Abstract
Protein kinases play an important role in the maintenance of homeostasis between cell survival and apoptosis. Deregulation of these kinases leads to various pathological manifestations, such as cancer and neurodegenerative diseases. The MST1 encodes a serine/threonine kinase that is activated upon apoptotic stimulation, which in turn phosphorylates its downstream targets, Histone H2B and FOXO. However, the upstream regulators of MST1 kinase have been poorly studied. In this study, we report that JNK (c-Jun N-terminal kinase) phosphorylates MST1 at serine 82, which leads to the enhancement of MST1 activation. Accordingly, the activation of MST1 phosphorylates FOXO3 at serine 207 and promotes cell death. The inhibition of JNK kinase per se attenuates MST1 activity and nuclear translocation as well as MST1-induced apoptosis. We also find the S82A (serine mutated to alanine) diminishes MST1 activation and its effect on the FOXO transcription activity. Collectively, these findings define the novel feedback regulation of MST1 kinase activation by its putative substrate, JNK, with implication for our understanding of the signaling mechanism during cell death.
Keywords: Apoptosis, Cell/Apoptosis, Phosphorylation/Kinases/Serine-Threonine, Phosphorylation/MAPs, Signal Transduction/Protein Kinases, Signal Transduction/Protein Kinases/MAP
Introduction
Mammalian sterile 20-like kinase 1 (MST1),4 a mammalian homolog of Drosophila Hippo, contains a Ste20-related kinase catalytic domain in the N-terminal region and a regulatory domain at the C terminus (1). In Drosophila, recent studies showed that Hippo restricts cell growth and cell proliferation and promotes cell death by interacting with Salvador (Sav) and Warts (Wts), which result in inhibition of transcription and/or degradation of cyclin E and DIAPs (2, 3) through phosphorylating Yorkie (4). The non-catalytic tail of MST1 has been shown to be cleaved by caspase-3 upon various apoptotic stimuli such as death receptor triggering by CD95/FasL and treatments with staurosporine (STS), ceramide, as well as heat shock and arsenite. The N terminus of MST1 translocates to the nucleus, where it promotes chromatin condensation and herein apoptosis (5–7). In addition, MST1 cleavage and induced apoptosis were also observed upon overexpression (8). Asp-326 and Asp-349 has been reported to be the two major cleavage sites, and the mutation of the cleavage sites attenuates its kinase activation, nuclear translocation, and ability to induce apoptosis (7, 9). In mammals, it has been shown that MST1 apparently activates JNK and p38MAPK kinase pathways through MKK4/MKK7 and MKK3/MKK6, respectively (6). Recently it was reported that activation of the JNK kinase pathway is essential and sufficient for MST1-mediated chromatin condensation and apoptosis (10), and the dominant-negative mutant of JNK, not dominant-negative p38 or the p38 inhibitor inhibited MST1-induced caspase activation and cell death (11).
Recently, the Allis group (8, 12) reported that MST1 induces apoptosis by phosphorylating histone H2B on a conserved site, serine 14 in mammalian cells and serine 10 in Saccharomyces cerevisiae. MST1 has also been implicated in the control of neuronal cell death via phosphorylating FOXO at serine 207 and inducing the FOXO-dependent transcription and cell death (13, 14). Recently threonine 183 in subdomain VIII of MST1 has been defined as a primary site for the phosphoactivation, and autophosphorylation of threonine 183 within the MST1 kinase domain is essential for kinase activation (15).
Raf-1, an inhibitory regulator for MST2, could prevent dimerization and recruit a phosphatase to MST2, but the inhibition is independent of Raf-1 kinase activity (16). Interestingly, EGF stimulation has been observed to cause a transient drop of MST1 kinase activity (17). However, the upstream signaling regulator(s) of MST/Hippo is still largely unknown. In this study, we demonstrate that MST1 is regulated by stress kinase JNK. JNK phosphorylates MST1 at serine 82, which leads to enhancement of MST1 cleavage and kinase activation as well as nuclear translocation. Consistent with these results, we also found that the inhibition of JNK kinase activity diminishes MST1-FOXO3 pro-apoptotic signaling. The S82A (serine to alanine mutation) form of MST1, which cannot be phosphorylated by JNK, confers a lower kinase activity and pro-apoptotic ability compared to that of wild-type MST1. Collectively, our findings suggest that JNK plays a critical role in the feedback regulation of the MST1 pro-apoptotic signaling cascade.
EXPERIMENTAL PROCEDURES
Reagents and Cell Culture
Staurosporine and anti-FLAG antibody (M2) were obtained from Sigma. Anti-GFP antibody, DMEM, and fetal bovine serum were purchased from Invitrogen (Grand Island, NY). Anti-phospho-JNK (p54/44), anti-phospho-Thr-183 MST1, and anti-Erk1/2 were obtained from Cell Signaling Technology (Beverly, MA). The JNK inhibitor SP600125 was from Calbiochem Co. (San Diego, CA). COS7, OV8, and 293T cells as well as several tumor cell lines were cultured at 37 °C and 5% CO2 in DMEM supplemented with 10% fetal bovine serum. The cells were seeded in 60-mm Petri dishes at a density of 0.5 × 106 cells per dish.
Expression Constructs
3×FLAG-tagged Mst1 constructs in the pCMV5 expression vector were constructed using the human fetal Marathon-Ready cDNA library (Clontech), and human MST1-specific primers are: cgcaagcttatggagacggtacagctgag (5′ primer) and cgctctagatgctcagaagttttgttg (3′ primer). Mst1 S82A was constructed by polymerase chain reaction mutagenesis. pSIREN-RetroQ-dsRed.siJNK-A and pSIREN-RetroQ-dsRed.siJNK-B were designed by selecting two target sequences, which are shared by different isoforms of both JNK1 and JNK2, GATCATGAAAGAATGTCCTACC and CATGAAAGAATGTCCTACCTTC. MST1 hpRNA: G GGC ACT GTC CGA GTA GCC AGC.
Immunoprecipitation and Immunoblotting
Cells were lysed in a buffer containing 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% (v/v) glycerol, 1% Nonidet P-40, 2 mm phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin and leupeptin, 2 mm benzamidine, 20 mm NaF, 10 mm NaPPi, 1 mm sodium vanadate, and 25 mm β-glycerophosphate. Lysates were centrifuged at 12,000 × g for 15 min at 4 °C prior to immunoprecipitation or Western blot. Aliquots of the cell lysates were analyzed for protein expression and enzyme activity. For immunoprecipitation, lysates were precleared with protein A-protein G (2:1)-agarose beads at 4 °C for 20 min. Following the removal of the beads by centrifugation, lysates were incubated with appropriate antibodies in the presence of 15 μl of protein A-protein G (2:1)-agarose beads for at least 2 h at 4 °C. The beads were washed with buffer containing 50 mm Tris-HCl, pH 7.5, 0.5 m LiCl, and 0.5% Triton X-10, twice with phosphate-buffered saline, and once with buffer containing 10 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 10 mm MnCl2, and 1 mm dithiothreitol, all supplemented with 20 mm β-glycerophosphate and 0.1 mm sodium vanadate. The immunoprecipitates were subjected to in vitro kinase assay or Western blotting analysis. Protein expression was determined by probing Western blots of immunoprecipitates or total cell lysates with the appropriate antibodies as noted in the figure legends. Detection of antigen-bound antibody was carried out with the ECL Western blotting analysis system (Amersham Biosciences).
In Vitro Kinase Assay
Protein kinase assays were carried out as described (14). Briefly, reactions were carried out in the presence of 10 μCi of [γ-32P]ATP (PerkinElmer Life Sciences) and 3 μm cold ATP in 30 μl of buffer containing 20 mm HEPES (pH 7.4), 10 mm MgCl2, 10 mm MnCl2, and 1 mm dithiothreitol. 1 μg of histone H2B basic protein or recombinant GST-fused protein was used as the exogenous substrate. After incubation at room temperature for 30 min, the reaction was stopped by adding protein-loading buffer, and proteins were separated on SDS-PAGE gels. Each experiment was repeated three times, and the relative amounts of incorporated radioactivity were determined by autoradiography.
Luciferase Assay
For the luciferase reporter assay, 293T cells were seeded in 24-well plates. 3×IRS-luciferase reporter, pR-tk, and indicated plasmids were cotransfected as described previously (14). Thirty-six hours after transfection, cells were lysed, and luciferase activity was assayed using the luciferase assay kit obtained from Promega according to the manufacturer's instructions. All luciferase activities were normalized to Renilla.
In Vivo [32P] Pi Labeling
COS7 cells were co-transfected with active JNK1 and MST1 and labeled with [32P]Pi (0.5 mCi/ml) in phosphate- and serum-free DMEM medium for 4 h. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibody. The immunoprecipitates were separated by 7.5% SDS-PAGE and transferred to membranes. The phosphorylated MST1 band was visualized by autoradiography. The expression of transfected MST1 was detected with anti-FLAG antibody.
MBP in-Gel Kinase Assay
Cells were seeded in 60-mm plates and transfected with different MST1 constructs. After 36 h of the transfection, cells were lysed for 15 min on ice with lysis buffer (20 mm Hepes pH 7.4), 2 mm EGTA, 50 mm glycerophosphate, 1% Triton X-100, 10% glycerol, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mm Na2VO4). The cell debris was removed by microcentrifugation at 14,000 × g for 15 min at 4 °C. The FLAG immunoprecipitates were loaded onto a 10% SDS-polyacrylamide gel that had been polymerized in the presence of 0.2 mg/ml bovine brain MBP. After running, the gel was washed twice at room temperature for 30 min with 100 ml of buffer A (50 mm Hepes pH 7.6, 5 mm 2-mercaptoethanol) containing 20% isopropyl alcohol. The gel was then washed twice at room temperature with buffer A and twice with buffer A containing 6 m urea. Renaturation was achieved by sequentially washing the gel twice for 30 min at 4 °C with buffer A containing 3 m urea, 1.5 m urea, 0.75 m urea, and buffer A alone. After an overnight wash in buffer A with 0.05% Tween 20 (v/v), the gel was washed twice and equilibrated at 30 °C for 30 min in kinase buffer (20 mm Hepes pH 7.6, 20 mm MgCl2, 2 mm dithiothreitol) prior to the addition of 20 μm Mg/ATP and 100 μCi of [32P]ATP and incubation for at 30 °C for 30 min. The reaction was stopped, and unincorporated [32P]ATP was removed by washing with 100 ml of 5% trichloroacetic acid (w/v) and 1% NaPPi (w/v) at room temperature until the washing solution has no radioactivity, and the gel was dried prior to autoradiography.
Cell Death Assay (TUNEL Assay)
Cells were seeded into 60-mm dishes and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum for 24 h, treated with 0.1 μm STS. Apoptosis was determined by Tunel assay using an in situ cell death detection kit (Roche Applied Science, Indianapolis, IN). These experiments were performed in triplicate.
RESULTS AND DISCUSSION
JNK Activity Is Required for MST1 Activation, Cleavage, and Nuclear Translocation
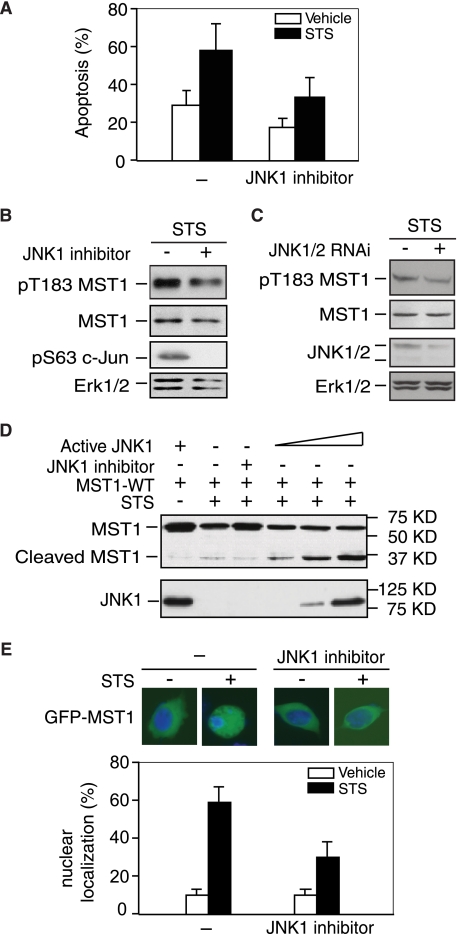
JNK is a member of the stress-response MAPK superfamily, which are potently activated by a variety of physiological stimuli. Many studies have indicated that JNK is a regulator of the initiation of cell death (18). JNK activation induces caspase-dependent MST1 cleavage and activation, indicating JNK might act as an upstream regulator for MST1 kinase activation, even though there have already been many reports suggesting JNK is indeed one of the MST1 substrates (11, 19, 20). Previous studies showed that JNK1 induced cell death through phosphorylation of its downstream in a transcription-independent manner (10), and Becker et al. (21) showed that JNK can promote neuronal cell death through phosphorylating BIM at serine 65. Consistent with the observation, we observed that JNK inhibitors could decrease MST1 overexpression-induced cell death upon STS treatment (Fig. 1A). Then we sought to examine whether JNK1 directly regulates MST1 activity and cleavage. Immunoblotting analysis in Fig. 1, B and C showed that inhibition of JNK by using JNK inhibitor or JNK RNAi, reduced MST1 autophosphorylation at Thr-183, and JNK inhibition also attenuates caspase-mediated cleavage whereas active JNK1 enhanced MST1 cleavage in response to STS stimulation (Fig. 1D). Fluorescent microscopy analysis also shows that JNK1 inhibitor reduced STS-induced MST1 nuclear translocation (Fig. 1E). These data suggest that JNK1 might act as an upstream activator for MST1.
FIGURE 1.
JNK activity is required for MST1 activation, cleavage, and nuclear translocation. A, ovarian cancer cell line OV8 overexpressed with wild-type MST1 and treated with or without staurosporine (20 nm, 24 h) and JNK inhibitor (SP600125) and cell death was analyzed by TUNEL staining. Cell death was significantly reduced in cells treated with JNK inhibitor (n = 3, p < 0.05, ANOVA followed by Fisher's PLSD post-hoc test). B, lysates of 293T cells transfected with wild-type MST1 and treated with JNK inhibitor in the presence of STS stimulation were blotted with the pT183 MST1, FLAG, or pS63 c-Jun antibody, and Erk1/2 expression was used as the loading control. JNK inhibition reduced STS-induced MST1 autophosphorylation. C, lysates of 293T cells transfected with JNK1/2 RNAi or control vector and treated with STS (0.1 μm, 3 h) were blotted with pT183 MST1, FLAG, JNK1/2, or Erk1/2 antibody. JNK knock-down reduces MST1 activation upon STS treatment. D, lysates of COS7 cells transfected with FLAG-tagged MST1 and different amounts of active JNK1 (MKK fused JNK1) or its control vector in the presence of STS or JNK inhibitor as indicated in the figure were immunoblotted with the anti-FLAG or JNK1 antibody. Active JNK induces MST1 cleavage, and JNK inhibition decreases MST1 cleavage. E, COS7 cells transfected with GFP-MST1 and treated with STS in the absence or presence of JNK inhibitor were analyzed by fluorescence microscopy. Representative images are shown. Quantification of results is shown below, and JNK inhibitor significantly decreased GFP-MST1 nuclear translocation upon STS treatment. For each experiment, 100 cells were counted (n = 3; p < 0.01, ANOVA followed by Fisher's PLSD post-hoc test).
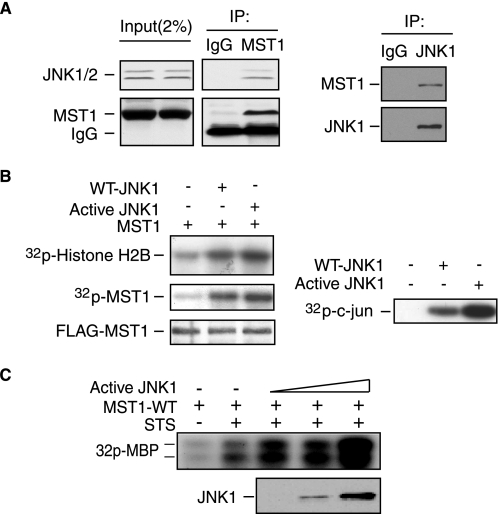
JNK Interacts with MST1 and Activates MST1 Kinase Activity in Vitro and in Vivo
An immunoprecipitation experiment shows that MST1 and JNK1/2 can interact with each other (Fig. 2A), indicating that MST1 and JNK could form a physical complex. To further delineate the functional interaction between JNK and MST1, in vitro kinase assay was performed and showed that active JNK1 significantly enhanced the kinase activity of MST1 (Fig. 2B) by using the histone H2B as the substrates. Immunoprecipitated MST1 from COS7 cells co-transfected with active JNK1 and MST1-WT was subjected to MBP in-gel kinase assay, indicating that JNK also enhances STS-induced MST1 kinase activation (Fig. 2C). Thus, the activation of JNK kinase is essential for MST1 activation in vitro and in vivo.
FIGURE 2.
JNK interacts with MST1 and increases MST1 kinase activity. A, lysates of COS7 cells were immunoprecipitated with the anti-MST1 antibody (left panel) or anti-JNK1 antibody (right panel) or the control IgG, and were blotted with anti-JNK1/2 (left) or anti-MST1 (right) antibody. MST1 interacts with JNK. B, lysates of COS7 cells transfected with FLAG-MST1 expression plasmids together with an expression vector encoding WT-JNK1, active JNK1, or the control vector were immunoprecipitated with anti-FLAG antibody and subjected to in vitro MST1 hot kinase assay by using histone H2B as the substrate (left). MST1 autophosphorylation and expression are shown in the lower panels. JNK activity was determined by JNK kinase assay using recombinant GST-c-Jun as the substrate (right panel). JNK activation increased the MST1 activity in vitro. C, MBP in-gel kinase assay was performed using the immunoprecipitates from COS7 cells transfected with MST1 together with active JNK1 in the presence of STS stimulation. The active JNK expression level is shown in the lower panel. JNK enhances STS-induced MST1 activation.
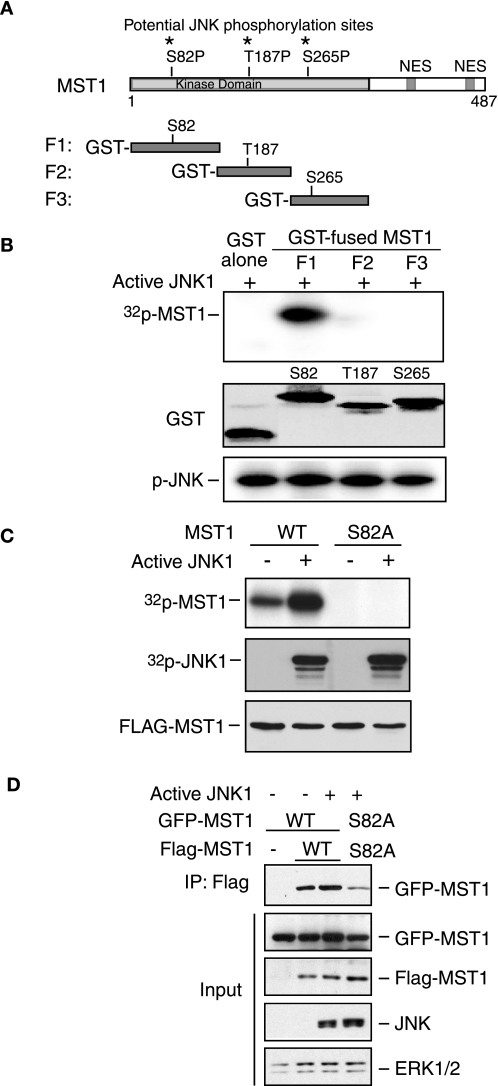
JNK Phosphorylates MST1 at Serine 82 in Vitro and in Vivo
To characterize the phosphorylation of MST1 by JNK, we used recombinant active JNK kinase for the in vitro kinase assay by incubating different GST-fused fragments of MST1 as the substrates, which were designed based on the consensus phosphorylation site of JNK (S/T-proline) (Fig. 3A). The in vitro JNK kinase assay suggests that Ser-82 on MST1 was the major phosphorylation site by JNK (Fig. 3B). To determine the function of JNK activation on MST1 kinase activity, we co-transfected MST1-WT or -S82A with active JNK1 in COS7 cells, and the in vivo orthophosphate labeling was performed, showing that active JNK can phosphorylate MST1-WT but not MST1-S82A (Fig. 3C). It has been reported that the C-terminal region of MST1 participates in protein dimerization and mediates MST1 activation onto the substrates, such as FOXO1 (22). We sought to examine whether Ser-82 phosphorylation might affect its homodimerization. The co-immunoprecipitation data showed that S82A, a non-phosphorylated mutant MST1 lowers MST1 dimerization as well as the autophosphorylation, indicating Ser-82 phosphorylation by active JNK enhances MST1 dimerization (Fig. 3D). Collectively, JNK can directly phosphorylate MST1 at serine 82 in vitro and in vivo, and phosphorylation enhances the homodimerization.
FIGURE 3.
JNK phosphorylates MST1 at serine 82. A, three potential JNK phosphorylation sites in the N terminus of MST1 are indicated. NES, nuclear- exporting sequence. B, in vitro JNK kinase assay was performed by incubating the recombinant active JNK with different GST-fused MST1 fragments (P1, P2, and P3) or GST only as substrate in the presence of [32P]ATP. The reaction was analyzed by SDS-PAGE followed by autoradiography. GST protein expression of MST1 and autophosphorylation of JNK are shown in the middle panel and lower panel, respectively. The phosphorylation site of MST1 by JNK kinase is in the P1 fragment. C, in vivo labeling was performed according to the “Experimental Procedures” after co-transfection with active JNK and MST1 in COS7 cells. The membrane was exposed to X-films after electrophoresis (upper panel). The expression of JNK or MST1 is shown in the lower panels. D, lysates of 293T cells transfected with GFP-MST1 (WT or S82A) and FLAG-MST1 (WT or S82A) together with an expression vector encoding active JNK1 or the control vector were immunoprecipitated with anti-FLAG antibody and blotted with GFP antibody. The input was blotted with GFP, FLAG, JNK, or Erk1/2 antibody. MST-S82A mutants confer a lower capability of dimerization.
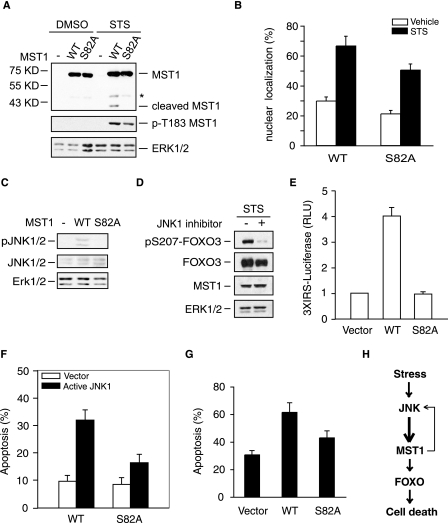
JNK-enhancing MST1-FOXO Pro-apoptotic Signaling Pathway Is Serine 82 Phosphorylation-dependent
The ability of JNK to phosphorylate MST1 at serine 82 led us next to determine the functional consequences of the MST1 phosphorylation. By using the MST1-S82A, a non-phosphorylatable mutant, we found that MST1-S82A confers a lower cleavage and autophosphorylation level upon STS stimulation compared with MST1-WT (Fig. 4A). Consistent with this, there is less nuclear translocation in the S82A-MST1-transfected cells than that of wild-type MST1 cells (Fig. 4B). Furthermore, MST1-S82A cannot induce JNK activation (Fig. 4C) and similarly, knock-down of MST1 using RNAi reduces JNK activation (supplemental Fig. S1). Taken together, this suggests that Ser-82 phosphorylation of MST1 is important for the activation of MST1 signaling. Because MST1-FOXO signaling is required for stress-induced cell death (13), we further assessed the effect of JNK-induced phosphorylation of MST1 at serine 82 on the MST1-FOXO3 signaling cascade. By examining the FOXO3 phosphorylation at Ser-207, which is reported as the phosphorylation site by MST1 (13), we showed that the JNK inhibition reduces MST1-induced FOXO3 phosphorylation (Fig. 4D). We further characterized the effect of Ser-82 phosphorylation on the transcriptional activation of FOXO. Compared with MST1-WT, MST1-S82A had a significantly lower ability to trigger the expression of the FOXO responsive reporter gene (Fig. 4E and supplemental Fig. S2). The apoptosis data illustrate that active JNK or STS treatment induces more cell death when co-transfected with MST1-WT than MST1-S82A (Fig. 4, F and G), indicating that the serine 82 phosphorylation of MST1 by JNK kinase is important for full activation of MST1 kinase cascade during cell death. Taken together, JNK is not only a downstream target of MST1 as described before, but also one of the upstream targets, which could provide up-regulation feedback through MST1 cleavage, nuclear translocation, and kinase activation upon stress stimulation through phosphorylation at Ser-82.
FIGURE 4.
Serine 82 phosphorylation is required for the activation of the MST1 signaling cascade in the cell death. A, lysates of 293T cells, transfected with FLAG-tagged WT- or S82A-MST1 and treated with or without STS, were immunoblotted with the FALG (top panel), pT183-MST1(middle panel), or Erk1/2 antibody (lower panel). Asterisk indicates the nonspecific band. The serine 82 to alanine mutation reduces the cleavage and activation of MST1. B, COS7 cells transfected with GFP-MST1 WT or S82A and treated with STS were analyzed as in Fig. 1E. S82A decreased GFP-MST1 nuclear translocation upon STS treatment (p < 0.05). C, lysates of 293T cells transfected with WT- or S82A-MST1 or the control vector were immunoblotted with the pJNK1/2 (top panel), JNK1/2 (middle panel), or Erk1/2 antibody (lower panel). The serine 82 to alanine mutation reduces the activation of JNK. D, lysates of 293T cells transfected MST1 together with FOXO3 in the treatment of JNK inhibitor or vehicle in the presence of STS were immunoblotted with the pS207-FOXO3 (top panel), GFP (FOXO3), FLAG (MST1), or Erk1/2 antibody. The inhibition of JNK kinase decreases the MST1-induced FOXO3 phosphorylation. E, cells transfected with 3XIRS luciferase plasmid and tk-Renilla reporter together with FOXO3 expression plasmid, and an expression plasmid encoding WT-MST1, S82A-MST1, or the control vector were subjected to the luciferase assay. Shown is the mean ± S.E. of the normalized fly/Renilla luciferase values relative to the control (ANOVA; p < 0.001, n = 3). The MST1-FOXO-mediated gene transcription is significantly reduced in S82A-MST1-transfected cells. F, COS7 cells were transfected with WT- or S82A-MST1 plasmid together with active JNK1 or the control vector, and cell death was analyzed as in Fig. 1A. Active JNK increases the WT-MST1, but not the S82A-MST1-induced cell death (Student's t test, p < 0.01, n = 3). G, COS7 cells were transfected with WT- or S82A-MST1 plasmid together or with the control vector in the presence of STS treatment, and cell death was analyzed as in Fig. 1A. STS induced less cell death in the S82A-MST1-transfected cells compared with WT-MST1 (p < 0.05). H, model of the JNK-MST1 signaling pathway.
In this study, we have identified an upstream regulator of MST1 kinase and uncovered a new substrate of stress kinase JNK in the apoptotic signaling pathway. Our major findings are: 1) JNK phosphorylates the N-terminal of MST1 at serine 82 in vitro and in vivo. 2) The JNK-induced MST1 phosphorylation promotes caspase-mediated MST1 cleavage, STS-induced MST1 kinase activity, and nuclear translocation as well as dimerization. 3) Inhibition of JNK activation diminishes MST1 cleavage and activity as well as nuclear translocation. 4) JNK-MST1 signaling pathway plays an important role in cell death. Collectively, we have identified MST1 as a novel substrate of stress-activated kinase JNK in the apoptosis signaling pathway (see model in Fig. 4H), and we have elucidated JNK as an upstream regulator of the MST1-FOXO signaling pathway during the stress-induced cell death, which supports the concept that JNK induced cell death independently of c-Jun transactivation (10).
The identification of JNK1-induced MST1 phosphorylation and activation supports the growing concepts that JNK1 is a major stress mediator (18). Intriguingly, the disruption of 14-3-3 proteins from the pro-apoptotic proteins plays a vital role in determining the balance between life and death of cells (13, 23–26). Thus a number of kinases, including Cdk1, MST1, and JNK1, trigger the dissociation of 14-3-3 proteins from apoptotic proteins including BH3-only proteins BAD and BAX, and the transcription factor FOXO as well as c-abl (13, 14, 24, 27–29). Cdk1 promotes cell death through phosphorylating BAD and FOXO1 (23, 24). Thus, Cdk1 and MST1 seem to converge on the activation of pro-apoptotic transcription factor FOXO1 by phosphorylating two distinct sites, serine 249 and serine 212, respectively, both of which promote the dissociation of FOXO1 from 14-3-3 proteins (13, 24). JNK1 also disrupts the association of 14-3-3 and other proteins including c-Abl and FOXO (29, 30). However, JNK1 appears to phosphorylate 14-3-3 proteins rather than directly phosphorylating FOXO. Consistent with this, our data support that JNK increases FOXO-14-3-3 disruption by enhancing MST1 kinase activity in addition to direct regulation of 14-3-3 proteins.
The identification of JNK as a feedback regulator of MST1 during stress-induced cell death further builds a case that MST1 is a key player in cell death. It will be important in future studies to explore the role of the JNK-MST1 signaling pathway in brain development and oxidative stress-induced neuronal cell death and to investigate the possibility of JNK and/or MST1 inhibition as a therapeutic application for related neurological diseases.
Supplementary Material
Acknowledgments
We thank R. Davis for providing the active JNK1 construct, Z. H. Xu for the JNK1/2 RNAi constructs, and members of the Yuan laboratory for critical reading of the manuscript.
This work was supported by the National Science Foundation of China (30870792) and the Ministry of Science and Technology of China (973-2009CB918704).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- MST1
- mammalian STE20-like kinase 1
- JNK
- c-Jun N-terminal kinase
- HA
- hemagglutinin
- PBS
- phosphate-buffered saline
- DMEM
- Dulbecco's modified Eagle medium
- STS
- staurosporine
- MAPK
- mitogen-activated protein kinase
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling
- ANOVA
- analysis of variance
- WT
- wild type
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Creasy C. L., Ambrose D. M., Chernoff J. (1996) J. Biol. Chem. 271, 21049–21053 [DOI] [PubMed] [Google Scholar]
- 2.Harvey K. F., Pfleger C. M., Hariharan I. K. (2003) Cell 114, 457–467 [DOI] [PubMed] [Google Scholar]
- 3.Wu S., Huang J., Dong J., Pan D. (2003) Cell 114, 445–456 [DOI] [PubMed] [Google Scholar]
- 4.Huang J., Wu S., Barrera J., Matthews K., Pan D. (2005) Cell 122, 421–434 [DOI] [PubMed] [Google Scholar]
- 5.Taylor L. K., Wang H. C., Erikson R. L. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10099–10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves J. D., Gotoh Y., Draves K. E., Ambrose D., Han D. K., Wright M., Chernoff J., Clark E. A., Krebs E. G. (1998) EMBO J. 17, 2224–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K. K., Murakawa M., Nishida E., Tsubuki S., Kawashima S., Sakamaki K., Yonehara S. (1998) Oncogene 16, 3029–3037 [DOI] [PubMed] [Google Scholar]
- 8.Cheung W. L., Ajiro K., Samejima K., Kloc M., Cheung P., Mizzen C. A., Beeser A., Etkin L. D., Chernoff J., Earnshaw W. C., Allis C. D. (2003) Cell 113, 507–517 [DOI] [PubMed] [Google Scholar]
- 9.Graves J. D., Draves K. E., Gotoh Y., Krebs E. G., Clark E. A. (2001) J. Biol. Chem. 276, 14909–14915 [DOI] [PubMed] [Google Scholar]
- 10.Ura S., Nishina H., Gotoh Y., Katada T. (2007) Mol. Cell. Biol. 27, 5514–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ura S., Masuyama N., Graves J. D., Gotoh Y. (2001) Genes Cells 6, 519–530 [DOI] [PubMed] [Google Scholar]
- 12.Ahn S. H., Cheung W. L., Hsu J. Y., Diaz R. L., Smith M. M., Allis C. D. (2005) Cell 120, 25–36 [DOI] [PubMed] [Google Scholar]
- 13.Lehtinen M. K., Yuan Z., Boag P. R., Yang Y., Villén J., Becker E. B., DiBacco S., de la Iglesia N., Gygi S., Blackwell T. K., Bonni A. (2006) Cell 125, 987–1001 [DOI] [PubMed] [Google Scholar]
- 14.Yuan Z., Lehtinen M. K., Merlo P., Villén J., Gygi S., Bonni A. (2009) J. Biol. Chem. 284, 11285–11292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glantschnig H., Rodan G. A., Reszka A. A. (2002) J. Biol. Chem. 277, 42987–42996 [DOI] [PubMed] [Google Scholar]
- 16.O'Neill E., Rushworth L., Baccarini M., Kolch W. (2004) Science 306, 2267–2270 [DOI] [PubMed] [Google Scholar]
- 17.Creasy C. L., Chernoff J. (1995) Gene 167, 303–306 [DOI] [PubMed] [Google Scholar]
- 18.Ip Y. T., Davis R. J. (1998) Curr. Opin. Cell Biol. 10, 205–219 [DOI] [PubMed] [Google Scholar]
- 19.Hao W., Takano T., Guillemette J., Papillon J., Ren G., Cybulsky A. V. (2006) J. Biol. Chem. 281, 3075–3084 [DOI] [PubMed] [Google Scholar]
- 20.Dan I., Watanabe N. M., Kusumi A. (2001) Trends Cell Biol. 11, 220–230 [DOI] [PubMed] [Google Scholar]
- 21.Becker E. B., Bonni A. (2006) Neuron 49, 655–662 [DOI] [PubMed] [Google Scholar]
- 22.Anand R., Kim A. Y., Brent M., Marmorstein R. (2008) Biochemistry 47, 6719–6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi Y., Lehtinen M., Donovan N., Bonni A. (2002) Mol. Cell 9, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 24.Yuan Z., Becker E. B., Merlo P., Yamada T., DiBacco S., Konishi Y., Schaefer E. M., Bonni A. (2008) Science 319, 1665–1668 [DOI] [PubMed] [Google Scholar]
- 25.Donovan N., Becker E. B., Konishi Y., Bonni A. (2002) J. Biol. Chem. 277, 40944–40949 [DOI] [PubMed] [Google Scholar]
- 26.Wang P., Cao X., Nagel D. J., Yin G. (2007) Neurosci. Lett. 415, 248–252 [DOI] [PubMed] [Google Scholar]
- 27.Lehtinen M. K., Bonni A. (2008) Curr. Mol. Med. 8, 313–318 [DOI] [PubMed] [Google Scholar]
- 28.Tsuruta F., Sunayama J., Mori Y., Hattori S., Shimizu S., Tsujimoto Y., Yoshioka K., Masuyama N., Gotoh Y. (2004) EMBO J. 23, 1889–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida K., Yamaguchi T., Natsume T., Kufe D., Miki Y. (2005) Nat. Cell Biol. 7, 278–285 [DOI] [PubMed] [Google Scholar]
- 30.Sunayama J., Tsuruta F., Masuyama N., Gotoh Y. (2005) J. Cell Biol. 170, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.