Abstract
Langerin is categorized as a C-type lectin selectively expressed in Langerhans cells, playing roles in the first line of defense against pathogens and in Birbeck granule formation. Although these functions are thought to be exerted through glycan-binding activity of the C-type carbohydrate recognition domain, sugar-binding properties of Langerin have not been fully elucidated in relation to its biological functions. Here, we investigated the glycan-binding specificity of Langerin using comprehensive glycoconjugate microarray, quantitative frontal affinity chromatography, and conventional cell biological analyses. Langerin showed outstanding affinity to galactose-6-sulfated oligosaccharides, including keratan sulfate, while it preserved binding activity to mannose, as a common feature of the C-type lectins with an EPN motif. By a mutagenesis study, Lys-299 and Lys-313 were found to form extended binding sites for sulfated glycans. Consistent with the former observation, the sulfated Langerin ligands were found to be expressed in brain and spleen, where the transcript of keratan sulfate 6-O-sulfotransferase is expressed. Moreover, such sulfated ligands were up-regulated in glioblastoma relative to normal brain tissues, and Langerin-expressing cells were localized in malignant brain tissues. Langerin also recognized pathogenic fungi, such as Candida and Malassezia, expressing heavily mannosylated glycans. These observations provide strong evidence that Langerin mediates diverse functions on Langerhans cells through dual recognition of sulfated as well as mannosylated glycans by its uniquely evolved C-type carbohydrate-recognition domain.
Keywords: Carbohydrate/Function, Glycoproteins, Glycoproteins/Carbohydrates, Glycosylation, Immunology/Innate Immunity, Receptors/Oligosaccharides
Introduction
Langerhans cells (LCs)2 are a subset of dendritic cells resident in an immature state in skin epidermis and mucosal epithelium. They have been considered to play a sentinel role through their specialized function in antigen capture and migratory capacity to secondary lymphoid tissue to initiate specific immunity (1). LCs are characterized by the presence of a cytoplasmic organelle, Birbeck granules (BGs), and the cell surface LC-specific receptor, Langerin (CD207) (2, 3).
Langerin was first identified as a molecule specifically expressed in LCs, which is recognized by LC-specific mAb DCGM4 (2). Langerin belongs to group II C-type lectins, most of which have been reported to recognize glycan ligands in a Ca2+-dependent manner. Langerin is a 37.5-kDa (328 amino acids) type II transmembrane receptor consisting of an N-terminal short cytoplasmic domain, a transmembrane domain, a neck region, and a C-terminal C-type carbohydrate-recognition domain (CRD) (2). The cytoplasmic domain contains a proline-rich motif (WPREPPP) as a potential signal transduction site. The CRD contains an EPN (Glu-Pro-Asn) motif characteristic of C-type lectins with mannose (Man) specificity (4). Mannan-binding protein and dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) are representative members of this Man-specific subfamily.
Langerin functions as an endocytic receptor, which has been believed to be involved in self-defense against invading pathogens. Langerin is expressed on the cell surface and has the ability to uptake (5), process, and present antigens to T cells through the major histocompatibility complex I and II pathways (6), although it lacks a typical tyrosine-based internalization motif (2). Recently, Langerin was reported to have activity to prevent human immunodeficiency virus-1 (HIV-1) transmission (7). Indeed, Langerin binds to HIV-1 through gp120 with mannosylated glycans and uptakes into the BGs, resulting in HIV-1 degradation and viral clearance.
Langerin also functions as an essential component of the induction of BG formation as proved by the following findings: (a) inside the cell, Langerin exists mainly in the BGs (2); (b) transient transfection of Langerin cDNA into the fibroblastic mouse cell line induces BG formation (1); and (c) disruption of the Langerin gene abolishes the BGs in LCs (8). Interestingly, the C-type CRD of Langerin is essential for the BG formation, because the activity is abrogated by eliminating the CRD (2). In agreement with this observation, calcium deprivation results in unzippering of the BGs and transformation into vesicles (3). A recent report also demonstrated that antibody binding to the Langerin CRD induces global rearrangements of LC morphology (9). Undoubtedly, the Langerin CRD is essential for its biological functions in LCs. However, its sugar-binding specificity in relation to diverse functions in LCs remains to be elucidated.
In terms of sugar-binding specificity, two studies have been reported. Stambach et al. analyzed the glycan-binding specificity of Langerin by solid phase and glycoprotein blotting assays, showing that the Langerin CRD binds to Man, fucose, and N-acetylglucosamine (GlcNAc) similar to other C-type lectins with the EPN motif, and that the oligomeric structure formed via the neck region is required for sufficient binding (10). More recently, Galustian et al. reported the glycan-binding specificity of Langerin using carbohydrate microarray (11). In contrast to the report by Stambach et al., Langerin was shown to bind to sulfated Lex-related glycans, whereas only weak binding was observed for high mannose-type N-glycans. Therefore, current understanding about sugar-binding specificity of Langerin has been rather confused. More systematic analysis is necessary to elucidate sugar-binding properties of Langerin in relation to its biological functions on LCs.
Here, we analyzed glycan-binding specificity of Langerin using glycoconjugate microarray (12), frontal affinity chromatography (FAC) (13, 14), and cell biological assays. We demonstrate that Langerin is a unique receptor with dual specificity to sulfated and mannosylated glycans via a single C-type CRD. The binding mechanism and putative functions of the dual specificity of Langerin are also discussed in this study.
EXPERIMENTAL PROCEDURES
Materials
FITC-labeled rabbit anti-donkey IgG (H+L), Cy3-labeled donkey anti-human Fcγ, peroxidase-labeled goat anti-human IgG (H+L), Cy3-labeled donkey anti-mouse IgG (H+L), and Cy3-labeled streptavidin were purchased from Jackson ImmunoResearch (West Grove, PA). FITC-labeled rat anti-CD3 and anti-CD19 mAbs were purchased from BD Biosciences. Mouse anti-human Langerin mAb (12D6, Novocastora) was purchased from Mitsubishi Chemical Medience Corp. (Tokyo, Japan). N-Acetyllactosamine (LacNAc)-polyacrylamide (PAA)-biotin, 6-sulfo-N-acetylglucosamine (GlcNAc)-PAA- biotin, 6-sulfo-LacNAc-PAA-biotin, and 6′-sulfo-LacNAc-PAA-biotin probes were purchased from Glycotech (Gaithersburg, MD). Anti-highly sulfated keratan sulfate mAb (5D4) was purchased from Seikagaku Co. (Tokyo, Japan). Arthrobacter ureafaciens sialidase was purchased from Roche Diagnostics (Tokyo, Japan). Tissue microarrays of astrocytic tumors were purchased from Cybrdi, Inc. (Frederick, MD). Tissue specimens of gliomas were also obtained from the Kumamoto University Hospital. Marathon Ready® cDNAs of various human tissues were purchased from Takara (Shiga, Japan). The human Langerin (#4692155), KS6ST (#4814499), and GlcNAc6ST (#4562846) cDNAs were purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Protein A-Sepharose 4 Fast Flow was purchased from GE Amersham Biosciences. Pyridylaminated (PA) bovine corneal KS (KS-I) and shark cartilaginous KS (KS-II) were generous gifts from Seikagaku Co.
Fungi
Saccharomyces cerevisiae (Institute of Food Microbiology number = 49922), Candida albicans (40009), Candida glabrata (40018), Candida guilliermondii (46828), Candida kefyr (46842), Candida krusei (46839), Candida parapsilosis (52607), Candida tropicalis (40065), Malassezia furfur (52635), Malassezia pachydermatis (48586), and Cryptococcus neoformans (B4500) were obtained from the National BioResource Project. S. cerevisiae, Candida species, and C. neoformans were cultured in YPD medium (10% Tryptone, 5% dried yeast extract, 10% glucose, pH 5.8). Malassezia species were cultured in Malassezia medium (10% Tryptone, 10% dried yeast extract, 20% glucose, 0.5% Tween 80).
Langerin-Fc Fusion Protein
The CRD region of human Langerin (193–328 amino acids) was amplified by PCR using specific primer sets (forward and reverse, 5′-CTCGAGATGACTGTGGAGAAGGAGGCCCCTGATGCG-3′ and 5′-GATATCCGGTTCTGATGGGACATAGGGTCGCTTACA-3′), and ligated into a pSecTag/FRT/V5-His vector (Invitrogen Japan K.K., Tokyo, Japan). The Fc region of human IgG1 was then inserted into the C terminus of the Langerin CRD sequence via AgeI and PmeI sites. Site-directed mutagenesis of Langerin-Fc was also performed using a Genetailor site-directed mutagenesis system (Invitrogen). The primers used for construction of Langerin-Fc mutants were as follows: for E285A, 5′-GAGGTTCTGGATTCCAGGTGCGCCCAACAAT-3′ (sense) and 5′-CACCTGGAATCCAGAACCTCGCACTTTGGA-3′ (antisense); for N287A, 5′-TCTGGATTCCAGGTGAGCCCGCCAATGCTGGG-3′ (sense) and 5′-GGGCTCACCTGGAATCCAGAACCTCGCACT-3′ (antisense); for K299A, 5′-ATGAACACTGTGGCAATATAGCGGCTCCCTCAC-3′ (sense) and 5′-TATATTGCCACAGTGTTCATTGTTCCCAGC-3′ (antisense); and for K313A, 5′-GGAATGATGCCCCATGTGACGCAACGTTTCTTT-3′ (sense) and 5′-GTCACATGGGGCATCATTCCAGGCCTGAAG-3′ (antisense). The expression constructs were transiently transfected into HEK293T cells cultured in Opti-MEM (Invitrogen) supplemented with 5% low IgG fetal bovine serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) by Lipofectamine LTX (Invitrogen) followed by the manufacturer's procedure. Fusion proteins secreted into medium were purified by affinity chromatography on Protein A-Sepharose 4 Fast Flow (Amersham Biosciences).
Preparation of CHO Cells Stably Expressing Full-length Langerin
The full-length human Langerin (CD207) gene (1–328 amino acids) was amplified by PCR using specific primer sets (forward and reverse, 5′-CTCGAGATGACTGTGGAGAAGGAGGCCCCTGATGCG-3′ and 5′-GATATCCGGTTCTGATGGGACATAGGGTCGCTTACA-3′) and ligated into a pcDNA5/FRT/V5-His vector (Invitrogen). The expression vector was transfected into an Flp-In CHO cell line (Invitrogen) by Lipofectamine LTX. CHO cells stably expressing the full-length Langerin were selected with 0.5 mg/ml hygromycin B (Invitrogen).
Preparation of Sulfotransferase Expression Vectors
The full-length cDNA of KS6ST (CHST1, keratan sulfate Gal-6-sulfotransferase), GlcNAc6ST (CHST2, carbohydrate N-acetylglucosamine-6-O-sulfotransferase 2), and I-GlcNAc6ST (CHST5, intestinal N-acetylglucosamine 6-O-sulfotransferase) were ligated into pcDNA3.1 (+) or (−) vectors (Invitrogen) via EcoRV sites.
Glycoconjugate Microarray
The sugar-binding specificity of Langerin was analyzed by glycoconjugate microarray as described previously (12). Briefly, Langerin-Fc chimera (1 μg/ml) was precomplexed with Cy3-labeled donkey anti-human Fcγ (1.4 μg/ml) in TBS-T (10 mm Tris-HCl, pH 7.4, containing 150 mm NaCl (TBS), 1% (v/v) Triton X-100) containing 1 mm CaCl2, and then applied onto a glycoconjugate microarray (version 4.2). As negative control experiments, binding was also analyzed in TBS-T containing 10 mm EDTA. After incubation at 20 °C overnight, binding was detected by the evanescent-field fluorescence-assisted scanner, SC-profiler (GP Biosciences Ltd., Yokohama, Japan) under Cy3 mode. Culture supernatants containing Langerin-Fc were precomplexed with Cy3-labeled anti-human Fcγ in the presence or absence (control) of inhibitors and applied onto the glycoconjugate microarray. 50 mm of Man and Glc, and 5 μg/ml 6′-sulfo-LacNAc-PAA (6′-sulfo-LacNAc), 6-sulfo-LacNAc-PAA (6-sulfo-LacNAc), and LacNAc-PAA (LacNAc), and 10 mm EDTA were used as inhibitors. Glycans used for glycoconjugate microarray are shown in supplemental Fig. 1 and supplemental Table 1.
Binding of Langerin-Fc to CHO Cells Transfected with Sulfotransferase cDNA
CHO cells (1 × 105) were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) in 6-well plates at 37 °C. After 2 days, sulfotransferase expression vectors (2.5 μg) were transfected into CHO cells using Lipofectamine LTX (6.25 μl, Invitrogen) in accordance with the manufacturer's procedure. Cells were recovered, resuspended in TSA buffer (TBS containing 10 mg/ml bovine serum albumin, 2 mm CaCl2, and 2 mm MgCl2), and incubated with or without 25 milliunits of A. ureafaciens sialidase at 37 °C for 30 min. After washing with TSA buffer, cells were incubated with 20 μg/ml Langerin-Fc chimera precomplexed with 20 μg/ml Cy3-labeled donkey anti-human Fcγ on ice for 1 h. As negative controls, cells were also incubated with Langerin-Fc chimera in EDTA buffer (TBS containing 10 mg/ml bovine serum albumin and 5 mm EDTA). Flow cytometry data were acquired on a FACSCanto-II cytometer (BD Biosciences) and analyzed using FACSDiva and FlowJo software.
Probe Binding Assay
Langerin-CHO cells (2 × 105) were cultured in 6-well plates for 2 days at 37 °C. After washing with TSA buffer, cells were incubated with glycoside-PAA probes (10 μg/ml) precomplexed with Cy3-streptavidin (10 μg/ml) on ice for 1 h. Cells were then recovered with trypsin, and flow cytometry data were acquired on a FACSCanto-II cytometer and analyzed using FACSDiva and FlowJo. As a negative control, probe binding was also analyzed in the presence of EDTA.
Probe Internalization Assay
Langerin-CHO cells (2 × 105) were cultured in 6-well plates for 2 days at 37 °C. After washing with TSA buffer, cells were incubated with glycoside-PAA probes (20 μg/ml) precomplexed with Cy3-streptavidin (20 μg/ml) in TSA buffer at 37 °C for 1 h to trigger internalization. Cells were then recovered with trypsin, and the internalized fluorescence was detected by flow cytometry. Flow cytometry data were acquired on a FACSCanto-II cytometer and analyzed using FACSDiva and FlowJo. Negative control experiments were also performed using EDTA buffer.
FAC
FAC was performed as described previously (13, 14). Briefly, Langerin-Fc fusion proteins were immobilized on Protein A-Sepharose 4 Fast Flow at a concentration of 12 mg/ml, and the resulting lectin-immobilized gel was packed into a miniature column (inner diameter, 2 mm; length, 10 mm, bed volume, 31.4 μl, Shimadzu) and connected to an automated FAC system (FAC-1). A panel of 142 PA-glycans was successively injected into the columns by the auto-sampling system, and elution of the PA-glycans was detected by fluorescence (excitation, 310 nm; emission, 380 nm). The elution front of each PA-glycan relative to that of an appropriate control (lactose-PA), referred to as V − V0, was then determined. Glycans used for FAC are shown in supplemental Fig. 2. To determine Kd values, Bt (effective ligand content) was assumed to be 50% of the immobilized Langerin-Fc.
Real-time PCR
Real-time PCR was performed using an ABI Prism 7700 Sequence Detection System (Invitrogen) as previously described (15). Standard curves for the KS6ST gene and the human glyceraldehyde-3-phosphate dehydrogenase gene, as an endogenous control, were generated by serial dilution of each plasmid DNA. Data are shown as the relative amount of KS6ST transcript normalized by the amount of glyceraldehyde-3-phosphate dehydrogenase transcript in the same cDNA.
Binding of Langerin-Fc to Mouse Splenocytes
Mouse spleen was ground between two frosted glass slides and passed through a cotton-plugged Pasteur pipette. Erythrocytes were lysed with BD Pharmingen Cell Lysis Buffer (BD Biosciences). Splenocytes (1 × 106) were incubated with or without 25 milliunits of A. ureafaciens sialidase (Roche Diagnostics K.K.) in TSA buffer at 37 °C for 30 min. After washing with TSA buffer, cells were then incubated with Langerin-Fc fusion proteins (20 μg/ml) precomplexed with Cy3-labeled donkey anti-human Fcγ (20 μg/ml) on ice for 1 h. Cells were also stained with FITC-labeled rat anti-CD3 and anti-CD19 mAbs. Flow cytometry data were acquired on a FACSCanto-II cytometer and analyzed using FACSDiva and FlowJo.
Tissue Staining
Brain tissue paraffin-embedded sections were obtained from Cybrdi, Inc. and the Kumamoto University Hospital (Kumamoto, Japan). After deparaffinization with xylene and rehydration with ethanol, tissue sections were immersed in methanol containing 0.3% hydrogen peroxide for 30 min to inhibit endogenous peroxidase activity. For Langerin immunostaining, tissue sections were pretreated with microwave to unmask antigen and then incubated with anti-Langerin mAb (12D6). Tissue sections were then stained using Histofine® Simple Stain MAX-PO (M) secondary antibody (Nichirei Biosciences, Inc., Tokyo, Japan). The immunoreactions were visualized using a diaminobenzidine substrate kit (Vector Labs, Burlingame, CA) (brown) and counterstained with hematoxylin (blue). Tissue sections were also stained with Langerin-Fc (20 μg/ml) precomplexed with peroxidase-conjugated goat anti-human-IgG (10 μg/ml).
Binding of Langerin-Fc to Live Fungi
Langerin-Fc fusion proteins (0.5 μg/ml) were precomplexed with Cy3-labeled anti-human Fcγ (0.5 μg/ml) in TSA buffer at room temperature for 30 min and then incubated with 1 × 105 of live fungi on ice for 1 h. After washing with TSA buffer, flow cytometry data were acquired on a FACSCanto-II cytometer and analyzed using FACSDiva and FlowJo. Langerin-Fc was also incubated with fungi in EDTA buffer.
RESULTS
Glycoconjugate Microarray Screening of Langerin-Fc Chimera
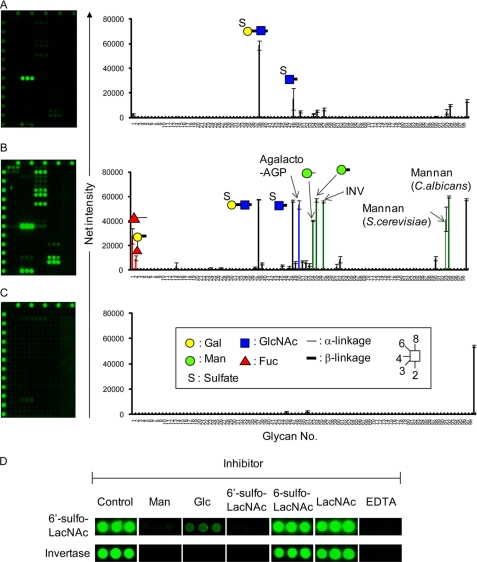
Langerin is a type II transmembrane protein containing a single extracellular C-type CRD and a cytoplasmic domain. To examine its glycan-binding specificity, the soluble Langerin-Fc chimera was generated by cloning the CRD into the pSecTag/FRT/V5-His vector followed by insertion of the Fc portion of human IgG1 at the C terminus of the Langerin CRD. Using the Langerin-Fc chimera, potential glycan ligands were first screened using the recently developed glycoconjugate microarray comprising 98 immobilized compounds (supplemental Fig. 1 and supplemental Table 1) (12). Langerin-Fc chimera was precomplexed with Cy3-labeled donkey anti-human Fcγ antibody and then incubated with glycoconjugate microarray. Binding was detected using the evanescent-field fluorescence-assisted scanner. At a low gain (Fig. 1A), Langerin-Fc exhibited highly selective binding to 6′-sulfo-LacNAc (38, [6-SO4]Galβ1–4GlcNAc), whereas no binding was observed for either its position isomer, 6-sulfo-LacNAc (37, Galβ1-4[6-SO4]GlcNAc), or unsulfated form (35, Galβ1-4GlcNAc). A weak, but significant signal was also observed for 6-sulfated GlcNAc (48, [6-SO4]GlcNAc), suggesting that sulfate at the C-6 of the non-reducing end sugar might be important for Langerin recognition. On the other hand, at a high gain (Fig. 1B), extensive binding to a certain set of glycans with Man (shown in green), GlcNAc (shown in blue), and fucose (shown in red) was observed, in agreement with the previous report (10): that is, Langerin-Fc bound to heavily mannosylated glycans such as αMan (54) and βMan polymers (55), yeast invertase (57), and mannan from S. cerevisiae and C. albicans (93 and 94). Notably, no binding was observed for a typical endogenous glycoprotein, thyroglobulin (28 and 46) expressing high mannose-type N-glycans, indicating that mannose multivalency and geometry, characteristic of exogenous organisms, might be important for Langerin recognition. A similar observation has been reported for mannan-binding protein (16). Langerin-Fc also exhibited binding to agalacto-AGP (50) containing highly branched agalactosylated N-glycans as well as fucosylated glycans (1 and 2), but to a much lesser extent. Binding signals were abolished in the presence of EDTA (Fig. 1C), indicating that the results are due to specific interactions via the C-type CRD. To further clarify whether the binding of Langerin to sulfated and mannosylated glycans is mediated by the same binding site, we then performed inhibition assay. As shown in Fig. 1D, binding of Langerin-Fc to both 6′-sulfo-LacNAc and yeast invertase was abrogated in the presence of either 50 mm Man or 5 μg/ml 6′-sulfo-LacNAc-PAA, but no inhibitory effect was observed for 5 μg/ml 6-sulfo-LacNAc-PAA and LacNAc-PAA, consistent with the results obtained in Fig. 1 (A and B). These results indicate that dual specificity of Langerin to sulfated and mannosylated glycans share the same ligand binding site.
FIGURE 1.
Analysis of the glycan-binding specificity of Langerin-Fc chimera by glycoconjugate microarray. Langerin-Fc fusion proteins were precomplexed with Cy3-labeled anti-human Fcγ in TBS-T containing 1 mm CaCl2 at room temperature for 15 min and directly applied to glycoconjugate microarray (version 4.2). After incubation at 20 °C overnight, binding was detected by the evanescent-field fluorescence-assisted scanner, SC-profiler at low (gain 60, A) and high gains (gain 120, B). As a negative control, binding was also analyzed in the presence of 10 mm EDTA (gain 120, C). D, inhibition assay. Culture supernatants, containing Langerin-Fc precomplexed with Cy3-labeled anti-human Fcγ in the presence or absence (control) of inhibitors, were applied onto glycoconjugate microarray. 50 mm of Man and Glc, and 5 μg/ml 6′-sulfo-LacNAc-PAA (6′-sulfo-LacNAc), 6-sulfo-LacNAc-PAA (6-sulfo-LacNAc), and LacNAc-PAA (LacNAc), and 10 mm EDTA were used as inhibitors. Binding of Langerin-Fc to invertase and 6′-sulfo-LacNAc are shown. Glycans used for glycoconjugate microarray are shown in supplemental Fig. 1 and supplemental Table 1.
Lys-299 and Lys-313 Form Extended Binding Sites for the Recognition of Sulfated Glycans
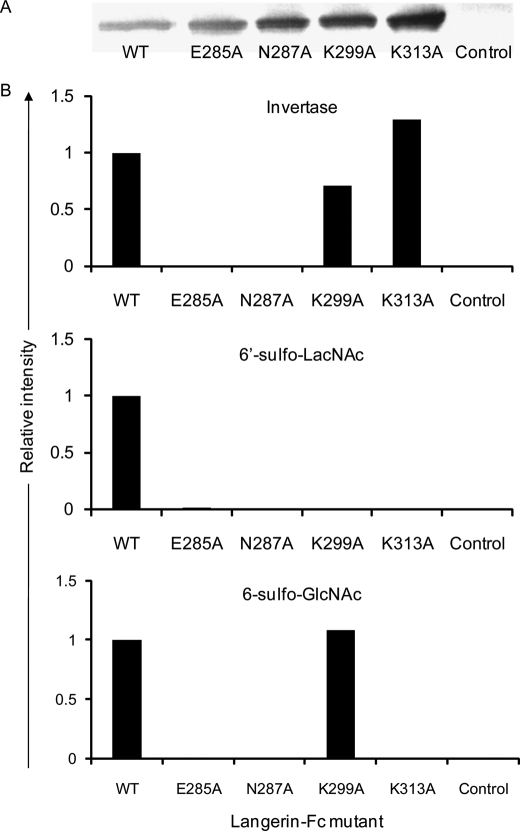
Structurally, the Langerin CRD exhibits some differences from other known C-type CRDs having the EPN motif, such as DC-SIGN (9): two lysine residues, Lys-299 and Lys-313, are present in the Langerin CRD, but not in the DC-SIGN CRD (supplemental Fig. 3) (9, 17). The presence of such positively charged amino acids in the CRD is likely involved in the formation of salt bridges with negatively charged sulfated ligands (9, 17). Expression plasmids of WT and mutant forms of Langerin-Fc were transfected into HEK293T cells, and the resulting culture supernatants were analyzed by glycoconjugate microarray with the aid of a Cy3-labeled secondary antibody. The expression of WT and mutant forms of Langerin-Fc in the culture supernatants was confirmed by Western blotting using peroxidase-labeled goat anti-human IgG (H+L) (Fig. 2A). As shown in Fig. 2B, binding of Langerin-Fc to both invertase and 6′-sulfo-LacNAc was abolished by mutation of either Glu-285 or Asn-287, which are components of the EPN motif. Interestingly, binding of Langerin-Fc to 6′-sulfo-LacNAc was also canceled by mutation of either Lys-299 or Lys-313, whereas binding of Langerin-Fc to 6-sulfo-GlcNAc was abolished by mutation of Lys-313, but not Lys-299. No effect of either mutation was observed on the binding to invertase. These results demonstrate that Lys-299 and Lys-313 form extended binding sites for the recognition of sulfated glycans. Binding mechanism of Langerin to 6′-sulfo-LacNAc and 6-sulfo-GlcNAc might be slightly different.
FIGURE 2.
Lys-299 and Lys 313 are extended binding sites for the recognition of sulfated glycans. A, WT and mutant forms of Langerin-Fc expression vectors were transfected into HEK293T cells, and 1 ml of the resulting culture supernatants was incubated with 10 μl of Protein A-Sepharose. The gels were then incubated with SDS sample buffer, and the eluted fractions were run on SDS-PAGE and blotted with peroxidase-labeled goat anti-human IgG (H+L). B, culture supernatants of WT and mutant forms of Langerin-Fc were precomplexed with Cy3-labeled anti-human Fcγ and analyzed by glycoconjugate microarray. Binding of WT and mutant forms of Langerin-Fc to invertase, 6′-sulfo-LacNAc, and 6-sulfo-GlcNAc are shown relative to WT.
KS6ST Generates Langerin Ligands
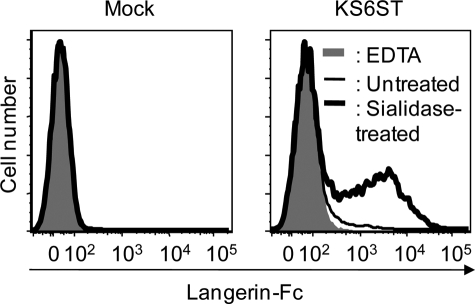
Having shown that Langerin-Fc chimera recognize 6′-sulfo-LacNAc as a preferred glycan ligand, we then examined whether KS6ST, which has activity to transfer sulfate to the C-6 of Gal of LacNAc on glycoproteins (18), is responsible for the generation of a Langerin ligand. CHO cells were transfected with the KS6ST expression plasmid with or without sialidase pretreatment, and binding of Langerin-Fc was analyzed by flow cytometry. As shown in Fig. 3, Langerin-Fc bound to sialidase-treated CHO cells transfected with KS6ST (bold line), whereas only weak binding was observed before sialidase pretreatment (thin line). This observation indicates that Langerin recognizes 6′-sulfo-LacNAc but not its sialylated form. Combined with the results obtained by glycoconjugate microarray, it is likely that Langerin recognizes 6′-sulfo-LacNAc on glycoproteins expressed at the cell surface, in which synthesis KS6ST is involved.
FIGURE 3.
KS6ST generates sulfated Langerin ligands. Untreated (thin line) and sialidase-treated CHO cells (bold line) transfected with or without a KS6ST (CHST1, keratan sulfate Gal-6-sulfotransferase) expression vector was incubated with Langerin-Fc chimera precomplexed with Cy3-labeled anti-human Fcγ in TSA buffer on ice for 1 h. After washing, cells were analyzed by flow cytometry. Binding of Langerin-Fc in the presence of 5 mm EDTA was taken as negative controls (gray).
Langerin Mediates Binding to and Uptake of 6′-Sulfo- LacNAc
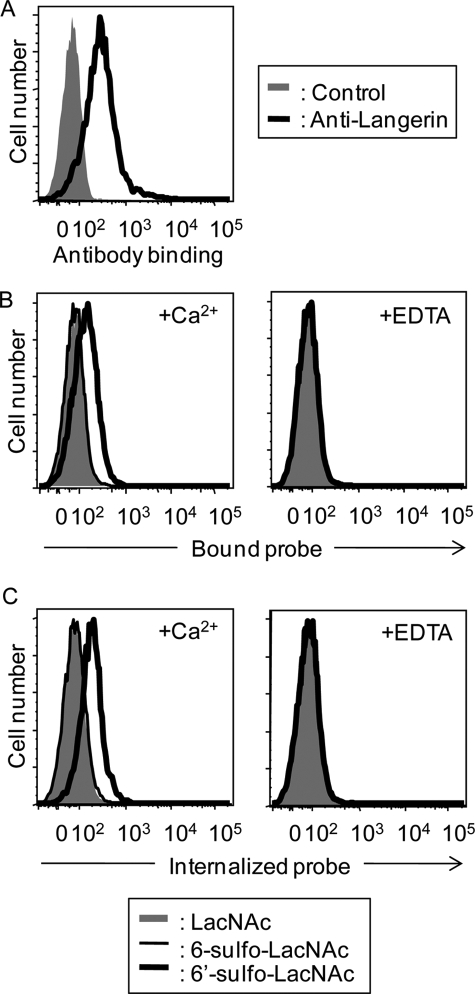
To assess the binding of Langerin to 6′-sulfo-LacNAc under more physiological conditions, we examined whether the full-length Langerin expressed at the cell surface also binds to this structure. For this purpose, CHO cells stably expressing Langerin (Langerin-CHO) were first established (Fig. 4A), and binding of biotin-labeled PAA probes precomplexed with Cy3-streptavidin to Langerin-CHO cells was detected by flow cytometry (Fig. 4B). The 6′-sulfo-LacNAc-PAA-biotin probe bound to Langerin-CHO cells (Fig. 4B, bold line), whereas no binding was observed with either 6-sulfo-LacNAc-PAA-biotin (Fig. 4B, thin line) or LacNAc-PAA-biotin probes (Fig. 4B, gray). Therefore, Gal-6-sulfate is considered to be essential for Langerin binding, in agreement with the results obtained by glycoconjugate microarray. Binding of the 6′-sulfo-LacNAc-PAA-biotin probe to Langerin-CHO cells was cancelled in the presence of EDTA, indicating that Langerin binding is dependent on the C-type CRD.
FIGURE 4.
Langerin expressed at the cell surface mediates binding to and uptake of 6′-sulfated LacNAc. A, expression of the full-length Langerin on CHO cells. Langerin-CHO cells were stained with goat anti-Langerin IgG followed by FITC-labeled anti-goat IgG (black line). Control represents staining using the secondary antibody (FITC-labeled anti-goat IgG) only (gray). B, probe binding assay. Glycoside-PAA probes precomplexed with Cy3-labeled streptavidin were incubated with Langerin-CHO cells on ice for 1 h in the presence of 2 mm CaCl2 and 2 mm MgCl2 (left panel, +Ca2+). Probe binding to Langerin-CHO cells was also analyzed in the presence of 5 mm EDTA (right panel, +EDTA). Bound fluorescence was analyzed by flow cytometry. C, probe internalization assay. Glycoside-PAA probes precomplexed with Cy3-labeled streptavidin were incubated with Langerin-CHO cells in the presence of 2 mm CaCl2 and 2 mm MgCl2 (left panel, +Ca2+) at 37 °C for 1 h. Probe internalization was also analyzed in the presence of 5 mm EDTA (right panel, +EDTA). Internalized fluorescence was analyzed by flow cytometry.
One of the roles of Langerin expressed on LCs is to bind antigens and internalize them into the BGs. Therefore, we next investigated whether Langerin could endocytose bound 6′-sulfo-LacNAc-PAA-biotin probes into intracellular compartments. For this purpose, Langerin-CHO cells were incubated with the biotin-labeled 6′-sulfo-LacNAc-PAA-biotin probe precomplexed with Cy3-streptavidin via a biotin tag at 37 °C for 60 min. After this internalization process, residual cell surface probes were removed by 5 mm EDTA, and internalized Cy3-labeled probes were detected by flow cytometry. As shown in Fig. 4C, internalization of the 6′-sulfo-LacNAc-PAA-biotin probe was detected after 60 min of incubation. On the other hand, apparently no internalization was observed with either 6-sulfo-LacNAc-PAA-biotin or LacNAc-PAA-biotin used as negative controls. These results demonstrate that 6′-sulfo-LacNAc serves as a glycan ligand for Langerin.
Langerin Recognizes Keratan Sulfate
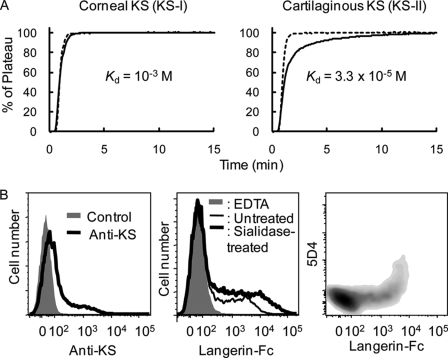
Keratan sulfate (KS) is a Gal-6-sulfated oligosaccharide consisting of a linear polymer of LacNAc, (Galβ1–4GlcNAcβ1–3)n that is sulfated to a varying degree on the C-6 of either GlcNAc or Gal residues. Therefore, it is possible that KS serves as an endogenous ligand for Langerin. To prove this, binding of Langerin to KS was first investigated by FAC using two types of natural KS polymers, bovine corneal KS (KS-I) and shark cartilaginous KS (KS-II). KS-II is more extensively sulfated than KS-I (19). In Fig. 5A, Langerin showed much stronger affinity to highly sulfated KS-II (Kd = 3.3 × 10−5 m) than KS-I (Kd = 10−3 m). Similarly, no binding signal was detected for KS-I (91) by glycoconjugate microarray (Fig. 1). Weak, but significant binding was also observed for a KS-related glycan, 922 ([6S]Galβ1- 4GlcNAcβ1–3[6S]Galβ1–4GlcNAc, Kd = 7 × 10−2 m). On the other hand, Langerin showed no binding to other glycans (for the list of standard 142 glycans, see supplemental Fig. 2) representing N-glycans, O-glycans, glycolipid-type glycans, and milk oligosaccharides, including 3′-sulfolactose (918). Binding affinity to KS-II was found to be 100-fold higher than to high mannose-type N-glycans (Kd > 10−3) under the current experimental condition. It seems that Langerin requires multivalency for the recognition of mannosylated glycans.
FIGURE 5.
Langerin recognizes KS. A, binding of Langerin-Fc chimera to KS analyzed by FAC. Dotted lines represent PA-labeled lactose used as negative controls. Solid lines represent PA-labeled corneal (left panel) and cartilaginous KS (right panel). B, CHO cells transfected with both KS6ST and GlcNAc6ST cDNA expression vectors were stained with anti-highly sulfated KS mAb (5D4) followed by incubation with FITC-labeled donkey anti-mouse IgG (left panel), Langerin-Fc chimera precomplexed with Cy3-labeled anti-human Fcγ (middle panel), and double stained with both 5D4 and Langerin-Fc chimera (right panel) with or without sialidase pretreatment. Control represents staining using the secondary antibody (Cy3-labeled donkey anti-mouse IgG) only (left panel, gray). As a negative control, binding of Langerin-Fc was also analyzed in the presence of 5 mm EDTA (middle panel, gray).
We then investigated whether Langerin binds to highly sulfated KS expressed at the cell surface. For this purpose, we generated KS-expressing cells by double transfection with both KS6ST and GlcNAc6ST into CHO cells. In the KS synthesis, KS6ST has activity to transfer sulfate to the C-6 of Gal, whereas GlcNAc6ST acts to transfer sulfate to the C-6 of GlcNAc. As shown in Fig. 5B, left panel, double transfected-CHO cells were stained with 5D4 mAb, which is specific for highly sulfated KS. Therefore, the double- transfected cells are considered to produce KS on cell surfaces. Langerin-Fc chimera exhibited binding to the double-transfected CHO cells even without sialidase treatment (Fig. 5B, middle panel). Upon sialidase treatment, however, much enhanced binding was observed, consistent with the previous experiment showing sialylation inhibits Langerin binding (Fig. 3). Although Langerin-Fc bound to both 5D4-positive and -negative CHO cells, the lectin gave stronger binding on 5D4-positive CHO cells (Fig. 5B, right panel). Similar results were obtained by cotransfection with KS6ST and I-GlcNAc6ST (CHST5, intestinal N-acetylglucosamine 6-O-sulfotransferase) (data not shown) (20). Therefore, it is strongly suggested that cells expressing highly sulfated KS serve as target ligands for Langerin.
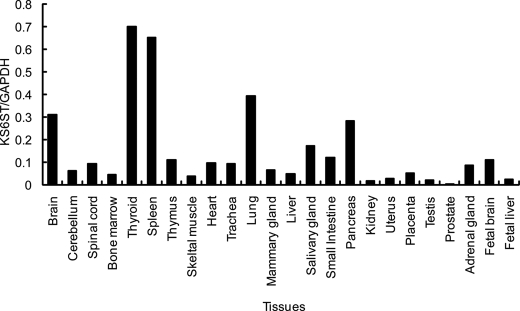
Quantitative Real-time PCR Analysis of the KS6ST Transcript in Human Tissues
Having shown that KS6ST is responsible for transferring sulfate to the C-6 of Gal recognized by Langerin, we performed quantitative real-time PCR analysis to see which human tissues express KS6ST mRNA. Previously, KS6ST mRNA was reported to be expressed in brain (21) and spleen (18) analyzed by Northern blotting. Consistently, the expression of KS6ST mRNA was high in brain and spleen (Fig. 6), and expression was also detected in lung, pancreas, and thyroid tissues.
FIGURE 6.
Quantitative real-time PCR analysis of the KS6ST transcript in human tissues. Data are shown relative to the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript.
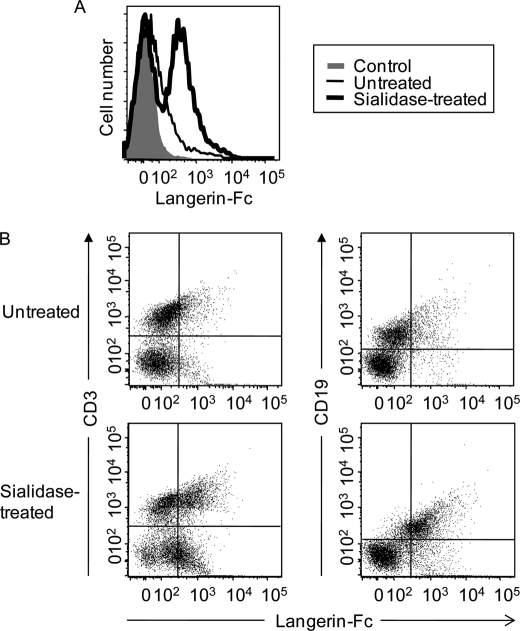
Langerin Ligands Are Expressed in Mouse Spleen
Because spleen was one of the dominant tissues expressing KS6ST mRNA, we analyzed expression of Langerin ligands using mouse splenocytes and Langerin-Fc chimera as a probe. As shown in Fig. 7A, Langerin-Fc chimera bound to splenocytes even without sialidase pretreatment. Approximately 50% of splenocytes were estimated to be positive for Langerin binding. However, the binding was strongly increased with the sialidase pretreatment, again confirming that the sialylation event is inhibitory to some degree on Langerin binding to sulfated ligands. Langerin-Fc bound to both CD3+ T and CD19+ B cells even without sialidase treatment, and the binding was enhanced after sialidase treatment, indicating that sulfated Langerin ligands are expressed on both intact CD3+ T and CD19+ B cells (Fig. 7B).
FIGURE 7.
Binding of Langerin-Fc to mouse splenocytes. A, mouse splenocytes were incubated with Langerin-Fc chimera precomplexed with Cy3-labeled anti-human Fcγ with or without sialidase pretreatment. B, mouse splenocytes were double stained with FITC-labeled anti-CD3/Langerin-Fc and FITC-labeled anti-CD19/Langerin-Fc with or without sialidase pretreatment.
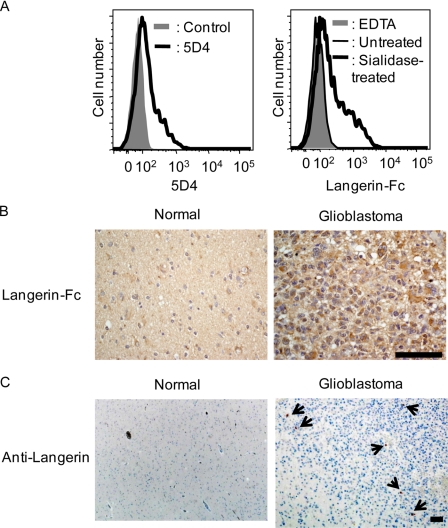
Brain Glioblastoma Is a Candidate Target for Langerin
Recently, an LN229 glioblastoma cell line was shown to express 5D4-positive highly sulfated KS (22). If our assumption that highly sulfated KS is an endogenous ligand for Langerin is the case, such glioblastoma cells should be targets for Langerin recognition. Thus, we examined the binding ability of Langerin to KS-expressing glioblastoma cells by flow cytometry. As shown in Fig. 8A, left panel, LN229 cells were stained with 5D4 mAb, confirming that highly sulfated KS is indeed expressed on the cells (22). Langerin-Fc chimera bound to LN229 cells with sialidase pretreatment, whereas little or no binding was observed for untreated LN229 cells (Fig. 8A, right panel) consistent with the previous observations (Fig. 3). The results provide the possibility that glioblastoma cells are target cells for Langerin in vivo.
FIGURE 8.
Glioblastoma is a candidate target for Langerin. A, LN229 glioblastoma cells were stained with 5D4 followed by incubation with Cy3-labeled donkey anti-mouse IgG (left panel) and Langerin-Fc chimera precomplexed with Cy3-labeled anti-human Fcγ (right panel) with or without sialidase pretreatment. Data were analyzed by flow cytometry. B, normal brain (left panel) and glioblastoma tissue sections (right panel) were stained with Langerin-Fc chimera. C, normal brain (left panel) and glioblastoma tissue sections (right panel) were stained with anti-Langerin mAb (12D6). Bar, 50 μm.
KS6ST mRNA as well as its product, KS, is expressed in brain (19, 21) (Fig. 6). Recently, the expression of 5D4-positive highly sulfated KS was reported to be up-regulated depending on the malignancy of astrocytic tumors (23). Because Langerin bound to LN229 glioblastoma cells (Fig. 8A), we hypothesized that glioma cells could be an endogenous target for Langerin. Brain tissue sections were incubated with Langerin-Fc chimera, and the binding was observed by microscopy. As shown in Fig. 8B, left panel, normal brain tissues were weakly, but significantly, stained with Langerin-Fc. On the other hand, greatly enhanced staining was observed for grade IV astrocytic tumors (Fig. 8B, right panel), where prominent staining was detected at the cell surface of tumor cells. Because the staining was inhibited by EDTA, the binding is attributed to the C-type CRD (data not shown). These results indicate that Langerin ligands are expressed at a basal level even in normal brain tissues, whereas their expression is dramatically up-regulated in malignancy.
We then examined the expression of Langerin in normal brain and glioma tissues using anti-human Langerin mAb (12D6) (Fig. 8C). From a histopathological viewpoint, glioma are classified into grades I to IV based on the degree of malignancy. Glioma includes astrocytoma, oligodendrocytoma, ependymoma, and oligoastrocytoma. Among them, astrocytoma is the most common. Langerin-expressing cells were detected in two clinical cases, including astrocytoma and malignant ependymoma, but not in normal brain tissues. In grade IV astrocytoma (glioblastoma), Langerin-expressing cells were diffusely localized in vascular-rich stromal tissues (Fig. 8C, right panel). Morphologically, however, Langerin-expressing cells are not tumor cells, but are probably dendritic cell-like cells infiltrated from blood vessels.
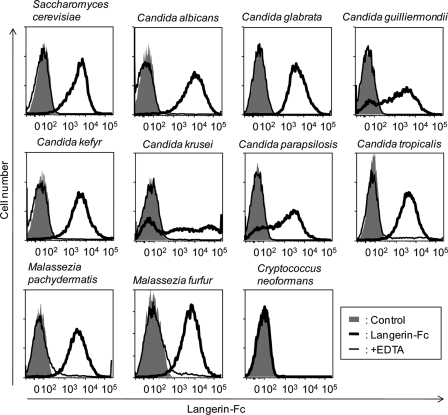
Langerin Recognizes Live Pathogenic Fungi
So far, we have described sulfated glycans as the most possible Langerin ligands. However, Langerin-Fc chimera also bound to heavily mannosylated ligands, such as yeast mannan and invertase analyzed by glycoconjugate microarray (Fig. 1). In this regard, LCs basically residing in skin are believed to be involved in sensing and capturing invading pathogens for efficient antigen presentation to naïve T cells present in draining lymph node. To investigate this point from a glycobiological viewpoint, we extended analysis by examining whether Langerin recognizes live pathogenic fungi, such as Candida, Malassezia, and Cryptococcus, by flow cytometry. As shown in Fig. 9, Langerin-Fc chimera precomplexed with Cy3-labeled donkey anti-human Fcγ strongly bound to fungi in a Ca2+-dependent manner, which include both pathogenic (Candida and Malassezia) and non-pathogenic (Saccharomyces) species. On the other hand, no significant binding was observed for pathogenic Cryptococcus neoformans. These results indicate that Langerin recognizes extensive categories of fungi, consistent with an intrinsic role of LCs in anti-fungal immunity.
FIGURE 9.
Binding of Langerin-Fc to live pathogenic fungi. Langerin-Fc fusion proteins (0.5 μg/ml) were precomplexed with Cy3-labeled anti-human Fcγ (0.5 μg/ml) in TSA buffer at room temperature for 30 min and then incubated with 1 × 105 of fungi at 4 °C for 1 h (bold line). Binding was analyzed by flow cytometry. Langerin-Fc was also incubated with fungi in the presence of EDTA (thin line). Control represents staining using Cy3-labeled anti-human Fcγ only (gray).
DISCUSSION
There are lines of evidence that sulfation of cell surface glycoproteins plays critical roles in cell-cell communications. GlcNAc 6-sulfate is involved in several recognition phenomena, including selectin-mediated lymphocyte homing (24), activation of CD44-mediated cellular interaction (25), and dendritic cell function (26). GlcNAc 6-sulfate even serves as a ligand for siglecs, such as CD22 (Siglec-2) (27, 28) and Siglec-9 (29). In contrast, the functional importance of Gal 6-sulfate is much less understood, although Gal-6-sulfated sialyl LewisX was shown to be a candidate ligand for Siglec-8 (30) and its mouse paralog, Siglec-F (29, 31). In 2004, Galustian et al. (11) reported binding activity of Langerin to sulfated LewisX glycans, although the importance of sulfation on Langerin recognition has not been fully elucidated. In this study, we have obtained clear evidence that Langerin recognizes Gal-6-sulfated lactosamine (6′-sulfo-LacNAc) and polylactosamine (KS), although Langerin is a C-type lectin with the consensus EPN motif predicting basic specificity to Man. Sialylation clearly inhibited Langerin binding to Gal-6-sulfated glycans unlike the results obtained by Galustian et al. showing that Langerin binds to both sialylated and non-sialylated Gal-6-sulfated glycans (11). The two lysine residues, Lys-299 and Lys-313, were found to be involved in the recognition of sulfated ligands. These positively charged amino acids in the CRD are likely involved in the formation of salt bridges with negatively charged sulfated ligands. Further studies are required to understand the more precise binding mechanism of Langerin to its sulfated ligands.
Langerin exhibited strong binding to KS. KS was first identified in bovine cornea in 1953 (32) and was later shown to be expressed in cartilage, bone, and the central nervous system (19). In fact, KS is expressed on a subpopulation of microglia (33) and spinal cord (34). Interestingly, KS synthesis is up-regulated in the lesions on central nervous system injury (35). Recently, 5D4-positive highly sulfated KS was reported to be up-regulated depending on the malignancy of astrocytic tumors (22). Therefore, Gal-6 sulfation is considered to be closely involved in brain biology. In this report, we showed that Langerin ligands are strongly expressed in malignant astrocytic tumors. However, Langerin also binds to heavily mannosylated glycans such as mannan but not to glycoproteins containing human-type high mannose-type N-glycans such as thyroglobulin (Fig. 1). Increased expression of heavily mannosylated glycans in glioblastoma has not been reported. Therefore, it is reasonable to speculate that Langerin binds to glioblastoma tissues via Gal-6-sulfated glycans. Consistently, Langerin bound to the KS-expressing LN229 glioblastoma cell line after sialidase treatment, most probably via Gal-6-sulfated glycans. In LN229, Gal-6-sulfated glycans were sialylated. However, glycans expressed on tissues are much more heterogeneous. Therefore, it is not surprising that non-sialylated Gal-6-sulfated glycans are expressed in the glioblastoma tissues. Coincidently, Langerin-expressing cells were found to be localized in glioma, but not in normal brain tissues. Therefore, LCs could interact with glioma cells via the Langerin C-type CRD. The consequences of the interactions between LCs and astrocytic tumors were not studied in this report, but it could induce anti-tumor immunity or immune tolerance. Further studies are essential to understand the functions of Gal-6-sulfated glycans in the brain. Generation of antibody specific for Gal-6 sulfation would be helpful for this purpose.
The interactions between C-type lectins and tumor-associated glycans have previously been described. Mannan-binding protein recognizes human colorectal carcinoma cells through clusters of tandem repeats of the Lewisb/Lewisa epitopes, resulting in anti-tumor immunity (36, 37). DC-SIGN was also shown to recognize colorectal cancer cells through the recognition of LewisX and LewisY antigens present on carcinoembryonic antigen, whereas normal colon tissues with low levels of LewisX and LewisY antigens do not interact with DC-SIGN (38, 39). Macrophage galactose-binding lectin was reported to recognize Tn antigens present on MUC1, which are tumor-associated glycosylation in colon carcinoma (40). Therefore, it is possible that Langerin may be a novel member of C-type lectins, which recognize tumor-specific glycosylation and is involved in anti-tumor immunity.
Langerin also exhibited binding to heavily mannosylated glycans such as mannan, which is a major structural component of fungal cell walls. Previously, Takahara et al. (41) reported that Langerin mediates the uptake of C. albicans. In this study, we have confirmed the binding ability of Langerin to live pathogenic fungi. Langerin was found to recognize a series of Candida species as well as Malassezia furfur in a Ca2+-dependent manner, whereas no binding was observed for C. neoformans. Indeed, there is a striking difference between the structures of mannosylated glycans in their cell walls, e.g. between C. albicans (42) and C. neoformans (43): C. albicans expresses N- and O-linked mannoproteins, whereas C. neoformans expresses glucuronoxylomannan as a major component. Together with the observation that Langerin exhibits no binding to thyroglobulin expressing high mannose-type N-glycans, mannose multivalency, geometry, and/or spatial distribution might be important factors for Langerin recognition (44).
In this study, Langerin was shown to bind to sulfated as well as mannosylated glycans. We have also demonstrated its potential functions on glioma biology and anti-fungal immunity to be exerted through the C-type CRD. The mannose receptor expressed on macrophages, a member of the C-type lectin, is known to bind to both sulfated and mannosylated glycans. In this case, however, such dual specificities are attributed to distinct CRDs, of which the evolutionary origins are different: the former is performed by an R-type CRD, whereas the latter is performed by consecutive C-type CRDs present in a single polypeptide. In this context, Langerin is extremely unique among C-type lectins, recognizing both sulfated and mannosylated glycans via a single C-type CRD. Such specificity of Langerin should be directly involved in the functions of LCs.
Supplementary Material
Acknowledgments
We thank M. Fukumura, Y. Kubo, and K. Kiyohara for technical assistance.
This work was supported in part by the New Energy and Industrial Technology Development Organization, grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan (to H. T.), and the National BioResource Project in Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Table 1.
- LC
- Langerhans cell
- CHO
- Chinese hamster ovary
- CRD
- carbohydrate recognition domain
- DC-SIGN
- dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin
- FAC
- frontal affinity chromatography
- Gal
- d-galactose
- KS
- keratan sulfate
- KS-I
- bovine corneal KS
- KS-II
- shark cartilaginous KS
- LacNAc
- N-acetyllactosamine
- Man
- d-mannose
- PA
- pyridylamino
- BG
- Birbeck granule
- mAb
- monoclonal antibody
- HIV-1
- human immunodeficiency virus, type 1
- FITC
- fluorescein isothiocyanate
- PAA
- polyacrylamide.
REFERENCES
- 1.Romani N., Holzmann S., Tripp C. H., Koch F., Stoitzner P. (2003) APMIS 111, 725–740 [DOI] [PubMed] [Google Scholar]
- 2.Valladeau J., Ravel O., Dezutter-Dambuyant C., Moore K., Kleijmeer M., Liu Y., Duvert-Frances V., Vincent C., Schmitt D., Davoust J., Caux C., Lebecque S., Saeland S. (2000) Immunity 12, 71–81 [DOI] [PubMed] [Google Scholar]
- 3.Valladeau J., Dezutter-Dambuyant C., Saeland S. (2003) Immunol. Res. 28, 93–107 [DOI] [PubMed] [Google Scholar]
- 4.Weis W. I., Taylor M. E., Drickamer K. (1998) Immunol. Rev. 163, 19–34 [DOI] [PubMed] [Google Scholar]
- 5.McDermott R., Bausinger H., Fricker D., Spehner D., Proamer F., Lipsker D., Cazenave J. P., Goud B., De La Salle H., Salamero J., Hanau D. (2004) J. Invest. Dermatol. 123, 72–77 [DOI] [PubMed] [Google Scholar]
- 6.Idoyaga J., Suda N., Suda K., Park C. G., Steinman R. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1524–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Witte L., Nabatov A., Pion M., Fluitsma D., de Jong M. A., de Gruijl T., Piguet V., van Kooyk Y., Geijtenbeek T. B. (2007) Nat. Med. 13, 367–371 [DOI] [PubMed] [Google Scholar]
- 8.Kissenpfennig A., Aït-Yahia S., Clair-Moninot V., Stössel H., Badell E., Bordat Y., Pooley J. L., Lang T., Prina E., Coste I., Gresser O., Renno T., Winter N., Milon G., Shortman K., Romani N., Lebecque S., Malissen B., Saeland S., Douillard P. (2005) Mol. Cell Biol. 25, 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thépaut M., Valladeau J., Nurisso A., Kahn R., Arnou B., Vivès C., Saeland S., Ebel C., Monnier C., Dezutter-Dambuyant C., Imberty A., Fieschi F. (2009) Biochemistry 18, 2684–2698 [DOI] [PubMed] [Google Scholar]
- 10.Stambach N. S., Taylor M. E. (2003) Glycobiology 13, 401–410 [DOI] [PubMed] [Google Scholar]
- 11.Galustian C., Park C. G., Chai W., Kiso M., Bruening S. A., Kang Y. S., Steinman R. M., Feizi T. (2004) Int. Immunol. 16, 853–866 [DOI] [PubMed] [Google Scholar]
- 12.Tateno H., Mori A., Uchiyama N., Yabe R., Iwaki J., Shikanai T., Angata T., Narimatsu H., Hirabayashi J. (2008) Glycobiology 18, 789–798 [DOI] [PubMed] [Google Scholar]
- 13.Tateno H., Nakamura-Tsuruta S., Hirabayashi J. (2007) Nat. Protoc. 2, 2529–2537 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura-Tsuruta S., Kominami J., Kamei M., Koyama Y., Suzuki T., Isemura M., Hirabayashi J. (2006) J. Biochem. 140, 285–291 [DOI] [PubMed] [Google Scholar]
- 15.Sato T., Sato M., Kiyohara K., Sogabe M., Shikanai T., Kikuchi N., Togayachi A., Ishida H., Ito H., Kameyama A., Gotoh M., Narimatsu H. (2006) Glycobiology 16, 1194–1206 [DOI] [PubMed] [Google Scholar]
- 16.Weis W. I., Drickamer K. (1996) Annu. Rev. Biochem. 65, 441–473 [DOI] [PubMed] [Google Scholar]
- 17.Chatwell L., Holla A., Kaufer B. B., Skerra A. (2008) Mol. Immunol. 45, 1981–1994 [DOI] [PubMed] [Google Scholar]
- 18.Torii T., Fukuta M., Habuchi O. (2000) Glycobiology 10, 203–211 [DOI] [PubMed] [Google Scholar]
- 19.Funderburgh J. L. (2000) Glycobiology 10, 951–958 [DOI] [PubMed] [Google Scholar]
- 20.Akama T. O., Nakayama J., Nishida K., Hiraoka N., Suzuki M., McAuliffe J., Hindsgaul O., Fukuda M., Fukuda M. N. (2001) J. Biol. Chem. 276, 16271–16278 [DOI] [PubMed] [Google Scholar]
- 21.Fukuta M., Inazawa J., Torii T., Tsuzuki K., Shimada E., Habuchi O. (1997) J. Biol. Chem. 272, 32321–32328 [DOI] [PubMed] [Google Scholar]
- 22.Hayatsu N., Ogasawara S., Kaneko M. K., Kato Y., Narimatsu H. (2008) Biochem. Biophys. Res. Commun. 368, 217–222 [DOI] [PubMed] [Google Scholar]
- 23.Kato Y., Hayatsu N., Kaneko M. K., Ogasawara S., Hamano T., Takahashi S., Nishikawa R., Matsutani M., Mishima K., Narimatsu H. (2008) Biochem. Biophys. Res. Commun. 369, 1041–1046 [DOI] [PubMed] [Google Scholar]
- 24.Uchimura K., Kadomatsu K., El-Fasakhany F. M., Singer M. S., Izawa M., Kannagi R., Takeda N., Rosen S. D., Muramatsu T. (2004) J. Biol. Chem. 279, 35001–35008 [DOI] [PubMed] [Google Scholar]
- 25.Delcommenne M., Kannagi R., Johnson P. (2002) Glycobiology 12, 613–622 [DOI] [PubMed] [Google Scholar]
- 26.Schäkel K., Kannagi R., Kniep B., Goto Y., Mitsuoka C., Zwirner J., Soruri A., von Kietzell M., Rieber E. (2002) Immunity 17, 289–301 [DOI] [PubMed] [Google Scholar]
- 27.Kimura N., Ohmori K., Miyazaki K., Izawa M., Matsuzaki Y., Yasuda Y., Takematsu H., Kozutsumi Y., Moriyama A., Kannagi R. (2007) J. Biol. Chem. 282, 32200–32207 [DOI] [PubMed] [Google Scholar]
- 28.Blixt O., Head S., Mondala T., Scanlan C., Huflejt M. E., Alvarez R., Bryan M. C., Fazio F., Calarese D., Stevens J., Razi N., Stevens D. J., Skehel J. J., van Die I., Burton D. R., Wilson I. A., Cummings R., Bovin N., Wong C. H., Paulson J. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17033–17038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campanero-Rhodes M. A., Childs R. A., Kiso M., Komba S., Le Narvor C., Warren J., Otto D., Crocker P. R., Feizi T. (2006) Biochem. Biophys. Res. Commun. 344, 1141–1146 [DOI] [PubMed] [Google Scholar]
- 30.Bochner B. S., Alvarez R. A., Mehta P., Bovin N. V., Blixt O., White J. R., Schnaar R. L. (2005) J. Biol. Chem. 280, 4307–4312 [DOI] [PubMed] [Google Scholar]
- 31.Tateno H., Crocker P. R., Paulson J. C. (2005) Glycobiology 15, 1125–1135 [DOI] [PubMed] [Google Scholar]
- 32.Meyer K., Linker A., Davidson E. A., Weissmann B. (1953) J. Biol. Chem. 205, 611–616 [PubMed] [Google Scholar]
- 33.Bertolotto A., Agresti C., Castello A., Manzardo E., Riccio A. (1998) J. Neuroimmunol. 85, 69–77 [DOI] [PubMed] [Google Scholar]
- 34.Jander S., Stoll G. (1996) Glia 18, 255–260 [DOI] [PubMed] [Google Scholar]
- 35.Jones L. L., Tuszynski M. H. (2002) J. Neurosci. 22, 4611–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y., Uemura K., Oka S., Kozutsumi Y., Kawasaki N., Kawasaki T. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terada M., Khoo K. H., Inoue R., Chen C. I., Yamada K., Sakaguchi H., Kadowaki N., Ma B. Y., Oka S., Kawasaki T., Kawasaki N. (2005) J. Biol. Chem. 280, 10897–10913 [DOI] [PubMed] [Google Scholar]
- 38.van Gisbergen K. P., Aarnoudse C. A., Meijer G. A., Geijtenbeek T. B., van Kooyk Y. (2005) Cancer Res. 65, 5935–5944 [DOI] [PubMed] [Google Scholar]
- 39.Nonaka M., Ma B. Y., Murai R., Nakamura N., Baba M., Kawasaki N., Hodohara K., Asano S., Kawasaki T. (2008) J. Immunol. 180, 3347–3356 [DOI] [PubMed] [Google Scholar]
- 40.Saeland E., van Vliet S. J., Bäckström M., van den Berg V. C., Geijtenbeek T. B., Meijer G. A., van Kooyk Y. (2007) Cancer Immunol. Immunother. 56, 1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahara K., Yashima Y., Omatsu Y., Yoshida H., Kimura Y., Kang Y. S., Steinman R. M., Park C. G., Inaba K. (2004) Int. Immunol. 16, 819–829 [DOI] [PubMed] [Google Scholar]
- 42.Nelson R. D., Shibata N., Podzorski R. P., Herron M. J. (1991) Clin. Microbiol. Rev. 4, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherniak R., Sundstrom J. B. (1994) Infect. Immun. 62, 1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor M. E., Drickamer K. (2009) Glycobiology 19, 1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.