Abstract
Calcineurin is a protein phosphatase that is uniquely regulated by sustained increases in intracellular Ca2+ following signal transduction events. Calcineurin controls cellular proliferation, differentiation, apoptosis, and inducible gene expression following stress and neuroendocrine stimulation. In the adult heart, calcineurin regulates hypertrophic growth of cardiomyocytes in response to pathologic insults that are associated with altered Ca2+ handling. Here we determined that calcineurin signaling is directly linked to the proper control of cardiac contractility, rhythm, and the expression of Ca2+-handling genes in the heart. Our approach involved a cardiomyocyte-specific deletion using a CnB1-LoxP-targeted allele in mice and three different cardiac-expressing Cre alleles/transgenes. Deletion of calcineurin with the Nkx2.5-Cre knock-in allele resulted in lethality at 1 day after birth due to altered right ventricular morphogenesis, reduced ventricular trabeculation, septal defects, and valvular overgrowth. Slightly later deletion of calcineurin with the α-myosin heavy chain Cre transgene resulted in lethality in early mid adulthood that was characterized by substantial reductions in cardiac contractility, severe arrhythmia, and reduced myocyte content in the heart. Young calcineurin heart-deleted mice died suddenly after pressure overload stimulation or neuroendocrine agonist infusion, and telemetric monitoring of older mice showed arrhythmia leading to sudden death. Mechanistically, loss of calcineurin reduced expression of key Ca2+-handling genes that likely lead to arrhythmia and reduced contractility. Loss of calcineurin also directly impacted cellular proliferation in the postnatal developing heart. These results reveal multiple mechanisms whereby calcineurin regulates cardiac development and myocyte contractility.
Keywords: Calcium/Calcineurin, Development Differentiation, Diseases/Muscular, Genetics/Mouse, Phosphorylation/Phosphatases/Serine-Threonine, Signal Transduction/Calcium
Introduction
Calcineurin (protein phosphatase 2B) is a Ca2+-calmodulin-activated, serine/threonine protein phosphatase that responds to sustained elevations in intracellular Ca2+ (1, 2). Once activated, calcineurin directly dephosphorylates nuclear factor of activated T cells (NFAT)4 transcription factors within the cytoplasm, promoting their translocation into the nucleus and the activation of gene expression (2). Calcineurin-NFAT signaling is critically involved in regulating a diverse range of biologic processes including T lymphocyte development and reactivity, development of the nervous and vascular systems, fiber-type switching in skeletal muscle, development of heart valves, development of bone, and the control of cardiac hypertrophy (1–3). Although NFAT transcription factors are critical downstream effectors of calcineurin signaling in vertebrates, a number of important functions of calcineurin are independent of NFAT. For example, calcineurin dephosphorylates various synaptic proteins and contributes to vesicle processing in neurons (4–6). Calcineurin can also regulate myocyte enhancer factor-2 (MEF-2) transcriptional activity, activity of the ATP-sensitive K+ channel Kir6.1, the mitochondrial regulatory factor dynamin-related protein 1 (Drp1), the proapoptotic Bcl-2 family member Bad, the inositol 1,4,5-trisphosphate receptor to modulate Ca2+ release, the kinesin heavy chain to regulate insulin secretion, and many other proteins as well (7–12).
Calcineurin consists of a 57–61-kDa catalytic subunit (CnA) and a 16–19-kDa EF-hand regulatory subunit (CnB). CnA and CnB are obligate heterodimers such that if CnB is missing, CnA becomes unstable and is degraded (13, 14). There are three genes that encode the CnA catalytic subunit (CnAα, CnAβ, and CnAγ) and two genes that encode the CnB regulatory subunit (CnB1 and CnB2). The mammalian heart only expresses CnAα, CnAβ, and CnB1, whereas CnAγ and CnB2 expression are restricted to the brain and testis (2, 15). Mice with single disruption of CnAα or CnAβ are each capable of generating viable adults (16, 17), although CnAα/β double nulls that lack all calcineurin activity are early embryonic lethal (18). Mice lacking the regulatory subunit gene CnB1 are also lethal during embryonic life as they lack all calcineurin activity (19). Hence, loxP-targeted CnB1 mice have been generated as a means of deleting all calcineurin activity from any desired tissue. For example, Cre-LoxP-mediated deletion of CnB1 from skeletal muscle (>90% deletion of all calcineurin) reduced the slow/oxidative fiber-type program and resulted in a comparable loss of CnA protein (14). The CnB1-LoxP-targeted mouse has also been used to disrupt all calcineurin activity early in development of T-cells, endothelial cells, skin, neural crest cells, lung, urinary tract, and pancreatic β-cells (20–26). These various tissue-specific deletion approaches have revealed critical functions for calcineurin signaling in regulating cellular viability, differentiation, and proliferation. Here we investigated the requirement of calcineurin signaling within the cardiac myocyte of both the developing and the adult heart.
MATERIALS AND METHODS
Animal Models
All experimental procedures with animals were approved by the Institutional Animals Care and Use Committee. CnB1-LoxP (fl)-targeted mice were described previously (20). CnB1WT/WT and CnB1fl/fl mice were crossed with the Nkx2.5-Cre knock-in allele and the cardiac-specific cardiac troponin T (cTnT)-Cre and α-myosin heavy chain (αMHC)-Cre transgenic lines (27–29). The cardiac-specific NFAT-luciferase reporter transgenic mice were described elsewhere and were back-crossed for at least six generations into the C57BL/6 background (30). The transverse aortic constriction (TAC) surgical procedure for cardiac pressure overload stimulation was previously described (30). Cardiac ventricular performance and chamber dimensions were assayed by trans-thoracic echocardiography (31). Alzet minipumps (Durect Corp., Cupertino CA) were implanted subcutaneously following a routine surgical procedure (32). They released angiotensin II (Ang) and phenylephrine (PE) (respectively, 432 μg/kg/day and 100 mg/kg/day dissolved in 150 mm NaCl, (0.01 n acetic acid) or isoprenaline (60 mg/kg/day in phosphate-buffered saline) for 2 weeks. The physiological hypertrophy response was assessed by a 3-week protocol of forced swimming (30). Continuous electrocardiogram (ECG) recordings were made by radiotelemetry using Data Sciences International model ETA-F20 biopotential implants (St. Paul, MN). The devices were implanted intra-abdominally under isoflurane anesthesia, and the leads were secured subcutaneously at the right shoulder and left abdominal wall (Lead II). ECG recordings were made continuously for up to 7 days at a sampling rate of 2 kHz using an AD Instruments PowerLab data acquisition unit. Mice were sacrificed by CO2 asphyxiation, and heart weights were determined after atria removal. For quantification of ventricular proliferation, 3 day-old neonates were injected intraperitoneal with bromodeoxyuridine (BrdUrd) labeling reagent according to the manufacturer's instructions (Zymed Laboratories Inc., San Francisco, CA). Adult cardiomyocytes were isolated from 5-week-old mice as described previously (33).
Sarcomere Contractility and Ca2+ Transients
Sarcomere length measurements and Ca2+ transients were made using a PTI system (Photon Technology International, Birmingham, NJ) following a previously described procedure (34). Briefly, freshly isolated adult cardiomyocytes were cultured for 2 h in plating medium (medium 199 containing 1.8 mm Ca2+, 1% penicillin and streptomycin, 5% bovine calf serum, 1 mg/ml 2,3-butanedione 2-monoxine) and were switched 30 min before the experiment to culture medium (medium 199, 1% penicillin and streptomycin, 1 μg/ml bovine serum albumin). For measurements, myocytes were electrically stimulated at 60 volts at a frequency of 0.5 Hz. Myocyte contraction was measured using video edge detection and averaged over 10 contraction cycles. Myocytes used for Ca2+ transients were first incubated for 15 min in culture medium containing 5 μm Fura-2 AM (Molecular Probes, Invitrogen). Ca2+ transients were averaged over 10 cycles, and kinetics were analyzed as described previously (34).
Cell Culture and Infections
Primary mouse embryonic fibroblasts (MEFs) were obtained from embryonic day 13.5 CnB1-LoxP embryos and cultured as described previously (35). Passage 4 MEFs were infected for 24 h with β-galactosidase, Cre, constitutively active CnA (ΔCnA), or NFAT-luciferase reporter adenoviruses. After infection, MEFs were switched to Dulbecco's modified Eagle's medium containing 0.5% heat-inactivated bovine calf serum for 72 h before experiments.
Histological Analysis, Immunostaining, and Electron Microscopy
Hearts were collected at the indicated time, fixed in 10% formalin (phosphate-buffered saline-buffered), dehydrated, and embedded in paraffin. Global heart architecture was determined from longitudinal 5-μm deparaffinized sections stained with hematoxylin and eosin. Fibrosis and glycogen heart content were respectively detected with Masson's trichrome and periodic acid-Schiff staining. For cell area measurements, 5-μm heart sections were deparaffinized and stained with fluorescein isothiocyanate- or TRITC-labeled lectin (Sigma) and TO-PRO-3 iodine (Molecular Probes, Invitrogen). Five-week-old isolated adult myocytes were immediately fixed with 4% paraformaldehyde, plated on polylysine-coated slides (Sigma), and incubated for 1 h with bisbenzimide (100 μg/ml) to show nuclei. A minimum of 500 myocytes from three different animals was quantified for each experimental group using ImageJ 1.33 software (Scion Corp., Frederick, MD). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) was measured 1 day after TAC or 3 days of isoprenaline stimulation using the TMR Red in situ detection kit according to the manufacturer's instructions (Roche Diagnostics). Membranes and nuclei were counterstained with fluorescein isothiocyanate-labeled lectin and TO-PRO-3 iodine (31). A minimum of 100,000 myocytes from three different animals was analyzed for each experimental group. Quantification of ventricular proliferation was performed as described previously (36). Briefly, 3-day-old hearts were harvested after 4 h of BrdUrd labeling. Cardiomyocyte BrdUrd incorporation was identified on 5-μm paraffin sections labeled with an antibody against BrdUrd (1/50, Molecular Probes, Invitrogen) and GATA4 (1/50, Santa Cruz Biotechnology, Santa Cruz, CA). Anti-phospho-Ser-10-histone H3 staining (1/50, Upstate Biotechnology, Billerica, MA) and anti-CD31 (1/100, Chemicon) staining were performed as described previously (36, 37). For transmission electron microscopy, embryonic day 18.5 CnB1fl/flNkx2.5-Cre embryos were fixed in glutaraldehyde and cacodylate, embedded in epoxy resin and sectioned, and counterstained with uranyl acetate and lead citrate as described previously (38).
NFAT Reporter Assays
Hearts from NFAT-luciferase reporter transgenic mice or NFAT-luciferase-infected MEFs were homogenized in lysis buffer (100 mm KPO4, 0.5% Nonidet P-40, 1 mm dithiothreitol) and luciferase activity was assayed in luciferase buffer (100 mm Tris HCl pH 7.5, 10 mm magnesium acetate, 1 mm EDTA, 0.1 mm luciferin, 0.3 mm ATP). Luciferase activity was expressed as relative light units per microgram of protein (30).
Western Blotting
For Western blotting, hearts were homogenized in a modified radioimmune precipitation buffer (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 4% glycerol, 0.5 mm Na2S2O5, 1% Triton X-100, 0.1% sodium deoxycholate, 0.05% SDS) containing protease inhibitors and phosphatase inhibitor cocktails I and II (Roche Diagnostics) and centrifuged at 12,000 × g for 15 min. The antibody directed against calcineurin A (α/β isoforms) was from Chemicon (Billerica, MA). Anti-CnB and anti-phospho-phospholamban-S16 (PLN) antibodies were purchased from Upstate Biotechnology. Antibody directed against PLN was from Affinity BioReagents, anti-phospho-PLN-T17 was from Badrilla (Leeds, UK), anti-α1c (L-type Ca2+ channel pore subunit) was from Alomone Labs (Jerusalem, Israel), and anti-Na+/Ca2+ exchanger-1 (NCX1) was from Swant (Bellinzona, Switzerland). Anti-sarcoplasmic reticulum Ca2+ ATPase (SERCA2), anti-ryanodine receptor-2 (RyR2), and anti-glyceraldehyde-3-phosphate dehydrogenase antibodies were purchased from Santa Cruz Biotechnology.
RT-PCR
One microgram of DNase I-treated RNA was subjected to semiquantitative RT-PCR using SuperScript III (Invitrogen) following the manufacturer's instructions. Reactions consisted of an initial RT reaction of 50 min at 50 °C followed by amplification of 26 PCR cycles (94 °C for 20 s; 54 °C for 40 s, 72 °C for 45 s). Primers used were for α1c (5′-acacagccaataaagccctcctg-3′; 5′-ggccagcttcttcctctcctt-3′); L-type Ca2+ channel accessory subunit β2 (β2) (5′-ggttcggcagactcctacac-3′; 5′-tccgatttcacagccttctt-3′); NCX1 (5′-cacccaacactgccaccataac-3′; 5′-ctccatgatgccaatgctc-3′); β2-adrenergic receptor (β2AR) (5′-cgtcctgcattgtgtctttctacg-3′; 5′-agccgttcccataggtttcg-3′); SERCA2 (5′-ctccatctgcctgtccatgt-3′; 5′-gaagcggttactccagtattgc-3′); RyR2 (5′-aaggagaacacttcccgtacgagc-3′; 5′-aaagaggcctgcttgcgacaga-3′); PLN (5′-actgtgacgatcaccgaagc-3′; 5′-cagcagcagacatatcaagatg-3′); and L7 (5′-gaagctcatctatgagaaggc-3′; 5′-aagacgaaggagctgcagaac-3′).
Cardiac Fiber Mechanical Experiments
Cardiac fibers from 6-week-old mice were dissected from papillary muscles and permeabilized for 30 min in saponin (50 μg/ml) as described previously (39). Briefly, fibers were mounted on a stainless steel hook connected to a force transducer (AE 801, Microelectronics, Horton, Norway) and were immersed in chambers containing a stepwise Ca2+ adjustable solution (10 mm EGTA, 40 mm BES (pH 7.1), 1 mm free Mg2+, 10 mm taurine, 3 mm K2HPO4, 0.5 mm dithiothreitol, 3.16 mm ATP, 160 mm potassium methanesulfonate, 6% dextran). Sarcomere length was measured at 22 °C by laser diffraction and adjusted to maximum (2.1–2.2 μm). Tension (expressed in millinewtons mm−2) was measured at free Ca2+ concentration, 1 nm (resting force), and 31.6 μm (active force).
Fibrosis and Glycogen Quantification
Blue collagen deposition in the heart stained by Masson's trichrome or periodic acid-Schiff pink glycogen staining were quantified using MetaMorph 6.1 software (Universal Imaging Corp.) as described previously (14). A total of five pictures covering the entire heart section were analyzed in each of at least three mice per experimental group (magnification ×100).
Statistical Analysis
Means ± S.E. are presented for all data analysis. Differences between two experimental groups with normal distribution were analyzed by unpaired Student's t test; otherwise Mann-Whitney U test was applied (SigmaStat 3.5 software). Parametric analysis of variance (coupled to Student's Newman-Keuls post hoc test) was applied to normally distributed values. Differences between non-normally distributed groups were tested with Kruskal-Wallis analysis of variance (Dunn's post hoc test) (SigmaStat 3.5 software). p < 0.05 was considered significant.
RESULTS
Deletion of CnB1 from the Early Heart Results in Perinatal Lethality
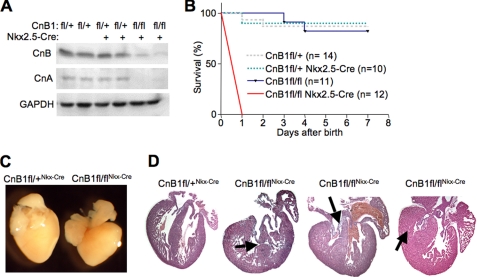
We have previously shown that CnAβ−/− mice, which have a 70% reduction in total calcineurin activity in the heart, are viable with no gross cardiovascular abnormalities, presumably given sufficient residual activity due to the presence of the CnAα gene. Here we used the CnB1-LoxP-targeted mouse as a means of deleting nearly all calcineurin activity in the heart in conjunction with various cardiac Cre-expressing lines. The Nkx2.5-Cre knock-in line was first employed given its robust and early expression in the developing heart field of the embryo (27). This cross resulted in a near complete deletion of all calcineurin A/B protein in the heart at 1 day of age (Fig. 1A). Remarkably, CnB1fl/flNkx2.5-Cre mice survived to birth and were present at predicted Mendelian ratios, suggesting that calcineurin signaling was not required for cardiac myocyte differentiation. However, CnB1fl/flNkx2.5-Cre mice all perished within 1 day of birth, whereas all other genotypes from the same parental crosses survived normally into adulthood (Fig. 1B). Pathologic dissection of 1-day-old CnB1fl/flNkx2.5-Cre neonates showed no gross abnormalities outside the heart, although the heart was noticeably altered in morphology (Fig. 1C). Histological assessment of hearts from these neonates showed a spectrum of profound defects that included a failure of the right ventricle to cavitate, lack of right ventricular trabeculation, septal defects, and valvular overgrowth (Fig. 1D).
FIGURE 1.
CnB1fl/flNkx2.5-Cre newborns die within 1 day of birth. A, Western blotting for CnB and pan-CnA in hearts of newborn CnB1fl/flNkx2.5-Cre, CnB1fl/+Nkx2.5-Cre, and CnB1fl/+ littermates. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was run as a loading control. Representative Western blot of three independent experiments is shown. B, survival rates of CnB1fl/flNkx2.5-Cre, CnB1fl/fl, CnB1fl/+Nkx2.5-Cre, and CnB1fl/+ mice. Eight litters were genotyped at day 0 and monitored every day for a week (p < 0.001). C, representative photograph of CnB1fl/flNkx2.5-Cre and CnB1fl/+Nkx2.5-Cre newborn hearts. D, hematoxylin and eosin staining of longitudinal newborn heart sections from CnB1fl/flNkx2.5-Cre and CnB1fl/+Nkx2.5-Cre neonates. Arrows indicate a septo-ventricular defect, thickened tricuspid valve leaflets, and a right ventricle showing an absence of trabeculation (from left to right).
Significant Deletion of CnB1 from the Adult Heart Results in Altered Function and Eventual Lethality
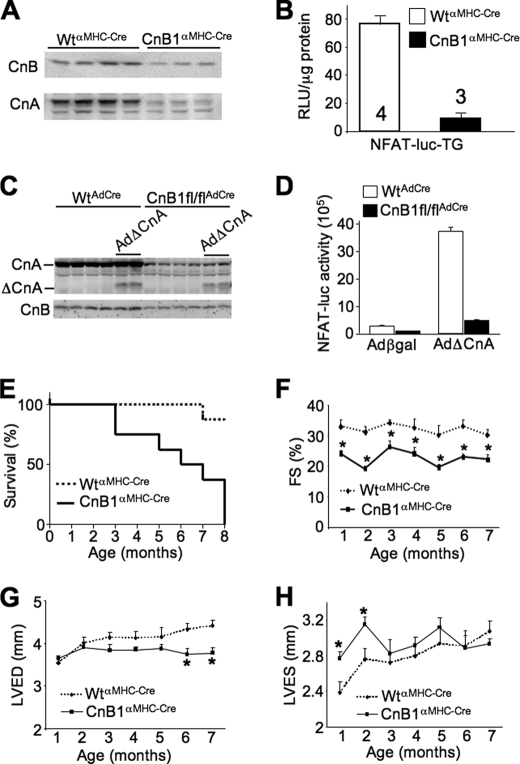
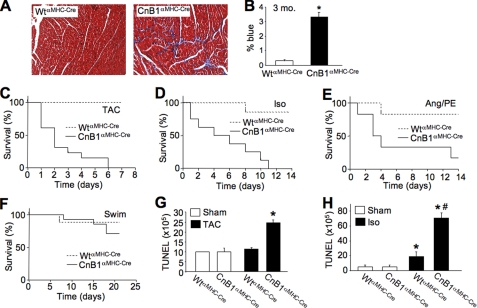
CnB1-LoxP mice were also crossed with αMHC-Cre transgenic mice as a means of deleting calcineurin during postnatal development so that effects in the mature heart could be investigated. By 2 weeks of age, this strategy resulted in 70–80% deletion of calcineurin A/B protein in the heart (Fig. 2A). The αMHC-Cre transgene continues to express in the juvenile and adult heart so that deletion becomes even more extensive over time. To more accurately quantify this effect, we crossed CnB1fl/flαMHC-Cre mice with NFAT-luciferase reporter transgenic mice. Hearts analyzed from CnB1fl/flαMHC-Cre mice showed a greater than 85% reduction in total calcineurin-NFAT activity when compared with αMHC-Cre-only controls at 2 months of age (Fig. 2B). To determine whether loss of CnB1 truly cripples CnA function in vivo, we analyzed CnA protein stability and NFAT-luciferase activity in CnB1-deleted MEFs. An adenovirus expressing Cre recombinase was used to delete CnB1 from MEFs containing the LoxP-targeted homozygous alleles, which reduced CnA and CnB1 protein levels by ≈90% (Fig. 2C). Simultaneous infection with an adenovirus expressing the activated (truncated) form of CnA showed relatively stable expression even in the absence of CnB1 (Fig. 2C). However, activated CnA expression was essentially unable to significantly induce NFAT-luciferase activity in CnB1-deleted MEFs (Fig. 2D). These results suggest that loss of CnB1 blocks all CnA-NFAT activity. With respect to the gene-targeted mice, the dramatic reduction in total calcineurin activity in cardiac myocytes was associated with 50% lethality by 6 months of age and complete lethality by 8 months (Fig. 2E). Echocardiographic assessment of cardiac ventricular performance showed significantly depressed function even at 1 month of age in CnB1fl/flαMHC-Cre mice that persisted until they perished (Fig. 2F). Impaired cardiac function was associated with left ventricular dilation at young ages and reduced relaxation of the left ventricle in older mice (Fig. 2, G and H). Other indexes of heart disease were also present, such as significant cardiac fibrosis (Fig. 3, A and B). Moreover, CnB1fl/flαMHC-Cre mice were highly sensitive to cardiac stress stimulation, resulting in complete lethality after only 6 days of pressure overload stimulation by TAC, whereas all control mice survived this time period and beyond (Fig. 3C). Isoprenaline (Iso) infusion in Alzet minipumps also resulted in complete lethality in CnB1fl/flαMHC-Cre mice after 11 days, whereas Ang/PE infusion was similarly lethal when compared with little effect on controls (Fig. 3, D and E). In contrast, physiologic stimulation on the heart through swimming exercise did not induce significant lethality when compared with controls (Fig. 3F).
FIGURE 2.
CnB1fl/flαMHC-Cre mice develop heart failure early in life. A, representative Western blots for CnB and pan-CnA protein levels in 2-week-old WTαMHC-Cre and CnB1fl/flαMHC-Cre hearts (n = 3 independent experiments). B, quantification of luciferase activity in hearts from 2-month-old WTαMHC-Cre (n = 4) and CnB1fl/flαMHC-Cre (n = 3) mice carrying the NFAT luciferase reporter transgene (NFAT-luc-TG). RLU, relative light units. C, Western blots for CnB, pan-CnA, and activated CnA (Δ) protein levels in WT and CnB1fl/fl MEFs-infected with AdCre and AdΔCnA. D, quantification of luciferase activity in AdNFAT-luciferase reporter-infected WT and CnB1fl/fl MEFs with AdCre infection and Ad-β-galactosidase (Adβgal, control) or AdΔCnA. E, mortality rate of CnB1fl/flαMHC-Cre (initially n = 8) and WTαMHC-Cre (initially n = 8) mice (p < 0.001). F, monthly echocardiographic analysis of ventricular fractional shortening (FS) in CnB1fl/flαMHC-Cre and WTαMHC-Cre mice (initially n = 8 mice in each group) (*, p < 0.05). G, monthly echocardiographic analysis of left ventricular end diastolic dimension (LVED) in CnB1fl/flαMHC-Cre and WTαMHC-Cre mice (initially n = 8 mice in each group) (*, p < 0.05). H, monthly echocardiographic analysis of left ventricular end systolic dimension (LVES) in CnB1fl/flαMHC-Cre and WTαMHC-Cre mice (initially n = 8 mice in each group) (*, p < 0.05).
FIGURE 3.
Cardiac pathology and stress-induced sudden death in CnB1fl/flαMHC-Cre mice. A, Masson's trichrome staining of heart histological sections from 3-month old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice. B, quantification of fibrosis in Masson's trichrome-stained histological heart sections described in A (n = 3 mice in each group, *, p < 0.05). C, survival rate in 2-month-old WTαMHC-Cre (n = 4) and CnB1fl/flαMHC-Cre mice (n = 7) subjected to TAC (p < 0.0001). D, survival rate in 2-month-old WTαMHC-Cre (n = 7) and CnB1fl/flαMHC-Cre (n = 9) mice implanted with Alzet minipumps releasing Iso (60 mg/kg/day, p < 0.05). E, survival rate in 2-month-old WTαMHC-Cre (n = 6) and CnB1fl/flαMHC-Cre mice (n = 6) implanted with Ang/PE containing Alzet minipumps (Ang/PE, respectively, 432 μg/kg/day and 100 mg/kg/day, p < 0.03). F, survival curve in 2-month old WTαMHC-Cre (n = 8) and CnB1fl/flαMHC-Cre mice (n = 10) subjected to 3 weeks of daily swimming. G and H, immunohistochemical assessment of apoptosis by TUNEL in the hearts of WTαMHC-Cre and CnB1fl/flαMHC-Cre mice subjected to 1 day of TAC (G) or Iso infusion (H) (at least 100,000 nuclei were counted; n = 3 mice in each group, *, p < 0.05 versus sham; #, p < 0.05 versus WT Iso).
However, worsening of heart failure did not appear to underlie lethality in CnB1fl/flαMHC-Cre mice with aging as pulmonary edema was not present, the reduced state of cardiac function remained stable, body weight remained stable, and cardiac function did not worsen 48 h after TAC (data not shown). Although histological analysis of TUNEL showed significantly more cell death in the hearts of CnB1fl/flαMHC-Cre mice after both TAC and Iso stimulation (Fig. 3, G and H), this was likely due to reduced contractility leading to compensatory increases in neurohumoral stimulation. Taken together these results suggest that hearts from CnB1fl/flαMHC-Cre mice have a unique pathologic profile whereby they maintain poor function long term without failure but are more prone to sudden death at baseline and with pathologic stimulation.
CnB1fl/flαMHC-Cre Mice Show Dramatic Alterations in Cardiac Conduction and Ion-handling Genes
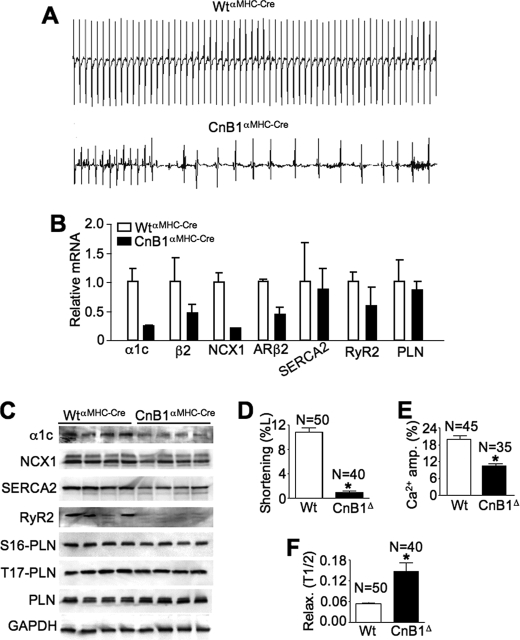
Although CnB1fl/flαMHC-Cre mice had increased cardiomyocyte TUNEL levels after TAC and Iso stimulation, such an alteration is unlikely to explain acute lethality in these mice after TAC, Iso, and Ang/PE stimulation. We suspected that CnB1fl/flαMHC-Cre mice were dying suddenly, possibly due to arrhythmia. Hence, four control and four gene-deleted mice were instrumented with implantable telemetry devices for continuous ECG recordings over 7 days (5-month-old mice). All four control mice showed normal heart rhythm without disturbances, yet all four CnB1fl/flαMHC-Cre mice showed arrhythmias with few periods of stable rhythm (Fig. 4A). Importantly, two of the CnB1fl/flαMHC-Cre mice died during the 7 days of telemetric analysis, one of which was preceded by a period of massive arrhythmia (Fig. 4A). Interpretation of the ECGs showed first-degree atrioventricular nodal blockade, tachyarrhythmias, and low amplitude QRS complexes, suggesting diminished voltage in all CnB1fl/flαMHC-Cre mice studied (Fig. 4A).
FIGURE 4.
Arrhythmias and altered Ca2+-handling genes in CnB1fl/flαMHC-Cre mice. A, continuous telemetric ECG recordings in 5-month-old WTαMHC-Cre mice (top panel) and CnB1fl/flαMHC-Cre mice (lower panel). Representative traces are shown. B, semiquantitative RT-PCR analysis from hearts of 2-month-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (n = 2 animals in each group). Ribosomal protein L7 mRNA was used for normalization. ARβ2, adrenergic receptor β2. C, Western blots for proteins isolated from 1-month-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (n = 4 animals in each group). Glyceraldehyde-3-phosphate dehydrogenase(GAPDH) was used as a loading control. S16-PLN, Ser-16-PLN; T17-PLN, Thr-17-PLN. D, the percentage of change in length from rest (%L) in adult myocytes isolated from WTαMHC-Cre and CnB1fl/flαMHC-Cre (CnB1Δ) mice at 5 weeks of age (*, p < 0.01, number of cells analyzed is shown). E, quantitation of Ca2+ transient amplitude (amp.) normalized to baseline Fura-2 fluorescence in WTαMHC-Cre and CnB1fl/flαMHC-Cre cardiomyocytes (*, p < 0.01). F, time to 50% relaxation (Relax.) from peak contraction in isolated myocytes from WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (*, p < 0.01).
Given the observation of arrhythmia, we next investigated expression levels of ion-handling genes in the hearts of CnB1fl/flαMHC-Cre mice. This analysis showed prominent reductions in mRNA levels for the α pore-forming subunit (α1c) and β accessory subunit of the cardiac L-type Ca2+ channel, reductions in NCX1, and reductions in the β2-adrenergic receptor but no changes in SERCA2, RyR2, or PLN (Fig. 4B). At the protein level, we also observed a severe reduction in α1c, NCX1, SERCA2, and RyR2 by Western blots from heart extracts taken at 1 month of age in CnB1fl/flαMHC-Cre mice (Fig. 4C).
Diminished expression of Ca2+-handling proteins correlated with reductions in isolated myocyte function and the characteristics of the Ca2+ transient. For example, isolated myocytes from 5-week-old CnB1fl/flαMHC-Cre mice showed almost no contractile activity in culture when compared with WT control myocytes at the same age (Fig. 4D). Similarly, the amplitude of the Ca2+ transient was reduced by ∼50% (Fig. 4E), and the time to one-half relaxation was increased more than 2-fold (Fig. 4F). These results suggest that calcineurin activity is intimately involved in regulating ion-handling gene expression that is required for proper cardiac contractility and conduction.
Other Indices of Heart Disease in CnB1fl/flαMHC-Cre Mice
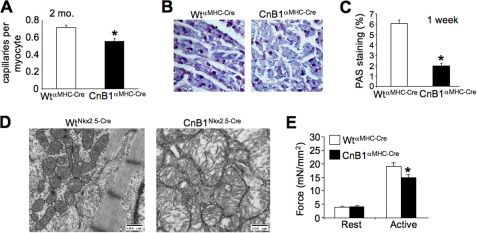
CnB1fl/flαMHC-Cre mice also showed reduced capillary content in the myocardium at 2 months of age and reduced glycogen storage revealed by periodic acid-Schiff staining from cardiac histological sections (Fig. 5, A–C). We also performed transmission electron microscopy from cardiac histological sections from day 18.5 CnB1fl/flNkx2.5-Cre embryos given their early and severe dysfunctional phenotype. The most obvious defect in these hearts was aberrant mitochondrial architecture characterized by swelling and a general loss of cristae organization, a hallmark of a failing heart (Fig. 5D). Finally, skinned fibers taken from hearts of CnB1fl/flαMHC-Cre mice showed a reduction in active force generation (Fig. 5E). These measurements were taken at a controlled level of Ca2+, suggesting that the myofilaments themselves generate less force in CnB1-deleted hearts, providing yet another mechanism whereby calcineurin might influence myocyte contractility. This defect in skinned myofiber tension development is hypothesized to also arise due to reductions in differentiated gene expression for select contractile proteins.
FIGURE 5.
Failure phenotype in heart-specific CnB1-deleted mice. A, quantification of capillaries per myocyte in cardiac histological sections by CD31 antibody staining in 2-month-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (n = 3 animals/group, *, p < 0.05). B, representative picture of glycogen content in heart histological sections from 1-week-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice stained with periodic acid-Schiff (PAS). C, quantitation of the staining shown in B (n = 2 animals/group, *, p < 0.05). D, transmission electron micrographs of heart histological sections from embryonic day 18.5 in CnB1fl/flNkx2.5-Cre (n = 2) and CnB1fl/+Nkx2.5-Cre embryos (n = 3). E, force developed by resting (pCa9, resting force) or saturating Ca2+ levels (pCa4.5, active force) from cardiac skinned papillary muscle fibers isolated from 5-week-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (for each genotype, n = 19 fibers from four different animals, *, p < 0.05).
Hearts from CnB1fl/flαMHC-Cre Mice Show Reduced Myocyte Content and Postnatal Cardiac Proliferation
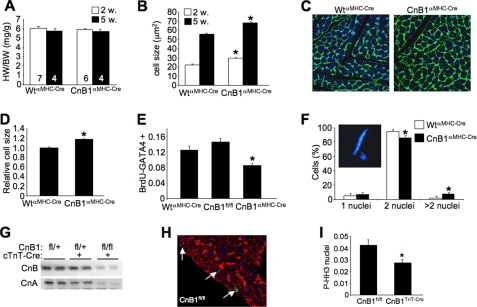
Although heart weight to body weight ratios did not vary in CnB1fl/flαMHC-Cre mice at 2 or 5 months of age, histological analyses of cardiomyocyte cross-sectional surface area were consistently larger at these same time points (Fig. 6, A–C). Because heart size was not different between CnB1fl/flαMHC-Cre and WTαMHC-Cre mice, these results suggest that fewer myocytes are present in the heart because each has to grow slightly larger to maintain end organ size. Indeed, isolation of adult myocytes from hearts of CnB1fl/flαMHC-Cre mice (5 weeks of age) showed that each myocyte was ∼17% larger than myocytes from control hearts (Fig. 6D). To more directly address this issue, we also measured DNA synthesis rates in the heart by BrdUrd incorporation in 3-day-old neonates of control and CnB1fl/flαMHC-Cre mice (Fig. 6E). The data show significantly reduced levels of myocytes that are GATA4 and BrdUrd positive in cardiac histological sections from CnB1fl/flαMHC-Cre mice, suggesting lower levels of cell cycle activity when calcineurin was deleted. Consistent with this phenotype of larger cells and altered cell cycle activity, we also observed a significantly larger fraction of myocytes with greater than two nuclei per cell and a corresponding minor but significant reduction in the content of binucleated adult myocytes (Fig. 6F). A representative myocyte with four nuclei (blue) is shown in the inset from a CnB1fl/flαMHC-Cre mouse. The alteration in proliferation and cellular content in the heart of CnB1-deleted mice was confirmed in another independent Cre-expressing transgene. We used the cTnT-Cre transgene given its early expression in the developing heart and exclusivity to cardiac myocytes (28). At 3 weeks of age, CnB1fl/flcTnT-Cre mice showed a 90% reduction in CnB and CnA protein in the heart when compared with untargeted mice. Analysis of cell cycle activity in these hearts by examination of phosphorylated histone H3 in 3-day-old mice revealed significantly less reactivity in CnB1fl/flcTnT-Cre mice when compared with controls, again suggesting that loss of CnB1 from the heart negatively affected cellular proliferation (Fig. 6, H and I).
FIGURE 6.
Altered cellular content in the hearts of CnB1-deleted mice. A, quantitation of heart weight normalized to body weight (HW/BW) at 2 and 5 weeks of age in WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (number of mice shown in the bars). B, quantitation of myocyte surface area from cardiac histological sections of 2- or 5-week-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (n > 500 myocytes/genotype, *, p < 0.05 versus WT of the same age). C, representative picture of cardiac histological sections from 5-week-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice stained with fluorescein isothiocyanate-lectin (green) and TO-PRO-3 iodine (blue for nuclei). D, quantitation of total relative surface area of isolated myocytes in a dish from dissociated hearts of 5-week-old WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (n > 500 myocytes for each genotype, *, p < 0.05). E, immunohistochemical quantitation of BrdUrd (BrdU) and GATA4 co-stained cardiomyocytes in histological sections expressed as a percentage of total GATA4 positive myocytes in 3-day-old WTαMHC-Cre control hearts (n = 4), CnBfl/fl littermate control hearts (n = 3), and CnB1fl/flαMHC-Cre hearts (n = 6) (*, p < 0.05 versus either control group). F, quantitation of the number of nuclei in 5-week-old disassociated cardiomyocytes from hearts of WTαMHC-Cre and CnB1fl/flαMHC-Cre mice (n > 1000/genotype, *, p < 0.05 versus WT). G, Western blotting for CnB and pan-CnA in 3-week-old CnB1fl/+, CnB1fl/+cTnT-Cre, and CnB1fl/flcTnT-Cre littermates. H, representative histological section from the heart of control CnB1fl/fl mice stained for phospho-histone H3 (green) antibody and TRITC-lectin (red). I, phospho-histone H3 (P-HH3) quantitation on immunostained heart histological sections of 3-day-old CnB1fl/fl (n = 4) and CnB1fl/flcTnT-Cre (n = 3) littermates expressed as a percentage of total number of cells (*, p < 0.05 versus WT).
DISCUSSION
In this study, we employed three different cardiac-expressing Cre transgenes to delete the CnB1 gene from the heart, each resulting in a slightly different failure phenotype. The Nkx2.5-Cre knock-in allele produced a very robust deletion of CnB1 from the heart, which resulted in a nearly complete loss of CnB and CnA protein from the heart as assayed at birth. The Nkx2.5-Cre allele is expressed very early in mouse heart development when the cardiac lineage is first specified, just before embryonic day 7.5 (27). This early and very robust deletion of calcineurin from the heart caused defective right ventricular development with septal defects and valvular overgrowth, a phenotypic spectrum that is completely consistent with lethality (40). Indeed, robust right ventricular function is required at birth and thereafter, coincident with the need to oxygenate blood in the lungs after birth. These results suggest that calcineurin-NFAT signaling is necessary for proper right ventricular morphogenesis and trabeculation. Moreover, calcineurin-NFAT signaling is already known to be required for valve formation as loss of Nfatc1 in gene-deleted mice causes prominent defects in valves and embryonic lethality (41, 42). More recently, the CnB1 gene was deleted from the endothelium of the heart and shown to disrupt valve formation as well (21). Because the Nkx2.5-Cre allele is not expressed in the endocardium, our results extend previous observations to suggest that calcineurin is required in myocardial cells to generate a morphologically correct valve, as well as for correct morphogenesis of the right ventricle.
Deletion of CnB1 with the αMHC-Cre transgene did not alter initial viability of the mice, most likely because calcineurin protein was not significantly lost until later in postnatal development. However CnB1fl/flαMHC-Cre mice died rapidly between 6 and 8 months of age with reduced function and prominent arrhythmia. Indeed, continuous monitoring with telemetry recorded arrhythmia events that directly preceded death in 5-month-old CnB1fl/flαMHC-Cre mice. Stressing these mice in early adulthood with two different agonists or pressure overload stimulation also resulted in acute lethality with features of sudden death. The baseline reduction in fractional shortening in the hearts of CnB1fl/flαMHC-Cre mice, as well as the reduction in function of isolated myocytes, is also likely due to a reduction in expression of ion-handling genes. This reduction in function likely triggers a neuroendocrine response that induces fibrosis, mitochondrial architectural alterations, reductions in glycogen stores, increased propensity toward apoptosis, and reduction in capillary density in the heart. Thus, most of the observed phenotypic alterations documented in these mice are likely secondary to the more proximal changes in Ca2+-handling genes and persistent arrhythmia. It is not clear whether loss of NFAT activity in the heart is directly responsible for this effect or one of many other targets of calcineurin that are NFAT-independent.
It is interesting to speculate that calcineurin-NFAT signaling are “hardwired” into the transcriptional control of ion-handling gene expression in the heart as such a mechanism could afford direct linkage between Ca2+ sensing by this phosphatase and the ability to change gene expression reflexively. Indeed, calcineurin-NFAT activation was directly tied to expression of SERCA2 in cardiac myocytes (43). Calcineurin signaling was also shown to up-regulate expression of the Ncx1 gene in cardiac myocytes (44–46). Finally, calcineurin also directly up-regulates L-type Ca2+ channel activity in cardiac myocytes, again suggesting that calcineurin signaling is important for both expression and activity of the Ca2+-handling machinery in a cardiac myocyte (47).
While this manuscript was in revision, Kelly and colleagues (48) published a similar analysis of CnB1-loxP-targeted mice crossed with the very same αMHC-Cre transgene. They also observed lethality in mice between 6 and 7 months of age, prominent reductions in ventricular performance, reduced contractility of isolated myocytes, and reduced Ca2+-cycling parameters. However, they did not observe arrhythmia in CnB1fl/flαMHC-Cre mice, nor did they find a reduction in expression of pivotal Ca2+-handling genes (48). These later two differences are intriguing because the arrhythmic phenotype and reduction in expression of Ca2+-handling genes (mRNA and proteins) were observed in every mouse analyzed at many different time points in our study. Arrhythmia and reduction in Ca2+-handling gene expression is also consistent with the observed phenotype of sudden death and prominently reduced contractility at the cellular, fiber, and whole heart level. It is uncertain why our two studies differ with respect to arrhythmia and expression of Ca2+-handling genes, although the broader aspects of the reported phenotypes were essentially identical.
Another potential direct effect associated with CnB1 deletion is a reduction in myocyte number and cell cycle activity in the heart. We observed reduced cell cycle activity in early postnatal hearts of CnB1fl/flαMHC-Cre and CnB1fl/flcTnT-Cre mice, as well as fewer myocytes contained within the adult hearts of CnB1fl/flαMHC-Cre mice. This observation is consistent with many other reports in the recent literature linking calcineurin signaling to cell cycle control and cellular proliferation. For example, deletion of CnB1 from the mesenchyme of the developing urinary tract reduced proliferation of smooth muscle cells and other cell types in the urinary tract, leading to multiple defects in development (25). Deletion of CnB1 from the β-cell lineage in the pancreas caused a reduction in β-cell proliferation, resulting in later hypoinsulinemia (26). Remarkably, overexpression of activated NFATc1 in these β-cells partially rescued the defects associated with CnB1 deletion, suggesting that NFAT factors are important downstream mediators of calcineurin signaling for cellular proliferation (26). More recently, calcineurin-NFAT signaling has been implicated in regulating tumor growth and cancer cell activity (reviewed in Ref. 49). Thus, one prominent mechanistic implication of CnB1 deletion from the heart is likely a direct link to cell cycle activity and the homeostasis of total myocyte content specified during development and early postnatal maturation of the heart. However, only a 20% increase in myocyte surface areas was observed, which is likely too minor of an effect to significantly impact heart function because each myocyte simply hypertrophies to compensate for the effect. Thus, it is unlikely that a reduction in myocyte content meaningfully impacts cardiac function and predisposition to failure.
It is more likely that the loss of calcineurin-NFAT signaling leads to primary defects in cardiac function and arrhythmia (death) by subtly altering the differentiated state of the cardiac myocyte such that select genes necessary for efficient myocyte contraction are not properly expressed. By comparison, deletion of CnB1 from keratinocytes altered their differentiated state, resulting in severe skin defects (22). Similarly, deletion of CnB1 from the developing lung caused respiratory failure from reduced maturation associated with deficient expression of surfactant, a lamellar body-associated protein, and the ABC transporter A3 (24). Deletion of CnB1 in the developing thymocytes altered positive selection and the proper differentiation of T-cells, whereas deletion of CnB1 from neural crest cells altered Schwann cell differentiation, leading to myelination defects (20, 23). Thus, loss of calcineurin-NFAT signaling is required for the proper differentiation of most tissues analyzed with the CnB1-loxP allele. In the heart, calcineurin was necessary for proper development and homeostasis in adulthood, most likely associated with alterations in differentiated gene expression.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant (to J. D. M.). This work was also supported by grants from the Howard Hughes Medical Institute and an international collaborative research grant in cardiovascular disease from the Fondation Leducq (to J. D. M.).
This article was selected as a Paper of the Week.
- NFAT
- nuclear factor of activated T-cells
- α1c
- L-type Ca2+ channel pore subunit
- β2a
- L-type Ca2+ channel accessory subunit
- αMHC
- α-myosin heavy chain
- PE
- phenylephrine
- Ang
- angiotensin II
- fl
- LoxP-targeted
- WT
- wild type
- BrdUrd
- bromodeoxyuridine
- CnA
- calcineurin A subunit
- CnB
- calcineurin B subunit
- cTnT
- cardiac troponin T
- ECG
- electrocardiogram
- NCX1
- Na+/Ca2+ exchanger-1
- MEF
- mouse embryonic fibroblasts
- PLN
- phospholamban
- RyR2
- ryanodine receptor
- SERCA2
- sarcoplasmic reticulum Ca2+ ATPase
- TAC
- transverse aortic constriction
- Iso
- isoprenaline
- RT
- reverse transcription
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- Ad
- adenovirus
- TRITC
- tetramethylrhodaminoisothiocyanate
- BES
- N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid.
REFERENCES
- 1.Wu H., Peisley A., Graef I. A., Crabtree G. R. (2007) Trends Cell Biol. 17, 251–260 [DOI] [PubMed] [Google Scholar]
- 2.Hogan P. G., Chen L., Nardone J., Rao A. (2003) Genes Dev. 17, 2205–2232 [DOI] [PubMed] [Google Scholar]
- 3.Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. (1998) Cell 93, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks B., McMahon H. T. (1998) Curr. Biol. 8, 740–749 [DOI] [PubMed] [Google Scholar]
- 5.Lai M. M., Hong J. J., Ruggiero A. M., Burnett P. E., Slepnev V. I., De Camilli P., Snyder S. H. (1999) J. Biol. Chem. 274, 25963–25966 [DOI] [PubMed] [Google Scholar]
- 6.Lai M. M., Luo H. R., Burnett P. E., Hong J. J., Snyder S. H. (2000) J. Biol. Chem. 275, 34017–34020 [DOI] [PubMed] [Google Scholar]
- 7.Mao Z., Wiedmann M. (1999) J. Biol. Chem. 274, 31102–31107 [DOI] [PubMed] [Google Scholar]
- 8.Orie N. N., Thomas A. M., Perrino B. A., Tinker A., Clapp L. H. (2009) Br. J. Pharmacol. 157, 554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cereghetti G. M., Stangherlin A., Martins de Brito O., Chang C. R., Blackstone C., Bernardi P., Scorrano L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H. G., Pathan N., Ethell I. M., Krajewski S., Yamaguchi Y., Shibasaki F., McKeon F., Bobo T., Franke T. F., Reed J. C. (1999) Science 284, 339–343 [DOI] [PubMed] [Google Scholar]
- 11.Cameron A. M., Steiner J. P., Roskams A. J., Ali S. M., Ronnett G. V., Snyder S. H. (1995) Cell 83, 463–472 [DOI] [PubMed] [Google Scholar]
- 12.Donelan M. J., Morfini G., Julyan R., Sommers S., Hays L., Kajio H., Briaud I., Easom R. A., Molkentin J. D., Brady S. T., Rhodes C. J. (2002) J. Biol. Chem. 277, 24232–24242 [DOI] [PubMed] [Google Scholar]
- 13.Klee C. B., Ren H., Wang X. (1998) J. Biol. Chem. 273, 13367–13370 [DOI] [PubMed] [Google Scholar]
- 14.Parsons S. A., Millay D. P., Wilkins B. J., Bueno O. F., Tsika G. L., Neilson J. R., Liberatore C. M., Yutzey K. E., Crabtree G. R., Tsika R. W., Molkentin J. D. (2004) J. Biol. Chem. 279, 26192–26200 [DOI] [PubMed] [Google Scholar]
- 15.Wilkins B. J., Molkentin J. D. (2004) Biochem. Biophys. Res. Commun. 322, 1178–1191 [DOI] [PubMed] [Google Scholar]
- 16.Bueno O. F., Brandt E. B., Rothenberg M. E., Molkentin J. D. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9398–9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B. W., Zimmer G., Chen J., Ladd D., Li E., Alt F. W., Wiederrecht G., Cryan J., O'Neill E. A., Seidman C. E., Abbas A. K., Seidman J. G. (1996) J. Exp. Med. 183, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons S. A., Wilkins B. J., Bueno O. F., Molkentin J. D. (2003) Mol. Cell. Biol. 23, 4331–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graef I. A., Chen F., Chen L., Kuo A., Crabtree G. R. (2001) Cell 105, 863–875 [DOI] [PubMed] [Google Scholar]
- 20.Neilson J. R., Winslow M. M., Hur E. M., Crabtree G. R. (2004) Immunity 20, 255–266 [DOI] [PubMed] [Google Scholar]
- 21.Chang C. P., Neilson J. R., Bayle J. H., Gestwicki J. E., Kuo A., Stankunas K., Graef I. A., Crabtree G. R. (2004) Cell 118, 649–663 [DOI] [PubMed] [Google Scholar]
- 22.Mammucari C., Tommasi di Vignano A., Sharov A. A., Neilson J., Havrda M. C., Roop D. R., Botchkarev V. A., Crabtree G. R., Dotto G. P. (2005) Dev. Cell 8, 665–676 [DOI] [PubMed] [Google Scholar]
- 23.Kao S. C., Wu H., Xie J., Chang C. P., Ranish J. A., Graef I. A., Crabtree G. R. (2009) Science 323, 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davé V., Childs T., Xu Y., Ikegami M., Besnard V., Maeda Y., Wert S. E., Neilson J. R., Crabtree G. R., Whitsett J. A. (2006) J. Clin. Invest. 116, 2597–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C. P., McDill B. W., Neilson J. R., Joist H. E., Epstein J. A., Crabtree G. R., Chen F. (2004) J. Clin. Invest. 113, 1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heit J. J., Apelqvist A. A., Gu X., Winslow M. M., Neilson J. R., Crabtree G. R., Kim S. K. (2006) Nature 443, 345–349 [DOI] [PubMed] [Google Scholar]
- 27.Moses K. A., DeMayo F., Braun R. M., Reecy J. L., Schwartz R. J. (2001) Genesis 31, 176–180 [DOI] [PubMed] [Google Scholar]
- 28.Jiao K., Kulessa H., Tompkins K., Zhou Y., Batts L., Baldwin H. S., Hogan B. L. (2003) Genes Dev. 17, 2362–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agah R., Frenkel P. A., French B. A., Michael L. H., Overbeek P. A., Schneider M. D. (1997) J. Clin. Invest. 100, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins B. J., Dai Y. S., Bueno O. F., Parsons S. A., Xu J., Plank D. M., Jones F., Kimball T. R., Molkentin J. D. (2004) Circ. Res. 94, 110–118 [DOI] [PubMed] [Google Scholar]
- 31.Oka T., Maillet M., Watt A. J., Schwartz R. J., Aronow B. J., Duncan S. A., Molkentin J. D. (2006) Circ. Res. 98, 837–845 [DOI] [PubMed] [Google Scholar]
- 32.Wilkins B. J., De Windt L. J., Bueno O. F., Braz J. C., Glascock B. J., Kimball T. F., Molkentin J. D. (2002) Mol. Cell. Biol. 22, 7603–7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakayama H., Chen X., Baines C. P., Klevitsky R., Zhang X., Zhang H., Jaleel N., Chua B. H., Hewett T. E., Robbins J., Houser S. R., Molkentin J. D. (2007) J. Clin. Invest. 117, 2431–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coutu P., Metzger J. M. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H601–H612 [DOI] [PubMed] [Google Scholar]
- 35.Baines C. P., Kaiser R. A., Sheiko T., Craigen W. J., Molkentin J. D. (2007) Nat. Cell Biol. 9, 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maillet M., Purcell N. H., Sargent M. A., York A. J., Bueno O. F., Molkentin J. D. (2008) J. Biol. Chem. 283, 31246–31255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heineke J., Auger-Messier M., Xu J., Oka T., Sargent M. A., York A., Klevitsky R., Vaikunth S., Duncan S. A., Aronow B. J., Robbins J., Crombleholme T. M., Molkentin J. D. (2007) J. Clin. Invest. 117, 3198–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fewell J. G., Osinska H., Klevitsky R., Ng W., Sfyris G., Bahrehmand F., Robbins J. (1997) Am. J. Physiol. 273, H1595–H1605 [DOI] [PubMed] [Google Scholar]
- 39.Joubert F., Wilding J. R., Fortin D., Domergue-Dupont V., Novotova M., Ventura-Clapier R., Veksler V. (2008) J. Physiol. 586, 5181–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turgeon B., Meloche S. (2009) Physiol. Rev. 89, 1–26 [DOI] [PubMed] [Google Scholar]
- 41.de la Pompa J. L., Timmerman L. A., Takimoto H., Yoshida H., Elia A. J., Samper E., Potter J., Wakeham A., Marengere L., Langille B. L., Crabtree G. R., Mak T. W. (1998) Nature 392, 182–186 [DOI] [PubMed] [Google Scholar]
- 42.Ranger A. M., Grusby M. J., Hodge M. R., Gravallese E. M., de la Brousse F. C., Hoey T., Mickanin C., Baldwin H. S., Glimcher L. H. (1998) Nature 392, 186–190 [DOI] [PubMed] [Google Scholar]
- 43.Anwar A., Taimor G., Korkususz H., Schreckenberg R., Berndt T., Abdallah Y., Piper H. M., Schlüter K. D. (2005) J. Mol. Cell. Cardiol. 39, 911–919 [DOI] [PubMed] [Google Scholar]
- 44.Shigekawa M., Katanosaka Y., Wakabayashi S. (2007) Ann. N.Y. Acad. Sci. 1099, 53–63 [DOI] [PubMed] [Google Scholar]
- 45.Jordan M. C., Quednau B. D., Roos K. P., Ross R. S., Philipson K. D., Nicholas S. B. (2002) Ann. N.Y. Acad. Sci. 976, 259–267 [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Nolan B., Kutschke W., Hill J. A. (2001) J. Biol. Chem. 276, 17706–17711 [DOI] [PubMed] [Google Scholar]
- 47.Tandan S., Wang Y., Wang T. T., Jiang N., Hall D. D., Hell J. W., Luo X., Rothermel B. A., Hill J. A. (2009) Circ. Res. 105, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaeffer P. J., Desantiago J., Yang J., Flagg T. P., Kovacs A., Weinheimer C. J., Courtois M., Leone T. C., Nichols C. G., Bers D. M., Kelly D. P. (2009) Am. J. Physiol. Heart Circ Physiol. 297, H1263–H1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medyouf H., Ghysdael J. (2008) Cell Cycle 7, 297–303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.