Abstract
Stable complexes among G proteins and effectors are an emerging concept in cell signaling. The prototypical Gβγ effector G protein-activated K+ channel (GIRK; Kir3) physically interacts with Gβγ but also with Gαi/o. Whether and how Gαi/o subunits regulate GIRK in vivo is unclear. We studied triple interactions among GIRK subunits 1 and 2, Gαi3 and Gβγ. We used in vitro protein interaction assays and in vivo intramolecular Förster resonance energy transfer (i-FRET) between fluorophores attached to N and C termini of either GIRK1 or GIRK2 subunit. We demonstrate, for the first time, that Gβγ and Gαi3 distinctly and interdependently alter the conformational states of the heterotetrameric GIRK1/2 channel. Biochemical experiments show that Gβγ greatly enhances the binding of GIRK1 subunit to Gαi3GDP and, unexpectedly, to Gαi3GTP. i-FRET showed that both Gαi3 and Gβγ induced distinct conformational changes in GIRK1 and GIRK2. Moreover, GIRK1 and GIRK2 subunits assumed unique, distinct conformations when coexpressed with a “constitutively active” Gαi3 mutant and Gβγ together. These conformations differ from those assumed by GIRK1 or GIRK2 after separate coexpression of either Gαi3 or Gβγ. Both biochemical and i-FRET data suggest that GIRK acts as the nucleator of the GIRK-Gα-Gβγ signaling complex and mediates allosteric interactions between GαiGTP and Gβγ. Our findings imply that Gαi/o and the Gαiβγ heterotrimer can regulate a Gβγ effector both before and after activation by neurotransmitters.
Keywords: Fluorescence Resonance Energy Transfer (FRET), G Proteins, Heterotrimeric G Proteins, Ion Channels, Potassium Channels
Introduction
It is believed that signaling via G protein-coupled receptors (GPCRs)5 occurs within multiprotein complexes that include GPCRs, G proteins, and effectors (1–3). The G protein-activated K+ channel (GIRK, Kir3), an important mediator of neuronal inhibition (4), is activated by the binding of Gβγ to the cytosolic N and C termini (NT and CT, respectively) of GIRK. Gβγ associates with the channel before and after receptor activation (5, 6). GIRK NT and CT segments also bind Gαi/o (see Ref. 7), but it is not clear whether and how Gαi/o regulates GIRK in vivo. Gα clearly plays a role in determining specificity of signaling from GPCR to GIRK (8, 9). In heterologous systems, expression of Gαi reduces GIRK basal activity (Ibasal) and increases the relative extent of activation by added or coexpressed Gβγ (10–12). Recently, we have demonstrated that this regulation is exerted by the GDP-bound form of Gαi, GαiGDP (12); no role for GαiGTP in GIRK gating could be assigned so far. We proposed that regulation of GIRK by Gαi relies upon the formation of the GαiGDP-Gβγ heterotrimer, which forms a persistent, dynamic signaling complex with GIRK to ensure proper gating with low Ibasal and high signal-to-background ratio upon Gβγ activation (11, 12).
We hypothesized that within a multiprotein signaling complex, the partner proteins alter each other's conformation and activity at various stages of the signaling process. Thus, Gαi and Gβγ may regulate the conformation of the channel both before and after activation. We explored this hypothesis in vitro and in vivo using biochemical, functional, and intramolecular Förster resonance energy transfer (i-FRET) methods. Our data reveal interdependent triple interactions among Gαi3, Gβγ, and GIRK subunits, which correlate with distinct conformational and gating states of the GIRK channel.
EXPERIMENTAL PROCEDURES
Additional details on all methods are available in the supplemental methods.
cDNA Constructs and Electrophysiology
The cDNAs used in this study were obtained or prepared using standard PCR-based procedures. G1NC was constructed as described (12), with an 8-amino acid linker GSTASGST replacing the transmembrane segment (amino acids 85–184). Doubly labeled (DL)-GIRK1 and DL-GIRK2 were created by fusing CFP to the NT and YFP to the CT of the GIRK subunit, via linkers (see supplemental methods). Other cDNA constructs were as in Ref. 12. Xenopus oocytes were injected with RNAs, and whole-cell currents were measured using standard two-electrode voltage clamp procedures at 20–22 °C, in the ND96 (low K+) solution (96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm HEPES, pH 7.5) or in a high K+ solution (see Fig. 3, hK) (24 mm K+, isotonically replacing NaCl in ND96) as described (12) (see supplemental methods). Differences in current amplitude, resulting from variations in GIRK expression, were corrected to channel expression, determined by the quantitation of YFP fluorescence as described (12). All currents were normalized to the control group.
FIGURE 3.
GIRK1 and IRK1 functionality. Aa, representative DL-G1/G2 currents measured in Xenopus oocytes. Horizontal bars indicate the exchange of the external solutions. Shift from low K+ ND96 (ND) to 24 mm K+ (hK) revealed the inward Ibasal, and the addition of 10 μm ACh induced additional current, IACh. The addition of 5 mm Ba2+ inhibited all GIRK currents. Ab, current-voltage curve obtained using a voltage ramp from −120 to +50 mV. The curve demonstrates the typical mild inward rectification of the GIRK1 channel. B, summary of GIRK currents (five experiments, n = 13–25 oocytes/group) corrected for the DL-G1 PM expression and normalized to Ibasal of DL-G1/G2. Bars with bold margins represent groups with coexpressed Gβγ. Ca, schematic presentation of the tetrameric DL-IRK1 channel (CT and NT of only two out of four subunits are shown). Cb, current-voltage curve obtained using a voltage ramp from −120 to +20 mV. Results are shown as mean ± S.E. *, p < 0.05, as compared with the control group.
Pulldown Assay
GST-fused Gαi3 and its mutants were purified as described (13). [35S]Methionine-labeled segments of GIRK1 (G1NC (G1N1–84C183–501); G1NYFP (YFP-N1–84); G1C (C184–501); G1C363–501 (C363–501)) were synthesized in rabbit reticulocyte lysate. Interaction among GST-fused Gαi3 or its mutants with the in vitro translated (ivt), [35S]methionine-labeled segments of GIRK1 was studied by pulldown on glutathione affinity beads with either GDP or GTPγS (see supplemental methods). The eluted proteins were separated on 12% polyacrylamide-SDS gels. The radioactive signals from protein bands of the gels were imaged and quantitated using PhosphorImager and the software ImageQuant (GE Healthcare). Western blots were performed using Gβ antibody (Santa Cruz Biotechnology) and ECL reagents from Pierce.
Imaging and Spectral FRET Analysis
Fluorescent signals were collected with the Zeiss 510 META confocal microscope and analyzed as described (14), with modifications (see supplemental methods). Briefly, two spectra were collected from the animal hemisphere of each oocyte, with 405-nm (CFP excitation) and 514-nm (YFP excitation) laser lines. Net FRET signal of the CFP/YFP-labeled channels was calculated in the YFP emission range (with the 405 nm excitation) by consecutive subtraction of a scaled CFP-only spectrum (giving the A ratio parameter) and then of the ratio A0, which reports the direct excitation of YFP by the 405-nm laser. Therefore,
 |
where
 |
Then
 |
Dynamic i-FRET
Ionic currents were recorded in ND96 solution at −80 mV (see supplemental methods). Oocytes were repetitively excited with the 405-nm laser every 40 s. An ∼150 × 100-μm region of the membrane area was imaged. The CFP and YFP fluorescence was collected using 470–500- and 505–550-nm band pass filters, respectively, and background signals were subtracted. YFP and CFP intensities at each point were normalized to an initial four measurements in each cell. This configuration allows leakage of CFP into the YFP recording window of ∼0.30. Because this leakage is purely optical and constant irrespective of the FRET changes, we did not correct for it (15). The ratio of normalized intensities was denoted FYFP/FCFP (16). Some photobleaching occurred during repetitive excitations, but it had negligible effects on the FYFP/FCFP (see Fig. 4). Cells showing >15% bleaching of YFP or CFP were discarded.
FIGURE 4.
i-FRET reveals distinct G protein-induced conformational changes in GIRK1 and GIRK2 but not in IRK1. The experiments were performed on heterotetrameric G1/G2 channels containing either DL-G1 (A) or DL-G2 (B) and homotetrameric IRK1 (C). For illustration, one double-labeled subunit (within the tetrameric channel) is depicted above each graph. A, summary of five independent experiments showing changes in i-FRET in DL-G1/G2 caused by Gαi3GA, Gαi3QL, Gβγ, and m-phosducin (+m-phosd). In each experiment, the (A − A0) parameter in each oocyte was normalized to the mean (A − A0) of the DL-GIRK1 (control) group. (A0 denotes the average A0 measured from more than seven oocytes in the same experiment.) B, summary of three independent experiments showing changes in i-FRET in G1/DL-G2 caused by Gαi3GA, Gαi3QL and Gβγ. C, summary of two experiments showing i-FRET in IRK1. Note: The G protein subunits or m-phosducin do not induce any changes in the i-FRET of IRK1. n.s., not significant. The number of oocytes is shown within bars. Statistical comparisons of multiple groups were done using one-way analysis of variance followed by pairwise Student-Newman-Keuls test. Results are shown as mean ± S.E. *, #, p < 0.05, **, ##, p < 0.01. n.s.: non-significant.
Statistical Analysis
Results are shown as mean ± S.E. (see also supplemental methods). Asterisks or pound signs in Figs. 1, 3, and 4 indicate statistically significant differences as follows: * or #, p < 0.05; ** or ##, p < 0.01; *** or ###, p < 0.001, as compared with the control group, unless specified differently.
FIGURE 1.
Gβγ enhances the interaction between Gαi3 and GIRK1. A, schematic depiction of the ivt synthesized GIRK1 fragments. B, pulldown of 35S-labeled ivt proteins by GST-Gαi3 in the presence of GDP or GTPγS, with or without Gβγ. Autoradiograms of one-sixtieth of the total loaded protein (upper panel, Input) and of the bound protein (lower panel, Binding) are shown. C, summary of binding of the ivt synthesized GIRK1 proteins (normalized to that of G1NC to Gαi3GDP) to GST-Gαi3, in the presence of either GDP or GTPγS, with or without the addition of 3 μg of purified Gβ1γ2. D, the interaction of Gαi3GA or Gαi3QL with purified Gβ1γ2, measured by Western blots as in E, in the presence of GDP or GTPγS. E, the interaction of G1NC with Gαi3GA or Gαi3QL, in the presence of either GDP or GTPγS, with or without Gβγ. The upper panel shows the summary of all experiments. Below the summary is a representative autoradiogram of pulled-down G1NC, and the bottommost panel shows Western blot of bound Gβ. In D and E, in each experiment, binding of Gβγ or G1NC was normalized to that measured with Gαi3QLGDP. Results are shown as mean ± S.E. *, p < 0.05; **, p < 0.01, as compared with the control group.
RESULTS
GIRK-Gα-Gβγ Triple Interactions in Vitro
We constructed a cDNA encoding the complete cytosolic domain of GIRK1, G1NC, in which the NT and CT were fused whereas the transmembrane region was replaced by a short linker (Fig. 1A), to test its interaction with the G proteins. Similar constructs were previously shown to have a strong propensity to form stable tetramers in solution (17, 18). The ivt 35S-labeled G1NC gave a single protein band on SDS gel, as expected (Fig. 1B). Moreover, G1NC bound purified Gβ1γ2 (12). We have recently reported (12) that Gβγ enhances the interaction of Gαi3GDP with the G1NC. Here, we further characterized the interaction of GIRK1 with Gαi3GDP and also with Gαi3GTP. We similarly produced ivt CT of GIRK1 (G1C), the distal half of the CT (G1C363–501) and YFP-fused NT of GIRK1, G1NYFP (Fig. 1A). A GST-fused Gαi3 bound ivt G1NC as well as separate G1C and G1NYFP, whereas YFP or the distal CT (G1C363–501) did not show detectable binding (Fig. 1, B and C). The latter observations confirm results obtained in a reciprocal configuration, with GST-fused channel parts and ivt Gαi3 (9).
The addition of purified Gβ1γ2 had no effect on the binding of GST-Gαi3 to separate GIRK1 N and C termini, in the presence of either GDP or GTPγS (Fig. 1B, summary in panel C). In contrast, the binding of G1NC to GST-Gαi3GDP was enhanced ∼9-fold by the addition of Gβ1γ2, corroborating the previous report (12). Unexpectedly, Gβγ also strongly enhanced the binding of G1NC to GST-Gαi3 in the presence of GTPγS (Fig. 1, B and C), a condition where GST-Gαi3 poorly binds Gβ1γ2 (supplemental Fig. S1). To alleviate the concern that a residual amount of Gαi3βγ heterotrimers remaining in GTPγS could bias the results, we constructed GST-fused versions of two well characterized Gαi3 mutants that mimic Gαi3GTP and Gαi3GDP, respectively: constitutively active Gαi3Q204L (Gαi3QL) and constitutively inactive Gαi3G203A (Gαi3GA) (see Ref. 12). GST-Gαi3GA showed the expected strong interaction with Gβ1γ2 in GDP and much less in GTPγS, as reported (19). GST-Gαi3QL showed some interaction with Gβ1γ2 in GDP (see also Ref. 20) but not in GTPγS (Fig. 1D, example in panel E).
Both GST-Gαi3GA and GST-Gαi3QL bound G1NC in the absence of Gβγ (Fig. 1E). Gβγ enhanced the binding of both Gαi3GA and Gαi3QL to G1NC, in GDP as well as in GTPγS, despite the great differences in their interaction with Gβγ (Fig. 1E). Thus, GIRK1 likely binds the active Gαi3 (Gαi3GTPγS and Gαi3QL) directly and not through Gβγ; by binding to GIRK1, Gβγ enhances this interaction.
G Protein Subunits Induce Non-identical Conformational Rearrangements in GIRK1 and GIRK2
i-FRET was previously used to detect conformational changes in membrane proteins (16). We created a doubly labeled GIRK1 subunit (DL-G1) by fusing CFP (the donor) and YFP (the acceptor) to the extremes of NT and CT of GIRK1, respectively. DL-G1 was coexpressed with the neuronal GIRK2 subunit in Xenopus oocytes to give DL-G1/G2 channels, and visualized in the plasma membrane (PM) using a confocal microscope (Fig. 2). A linear relation of YFP to CFP fluorescence in DL-G1 confirmed the expected CFP/YFP molar ratio of 1:1 (supplemental Fig. S2C). We tested the functionality of all our tagged clones (Fig. 3) and found that DL-G1/G2 displayed similar function to wild-type G1/G2. The DL-G1/G2 channel had the characteristic basal activity (Ibasal), was readily activated by acetylcholine (ACh) via a coexpressed muscarinic m2 receptor (m2R) and blocked by Ba2+ (Fig. 3Aa). Moreover, DL-G1/G2 exhibited the typical inward rectification (Fig. 3Ab). Importantly, the regulation of the channel by coexpressed Gαi3GA, Gαi3QL and the Gβγ-scavenging protein m-phosducin, was also identical to that of the wild-type G1/G2 channel (Fig. 3B) (12). We found that Gαi3GA strongly reduced Ibasal and when coexpressed with Gβγ, Gαi3GA did not reduce the total Gβγ-dependent current, Iβγ (as also observed for the wild-type channel). Thus, Gαi3GA enhanced the relative activation by coexpressed Gβγ (Rβγ) (supplemental Fig. S3A). Gαi3QL affected neither Ibasal, Iβγ, nor Rβγ. m-Phosducin reduced Ibasal but, in sharp contrast to Gαi3GA, also substantially diminished Iβγ, acting as a typical Gβγ scavenger (Fig. 3B and supplemental Fig. S3A) (11, 12).
FIGURE 2.
Imaging DL-GIRK1/2 at PM and assessing i-FRET by spectral FRET technique. A, schematic presentation of the DL-G1/G2 channel (the cytosolic domains of GIRK2 are not shown). Bottom insets, color-coded images (averaged emission spectrum) of three oocytes expressing DL-G1/G2 obtained with 514 nm (panel a) and 405 nm excitation (panel b). Objective ×5, zoom 0.7. B, spectral FRET. Shown are four images of oocytes expressing: 1, m2R-YFP excited with 514 nm; 2, m2R-YFP excited with 405 nm (white arrow points to the PM of the oocyte); 3, m2R-CFP excited with 405 nm; 4, DL-G1 excited with 405 nm. Objective ×20, zoom 2. White bar = 50 μm. C, normalized emission spectra of m2R-CFP (blue trace, 3) and DL-G1/G2 (green trace, 4) and the FFRET subtracted spectrum (red trace, 4-3). Numbers in C and D match images in panel B. The dichroic mirror is marked by a light gray area. FL, fluorescent. D, ratio A (black) of DL-G1/G2 and ratio A0 (gray) plotted against the emission wavelengths. Results are shown as mean ± S.E.
We quantified the i-FRET signal using the spectral FRET technique (14) (Fig. 2C and supplemental Fig. S2E). Each oocyte was excited at two wavelengths, 405 and 514 nm, emission spectra were collected (Fig. 2, B and C, and supplemental Fig. S2E), and parameters characterizing bleed-through and energy transfer (ratios A0 and A, respectively) were calculated. The extent of FRET is proportional to (A − A0). Ratios A and A0 were linear over a broad range of wavelengths, a testimony to the absence of optical and calculation artifacts (14) (Fig. 2D).
We found a strong basal i-FRET signal ((A − A0) = 0.28 ± 0.02) in DL-GIRK1/2 (Figs. 2D and 4A), indicating proximity between the CFP and YFP moieties. Coexpression of Gβγ significantly increased i-FRET (by ∼17%; Fig. 4A) concomitantly with the increase in channel activity (Fig. 3B). Inversely, coexpression of Gαi3GA or m-phosducin markedly decreased both i-FRET (by up to 20%) and Ibasal. Thus, low i-FRET in DL-G1/G2 correlates with non-conductive conformation(s), and high i-FRET corresponds to Gβγ-activated, open state(s) of the channel.
Coexpression of Gβγ with Gαi3-GA restored the active state with large Iβγ and with high i-FRET. In contrast, coexpression of Gβγ did not reverse the m-phosducin-induced reduction in i-FRET and in Iβγ (Figs. 3B and 4A), supporting the differentiation of the actions of Gαi3GA from those of a simple Gβγ scavenger (11). Expression of Gαi3QL did not significantly alter the basal i-FRET and currents, but further coexpression of Gβγ led to a strong increase in i-FRET (∼30%), significantly greater than under all other conditions (Fig. 4A; see supplemental Fig. S4 for complete statistical analysis).
Unlike GIRK1, Gβγ does not enhance Gαi3-GIRK2 interaction, and Gαi3 does not regulate Ibasal of homotetrameric GIRK2 (12). To assess whether Gαi3 and Gβγ confer conformational changes upon GIRK2 within the GIRK1/2 heterotetramer, we constructed a doubly labeled GIRK2 (DL-G2) as in GIRK1 (see “Experimental Procedures”). DL-G2 was expressed with wild-type GIRK1 to produce G1/DL-G2 heterotetramers, which showed adequate regulation by Gαi3 and Gβγ (supplemental Fig. S3B) and showed basal i-FRET of 0.25 ± 0.02 (n = 44). Similarly to DL-G1, i-FRET of DL-G2 was reduced by Gαi3GA (by ∼20%), an effect reversed by Gβγ (Fig. 4B). However, other parameters of i-FRET in the G1/DL-G2 heterotetramer were differently affected by the coexpressed G proteins. Coexpression of Gβγ did not increase i-FRET above basal level either in the presence or in the absence of Gαi3. Most interestingly, concomitant expression of Gαi3QL with Gβγ significantly reduced the basal FRET signal (by ∼26%), opposite to its effect on DL-G1.
As a control, we engineered a doubly labeled IRK1 (Kir2.1) channel, DL-IRK1 (Fig. 3C and supplemental Fig. S5). This inwardly rectifying K+ channel does not directly interact with, and is not regulated by, G proteins (21). DL-IRK1 exhibited PM localization, constitutively active gating, the typical strong inward rectification (Fig. 3, C and D) and strong basal i-FRET ((A − A0) = 0.35 ± 0.01). Coexpression of Gβγ, Gαi3GA, Gαi3QL, or m-phosducin caused no significant changes in i-FRET (Fig. 4C) or in channel currents. These results confirm the specificity of G protein regulation of conformational states of GIRK1/2 by G protein subunits, revealed by i-FRET.
Dynamic Structural Rearrangements in GIRK1/2 upon GPCR Activation
The changes in i-FRET caused by the presence of G protein subunits probably reflect the conformations of the channel at different activation steps. To verify that these static measurements reflect the conformations adopted by the channel during its physiological activation process, we monitored dynamic changes in i-FRET caused by activation of a GPCR (m2R). In these experiments, the electrophysiological response (K+ current) and fluorescence signals were collected simultaneously from individual oocytes (Fig. 5A).
FIGURE 5.
GPCR activation induces specific dynamic changes in i-FRET of DL-G1/G2. A, scheme of the experimental setup, where oocyte is both repeatedly imaged and electrophysiologically monitored by the two electrode voltage clamp method. B, top trace, representative electrophysiological recording made in a low K+ solution. The shaded area denotes ACh application. The middle trace shows averaged intensities in CFP (blue) and YFP (yellow) windows (n = 14 oocytes from four donor frogs). The bottom trace shows average FYFP/FCFP. C–E, control experiments. Each subfigure shows current traces recorded in representative oocytes (top) and mean FYFP/FCFP (bottom). C, oocytes expressing DL-G1/G2 were briefly perfused with ACh to verify the expression of the channels and receptor and then with 30 μm atropine (n = 8). D, the ACh-induced current and changes in i-FRET are abolished in oocytes expressing DL-G1/G2, pretreated with pertussis toxin (+PTX, n = 6). E, oocytes expressing DL-IRK1 show no electrophysiological and i-FRET response to 10 μm ACh (n = 10).
Oocytes expressing DL-GIRK1/2, m2R, and the wild-type Gαi3 were voltage-clamped at −80 mV and continuously perfused with ND96 solution. Concomitantly, a region of the oocyte membrane was excited with the excitation wavelength of the donor (405 nm) every 40 s. The emission signals of both donor and acceptor were recorded separately, and the FYFP/FCFP ratio was calculated. This ratiometric imaging method allows the monitoring of changes in the relative position of donor and acceptor fluorophores. For instance, reduction of the distance between donor and acceptor increases the energy transfer, causing an increase in YFP emission intensity concurrently with a decrease in CFP emission intensity and resulting in an increase in FYFP/FCFP ratio (16).
Under the recording conditions used, DL-G1/G2 showed considerable basal activity (11), corresponding to a mixture of “preactivated” states (see “Discussion”). The addition of 10 μm ACh (Fig. 5B, gray area) caused additional activation of GIRK (Fig. 5B, top red trace) and a concomitant increase in FYFP/FCFP ratio (Fig. 5B). When ACh was washed from the system, the channel reassumed its basal, preactivated FYFP/FCFP ratio and basal activity. These dynamic changes match our static i-FRET data (see “Discussion”). No change in FYFP/FCFP was seen in control experiments, where oocytes were exposed to 30 μm atropine, which completely inhibited the ACh-evoked K+ current (Fig. 5C). Importantly, pretreatment with pertussis toxin (PTX) completely inhibited both ACh-evoked currents and the ACh-induced change in i-FRET (Fig. 5D). The use of atropine rules out the involvement of the receptor in the induction of the change in conformation of the GIRK, and the latter is most probably caused by the direct interaction of the G proteins themselves. Finally, ACh did not induce any ionic currents or changes in FYFP/FCFP in DL-IRK1 channel (Fig. 5E).
DISCUSSION
Gβγ Is Crucial for Strong Interaction between GIRK1 and Gαi
Our biochemical results strongly support the idea of a preformed signaling complex of heterotrimeric Gαiβγ with GIRK1, initially based upon the finding of a strong interaction of Gαi1GDPβγ with the NT of GIRK1 in vitro (5). Indeed, Gβγ greatly enhanced the interaction of GIRK1 with Gαi3GDP under conditions favoring the formation of Gαβγ heterotrimers (Fig. 1) (12). Somewhat at odds with Huang et al. (5), our data suggest that both NT and CT are necessary for the formation of the strong GIRK1-Gαiβγ complex as the effect of Gβγ was not present in separate N and C termini. Further quantitative binding studies may be needed to understand the reason for this discrepancy.
A novel and unexpected finding was that Gβγ also enhanced the interaction of GIRK1 with Gαi3 and the constitutively active Gαi3QL in GTPγS, a condition when no Gαi3QL-Gβγ interaction was detected by pulldown (supplemental Fig. S1). Therefore, we suggest that the conformational change in GIRK1 induced by the binding of Gβγ allosterically improves the direct binding of Gαi3GTP to GIRK1. Alternatively, GIRK1 may still bind Gαi3GTP via Gβγ, utilizing a second Gβγ-binding site on Gαi3, distinct from the classical high affinity interaction site (22). In all, the biochemical data support the persistence of GIRK-Gαi/o-Gβγ signaling complexes, both before and after activation by agonist-bound GPCR. The continuous attachment of Gαi to GIRK1/2 would ensure a high local concentration of Gα and a diffusion-independent reassociation with Gβγ, which allows for fast termination of the physiological response upon removal of the agonist because of the fast kinetics of GαGDP-Gβγ binding (23).
i-FRET and GIRK Conformation
The available partial crystal structures of GIRK tetramers indicate proximity between the NT of one subunit to the mid-CT of an adjacent subunit (17, 18, 24). In a tetrameric DL-G1/G2 channel, i-FRET could potentially arise from proximity of CFP-NT of one DL-G1 subunit to the CT-YFP of an adjacent DL-G1. This would require a 1-1-2-2 arrangement of subunits in the tetramer, which is probably viable, by analogy with 1-1-4-4 in GIRK1/GIRK4, although 1-4-1-4 is preferable (25, 26). However, it is important to note that the proximal NTs (∼40 amino acids) and the distal CT (∼130 amino acids in GIRK1, amino acids 370–501; ∼45 amino acids in GIRK2) are omitted from the above crystal structures. The unique distal CT segment of GIRK1 is essential for the strong triple GIRK-Gαi-Gβγ interaction and for the differential regulation of GIRK1 and GIRK2 by Gαi and Gβγ (12). The flexible proximal NT and distal CT are probably long enough to allow for intrasubunit interactions. Such intrasubunit contacts were proposed to play an essential role in GIRK gating by Gβγ (27). Therefore, we interpret an increase in i-FRET as nearing of the NT and CT of either the same or adjacent GIRK subunits. The exact structural rearrangements remain to be determined. However, it is certain that changes in i-FRET reflect G protein-induced changes in conformation of the channel (Figs. 3C and 5B).
G Protein-regulated Conformational States of the GIRK Channel
Measurements of single-channel or population currents in native and chimeric GIRK channels demonstrated that heterotetrameric GIRK channels assume a number of closed and open conformations with a variable number of Gβγ molecules bound, from a closed, non-conducting state (interpreted as Gβγ-devoid), via intermediate states with one, two, or three Gβγ molecules bound and correspondingly increasing open probability (Po), to a fully Gβγ-activated state with four Gβγ bound and the highest Po (27–30). Analysis of i-FRET, when combined with biochemical and functional assays, extends our understanding of the rules and modes of G protein-GIRK interactions and of the regulation of channel conformation. A simplified schematic of some of the interactions and conformations is presented in Fig. 6.
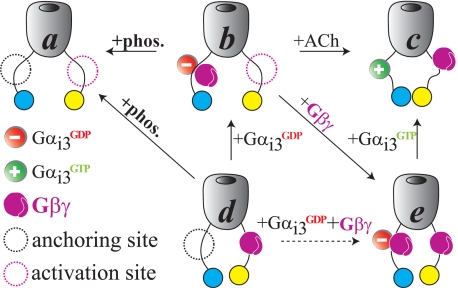
FIGURE 6.
The two-site model of GIRK regulation by Gαi and Gβγ. For simplicity, the GIRK channel is shown as if composed of only one GIRK1 subunit. The locations of anchoring and activation sites are shown arbitrarily. Distances between NT and CT are depicted schematically, where high i-FRET is interpreted to indicate proximity between the N and C termini (of the same or of adjacent subunits). Inversely, low i-FRET correlates with distancing of the termini. +phos., m-phosducin.
Preactivated (resting) states of GIRK1/2 probably reflect a mixture of conformations of GIRK1/2 channels, associated with a variable number of Gαiβγ heterotrimers and/or Gβγ that lack matching GαiGDP (Fig. 6, b and d), as suggested by functional data (11). Correspondingly, DL-G1/G2 and G1/DL- G2 expressed alone at high levels yield high Ibasal (10) and an intermediate i-FRET signal. A substantial basal GIRK activity has been reported in hippocampal and cortical neurons (31, 32); therefore, the preactivated states are physiologically relevant.
Overexpression of high doses of the G protein subunits or Gβγ scavengers shifts the channel population toward more homogenous states, some of which are detected by our i-FRET assay. 1) The first is Gβγ-free state, with low Ibasal (Fig. 6a). This state is promoted by m-phosducin, which removes Gβγ away from the channel (6, 11). It is characterized by low i-FRET and is depicted in Fig. 6 as having a large distance between NT and CT of GIRK1. 2) The second is Gβγ-activated state(s), with high i-FRET and GIRK currents, presumably with several (up to four in the full tetramer) Gβγ dimers bound at activation sites (Fig. 6, d and e). 3) Joint expression of Gβγ and Gαi3QL confers a conformation (Fig. 6c) characterized by the highest i-FRET in GIRK1 but the lowest i-FRET in GIRK2 (Fig. 3G). These opposite changes in i-FRET further point toward a difference in regulation of GIRK1 and GIRK2 subunits by Gαi and Gβγ (12). Neither GαiQL nor Gβγ alone confer such changes in i-FRET, indicating concomitant binding of Gαi3GTP and Gβγ to the heterotetrameric GIRK1/2.
Cells overexpressing Gβγ, Gβγ+GαiGA, or Gβγ+GαiQL show high constitutive, non-desensitizing GIRK currents (11, 12), clearly reporting the open states of the channel. Despite the different relative positions of GIRK N and C termini, these various open state(s) give similar whole-cell currents (but it remains to be seen whether they have distinct single-channel properties). The overall conformation of the channel in the presence of ACh should resemble that seen in static FRET experiments upon coexpression of Gβγ or, even more closely, Gβγ+Gαi3QL. Accordingly, dynamic activation of GIRK by ACh via coexpressed m2R caused an increase in both GIRK current and i-FRET in DL-G1/G2. The increase in i-FRET most probably corresponds to the opening of the channel because GIRK channels expressed in Xenopus oocytes show little ACh-induced desensitization over time periods of several minutes (33). Control experiments showed no changes in i-FRET upon exposure to the muscarinic antagonist atropine and after treatment with pertussis toxin, which prevents the activation of Gαi by GPCRs. Finally, no G protein, or GPCR, changes in i-FRET were observed in the double-labeled Gβγ-insensitive IRK1 channel. These control experiments demonstrate the authenticity and specificity of i-FRET changes caused by Gαi and Gβγ in GIRK1/2.
The low i-FRET and small GIRK currents observed after the expression of Gαi3GA are indistinguishable from those seen in Gβγ-free channels in the presence of m-phosducin, as if Gαi3GA removed Gβγ away from the channel. However, given the compelling evidence for a strong triple association between GIRK1, GαiGDP and Gβγ (Fig. 1) (5–7, 34), we hypothesize that the Gαi3GA-Gβγ heterotrimers, formed after expression of GαiGA, remain mostly associated with GIRK1/2. In support, we observe specific differences in the effects of Gαi3GA (but not the QL mutant) on i-FRET and the total GIRK current when coexpressed with Gβγ. This corroborates the hypothesis that it is the GDP-bound Gαi (or Gαiβγ heterotrimer) that regulates the basal activity of GIRK and “primes” the channel for activation by Gβγ (11, 12).
The new biochemical and i-FRET results, as well as previous functional observations, are compatible with a “two-site model” in which GIRK possesses an anchoring site for Gαi/o (and/or Gαi/oβγ) and a separate activation site, where the binding of Gβγ leads to channel opening (12) (Fig. 6). The assumption of separate binding sites for Gαi (or Gαβγ) and Gβγ is supported, although not proved, by the absence of competition among Gαi and Gβγ for binding to G1NC and G2NC under conditions that favor either the formation or the dissociation of Gαiβγ heterotrimers and by the more-than-additive character of i-FRET changes induced by GαiQL and Gβγ. The two sites may lie in proximity (or even partly overlap). According to the two-site model, the high basal activity of overexpressed GIRK1/2 is due to an excess of Gβγ associated with the channel, over Gαi (11, 12, 35). Under these conditions, some channels have Gβγ “uncompensated” by Gα and thus bound to the activation sites (Fig. 6d). The coexpressed Gαi3GA associates with Gβγ subunits, obstructing their interaction with the activation sites (hence the decrease in Ibasal and i-FRET). The newly formed Gαi3βγ heterotrimers are docked at the anchoring site (Fig. 6b). When more Gβγ is coexpressed, free Gβγ subunits bind at the activation sites, hence the high i-FRET and currents (Fig. 6e). The two-site hypothesis provides an economical description of a normal physiological situation; upon activation of GPCR, Gβγ detaches from Gαi and interacts with the activation site, whereas GαiGTP stays at the anchoring site (Fig. 6c). (Alternatively, Gβγ may stay at the same site, and it is Gαi that shifts to another location. Also, Gαi and Gβγ do not have to fully disengage (16, 36); a rearrangement followed by an exposure of a few crucial residues on Gβγ may be sufficient). Once GTP is hydrolyzed by Gα, Gβγ rebinds Gα and becomes docked again.
Conclusions
Coordinated biochemical, functional, and i-FRET data support the existence of a persistent, dynamic GIRK1/2-Gαi/o-Gβγ complex, both before and after activation by agonist-bound GPCRs. We propose that GIRK1 acts as the nucleator of the complex and that both active and inactive Gαi3 remain bound to the channel, ensuring fast and specific activation and termination of the signal. New in vitro and in vivo data reveal intricate triple interactions among GIRK1, Gαi3, and Gβγ and show that the presence of Gβγ is crucial to ensure strong GIRK1-Gαi interactions. At least some of the effects of Gβγ on GIRK1-Gαi interaction appear to be allosteric. Gαi and Gβγ induce mutually dependent conformational rearrangements in GIRK1/2, characterized by distinct changes in i-FRET in the GIRK subunits and often, but not always, by changes in channel activity.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM68493 (to N. D.) and GM60419 (to C. W. D.). This work was also supported by the United States Israel Binational Science Foundation Grant 01-122 (to N. D. and C. W. D.), Israel Science Foundation Grants 49/08 and 1396/05 (to N. D.), and a Segol Fellowship (to M. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental methods and supplemental Figs. S1–S5.
- GPCR
- G protein-coupled receptor
- GIRK
- G protein-activated K+ channel
- ivt
- in vitro translated
- CT
- C terminus
- NT
- N terminus
- GST
- glutathione S-transferase
- i-FRET
- intramolecular Förster resonance energy transfer
- CFP
- cerulean fluorescent protein
- YFP
- yellow fluorescent protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- ACh
- acetylcholine
- m2R
- m2 receptor
- DL
- doubly labeled
- PM
- plasma membrane.
REFERENCES
- 1.Dupré D. J., Robitaille M., Rebois R. V., Hébert T. E. (2009) Annu. Rev. Pharmacol. Toxicol. 49, 31–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doupnik C. A. (2008) J. Recept. Signal Transduct. Res. 28, 83–91 [DOI] [PubMed] [Google Scholar]
- 3.Dowal L., Provitera P., Scarlata S. (2006) J. Biol. Chem. 281, 23999–24014 [DOI] [PubMed] [Google Scholar]
- 4.Yamada M., Inanobe A., Kurachi Y. (1998) Pharmacol. Rev. 50, 723–760 [PubMed] [Google Scholar]
- 5.Huang C. L., Slesinger P. A., Casey P. J., Jan Y. N., Jan L. Y. (1995) Neuron 15, 1133–1143 [DOI] [PubMed] [Google Scholar]
- 6.Riven I., Iwanir S., Reuveny E. (2006) Neuron 51, 561–573 [DOI] [PubMed] [Google Scholar]
- 7.Clancy S. M., Fowler C. E., Finley M., Suen K. F., Arrabit C., Berton F., Kosaza T., Casey P. J., Slesinger P. A. (2005) Mol. Cell. Neurosci. 28, 375–389 [DOI] [PubMed] [Google Scholar]
- 8.Leaney J. L., Tinker A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5651–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanina T., Varon D., Peleg S., Rishal I., Porozov Y., Dessauer C. W., Keren-Raifman T., Dascal N. (2004) J. Biol. Chem. 279, 17260–17268 [DOI] [PubMed] [Google Scholar]
- 10.Peleg S., Varon D., Ivanina T., Dessauer C. W., Dascal N. (2002) Neuron 33, 87–99 [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein M., Peleg S., Berlin S., Brass D., Dascal N. (2007) J. Physiol. 581, 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinstein M., Peleg S., Berlin S., Brass D., Keren-Raifman T., Dessauer C. W., Ivanina T., Dascal N. (2009) J. Physiol. 587, 3473–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rishal I., Keren-Raifman T., Yakubovich D., Ivanina T., Dessauer C. W., Slepak V. Z., Dascal N. (2003) J. Biol. Chem. 278, 3840–3845 [DOI] [PubMed] [Google Scholar]
- 14.Zheng J., Varnum M. D., Zagotta W. N. (2003) J. Neurosci. 23, 8167–8175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto K., Nagai T., Miyawaki A., Hayashi Y. (2004) Nat. Neurosci. 7, 1104–1112 [DOI] [PubMed] [Google Scholar]
- 16.Vilardaga J. P., Bünemann M., Feinstein T. N., Lambert N., Nikolaev V. O., Engelhardt S., Lohse M. J., Hoffmann C. (2009) Mol. Endocrinol. 23, 590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida M., MacKinnon R. (2002) Cell 111, 957–965 [DOI] [PubMed] [Google Scholar]
- 18.Pegan S., Arrabit C., Zhou W., Kwiatkowski W., Collins A., Slesinger P. A., Choe S. (2005) Nat. Neurosci. 8, 279–287 [DOI] [PubMed] [Google Scholar]
- 19.Ogier-Denis E., Houri J. J., Bauvy C., Codogno P. (1996) J. Biol. Chem. 271, 28593–28600 [DOI] [PubMed] [Google Scholar]
- 20.Majumdar S., Ramachandran S., Cerione R. A. (2006) J. Biol. Chem. 281, 9219–9226 [DOI] [PubMed] [Google Scholar]
- 21.Stanfield P. R., Nakajima S., Nakajima Y. (2002) Rev. Physiol. Biochem. Pharmacol. 145, 47–179 [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Sengupta P., Guo Y., Golebiewska U., Scarlata S. (2009) J. Biol. Chem. 284, 16906–16913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea L. D., Neubig R. R., Linderman J. J. (2000) Life Sci. 68, 647–658 [DOI] [PubMed] [Google Scholar]
- 24.Inanobe A., Matsuura T., Nakagawa A., Kurachi Y. (2007) Channels 1, 39–45 [PubMed] [Google Scholar]
- 25.Silverman S. K., Lester H. A., Dougherty D. A. (1996) J. Biol. Chem. 271, 30524–30528 [DOI] [PubMed] [Google Scholar]
- 26.Tucker S. J., Pessia M., Adelman J. P. (1996) Am. J. Physiol. 271, H379–385 [DOI] [PubMed] [Google Scholar]
- 27.Sadja R., Alagem N., Reuveny E. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10783–10788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada M., Jahangir A., Hosoya Y., Inanobe A., Katada T., Kurachi Y. (1993) J. Biol. Chem. 268, 24551–24554 [PubMed] [Google Scholar]
- 29.Ivanova-Nikolova T. T., Nikolov E. N., Hansen C., Robishaw J. D. (1998) J. Gen. Physiol. 112, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemec J., Wickman K., Clapham D. E. (1999) Biophys. J. 76, 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Johnston D. (2005) J. Neurosci. 25, 3787–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiser O., Qian X., Ehlers M., Ja W. W., Roberts R. W., Reuveny E., Jan Y. N., Jan L. Y. (2006) Neuron 50, 561–573 [DOI] [PubMed] [Google Scholar]
- 33.Vorobiov D., Levin G., Lotan I., Dascal N. (1998) Pflugers Arch. 436, 56–68 [DOI] [PubMed] [Google Scholar]
- 34.Rebois R. V., Robitaille M., Galés C., Dupré D. J., Baragli A., Trieu P., Ethier N., Bouvier M., Hébert T. E. (2006) J. Cell Sci. 119, 2807–2818 [DOI] [PubMed] [Google Scholar]
- 35.Rishal I., Porozov Y., Yakubovich D., Varon D., Dascal N. (2005) J. Biol. Chem. 280, 16685–16694 [DOI] [PubMed] [Google Scholar]
- 36.Galés C., Van Durm J. J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., Bouvier M. (2006) Nat. Struct. Mol. Biol. 13, 778–786 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.