Abstract
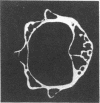
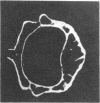
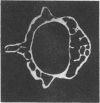
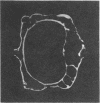
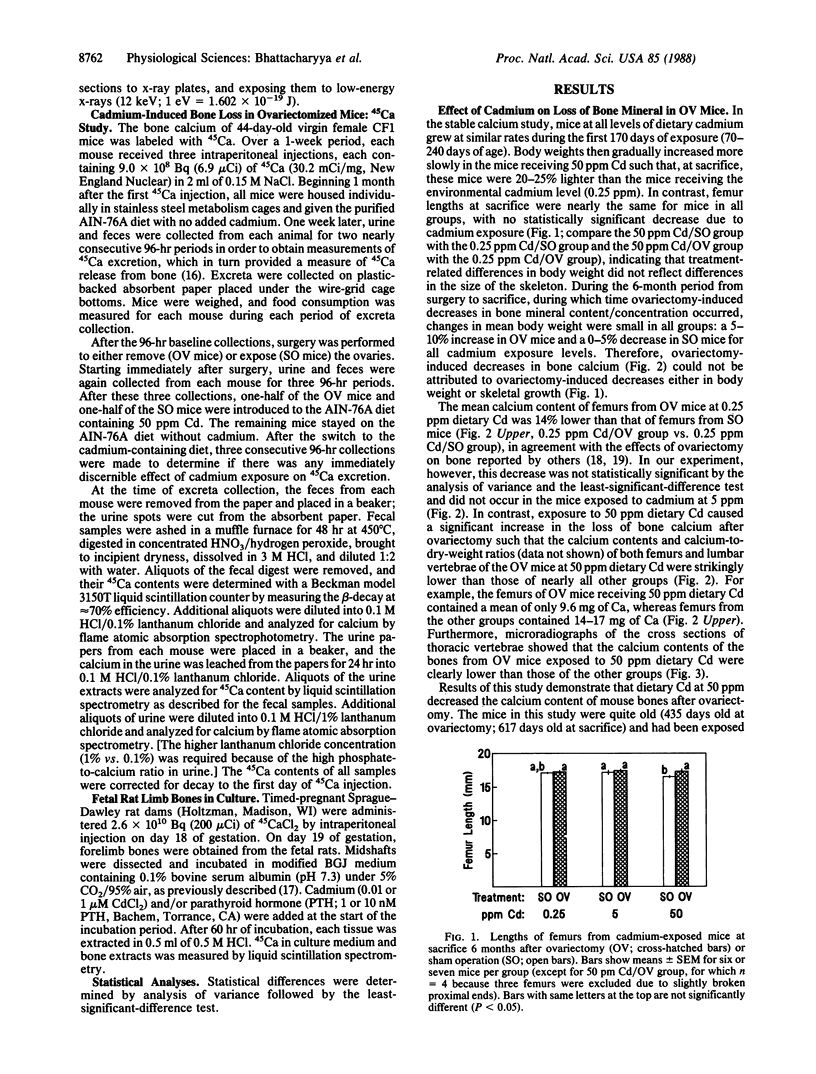
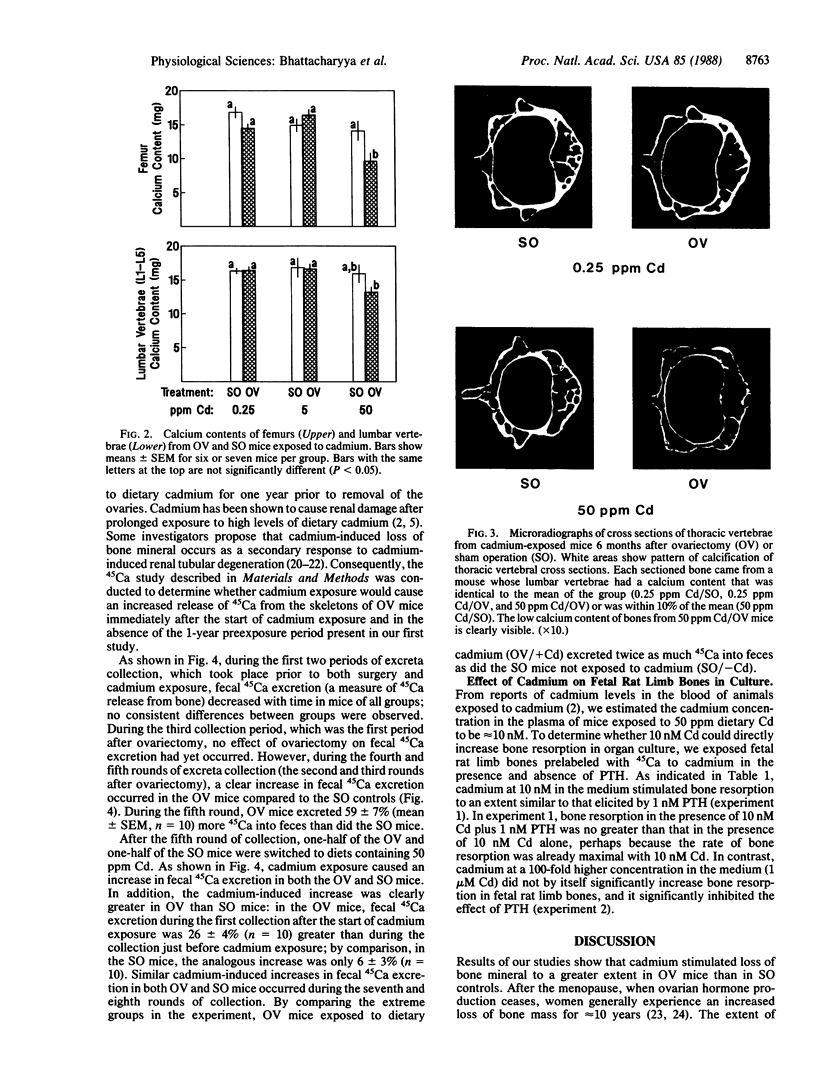
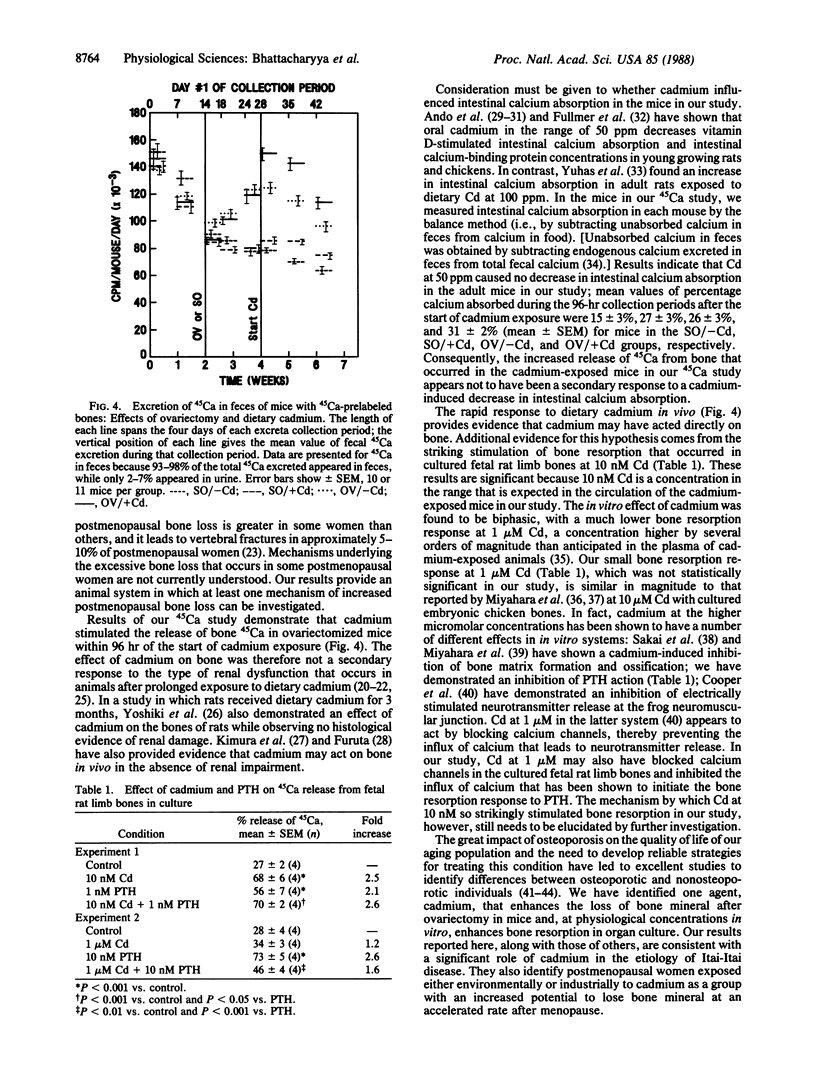
Loss of bone mineral after ovariectomy was studied in mice exposed to dietary cadmium at 0.25, 5, or 50 ppm. Results show that dietary cadmium at 50 ppm increased bone mineral loss to a significantly greater extent in ovariectomized mice than in sham-operated controls. These results were obtained from two studies, one in which skeletal calcium content was determined 6 months after ovariectomy and a second in which 45Ca release from 45Ca-prelabeled bones was measured immediately after the start of dietary cadmium exposure. Furthermore, experiments with 45Ca-prelabeled fetal rat limb bones in culture demonstrated that Cd at 10 nM in the medium, a concentration estimated to be in the plasma of mice exposed to 50 ppm dietary Cd, strikingly increased bone resorption, from 27 +/- 2% (mean +/- SEM) 45Ca release in cultures with no added cadmium to 68 +/- 6% release in cultures containing cadmium (n = 4). These in vitro results indicate that cadmium may enhance bone mineral loss by a direct action on bone. Results of the in vivo studies are consistent with a significant role of cadmium in the etiology of Itai-Itai disease among postmenopausal women in Japan and may in part explain the increased risk of postmenopausal osteoporosis among women who smoke.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloia J. F., Cohn S. H., Vaswani A., Yeh J. K., Yuen K., Ellis K. Risk factors for postmenopausal osteoporosis. Am J Med. 1985 Jan;78(1):95–100. doi: 10.1016/0002-9343(85)90468-1. [DOI] [PubMed] [Google Scholar]
- Ando M., Sayato Y., Osawa T. Studies on the disposition of calcium in bones of rats after continuous oral administration of cadmium. Toxicol Appl Pharmacol. 1978 Dec;46(3):625–632. doi: 10.1016/0041-008x(78)90308-3. [DOI] [PubMed] [Google Scholar]
- Ando M., Sayato Y., Tonomura M., Osawa T. Studies on excretion and uptake of calcium by rats after continuous oral administration of cadmium. Toxicol Appl Pharmacol. 1977 Feb;39(2):321–327. doi: 10.1016/0041-008x(77)90165-x. [DOI] [PubMed] [Google Scholar]
- Ando M., Shimizu M., Sayato Y., Tanimura A., Tobe M. The inhibition of vitamin D-stimulated intestinal calcium transport in rats after continuous oral administration of cadmium. Toxicol Appl Pharmacol. 1981 Dec;61(3):297–301. doi: 10.1016/0041-008x(81)90350-1. [DOI] [PubMed] [Google Scholar]
- Bernard A., Lauwerys R., Gengoux P. Characterization of the proteinuria induced by prolonged oral administration of cadmium in female rats. Toxicology. 1981;20(4):345–357. doi: 10.1016/0300-483x(81)90041-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya M. H., Whelton B. D., Peterson D. P., Carnes B. A., Moretti E. S., Toomey J. M., Williams L. L. Skeletal changes in multiparous mice fed a nutrient-sufficient diet containing cadmium. Toxicology. 1988 Jul;50(2):193–204. doi: 10.1016/0300-483x(88)90091-1. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Christensen M. S., Transbøl I. Bone mass in postmenopausal women after withdrawal of oestrogen/gestagen replacement therapy. Lancet. 1981 Feb 28;1(8218):459–461. doi: 10.1016/s0140-6736(81)91848-1. [DOI] [PubMed] [Google Scholar]
- Cooper G. P., Suszkiw J. B., Manalis R. S. Heavy metals: effects on synaptic transmission. Neurotoxicology. 1984 Fall;5(3):247–266. [PubMed] [Google Scholar]
- Daniell H. W. Osteoporosis of the slender smoker. Vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch Intern Med. 1976 Mar;136(3):298–304. doi: 10.1001/archinte.136.3.298. [DOI] [PubMed] [Google Scholar]
- Daniell H. W. Postmenopausal tooth loss. Contributions to edentulism by osteoporosis and cigarette smoking. Arch Intern Med. 1983 Sep;143(9):1678–1682. doi: 10.1001/archinte.143.9.1678. [DOI] [PubMed] [Google Scholar]
- Draper H. H., Bell R. R., Shin K. S. Influence of adult age on the skeletal response to phosphate and estrogen in rats. J Nutr. 1980 Apr;110(4):778–783. doi: 10.1093/jn/110.4.778. [DOI] [PubMed] [Google Scholar]
- Elinder C. G., Kjellström T., Lind B., Linnman L., Piscator M., Sundstedt K. Cadmium exposure from smoking cigarettes: variations with time and country where purchased. Environ Res. 1983 Oct;32(1):220–227. doi: 10.1016/0013-9351(83)90209-8. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S., Oku T., Wasserman R. H. Effect of cadmium administration on intestinal calcium absorption and vitamin D-dependent calcium-binding protein. Environ Res. 1980 Aug;22(2):386–399. doi: 10.1016/0013-9351(80)90150-4. [DOI] [PubMed] [Google Scholar]
- Furuta H. Cadmium effects on bone and dental tissues of rats in acute and subacute poisoning. Experientia. 1978 Oct 15;34(10):1317–1318. doi: 10.1007/BF01981442. [DOI] [PubMed] [Google Scholar]
- Gallagher J. C., Riggs B. L., Eisman J., Hamstra A., Arnaud S. B., DeLuca H. F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979 Sep;64(3):729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding A., Campbell D. Dietary NaCl loads promote calciuria and bone loss in adult oophorectomized rats consuming a low calcium diet. J Nutr. 1983 Jul;113(7):1409–1414. doi: 10.1093/jn/113.7.1409. [DOI] [PubMed] [Google Scholar]
- Itokawa Y., Abe T., Tabei R., Tanaka S. Renal and skeletal lesions in experimental cadmium poisoning: histological and biochemical approaches. Arch Environ Health. 1974 Mar;28(3):149–154. doi: 10.1080/00039896.1974.10666456. [DOI] [PubMed] [Google Scholar]
- Itokawa Y., Nishino K., Takashima M., Nakata T., Kaito H., Okamoto E., Daijo K., Kawamura J. Renal and skeletal lesions in experimental cadmium poisoning of rats. Histology and renal function. Environ Res. 1978 Apr;15(2):206–217. doi: 10.1016/0013-9351(78)90097-x. [DOI] [PubMed] [Google Scholar]
- Jensen J., Christiansen C., Rødbro P. Cigarette smoking, serum estrogens, and bone loss during hormone-replacement therapy early after menopause. N Engl J Med. 1985 Oct 17;313(16):973–975. doi: 10.1056/NEJM198510173131602. [DOI] [PubMed] [Google Scholar]
- Kajikawa K., Nakanishi I., Kuroda K. Morphological changes of the kidney and bone of rats in chronic cadmium poisoning. Exp Mol Pathol. 1981 Feb;34(1):9–24. doi: 10.1016/0014-4800(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Kimura M., Otaki N., Yoshiki S., Suzuki M., Horiuchi N. The isolation of metallothionein and its protective role in cadmium poisoning. Arch Biochem Biophys. 1974 Nov;165(1):340–348. doi: 10.1016/0003-9861(74)90172-6. [DOI] [PubMed] [Google Scholar]
- Lindgren J. U., Lindholm T. S. Effect of 1-alpha-hydroxyvitamin D3 on osteoporosis in rats induced by oophorectomy. Calcif Tissue Int. 1979 Apr 17;27(2):161–164. doi: 10.1007/BF02441179. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Forrest C., Baird C. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980 Nov 29;2(8205):1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- MacMahon B., Trichopoulos D., Cole P., Brown J. Cigarette smoking and urinary estrogens. N Engl J Med. 1982 Oct 21;307(17):1062–1065. doi: 10.1056/NEJM198210213071707. [DOI] [PubMed] [Google Scholar]
- Miyahara T., Miyakoshi M., Kozuka H. Effect of cadmium on bone resorption in cultured fetal bones. Bull Environ Contam Toxicol. 1980 Aug;25(2):294–297. doi: 10.1007/BF01985527. [DOI] [PubMed] [Google Scholar]
- Miyahara T., Miyakoshi M., Saito Y., Kozuka H. Influence of poisonous metals on bone metabolism. III. The effect of cadmium on bone resorption in tissue culture. Toxicol Appl Pharmacol. 1980 Sep 30;55(3):477–483. doi: 10.1016/0041-008x(80)90049-6. [DOI] [PubMed] [Google Scholar]
- Nomiyama K. Recent progress and perspectives in cadmium health effects studies. Sci Total Environ. 1980 Apr;14(3):199–232. doi: 10.1016/0048-9697(80)90024-8. [DOI] [PubMed] [Google Scholar]
- Nordberg M. Studies on metallothionein and cadmium. Environ Res. 1978 Jun;15(3):381–404. doi: 10.1016/0013-9351(78)90120-2. [DOI] [PubMed] [Google Scholar]
- Piscator M., Björck L., Nordberg M. Beta 2-microglobulin levels in serum and urine of cadmium exposed rabbits. Acta Pharmacol Toxicol (Copenh) 1981 Jul;49(1):1–7. doi: 10.1111/j.1600-0773.1981.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Riggs B. L. Pathogenesis of osteoporosis. Am J Obstet Gynecol. 1987 May;156(5):1342–1346. doi: 10.1016/0002-9378(87)90176-1. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Wahner H. W., Dunn W. L., Mazess R. B., Offord K. P., Melton L. J., 3rd Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981 Feb;67(2):328–335. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. H., Phillips T. E., Mavreas T. Bioassay of 1,25-dihydroxyvitamin D in human plasma purified by partition, alkaline extraction, and high-pressure chromatography. Anal Biochem. 1980 Feb;102(1):22–30. doi: 10.1016/0003-2697(80)90311-5. [DOI] [PubMed] [Google Scholar]
- Tsai K. S., Heath H., 3rd, Kumar R., Riggs B. L. Impaired vitamin D metabolism with aging in women. Possible role in pathogenesis of senile osteoporosis. J Clin Invest. 1984 Jun;73(6):1668–1672. doi: 10.1172/JCI111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki S., Yanagisawa T., Kimura M., Otaki N., Suzuki M. Bone and kidney lesions in experimental cadmium intoxication. Arch Environ Health. 1975 Nov;30(11):559–562. doi: 10.1080/00039896.1975.10666776. [DOI] [PubMed] [Google Scholar]
- Yuhas E. M., Miya T. S., Schnell R. C. Influence of cadmium on calcium absorption from the rat intestine. Toxicol Appl Pharmacol. 1978 Jan;43(1):23–31. doi: 10.1016/s0041-008x(78)80029-5. [DOI] [PubMed] [Google Scholar]