb+, Na+, and Tl+ (n = 3 each, not shown). The results indicate loss of channel function was due to the absence of measurable ion conduction.
Effect of Heterozygous Expression of G156R on Channel Conduction Properties
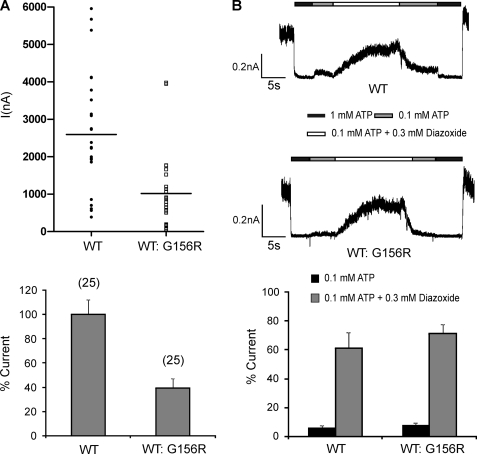
G156R is identified as a dominant heterozygous mutation; we asked how co-expression of this mutant subunit with a WT-Kir6.2 subunit at a 1:1 cDNA ratio affects overall channel activity and whether the functional phenotype under this condition correlates with clinical phenotype seen in patients. Current amplitudes measured from excised patches of cells simulating heterozygous expression of G156R were reduced by ∼60% compared with those from cells expressing pure WT channels (Fig. 2A, n = 25 each, p < 0.001). The heterozygous channels exhibited a response to diazoxide stimulation similar to WT channels (Fig. 2B). These results are consistent with the clinical phenotype in that channel function is reduced, but response to diazoxide remains.
FIGURE 2.
Simulated heterozygous expression of G156R. COSm6 cells were transfected with G156R and WT Kir6.2 cDNA at a 1:1 cDNA ratio (denoted as WT:G156R) together with SUR1 cDNA to simulate heterozygous expression of the mutant Kir6.2 in patients. A, current density was estimated from isolated membrane patches using patch pipettes with closely matched sizes. Twenty-five patches each of WT- or WT:G156R-expressing cells from three-independent experiments were recorded. Top, shown are amplitude distributions of ATP-sensitive potassium currents (I). Bottom, shown is the averaged current amplitude normalized to that of WT-expressing cells. B, shown is a comparison of diazoxide response in channels recorded from WT- and WT:G156R-expressing cells. Top, representative current traces show the stimulatory effect of diazoxide. Patches were exposed to K-INT solutions containing differing concentrations of ATP or diazoxide, as indicated by the bars above the current traces. Free Mg2+ concentration in all solutions was ∼1 mm. Bottom, shown is quantification of averaged diazoxide response. Channel activities observed in 0.1 mm ATP or 0.1 mm ATP + 0.3 mm diazoxide were expressed as the percent current of channel activity observed in K-INT solution (currents in 1 mm ATP were subtracted as background).
Because homomeric G156R channels express at a comparable level to WT channels at the cell surface, it is reasonable to assume that in simulated heterozygosity studies both subunits are incorporated into the surface channel complex and contribute to channel activity. However, a possibility remains that the channel activity and diazoxide response in patients and in simulated heterozygous expression result from haplosufficiency, i.e. the WT Kir6.2 accounts for all the channel activity and diazoxide response observed. To directly test if G156R-Kir6.2 forms functional heteromeric channels with WT-Kir6.2, we asked whether Kir6.2 tetramers containing one or more G156R mutant subunits give rise to KATP currents. To control the number of mutant subunits, we constructed a series of concatenated Kir6.2 tandem tetramers with 0–4 G156R subunits. Subunits were separated with a 12-amino acid linkers (Fig. 3A) spanning the C to N termini of adjacent subunits. This approach also allows us to assess the stoichiometry of G156R in blocking ion conduction in the Kir6.2 tetrameric pore. All tetrameric constructs produced proteins of the predicted size when transfected into COS cells, as shown by Western blots (Fig. 3B). Chemiluminescence assays were used to measure surface expression of the tandem channels in COS cells co-expressing SUR1 (Fig. 3C). Although all tandem channels were expressed at the cell surface, their expression levels were considerably lower than channels formed by SUR1 and monomeric Kir6.2 subunits. Macroscopic inside-out patch recordings showed that tandem channels containing all four WT Kir6.2 generated substantial ATP-sensitive currents. Tandem channels containing one G156R subunit also generated detectable currents but at a much reduced level compare with WT-tandem channels (only ∼10% of WT-tandem; Fig. 3D). In tandem channels containing two G156R mutant subunits, the average current fell to about 1% that of WT tandem channels, and no activity was seen with tandem channels containing three or four mutant subunits. These results demonstrate that channels containing a single G156R subunit are still able to conduct potassium current; however, more than two mutant subunits in the tetramer eliminate channel activity. The results support the idea that mutant and WT Kir6.2 subunits can form heteromeric channels at the cell surface where G156R subunit-containing channels contribute to the overall currents.
Functional Rescue of the G156R Mutation
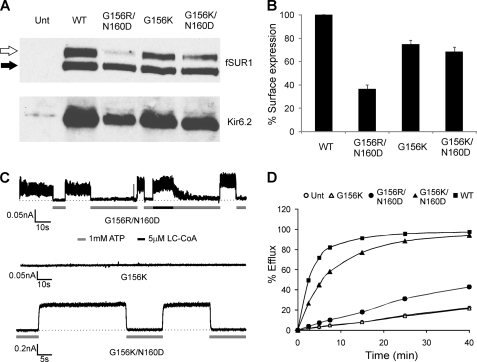
How does the G156R mutation affect ion conduction? One possibility is that this central glycine is essential for TM2 to undergo a bending conformational change to open the channel, as proposed for the bacterial potassium channel MthK (7). Alternatively, a charged amino acid at this position may directly interfere with the permeation pathway either by altering the electrostatic profile of the pore or by restricting conformational movement involved in gating. Homology modeling of a closely related channel Kir3.4 has suggested that the equivalent glycine and an asparagine about one helical turn down TM2 both face the pore and that the side chains of amino acids placed in these two positions interact (9). We, therefore, tested if the mutation of the equivalent asparagine at position 160 of Kir6.2 to a negatively charged aspartate (N160D) will alter manifestation of the G156R mutation effect on ion conduction. Charge pair mutant channels G156R/N160D had significantly reduced expression compared with the single mutation (Fig. 4, A and B). However, these channels exhibited measurable ionic currents and efflux in response to metabolic inhibition (Fig. 4, C and D). Channel activity in excised patches showed properties of KATP channels, with currents eliminated by 1 mm ATP and stimulated by 5 μm LC-CoA. A similar loss of channel activity was observed when Gly-156 was mutated to a lysine; however, KATP currents were again recovered when the G156K mutation was combined with the N160D mutation (Fig. 4, C and D). Moreover, replacing Asn-160 with glutamate (N160E) also restored ion conduction in the G156R or G156K background (Table 1). These results are consistent with the idea that the substituted amino acids at the 156 and 160 positions interact electrostatically to restore ion conduction. Charge reversal at the two positions (G156D/N160D or G156E/N160D) on the other hand did not yield functional channels (but see Fig. 6), although surface expression of G156D/N160R was ∼50% that of WT channels (Table 1). Furthermore, replacing the amino acid adjacent to Asn-160 or the amino acid predicted to be approximately one helical turn down from Asn-160 with an aspartate (A161D and L164D, respectively) failed to restore channel activity, suggesting that the orientation and distance of a negatively charged residue from G156R affects its ability to rescue the ion conduction defect caused by G156R (Table 1).
FIGURE 4.
Restoration of ion conduction in G156R or G156K mutant channels by a second site mutation N160D. A, shown are Western blots of SUR1 and Kir6.2 in COSm6 cells co-transfected with cDNAs for fSUR1 and one of the following Kir6.2s: WT, G156R/N160D, G156K, or G156K/N160D. B, surface expression of various channels was quantified using the chemiluminescence assay described under “Materials and Methods.” C, representative traces are shown of inside-out patch clamp recordings from cells expressing different mutant channels. In contrast to the G156R mutant shown in Fig. 1C, ATP-sensitive currents were readily detected from cells expressing the G156R/N160D mutant. Some activation by LC-CoA was also apparent based on the slow inhibition by ATP after LC-CoA exposure. The G156K mutation also abolished channel activity, and this defect was similarly overcome by introducing the second mutation N160D. D, 86Rb+ efflux assays show activation of the G156R/N160D and G156K/N160D mutant channels by metabolic inhibition as seen for WT channels, albeit to a lesser extent likely due to differences in expression levels and gating properties (see also Fig. 8 for ATP and MgADP responses). In contrast, no channel activity was detected for the G156K mutant, as with the G156R mutant shown in Fig. 1.
TABLE 1.
Mutant channel properties
| Channel | Surface expressiona | Patch activity | ATP sensitivity (IC50)b | Single channel conductancec |
|---|---|---|---|---|
| % | μm | Picosiemens | ||
| WT | 100 | Yes | 15.4 ± 2.5 | 75 ± 2 |
| N160D | N.D.d | Yes | 102.0 ± 17.1 | ∼75e |
| G156R/N160D | 37 ± 4 | Yes | 21.8 ± 4.3 | 67 ± 1 |
| G156R/N160E | 29 ± 2 | Yes | 12.3 ± 1.7 | 76 ± 2 |
| G156K/N160D | 69 ± 4 | Yes | 47.7 ± 7.3 | 18 ± 2 |
| G156K/N160E | 38 ± 3 | Yes | 7.2 ± 1.5 | 35 ± 3 |
| G156D/N160R | 56 ± 8 | Nof | ||
| G156D/N160K | ND | No | ||
| G156E/N160R | ND | No | ||
| G156E/N160D | ND | No | ||
| G156R/A161D | ND | No | ||
| G156R/L164D | ND | No |
a The value represents the means ± S.E. of 4–10 chemiluminescence experiments.
b ATP sensitivity was determined in K-INT solution containing 1 mm EDTA to minimize rundown; the IC50 values, therefore, tend to be higher than in those reported in studies by others where no EDTA was included in the K-INT solution. The value represents the mean ± S.E. of 4–6 patches.
c Values represent the averaged data of three recordings; error is S.E.
d ND, values not determined.
e Single channel conductance for N160D is from Shyng et al. (31).
f From three-seven patches.
FIGURE 6.
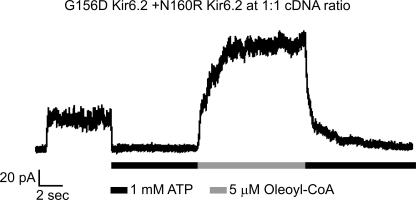
Evidence suggests functionally relevant intersubunit interactions between G156D and N160R. Shown is a representative inside-out patch clamp recording from COSm6 cells transfected with cDNA of SUR1 and a mixture of G156D Kir6.2 and N160R Kir6.2 at equal molar ratio. Patch was exposed to 1 mm ATP or 5 μm oleoyl-CoA as indicated. Of note, neither G156D Kir6.2 nor N160R Kir6.2 alone generated measurable currents when co-expressed with SUR1 (n = 10–15; not shown) (31). Also, placing G156D and N160R in the same subunit failed to generate potassium currents upon co-expression with SUR1 (Table 1).
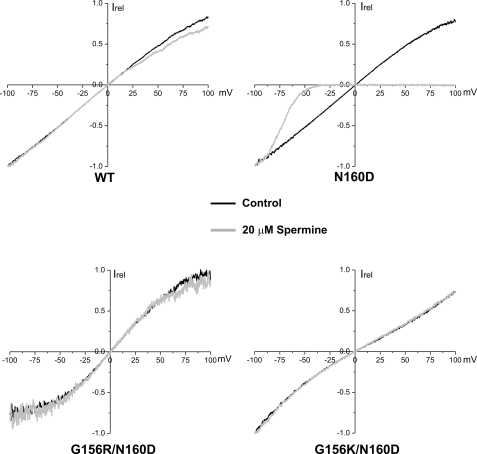
Mutation from asparagine to aspartate at the “rectification controller” position in Kir6.2 (N160D) converts KATP channels from weak to strong inward rectifiers. Prior studies have shown that under symmetrical K+ solution conditions in inside-out patch recording, the N160D mutant exhibits a nearly linear current-voltage relationship from −100 to +100 mV in the absence of Mg2+ or polyamines similar to WT channels; however, in the presence of Mg2+ or polyamines such as spermine, the outward currents at positive membrane potentials are diminished due to strong interaction between the aspartate residue and spermine, which blocks the pore (14, 31). If functional rescue of the G156R mutant by N160D results from electrostatic interactions between the two substituted amino acids, one would predict that the aspartate will no longer contribute to inward rectification, as it will not be available to interact with the positively charged polyamines. Indeed, inside-out excised patches of G156R/N160D and G156K/N160D channels showed weak rectification at positive potentials when exposed to 20 μm spermine (Fig. 5), similar to WT channels but in contrast to N160D channels. As a control, in the absence of polyamines none of the channels exhibited strong rectification (Fig. 5). Lack of rectification in the charge pair mutant channels indicates the positive charge of G156R or G156K prevents aspartate from stabilizing an interaction with a polyamine that enters the pore.
FIGURE 5.
The N160D mutation induces inward rectification on the WT- but not the G156R- or G156K-Kir6.2 background. COSm6 cells were transfected with cDNAs of SUR1 and one of the following Kir6.2s: WT, N160D, G156R/N160D, or G156K/N160D. Inside-out patches were subjected to a voltage-ramp protocol (membrane potential 100 to −100 mV) in control K-INT solution or K-INT solution containing 20 μm spermine. The pipette solution was K-INT. Representative experiments for each channel type are shown. Currents were normalized (Irel) to that seen at −100 mV. Although currents from N160D channels were completely blocked by spermine at positive membrane potentials, little block was observed for WT, G156R/N160D, or G156K/N160D channels. Similar results were obtained from four experiments.
Both Intra- and Intersubunit Interactions between G156R and N160D Occur in the Kir6.2 Tetramer
In the G156R/N160D mutant channels, restoration of ion conduction could be due to charge interactions within a single subunit or between two adjacent subunits. In the process of screening various Kir6.2 mutants with single or double charge mutations, we have made the intriguing observation that although individual G156D or N160R mutant Kir6.2 failed to give rise to measurable currents in the presence of SUR1 (n = 10–15 each; data not shown), co-expressing G156D and N160R Kir6.2 at a 1:1 cDNA ratio in the presence of SUR1 generated potassium currents inhibited by ATP and stimulated by LC-CoA (Fig. 6). The observation suggests G156D and N160R from different subunits could interact to restore channel function. To test this idea directly, we constructed a Kir6.2 tandem construct in which a G156R mutation or a N160D mutation was placed in the same subunit (G156R/N160D) or in adjacent subunits (G156R-N160D). Expression of mutant tandem constructs was judged by Western blots and formation of channel complexes with SUR1 judged by chemiluminescence assays and patch clamp recording (Fig. 7, A and B). Voltage-ramp experiments were again employed to assess spermine-induced inward rectification in these tandem mutant channels. Previous studies have shown that the extent of inward rectification in KATP channels becomes more pronounced as the number of N160D-containing subunits in the Kir6.2 tetramer increases (32, 33). Because only one N160D mutation is present in our mutant tandem channels, the positive membrane potentials were extended to +150 mV to observe spermine-induced block. Comparing the current-voltage relationship of WT×4, G156R-WT×3, N160D-WT×3, G156R/N160D-WT×3, and G156R-N60D-WT×2 tandem channels, it is apparent that the G156R and N160D arranged either in the same subunit or adjacent subunits exhibited less rectification than N160D-WT×3 channels at both 20 and 50 μm spermine concentrations tested (Fig. 7C). To better quantify the data, we calculated the current remaining in the presence of 50 μm spermine as a fraction of the current observed in K-INT control solution at +150 mV. This analysis allows for correction of current deviation from WT×4 channels at positive potentials in K-INT control solution that are caused by the mutations themselves and not spermine (Fig. 7D), as was observed previously by others (34). This analysis revealed clearly that spermine-induced reduction in current is greater in N160D-WT×3 channels than in channels where G156R is present in the same or adjacent subunit (Fig. 7E). These results led us to conclude that in the tetrameric Kir6.2 pore complex, G156R and N160D can form both intra- and intersubunit interactions to affect channel conduction.
Characterization of Gating Properties of Charge-pair Mutant Channels
Having observed restoration of potassium conduction in the charge-pair mutants (G156R/N160D, G156R/N160E, G156K/N160D, and G156K/N160E) we sought to further characterize the gating properties of these channels. First, single channel recordings were made using symmetrical K-INT, 1 mm EDTA solution to assess single channel conductance. These experiments show that whereas the G156R/N160D mutant channels have single channel conductance (67 ± 1 picosiemens (pS)) close to WT channels (75 ± 2 pS), the G156K/N160D and G156K/N160E mutants have significantly reduced single channel conductance (18 ± 2 and 35 ± 3 pS, respectively; Table 1), suggesting the Lys-156 may interact with Asp-160 differently than Arg-156 to influence K+ movement through the pore.
Next, we measured channel sensitivity to ATP. The ATP dose-response curves generated by fitting the data to a modified Hill equation (see the Fig. 8 legend) gave IC50 values for WT (15.4 ± 2.5 μm) and G156R/N160D (21.8 ± 4.3 μm)) that were not significantly different (p > 0.05; Fig. 8A, Table 1). However, the G156K/N160D mutant did show significantly reduced ATP sensitivity with an IC50 of 47.7 ± 7.3 μm (p < 0.01; Fig. 8A), as did the N160D mutation alone (IC50 = 102.0 ± 17.1 μm, p < 0.001) as reported before (31). The ATP sensitivity of G156R/N160E and G156K/N160E was also measured, with IC50 values being 12.3 ± 1.7 and 7.2 ± 1.5 μm, respectively; these values are close to the IC50 reported previously for N160E (17.7 μm) (31). Because some G156R/N160D patches tend to run down quickly during exposure to different concentrations of ATP, quantification of the dose-response for this mutant is not as accurate as in WT. To circumvent this problem, we also compared the extent of channel inhibition at an ATP concentration close to the IC50 of the WT. In these experiments, inside-out patches were briefly exposed to 20 μm ATP and a high concentration of ATP (1 mm) before the G156R/N160D mutant channels ran down significantly, and the residual activity during exposure was measured (Fig. 8B). G156R/N160D channels again showed similar levels of inhibition as WT, whereas G156K/N160D and N160D were both less inhibited. Finally, we examined the MgADP response of the G156R/N160D mutant channel, as this property is essential for physiological function of the channel in addition to proper conductance and ATP sensitivity (35). The G156R/N160D mutant was stimulated to an extent comparable with WT channels (Fig. 8C). These results show that the gating properties of G156R/N160D channels closely resemble those of WT channels and suggest they will sense metabolic changes similarly to WT channels.
DISCUSSION
Studies of KATP channel mutations identified in patients have significantly advanced our understanding of the structure-functional relationship of the channel and mechanisms by which mutations cause disease (23, 36). In this work we have described the effect of a Kir6.2 mutation G156R on KATP channel function. We show that the mutation causes loss of channel function by eliminating K+ conduction in homomeric mutant channels. Importantly, we demonstrate that the ion conduction defect caused by the G156R mutation can be suppressed by introducing a second mutation N160D at the inward-rectification controller site and that the resulting G156R/N160D channels exhibit nucleotide gating properties similar to WT channels. Moreover, using Kir6.2 tandem tetramer approach, we provide evidence that the interaction between G156R and N160D in the Kir6.2 tetramer can occur both within the same subunit and between two adjacent subunits. These findings have significant implications on not only the role of the Gly-156 residue in channel conduction and gating but also the spatial relationship of the inner pore helices. In addition, they illustrate how channel gating and conduction properties can be manipulated by altering the chemical interactions of pore residues.
G156R as a Heterozygous Mutation and Implications for Disease Mechanism
In a previous large-scale study of heterozygous KATP channel mutations identified in congenital hyperinsulinism including G156R of Kir6.2, we have found that the mutant channel subunits tend to express well at the cell surface as homomeric channels (28). It was suggested that these mutant channel subunits likely co-assemble with WT channel subunits to exert their gating effects in heterozygous patients. Our simulated heterozygosity and tandem tetramers studies provide evidence that this is indeed the case for G156R-Kir6.2. Under simulated heterozygous expression conditions, the activity of the ensemble heteromeric channels was significantly reduced (∼40% of that observed in cells transfected with WT channel subunits only) but well above that expected if homomeric WT channels were the sole conducting species in the surface channel population (estimated to be ∼6.25% assuming binomial distribution of heteromeric channels containing 0–4 WT subunits). We also showed that the ensemble heteromeric mutant channels exhibited a diazoxide response similar to WT channels. These observations are consistent with the clinical phenotype of the patients and provide a pathophysiological explanation for the G156R mutation where partial reduction of KATP channel activity would sustain a depolarized β-cell membrane potential base line compared with WT, leading to hyperinsulinemia and hypoglycemia. In addition, they suggest the G156R mutant subunit does not exert a complete dominant-negative effect in heteromeric channels, i.e. a single G156R in the Kir6.2 tetramer is insufficient to eliminate channel function, a notion directly supported by our result that concatenated Kir6.2 tetramer containing one G156R subunit forms ATP-sensitive potassium-conducting channels with SUR1. In tandem channels containing two G156R mutant subunits, channel activity dropped precipitously to a negligible level, and in channels with more than two G156R subunits, ion conduction was completely absent. It should be acknowledged that although the tandem tetramer provides a useful tool to address the stoichiometric effect of the mutation on channel function, it does not completely recapitulate the behavior of channels formed by Kir6.2 monomers. For example, channels formed by SUR1 and the Kir6.2 tandem tetramer have overall reduced surface expression and reduced ATP sensitivity, as has been reported previously for channels formed by Kir6.2 tandem dimers or SUR1-Kir6.2 tandem heteromers (32, 37, 38). Thus, one cannot use the current amplitudes observed from the tandem tetramer constructs to predict the channel activity expected for simulated heterozygous expression of G156R.
Implication on the Structural Role of Gly-156 of Kir6.2 in KATP Channel Gating
The Gly-156 residue in Kir6.2 corresponds to a highly conserved central glycine found in most potassium-selective channels (7, 9). Based on comparison of crystal structures of bacterial potassium channels in the open or closed conformation, it has been proposed that the glycine provides a flexible hinge to allow TM2 to bend during gating (7, 39). Although several subsequent structure-function studies seem to support this idea (40–42), it has become increasingly clear that such an interpretation might not apply to all potassium channels. Recent studies of a Kir3.4 channel have suggested that the corresponding central glycine does not play a hinge role in the pivoted bending of TM2 during gating; rather, the glycine residue, an amino acid lacking a side chain, appears necessary to prevent interactions with residues in its vicinity critical for gating (9, 10). Our results that the functional defect caused by the G156R mutation can be alleviated by a second site mutation, N160D, in the TM2 helix and that the resulting G156R/N160D channels show similar ligand-induced gating as WT are in agreement with the view that the central glycine does not play a necessary hinge role in KATP channel gating. In light of the findings presented here, an interesting and related question to address in the future is whether in Kir6.2 a lower glycine conserved in all Kir channels (G165 in Kir6.2) serves as a hinge as proposed by Kuo et al. (3) or the residue above the central glycine serves as the controller of TM2 bending as proposed for Kir3.4 (9).
Mechanisms by Which the N160D Mutation Restores Ion Conduction in G156R Mutant Channels
Potassium channel pores conduct cations by stabilizing electrostatics in the low dielectric environment of the membrane (43). Introducing fixed positive charges to the pore by G156R mutation in Kir6.2 would predictably destabilize cations within the central cavity. This could explain the complete absence of measurable conduction in G156R homomeric channels by cations with varying physical properties. On the other hand, introduction of a negatively charged side chain at a residue that faces the pore of Kir channels, for example N160D, can increase the binding rate and apparent affinity of oppositely charged molecules and ions; this is likely due to favorable electrostatics caused by an additional negative field that contributes greater positive charge stabilization (12, 44). Such a stabilization effect could explain how N160D restores ion conduction and gating in the G156R background. In this case, the negatively charged side chain of aspartate at 160 helps stabilize the positively charged side chain of arginine at 156 to sequester the adverse effect G156R imposes on ion conduction. This interpretation is strongly supported by our results that inward rectification expected of the N160D mutation is abrogated in the G156R/N160D mutant channels as one would predict if the negatively charged side chain of N160D is bound by the positively charged arginine to eliminate the strong binding site for spermine at positive potentials. That the G156R/N160E, G156K/N160D, and G156K/N160E charge pairs also yielded channels with measurable currents but attenuated inward rectification further support the idea that an electrostatic interaction occurs between the side chains of the substituted amino acids at these two positions. However, we note that rescue of channel function by introducing opposite charges into the KATP pore is position- and distance-dependent (Table 1); charge reversal at the two positions did not yield functional channels (but see discussion below), and substituting Ala-161 or the leucine one helical turn below Asn-160 with negatively charged amino acids failed to restore ion conduction in the G156R channel. Thus, it appears that the electrostatic environment immediately surrounding residue 156 would also affect the side chain orientation and its effect on ion conduction. Collectively, our data support the conclusion that a close electrostatic interaction between Arg-156 and Asp-160 is the mechanism underlying the restoration of ion conduction in the mutant channel. This mechanism is consistent with that proposed previously for a Kir3.4 channel in that the side chain of substituting amino acids at the equivalent central glycine can interact with residues in the vicinity, including those in the selectivity filter, the pore helix, and the TM2 helix (9, 10). In particular, the side chain of a lysine at this position has been proposed to interact with a conserved threonine in the selectivity filter and an asparagine one helical turn down TM2. It would be interesting to determine in future studies if G156R in Kir6.2 also interacts with residues in the selectivity filter to interfere with channel function.
Somewhat surprisingly, we discovered that co-expression of two Kir6.2 mutants, G156D and N160R, which when individually expressed did not give rise to measurable currents, recovered channel activity that was inhibited by ATP and stimulated by LC-CoA, characteristics of KATP channels (Fig. 6). Because the two mutations are not in the same subunit, this observation indicates restoration of channel activity via intersubunit interactions between the two mutant residues. This conclusion is further substantiated by the tandem tetramer experiments in which spermine-induced inward rectification was used to probe the intra- versus intersubunit interaction between G156R and N160D. Our results that both G156R/N160D-WT×3 (same subunit) and G156R-N160D-WTx2 (neighboring subunits) tandem tetramer channels showed attenuated spermine-induced inward rectification compared with N160D-WT×3 channels support the notion that G156R and N160D can interact not only within the same subunit but also from adjacent subunits to affect channel behavior (Fig. 7E). These results have the implications that the spatial arrangement of the inner pore helices must be such that the side chains of Arg-156 can form chemical interactions with Asp-160 in the same subunit as well as in the adjacent subunit. Curiously, the G156D/N160R combination in the same subunit did not restore channel activity (Table 1), suggesting that for this mutation pair, intersubunit interactions are favored. Future homology modeling and molecular dynamic simulation studies should take these implications into consideration.
Influence of the Chemical Environment of the Pore on Ligand-induced Gating
Analysis of channel ATP sensitivity revealed subtle differences among the various charge-pair mutants. Although the G156R/N160D channels have ATP sensitivity very similar to WT channels, the G156K/N160D channels exhibit lower ATP sensitivity (IC50 ∼3-fold higher than that of WT). The differences in gating between G156R/N160D and G156K/N160D suggest that although arginine and lysine share similar physical chemical properties, the structures of their side chains may impart differential interactions with aspartate. In this case, the arginine-aspartate interactions appear to suppress the effect of individual mutations on gating more completely than the lysine-aspartate interactions, possibly because the arginine-aspartate pair interaction is more stable in the Kir6.2 TM2 structure. Variation in ATP sensitivity was further observed in the G156R/N160E and the G156K/N160E mutation combinations (Table 1). Although we do not yet have sufficient information to explain the gating property of the various mutants in molecular detail, the results from this study illustrate that gating properties of the channel are subject to changes in the chemical environment of the pore. From a medicinal chemistry point of view, our study leads us to speculate that it may be possible to manipulate the chemistry of a mutant Kir6.2 pore to restore channel gating by ATP and MgADP such that it will be physiologically functional, as we have shown for the G156R/N160D channels.
Acknowledgments
We thank Dr. Yu-Wen Lin for data sharing, Dr. Thomas Baumann for use of glass electrode manufacturing equipment, and Dr. Qing Zhou and Emily Pratt for comments on the manuscript. We are grateful to the patients, the General Clinical Research Center staff, and the Children's Hospital of Philadelphia nurses.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK066485 (to S.-L. S.), F31NS065688 (to J. D. B.), and R01DK56268 (to C. A. S.).
- SUR
- sulfonylurea receptor
- LC-CoA
- long chain acyl-CoA
- WT
- wild type.
REFERENCES
- 1.Nichols C. G., Lopatin A. N. (1997) Annu. Rev. Physiol. 59, 171–191 [DOI] [PubMed] [Google Scholar]
- 2.Kuo A., Domene C., Johnson L. N., Doyle D. A., Vénien-Bryan C. (2005) Structure 13, 1463–1472 [DOI] [PubMed] [Google Scholar]
- 3.Kuo A., Gulbis J. M., Antcliff J. F., Rahman T., Lowe E. D., Zimmer J., Cuthbertson J., Ashcroft F. M., Ezaki T., Doyle D. A. (2003) Science 300, 1922–1926 [DOI] [PubMed] [Google Scholar]
- 4.Nishida M., MacKinnon R. (2002) Cell 111, 957–965 [DOI] [PubMed] [Google Scholar]
- 5.Yi B. A., Minor D. L., Jr., Lin Y. F., Jan Y. N., Jan L. Y. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11016–11023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haider S., Khalid S., Tucker S. J., Ashcroft F. M., Sansom M. S. (2007) Biochemistry 46, 3643–3652 [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y., Lee A., Chen J., Cadene M., Chait B. T., MacKinnon R. (2002) Nature 417, 523–526 [DOI] [PubMed] [Google Scholar]
- 8.Jin T., Peng L., Mirshahi T., Rohacs T., Chan K. W., Sanchez R., Logothetis D. E. (2002) Mol. Cell 10, 469–481 [DOI] [PubMed] [Google Scholar]
- 9.Rosenhouse-Dantsker A., Logothetis D. E. (2006) Biophys. J. 91, 2860–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenhouse-Dantsker A., Logothetis D. E. (2007) Channels 1, 189–197 [DOI] [PubMed] [Google Scholar]
- 11.Shang L., Tucker S. J. (2008) Eur. Biophys. J. 37, 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurata H. T., Phillips L. R., Rose T., Loussouarn G., Herlitze S., Fritzenschaft H., Enkvetchakul D., Nichols C. G., Baukrowitz T. (2004) J. Gen. Physiol. 124, 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hille B. (2001) Ion Channels of Excitable Membranes, 3rd Ed., pp. 150–154, 566–571, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 14.Lopatin A. N., Makhina E. N., Nichols C. G. (1994) Nature 372, 366–369 [DOI] [PubMed] [Google Scholar]
- 15.Yang J., Jan Y. N., Jan L. Y. (1995) Neuron 14, 1047–1054 [DOI] [PubMed] [Google Scholar]
- 16.Pegan S., Arrabit C., Zhou W., Kwiatkowski W., Collins A., Slesinger P. A., Choe S. (2005) Nat. Neurosci. 8, 279–287 [DOI] [PubMed] [Google Scholar]
- 17.Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. (1995) Science 270, 1166–1170 [DOI] [PubMed] [Google Scholar]
- 18.Aguilar-Bryan L., Clement J. P., 4th, Gonzalez G., Kunjilwar K., Babenko A., Bryan J. (1998) Physiol. Rev. 78, 227–245 [DOI] [PubMed] [Google Scholar]
- 19.Ashcroft F. M., Gribble F. M. (1998) Trends Neurosci. 21, 288–294 [DOI] [PubMed] [Google Scholar]
- 20.Nichols C. G. (2006) Nature 440, 470–476 [DOI] [PubMed] [Google Scholar]
- 21.Aguilar-Bryan L., Bryan J. (1999) Endocr. Rev. 20, 101–135 [DOI] [PubMed] [Google Scholar]
- 22.Ashcroft F. M., Gribble F. M. (1999) Diabetologia 42, 903–919 [DOI] [PubMed] [Google Scholar]
- 23.Huopio H., Shyng S. L., Otonkoski T., Nichols C. G. (2002) Am. J. Physiol. Endocrinol. Metab. 283, E207–E216 [DOI] [PubMed] [Google Scholar]
- 24.Aguilar-Bryan L., Bryan J., Nakazaki M. (2001) Recent Prog. Horm. Res. 56, 47–68 [DOI] [PubMed] [Google Scholar]
- 25.Dunne M. J., Cosgrove K. E., Shepherd R. M., Aynsley-Green A., Lindley K. J. (2004) Physiol. Rev. 84, 239–275 [DOI] [PubMed] [Google Scholar]
- 26.Glaser B. (2000) Semin. Perinatol. 24, 150–163 [DOI] [PubMed] [Google Scholar]
- 27.Stanley C. A. (2002) J. Clin. Endocrinol. Metab. 87, 4857–4859 [DOI] [PubMed] [Google Scholar]
- 28.Pinney S. E., MacMullen C., Becker S., Lin Y. W., Hanna C., Thornton P., Ganguly A., Shyng S. L., Stanley C. A. (2008) J. Clin. Invest. 118, 2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taschenberger G., Mougey A., Shen S., Lester L. B., LaFranchi S., Shyng S. L. (2002) J. Biol. Chem. 277, 17139–17146 [DOI] [PubMed] [Google Scholar]
- 30.Lin Y. W., MacMullen C., Ganguly A., Stanley C. A., Shyng S. L. (2006) J. Biol. Chem. 281, 3006–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shyng S., Ferrigni T., Nichols C. G. (1997) J. Gen. Physiol. 110, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shyng S., Nichols C. G. (1997) J. Gen. Physiol. 110, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C. W., Lin Y. W., Yan F. F., Casey J., Kochhar M., Pratt E. B., Shyng S. L. (2006) Diabetes 55, 1738–1746 [DOI] [PubMed] [Google Scholar]
- 34.Lu Z., MacKinnon R. (1995) Biochemistry 34, 13133–13138 [DOI] [PubMed] [Google Scholar]
- 35.Nichols C. G., Shyng S. L., Nestorowicz A., Glaser B., Clement J. P., 4th, Gonzalez G., Aguilar-Bryan L., Permutt M. A., Bryan J. (1996) Science 272, 1785–1787 [DOI] [PubMed] [Google Scholar]
- 36.Ashcroft F. M. (2005) J. Clin. Invest. 115, 2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clement J. P., 4th, Kunjilwar K., Gonzalez G., Schwanstecher M., Panten U., Aguilar-Bryan L., Bryan J. (1997) Neuron 18, 827–838 [DOI] [PubMed] [Google Scholar]
- 38.Lin Y. W., Jia T., Weinsoft A. M., Shyng S. L. (2003) J. Gen. Physiol. 122, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., MacKinnon R. (1998) Science 280, 69–77 [DOI] [PubMed] [Google Scholar]
- 40.Ding S., Ingleby L., Ahern C. A., Horn R. (2005) J. Gen. Physiol. 126, 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y., Lee A., Chen J., Ruta V., Cadene M., Chait B. T., MacKinnon R. (2003) Nature 423, 33–41 [DOI] [PubMed] [Google Scholar]
- 42.Magidovich E., Yifrach O. (2004) Biochemistry 43, 13242–13247 [DOI] [PubMed] [Google Scholar]
- 43.Roux B., MacKinnon R. (1999) Science 285, 100–102 [DOI] [PubMed] [Google Scholar]
- 44.Robertson J. L., Palmer L. G., Roux B. (2008) J. Gen. Physiol. 132, 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Y. W., Bushman J. D., Yan F. F., Haidar S., MacMullen C., Ganguly A., Stanley C. A., Shyng S. L. (2008) J. Biol. Chem. 283, 9146–9156 [DOI] [PMC free article] [PubMed] [Google Scholar]