Abstract
It is established that the adipocyte-derived cytokine adiponectin protects against cardiovascular and metabolic diseases, but the effect of this adipokine on macrophage polarization, an important mediator of disease progression, has never been assessed. We hypothesized that adiponectin modulates macrophage polarization from that resembling a classically activated M1 phenotype to that resembling alternatively-activated M2 cells. Peritoneal macrophages and the stromal vascular fraction (SVF) cells of adipose tissue isolated from adiponectin knock-out mice displayed increased M1 markers, including tumor necrosis factor-α, interleukin-6, and monocyte chemoattractant protein-1 and decreased M2 markers, including arginase-1, macrophage galactose N-acetyl-galactosamine specific lectin-1, and interleukin-10. The systemic delivery of adenovirus expressing adiponectin significantly augmented arginase-1 expression in peritoneal macrophages and SVF cells in both wild-type and adiponectin knock-out mice. In culture, the treatment of macrophages with recombinant adiponectin protein led to an increase in the levels of M2 markers and a reduction of reactive oxygen species and reactive oxygen species-related gene expression. Adiponectin also stimulated the expression of M2 markers and attenuated the expression of M1 markers in human monocyte-derived macrophages and SVF cells isolated from human adipose tissue. These data show that adiponectin functions as a regulator of macrophage polarization, and they indicate that conditions of high adiponectin expression may deter metabolic and cardiovascular disease progression by favoring an anti-inflammatory phenotype in macrophages.
Keywords: Adipocyte, Cytokine, Inflammation, Macrophage, Obesity, Adiponectin
Introduction
Obesity activates low grade inflammation that contributes to the pathogenesis of obesity-linked diseases, such as type 2 diabetes and atherosclerosis (1). Adipose tissue macrophages play an important role in the establishment of the chronic inflammatory state and metabolic dysfunction that is associated with obesity (2, 3). Recent findings show that adipose tissue macrophages from lean organisms express markers of the M2 or “alternatively activated” macrophage, whereas obesity leads to a reduction of these markers and an increase of genes associated with the M1 or “classically activated” macrophage (4). M1 macrophage polarization is associated with inflammation and tissue destruction, whereas the M2 macrophage has an anti-inflammatory phenotype that is associated with wound repair and angiogenesis.
Macrophages are polarized to the M1 state by interferon-γ and inducers of tumor necrosis factor-α (TNF-α)2, such as lipopolysaccharide (LPS). M1 macrophages up-regulate proinflammatory cytokines including TNF-α, interleukin (IL)-6, and IL-12, and they increase the production of reactive oxygen species (ROS) and nitrogen intermediates (5). In contrast, macrophages are polarized to the M2 state by IL-4 and IL-13. M2 macrophages up-regulate scavenger receptors, mannose receptor and IL-1 receptor antagonist. M2 cells also secrete the anti-inflammatory cytokine IL-10 and down-regulate the production of proinflammatory cytokines. These cells also up-regulate arginase-1, which metabolizes arginine to ornithine and polyamines, and thereby diminishes the inducible nitric-oxide synthase (iNOS) reaction (6). Furthermore, the “silent” ingestion of early apoptotic cell debris by macrophages will confer an anti-inflammatory M2-like phenotype that is associated with the production of anti-inflammatory mediators including IL-10 (7–9).
Adiponectin is a fat-derived, abundant plasma protein that is down-regulated in obesity and its complications including type 2 diabetes, hypertension, nonalcoholic steatohepatitis and atherosclerosis (10–13). A number of studies have established inverse correlations between circulating levels of adiponectin and the inflammatory markers C-reactive protein and IL-6 (14–17). Adiponectin has been shown to exert anti-inflammatory actions on multiple cell types and tissues. Studies in cell culture have shown that adiponectin affects the function of macrophages in a number of ways including the suppression of class A scavenger receptor expression (18), diminished NF-κB activation by Toll-like receptor (19), and up-regulation of the anti-inflammatory cytokine IL-10 (20). Although these studies document that adiponectin has anti-inflammatory effects on cultured macrophages, a systematic analysis of adiponectin action on macrophage phenotype has never been conducted nor have the actions of adiponectin on macrophage phenotype been assessed in vivo.
We hypothesized that adiponectin affects the phenotype of macrophages favoring an alternative state that promotes wound healing and the resolution of inflammation. In the present study, we investigated the role of adiponectin in the regulation of macrophage polarization in loss- and gain-of-function genetic models and in cultured mouse and human macrophages. Our results indicate that adiponectin functions as a direct regulator of macrophage phenotype favoring the switch from a proinflammatory M1-like state to an anti-inflammatory M2-like state.
EXPERIMENTAL PROCEDURES
Materials
Mouse arginase-1 antibody was purchased from BD Biosciences. Tubulin antibody was purchased from Oncogene. Quantitative real-time polymerase chain reaction primers are listed in supplemental Table 1. The enzyme-linked immunosorbent assay kit for murine TNF-α was from R&D Systems.
Preparation of Recombinant Adiponectin
Adiponectin cDNA was cloned by PCR from human adipose tissue cDNA and inserted into a mammalian cell expression vector. The adiponectin expression vector was transfected into Chinese hamster ovary cells by electroporation. High yield stably transfected clones were selected and up-scaled for production purposes. Cell culture supernatant was dialyzed against 1 mm CaCl2, 20 mm Tris-HCl, pH 7.5, and the protein was purified on a Source 15Q (GE Healthcare) column using a gradient of NaCl in 1 mm CaCl2, 20 mm Tris-HCl, pH 7.5. Adiponectin containing fractions were further purified on a Superdex 200 HR 26/60 using Tris-buffered saline with 5 mm CaCl2 as eluent. Endotoxin levels were determined by Limulus amoebocyte lysate-based kinetic turbidimetric tests (2.7 enzyme units/mg).
Isolation of Mouse Peritoneal Macrophages
Peritoneal macrophages were collected from 8–10-week-old male adiponectin knock-out and C57BL/6 mice by peritoneal lavage with 10 ml of phosphate-buffered saline, centrifuged at 1,000 rpm for 10 min, resuspended in RPMI1640 media supplemented 10% fetal bovine serum, and incubated on the 24-well plate for 1 h and then used for the experiments.
Mouse SVF Isolation
Epididymal fat pads from male adiponectin knock-out and C57BL/6 mice fed a normal or high fat diet were excised and minced in phosphate-buffered saline (PBS). Next, they were digested with 1 mg/ml collagenase Type 1 (Worthington Chemical Corporation) in PBS at 37 °C for 30 min. The cell suspension was filtered through a 100-μm filter and spun at 1,000 rpm centrifugation for 5 min to separate floating adipocytes from the SVF pellet.
In Vitro Peritoneal Macrophage Studies
Peritoneal macrophages from C57BL/6 mice were maintained in RPMI1640 supplemented 10% fetal bovine serum. Before each experiment, cells were placed in RPMI1640 media with 2% fetal bovine serum for 16 h for serum starvation. Experiments were performed by the addition of the indicated amount of recombinant adiponectin (30 μg/ml; Novo Nordisk), IL-4 (10 ng/ml; Sigma), or vehicle for 48 h.
Adenovirus-mediated Gene Transfer
4 × 108 plaque-forming units of adenovirus-producing adiponectin (Ad-APN) or adenovirus-producing β-galactosidase were injected into the tail vein of APN-KO or WT mice 5 days before collecting peritoneal macrophages and stromal vascular fractions.
Determination of mRNA Levels
Total RNA was prepared by Qiagen according to the manufacturer's suggested protocol, and cDNA was produced using ThermoScript real-time-PCR Systems (Invitrogen). Quantitative real-time PCR was performed on iCycler iQ Real-Time PCR Detection System (Bio-Rad) using SYBR Green I as a double-stranded DNA-specific dye (Applied Biosystems). We used the primers listed in supplemental Table 1.
Western Blot Analysis
Tissue samples were homogenized in lysis buffer. Immunoblot analysis was performed with antibodies at a 1:1,000 dilution, followed by incubation with a secondary antibody conjugated with horseradish peroxidase at a 1:5,000 dilution. An ECL Plus Western blotting detection kit (Amersham Biosciences) was used.
Human Subcutaneous Adipose Tissue Collection
Subcutaneous adipose tissue was collected via percutaneous needle biopsy or during gastric bypass surgery in consecutive obese adults (age ≥ 21 yr) with a body mass index ≥30 kg/m2 as described previously (21). Patients with unstable medical conditions such as active coronary syndromes, congestive heart failure, systemic infection, malignancy, or pregnancy were excluded. Abdominal subcutaneous fat biopsies were performed lateral to the umbilicus using standard sterile technique. Briefly, the region was carefully inspected for anatomical landmarks, draped, sterilely prepped using alcohol and betadine, and locally anesthetized with 2 ml of 2% lidocaine. Through a small superficial 0.5-cm skin incision, adipose tissue was collected via a 3-hole cannula needle. Subcutaneous abdominal tissue was also directly harvested in a cohort of bariatric subjects during planned operative procedure. Each subject provided a single biopsy specimen for analysis. All subjects gave written, informed consent, and the study was approved by the Boston University Medical Center Institutional Review Board.
Isolation of the SVF from Human Adipose Tissue
Human subcutaneous adipose tissue are excised, minced, and digested with 1 mg/ml collagenase Type 1 (Worthington Chemical Corporation) in PBS at 37 °C for 20 min. The cell suspension is filtered through a 100-μm filter and spun at 200 × g centrifugation for 5 min to separate floating adipocytes from the SVF pellet. The pellet containing SVF is incubated for 10 min in a red blood cell-lysing buffer (Sigma). Then, the cells were suspended in RPMI 1640 (Invitrogen) supplemented with 10% human type AB serum and incubated for 1 h at 37 °C in cell culture dishes. Nonadherent cells were removed by washing twice with PBS. The remaining adherent cells were cultured as SVF cells and then incubated with recombinant adiponectin for 24 h.
Isolation of Human Monocyte-derived Macrophages
Human monocyte-derived macrophages were isolated from peripheral blood by density gradient centrifugation. The cells were suspended in RPMI 1640 (Invitrogen) supplemented with 10% human type AB serum and incubated for 1 h at 37 °C in cell culture dishes. Nonadherent cells were removed by washing twice with PBS. The remaining adherent cells were cultured in the same medium. The medium was replaced every 2 days. Human monocyte-derived macrophages were cultured in the medium for 4 days and then incubated with recombinant adiponectin for 48 h.
Statistical Methods
Data are presented as mean ± S.E. Differences between groups were evaluated by the Student's t test or analysis of variance with Fisher's protected least significant difference (PLSD) test. A p value <0.05 denoted the presence of a statistically significant difference. All calculations were performed by using a standard statistical package (StatView for Windows, Version 5.0).
RESULTS
Peritoneal Macrophages from APN-KO Mice Exhibit Inflammatory Activation
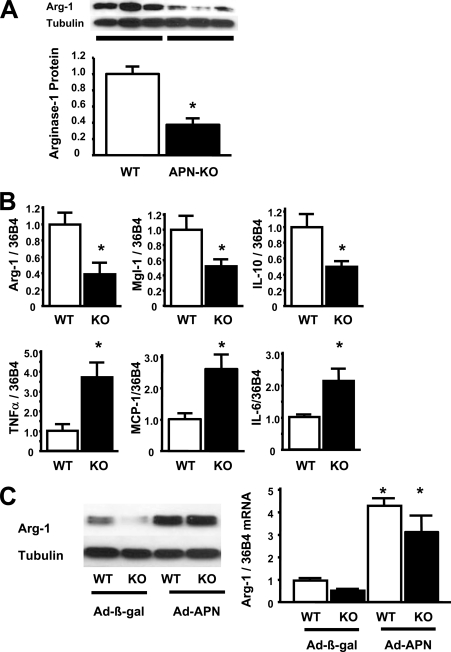
To investigate the phenotypic character of macrophages in the absence of adiponectin in vivo, we collected peritoneal macrophages from C57BL/6 (WT) and APN-KO mice. Peritoneal macrophages from APN-KO mice showed decreased protein levels of arginase-1, an anti-inflammatory M2 marker, in Western blot analysis (Fig. 1A). Transcript levels of M2 markers, including arginase- 1, Mgl-1 (macrophage galactose N-acetyl-galactosamine-specific lectin-1), and IL-10 were significantly lower in peritoneal macrophages from APN-KO mice than in those from WT mice (Fig. 1B). In contrast, mRNA levels of inflammatory M1 markers, including TNF-α, monocyte chemoattractant protein-1 (MCP-1), and IL-6 were significantly higher in macrophages from APN-KO mice compared with those from WT mice (Fig. 1B).
FIGURE 1.
Adiponectin deficiency promotes peritoneal macrophage activation. A, quantitative Western blot of arginase-1 and α-tubulin (Tubulin) in peritoneal macrophages from WT and APN-KO mice. B, the mRNA levels of anti-inflammatory M2 markers arginase-1, Mgl-1, and IL-10 and inflammatory M1 markers TNF-α, MCP-1, and IL-6 in quantitative RT-PCR methods. All results were normalized to 36B4. *, p < 0.05 versus WT (n = 6 in each group). C, Ad-β-galactosidase (Ad-β-gal) or Ad-APN (4 × 108 plaque-forming units each) was intravenously injected into WT and APN-KO mice 7 days prior to collection of peritoneal macrophages. The left panel shows Western blot of arginase-1 from WT and APN-KO (KO) mice after the injection of Ad-β-galactosidase or Ad-APN. The right panel shows the mRNA levels of arginase-1 from WT and KO mice after the injection of Ad-β-galactosidase or Ad-APN. *, p < 0.05 versus Ad-β-galactosidase-treated WT mice (n = 4 in each group). Data are presented as mean ± S.E.
To test whether the overexpression of adiponectin promotes macrophage M2 polarization in vivo, adenoviral vectors expressing adiponectin (Ad-APN) or β-galactosidase were injected intravenously in APN-KO and WT mice 5 days prior to peritoneal macrophage isolation. In these experimental groups of mice, circulating adiponectin levels were undetectable in Ad-β-galactosidase/APN-KO mice, 11.5 ± 1.2 μg/ml in Ad-β-galactosidase/WT mice, 23.3 ± 2.3 μg/ml in Ad-APN/APN-KO mice and 40.7 ± 1.6 μg/ml in Ad-APN-treated WT mice. Ad-APN treatment increased arginase-1 protein expression in peritoneal macrophages isolated from WT and APN-KO mice, as assessed by Western blot analyses. The mRNA levels of arginase-1 were also augmented in peritoneal macrophages from WT and KO mice following treatment with Ad-APN (Fig. 1C).
Stromal Vascular Fraction Cells and Lung Macrophages from APN-KO Mice Exhibit Inflammatory Activation
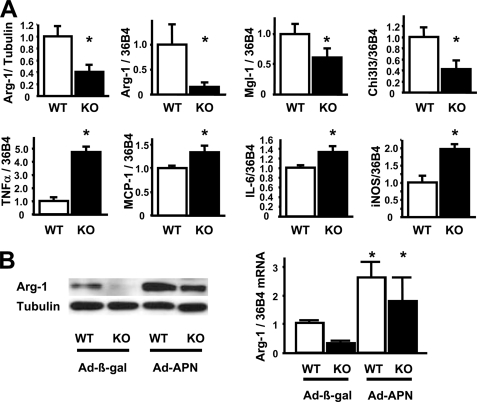
To investigate the effect of adiponectin on polarization of resident macrophages in adipose tissue, SVF cells were isolated from the epididymal fat pads of APN-KO and WT mice. The protein level of arginase-1 was significantly lower in SVF cells from APN-KO mice than in that from WT mice (Fig. 2A). Transcript markers of macrophage phenotype were also assessed in SVF cells. The expression levels of the M2 markers arginase-1, Mgl-1, and Chi3l3 (chitinase 3-like 3) were significantly lower in SVF cells from APN-KO mice than WT mice (Fig. 2A). Correspondingly, inflammatory M1 markers, including TNF-α, MCP-1, IL-6, and iNOS, were significantly higher in SVF cells from APN-KO mice compared with that from WT mice (Fig. 2A).
FIGURE 2.
Adiponectin deficiency promotes SVF cell activation. A, quantitative Western blot of arginase-1/α-tubulin (Tubulin) and the mRNA levels of anti-inflammatory M2 markers arginase-1, Mgl-1, and Chi3l3 and inflammatory M1 markers, TNF-α, MCP-1, IL-6, and iNOS in SVF cells from WT and APN-KO mice. All mRNA data were normalized to 36B4. *, p < 0.05 versus WT (n = 6 in each group). B, Ad-β-galactosidase (Ad-β-gal) or Ad-APN (4 × 108 plaque-forming units each) was intravenously injected into WT and APN-KO mice 7 days prior to collection of peritoneal macrophages. The left panel shows Western blot of arginase-1 from WT and APN-KO (KO) mice after the injection of Ad-β-galactosidase or Ad-APN. The right panel shows the mRNA levels of arginase-1 from WT and KO mice after the injection of Ad-β-galactosidase or Ad-APN. *, p < 0.05 versus Ad-β-galactosidase-treated WT mice (n = 4 in each group). Data are presented as mean ± S.E.
The effect of adiponectin gain-of-function genetic manipulation on adipose tissue macrophage polarization was also assessed. Systemic administration of Ad-APN increased arginase-1 protein expression in SVF cells from WT and APN-KO mice (Fig. 2B). Transcript levels of arginase-1 were also augmented in SVF cells from Ad-APN-treated WT and KO mice (Fig. 2B).
To generalize our findings to other populations of macrophages in mice, surface major histocompatibility complex class II expression was compared in lung macrophages from WT and APN-KO mice. For these studies, CD11c was used to mark alveolar macrophages in lung digests during flow cytometry analysis. As depicted in Table 1, results showed that major histocompatibility complex class II expression was significantly increased in alveolar macrophages from APN-KO mice, indicative of macrophage activation.
TABLE 1.
Lung macrophage activation in adiponectin-deficient mice
CD11c and major histocompatibility complex (MHC) class II cell surface expression were assessed by flow cytometry in whole lung digest from wild-type and adiponectin-deficient mice as described previously (25). Data are expressed as a percentage of total CD11c+ cells (average ± S.E., n = 3 in each group).
| Cell population | WT | APN-KO | p value |
|---|---|---|---|
| CD11c+, MHC class II− | 57% ± 21% | 16% ± 4% | <0.05 |
| CD11c+, MHC class II+ | 43% ± 8% | 84% ± 7% | <0.01 |
Adiponectin Directly Promotes an Anti-inflammatory Phenotype in Macrophages in Vitro
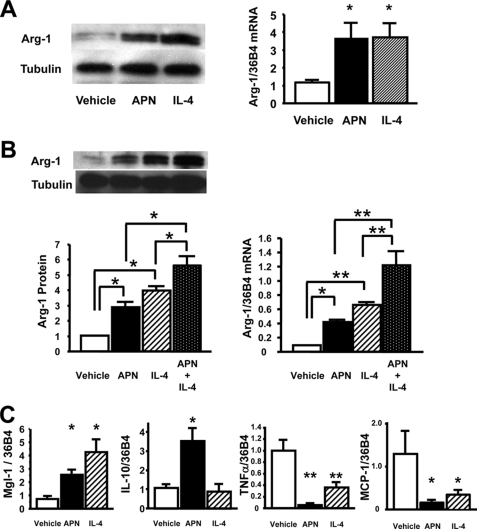
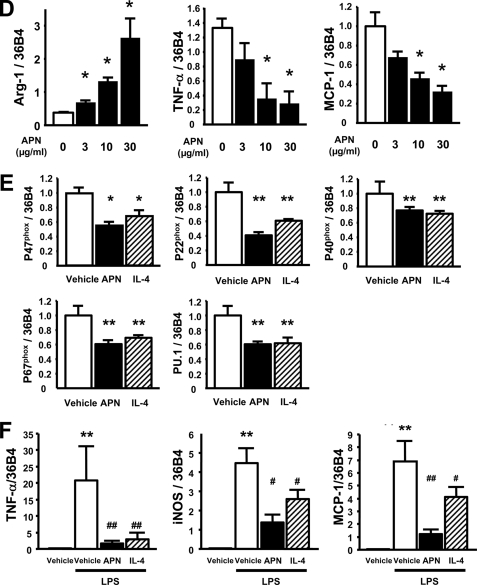
To investigate whether adiponectin acts directly on macrophages to control phenotype, an in vitro study was performed using peritoneal macrophages from WT mice. Treatment of macrophages with recombinant adiponectin protein for 48 h led to the marked up-regulation of arginase-1 expression at both protein and mRNA levels (Fig. 3A). The magnitude of arginase-1 up-regulation by adiponectin was similar to that seen with IL-4, a prototypical Th-2 cytokine. Co-treatment with APN and IL-4 increased arginase-1 protein and mRNA levels in an additive manner (Fig. 3B). Treatment of peritoneal macrophages with recombinant adiponectin increased transcript levels of other markers of the M2 phenotype including Mgl-1 and IL-10 (Fig. 3C). In comparison, IL-4 treatment increased transcript levels of Mgl-1 but not IL-10. Furthermore, treatment with adiponectin or IL-4 significantly decreased mRNA levels of the M1 markers TNF-α and MCP-1 (Fig. 3C). The responses of peritoneal macrophages to increasing concentrations of adiponectin are shown in Fig. 3D. Adiponectin activated arginase-1 expression and reduced TNF-α and MCP-1 expression in cultured peritoneal macrophages in a dose-dependent manner.
FIGURE 3.
Adiponectin promotes an anti-inflammatory phenotype in freshly isolated murine peritoneal macrophages. A, left panel, shows Western blot of arginase-1 and α-tubulin (Tubulin) under a 48-h treatment of recombinant APN (30 μg/ml) and IL-4 (10 ng/ml). The right panel shows mRNA levels of arginase-1. *, p < 0.05 versus vehicle (n = 4 in each group). The mRNA levels of arginase-1 were normalized to 36B4. B, left panel, shows Western blot of arginase-1 and tubulin under the treatment of APN, IL-4, or both. The right panel shows mRNA levels of arginase-1 under the treatment of APN, IL-4, or both. *, p < 0.05 and **, p < 0.01 (n = 4 in each group). The mRNA levels of arginase-1 were normalized to 36B4. C, the mRNA levels of anti-inflammatory M2 markers, Mgl-1 and IL-10 and inflammatory markers, TNF-α and MCP-1 in quantitative RT-PCR methods. *, p < 0.05 and **, p < 0.01 versus vehicle (n = 4 in each group). All results were normalized to 36B4. D, macrophage response to different levels of adiponectin in the culture media. *, p < 0.05 for APN 0 μg/ml treatment (n = 3 in each group). All results were normalized to 36B4. E, the mRNA levels of ROS-related genes under the treatment of APN or IL-4. *, p < 0.05 and **, p < 0.01 (n = 4 in each group). F, anti-inflammatory effects of APN and IL-4 against LPS-induced M1 phenotypic change in peritoneal macrophages. The mRNA levels of TNF-α, MCP-1, and iNOS in quantitative RT-PCR methods. **, p < 0.01 versus vehicle; #, p < 0.05 and ##, p < 0.01 versus LPS treatment (n = 4 in each group). All results were normalized to 36B4. Data are presented as mean ± S.E.
Because ROS production is linked to classical macrophage activation, a cytochrome c reduction assay was performed in the presence and absence of recombinant adiponectin. Adiponectin treatment attenuated ROS production in peritoneal macrophages from WT mice by 51.1 ± 10.2% (p < 0.01). Consistent with attenuated ROS reduction by adiponectin treatment, mRNA levels of the NADPH oxidase components P47phox, P22phox, P40phox, and P67phox were lower in adiponectin-treated peritoneal macrophages than those in vehicle-treated macrophages (Fig. 3E). Treatment of macrophages with IL-4 led to a similar reduction in the expression of these NADPH oxidase subunits. Furthermore, transcript levels of the transcriptional factor PU.1, a positive transcriptional regulator of the NADPH oxidase subunits, was also lower in both adiponectin- and IL-4-treated peritoneal macrophages than those in vehicle-treated macrophages (Fig. 3E).
To investigate the effects of adiponectin on expression of M1 markers in macrophages in response to inflammatory stimuli, we assessed mRNA levels of TNF-α, iNOS, and MCP-1 in peritoneal macrophages pretreated with adiponectin or IL-4 followed by 4-h stimulation of LPS. Pretreatments of APN or IL-4 blunted LPS-stimulated expression of TNF-α, iNOS, and MCP-1 in peritoneal macrophages (Fig. 3F).
Adiponectin Promotes an Anti-inflammatory Phenotype in Alveolar Macrophages
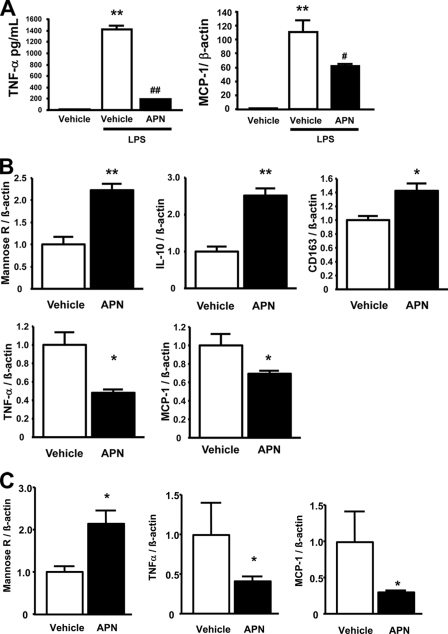
The anti-inflammatory action of adiponectin on macrophages was assessed in the MH-S murine alveolar macrophage cell line (Fig. 4A). Pretreatment with adiponectin suppressed LPS-induced expression of TNF-α protein and MCP-1 transcript levels. In contrast to findings in peritoneal macrophages and SVF cells, adiponectin did not induce arginase-1 expression in primary alveolar macrophages isolated from murine lung (supplemental Fig. 1) nor in an immortalized alveolar macrophage cell line (MH-S cells, data not shown).
FIGURE 4.
Adiponectin promotes an anti-inflammatory phenotype in human circulating monocyte-derived macrophages, SVF cells from human subcutaneous fat pads, and a murine alveolar macrophage cell line. A, anti-inflammatory effects of APN against LPS-induced M1 phenotypic change in MH-S cells, a murine alveolar macrophage cell line. TNF-α concentration in cell culture media as measured by enzyme-linked immunosorbent assay (R&D Systems). mRNA levels of MCP-1 by quantitative RT-PCR methods normalized to β-actin. **, p < 0.01 versus vehicle; #, p < 0.05 and ##, p < 0.01 versus LPS treatment (n = 3 in each group). B, the mRNA levels of anti-inflammatory M2 markers, mannose receptor (Mannose R), IL-10, and CD163 and inflammatory M1 markers, TNF-α and MCP-1 in quantitative RT-PCR methods. C, the mRNA levels of M2 marker, mannose receptor (mannose R), and M1 markers, TNF-α and MCP-1 in quantitative RT-PCR methods. All results were normalized to β-actin. *, p < 0.05; **, p < 0.01 versus vehicle (n = 4 in each group). Data are presented as mean ± S.E.
Adiponectin Promotes an Anti- inflammatory Phenotype in Human Monocyte-derived Macrophages and SVF Cells from Subcutaneous Fat
To corroborate the observation of adiponectin-induced macrophage polarization in mouse cells, the effects of recombinant adiponectin on M1/M2 markers in human monocyte-derived macrophages was also evaluated. Recombinant adiponectin treatment increased mRNA levels of the human M2 markers mannose receptor, IL-10, and CD163 (Fig. 4B). Adiponectin also decreased the proinflammatory M1 markers TNF-α and MCP-1. Finally, SVF cells were isolated from human subcutaneous fat and evaluated for their response to adiponectin. Recombinant adiponectin treatment increased mannose receptor transcript expression in human SVF cells and attenuated levels of TNF-α and MCP-1 (Fig. 4C).
DISCUSSION
In the present study, we have shown that peritoneal macrophages and SVF cells from adiponectin-deficient mice display an activated phenotype that is reminiscent of classically activated M1 cells. Conversely, adenovirus-mediated overexpression of adiponectin augments arginase-1 expression in peritoneal macrophages and SVF cells in WT as well as APN-KO mice. It is also shown that adiponectin can directly act on cultured macrophages to promote the expression of markers of the alternatively activated state. This direct activation of M2 markers and suppression of M1 markers was documented in macrophages isolated from circulation (human), peritoneal cavity (murine), or adipose tissue (human and murine).
Studies using lung macrophages suggest that these anti-inflammatory actions of adiponectin can be generalized to other tissue compartments. Phenotypically activated macrophages were increased in lungs of adiponectin-deficient mice, and APN effectively abrogated the response of cultured lung macrophages to LPS. In contrast, APN did not increase the expression of arginase-1 in lung macrophages. These findings are, however, consistent with published reports (42, 43) demonstrating that lung macrophages are phenotypically and functionally distinct from other macrophage populations. In this regard, the expression of arginase-1 must be tightly regulated in lung because its expression is linked to various pathological conditions, including asthma and pulmonary fibrosis (22, 23).
A number of reports have shown that adiponectin has beneficial effects on obesity-linked metabolic complications including insulin resistance (24, 19). Adiponectin also has protective actions on the heart (26, 27) and vasculature (28–30), and a deficiency in adiponectin will lead to an emphysema-like phenotype in lung (31) and ischemia-induced retinopathy (32). These pathological conditions all involve the activation of an acute or chronic inflammatory response. Thus, the ability of adiponectin to promote an alternative, anti-inflammatory phenotype in macrophages provides an attractive hypothesis to explain its protective effects in these diverse models of disease. These anti-inflammatory actions of adiponectin on macrophages, via an as-yet-unidentified mechanism, presumably augment its direct actions on tissues that are mediated by an up-regulation of AMP-activated protein kinase signaling (19, 28, 33).
It is generally assumed that adiponectin can regulate receptor-mediated intracellular signaling pathways in target tissues that control metabolic and cardiovascular protective pathways (19). However, there are a number of features about adiponectin that indicate that this cytokine functions as a modulator of immune function. For example, adiponectin accounts for 0.01% of the total plasma protein (10), whereas many growth factors and cytokines that interact with cell surface receptors to activate intracellular signaling cascades are present at levels that are lower by a factor of 1,000. Adiponectin is structurally similar to the collectin family of proteins, such as surfactant protein A, surfactant protein D, and C1q, which are abundantly expressed in the lung or circulation. Like adiponectin, these proteins possess a globular head and a collagenous tail, and they form stable trimers that undergo multimerization. The multimerization of collectin family proteins enables them to function as pattern recognition molecules and modulators of macrophage function (34). We have shown that adiponectin can regulate immune responses through its ability to promote the clearance of apoptotic cells by macrophages (35). In this process, adiponectin serves as a soluble bridge molecule between motifs on the surface of apoptotic cells and the phagocytic receptors on the surface of macrophages; a property that is also exhibited by collectin protein family members. Thus, the observation reported here, that adiponectin directly regulates macrophage phenotype, further supports the hypothesis that adiponectin functions as an important modulator of innate immunity.
Recent reports have shown that changes in T cell population in adipose tissue contribute to macrophage phenotype and inflammation in obese organisms (36–38). It has also been shown that cultured adipocytes produce the Th2 cytokines IL-13 and IL-4 (39). In the present study, we demonstrated that adiponectin is as effective as the Th2 cytokine IL-4 at conferring an anti-inflammatory phenotype to macrophages. The immune-regulatory properties of adiponectin are of interest in light of the notion that obesity leads to adipocyte death, which triggers the recruitment of macrophages (40). Thus, it is tempting to speculate that an impairment of macrophage phagocytic function, due in part to a reduction in adiponectin expression in obese organisms, can contribute to the inflammation of the fat pad. The lack of adiponectin may lead to the formation of “crown-like” structures of macrophages surrounding dead or dying adipocytes that is associated with obesity and diabetes (21, 40–41).
In summary, we show that adiponectin deficiency in mice leads to an activated macrophage phenotype. We also show that adiponectin acts on cultured murine and human macrophages to promote a switch to an anti-inflammatory phenotype. This regulatory feature may explain why low levels of adiponectin are linked with states of chronic inflammation. These data also indicate that the macrophage regulatory properties of adiponectin may significantly account for the protective actions of this cytokine in a variety of metabolic and cardiovascular disorders.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL81587, AG15052, and HL91949.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1, Fig. 1, and an additional reference.
- TNF-α
- tumor necrosis factor-α
- Ad-APN
- adenovirus-producing adiponectin
- IL
- interleukin
- APN-KO
- adiponectin knock-out
- iNOS
- inducible nitric-oxide synthase
- LPS
- lipopolysaccharide
- ROS
- reactive oxygen species
- SVF
- stromal vascular fraction
- WT
- wild-type
- PBS
- phosphate-buffered saline
- MCP
- monocyte chemoattractant protein-1.
REFERENCES
- 1.Shoelson S. E., Lee J., Goldfine A. B. (2006) J. Clin. Invest. 116, 1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neels J. G., Olefsky J. M. (2006) J. Clin. Invest. 116, 33–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., Chen H. (2003) J. Clin. Invest. 112, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon S. (2007) Eur. J. Immunol. 37, S9–S17 [DOI] [PubMed] [Google Scholar]
- 6.Odegaard J. I., Chawla A. (2008) Nat. Clin. Pract. Endocrinol. Metab. 4, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voll R. E., Herrmann M., Roth E. A., Stach C., Kalden J. R., Girkontaite I. (1997) Nature 390, 350–351 [DOI] [PubMed] [Google Scholar]
- 9.Savill J., Dransfield I., Gregory C., Haslett C. (2002) Nat. Rev. Immunol. 2, 965–975 [DOI] [PubMed] [Google Scholar]
- 10.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y. (1999) Biochem. Biophys. Res. Commun. 257, 79–83 [DOI] [PubMed] [Google Scholar]
- 11.Shapiro L., Scherer P. E. (1998) Curr. Biol. 8, 335–338 [DOI] [PubMed] [Google Scholar]
- 12.Cnop M., Havel P. J., Utzschneider K. M., Carr D. B., Sinha M. K., Boyko E. J., Retzlaff B. M., Knopp R. H., Brunzell J. D., Kahn S. E. (2003) Diabetologia 46, 459–469 [DOI] [PubMed] [Google Scholar]
- 13.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R. E., Tataranni P. A. (2001) J. Clin. Endocrinol. Metab. 86, 1930–1935 [DOI] [PubMed] [Google Scholar]
- 14.Ouchi N., Kihara S., Funahashi T., Nakamura T., Nishida M., Kumada M., Okamoto Y., Ohashi K., Nagaretani H., Kishida K., Nishizawa H., Maeda N., Kobayashi H., Hiraoka H., Matsuzawa Y. (2003) Circulation 107, 671–674 [DOI] [PubMed] [Google Scholar]
- 15.Engeli S., Feldpausch M., Gorzelniak K., Hartwig F., Heintze U., Janke J., Möhlig M., Pfeiffer A. F., Luft F. C., Sharma A. M. (2003) Diabetes 52, 942–947 [DOI] [PubMed] [Google Scholar]
- 16.Krakoff J., Funahashi T., Stehouwer C. D., Schalkwijk C. G., Tanaka S., Matsuzawa Y., Kobes S., Tataranni P. A., Hanson R. L., Knowler W. C., Lindsay R. S. (2003) Diabetes Care 26, 1745–1751 [DOI] [PubMed] [Google Scholar]
- 17.Esposito K., Pontillo A., Di Palo C., Giugliano G., Masella M., Marfella R., Giugliano D. (2003) Jama 289, 1799–1804 [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N., Kihara S., Arita Y., Nishida M., Matsuyama A., Okamoto Y., Ishigami M., Kuriyama H., Kishida K., Nishizawa H., Hotta K., Muraguchi M., Ohmoto Y., Yamashita S., Funahashi T., Matsuzawa Y. (2001) Circulation 103, 1057–1063 [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. (2006) J. Clin. Invest. 116, 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumada M., Kihara S., Ouchi N., Kobayashi H., Okamoto Y., Ohashi K., Maeda K., Nagaretani H., Kishida K., Maeda N., Nagasawa A., Funahashi T., Matsuzawa Y. (2004) Circulation 109, 2046–2049 [DOI] [PubMed] [Google Scholar]
- 21.Apovian C. M., Bigornia S., Mott M., Meyers M. R., Ulloor J., Gagua M., McDonnell M., Hess D., Joseph L., Gokce N. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1654–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North M. L., Khanna N., Marsden P. A., Grasemann H., Scott J. A. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 296, L911–L920 [DOI] [PubMed] [Google Scholar]
- 23.Endo M., Oyadomari S., Terasaki Y., Takeya M., Suga M., Mori M., Gotoh T. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 285, L313–L321 [DOI] [PubMed] [Google Scholar]
- 24.Scherer P. E. (2006) Diabetes 55, 1537–1545 [DOI] [PubMed] [Google Scholar]
- 25.Summer R., Fitzsimmons K., Dwyer D., Murphy J., Fine A. (2007) Am. J. Respir. Cell Mol. Biol. 37, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimentel D. R., Kumada M., Sato K., Schiekofer S., Ohashi K., Funahashi T., Colucci W. S., Walsh K. (2004) Nat. Med. 10, 1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata R., Sato K., Pimentel D. R., Takemura Y., Kihara S., Ohashi K., Funahashi T., Ouchi N., Walsh K. (2005) Nat. Med. 11, 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 29.Takaoka M., Nagata D., Kihara S., Shimomura I., Kimura Y., Tabata Y., Saito Y., Nagai R., Sata M. (2009) Circ. Res. 105, 906–911 [DOI] [PubMed] [Google Scholar]
- 30.Okamoto Y., Folco E. J., Minami M., Wara A. K., Feinberg M. W., Sukhova G. K., Colvin R. A., Kihara S., Funahashi T., Luster A. D., Libby P. (2008) Circ. Res. 102, 218–225 [DOI] [PubMed] [Google Scholar]
- 31.Summer R., Little F. F., Ouchi N., Takemura Y., Aprahamian T., Dwyer D., Fitzsimmons K., Suki B., Parameswaran H., Fine A., Walsh K. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1035–L1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higuchi A., Ohashi K., Kihara S., Walsh K., Ouchi N. (2009) Circ. Res. 104, 1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi K., Ouchi N., Sato K., Higuchi A., Ishikawa T. O., Herschman H. R., Kihara S., Walsh K. (2009) Mol. Cell Biol. 13, 3487–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeannin P., Jaillon S., Delneste Y. (2008) Curr. Opin. Immunol. 20, 530–537 [DOI] [PubMed] [Google Scholar]
- 35.Takemura Y., Ouchi N., Shibata R., Aprahamian T., Kirber M. T., Summer R. S., Kihara S., Walsh K. (2007) J. Clin. Invest. 117, 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) Nat. Med. 15, 914–920 [DOI] [PubMed] [Google Scholar]
- 37.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F., Maezawa Y., Drucker D. J., Engleman E., Winer D., Dosch H. M. (2009) Nat. Med. 15, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A. B., Benoist C., Shoelson S., Mathis D. (2009) Nat. Med. 15, 930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang K., Reilly S. M., Karabacak V., Gangl M. R., Fitzgerald K., Hatano B., Lee C. H. (2008) Cell Metab. 7, 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A. S., Obin M. S. (2005) J. Lipid Res. 46, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 41.Murano I., Barbatelli G., Parisani V., Latini C., Muzzonigro G., Castellucci M., Cinti S. (2008) J. Lipid Res. 49, 1562–1568 [DOI] [PubMed] [Google Scholar]
- 42.Murphy J., Summer R., Wilson A. A., Kotton D. N., Fine A. (2008) Am. J. Cell. Mol. Biol. 38, 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guth A. M., Janssen W. J., Bosio C. M., Crouch E. C., Henson P. M., Dow S. W. (2009) Am. J. Physiol. Lung Cell. Mol. Physiol. 396, L936–L946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.