Abstract
Enteropathogenic Escherichia coli, enterohemorrhagic E. coli, and Citrobacter rodentium belong to the family of attaching and effacing (A/E) bacterial pathogens. They intimately attach to host intestinal epithelial cells, trigger the effacement of intestinal microvilli, and cause diarrheal disease. Central to their pathogenesis is a type III secretion system (T3SS) encoded by a pathogenicity island called the locus of enterocyte effacement (LEE). The T3SS is used to inject both LEE- and non-LEE-encoded effector proteins into the host cell, where these effectors modulate host signaling pathways and immune responses. Identifying the effectors and elucidating their functions are central to understanding the molecular pathogenesis of these pathogens. Here we analyzed the type III secretome of C. rodentium using the highly sensitive and quantitative SILAC (stable isotope labeling with amino acids in cell culture)-based mass spectrometry. This approach not only confirmed nearly all known secreted proteins and effectors previously identified by conventional biochemical and proteomic techniques, but also identified several new secreted proteins. The T3SS-dependent secretion of these new proteins was validated, and five of them were translocated into cultured cells, representing new or additional effectors. Deletion mutants for genes encoding these effectors were generated in C. rodentium and tested in a murine infection model. This study comprehensively characterizes the type III secretome of C. rodentium, expands the repertoire of type III secreted proteins and effectors for the A/E pathogens, and demonstrates the simplicity and sensitivity of using SILAC-based quantitative proteomics as a tool for identifying substrates for protein secretion systems.
Keywords: Bacteria, Mass Spectrometry (MS), Protein Export, Protein Secretion, Proteomics, Citrobacter rodentium, Effectors, Quantitative Proteomics, Secretome, Type III Secretion System
Introduction
Type III secretion systems (T3SS)6 are protein secretion and translocation nanomachines widely employed by many important Gram-negative bacterial pathogens that cause a variety of significant diseases in both animals and plants (1, 2). These complex protein export apparatus consist of a series of multicomponent, ring-shaped protein structures spanning the bacterial envelope, the extracellular space, and the host plasma membrane. They facilitate the direct delivery of bacterial virulence proteins (effectors) to the interior of the host cell. A key component of the T3SS is the needle complex, comprised of a basal body with multiple rings, an inner rod, and an external, protruding needle (3). Proteins are secreted via a channel traversing the center of the needle complex.
A large number of proteins are secreted by T3SSs, although the exact number varies considerably among pathogens. There are at least 4 categories of protein substrates, based on their secretion hierarchy. The needle and inner rod components (early substrates), required for the type III secretion of later substrates, are secreted first via the basal body to complete the assembly of the needle complex (4). The needle complex then secretes the translocators (intermediate substrates) and effectors (late substrates). Because the translocators are needed for translocation of the effectors into host cells, they are presumably secreted ahead of the effectors. Some expression- and secretion-regulating proteins are also secreted via the T3SS in certain pathogens (1). Because the effectors play vital roles in mediating infections and diseases by modulating or subverting host immune responses and cellular structures and functions, their identification and function elucidation are critical to our understanding of the pathogenesis mechanisms of these pathogens.
The attaching/effacing (A/E) pathogens, including enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E. coli (EHEC), and the mouse pathogen Citrobacter rodentium, use a T3SS encoded by the locus of enterocyte effacement (LEE), a chromosomally located pathogenicity island, to colonize the host and cause disease (5, 6). There is considerable variation in the number of effector genes in the different A/E pathogens, ranging from as many as 41 in certain EHEC serotypes to as few as 21 in EPEC (7–10). C. rodentium infection of mice has been used extensively as a surrogate model for EPEC and EHEC infections of humans (11). Many type III secreted proteins and effectors have been identified over the years in the A/E pathogens using conventional molecular biology approaches, proteomics, and bioinformatics (6). Although many of the effectors are conserved among all the A/E pathogens, some of the effectors, such as Cif and EspFu/TccP (12–14), appear to be unique to only certain lineages, indicating plasticity in the repertoire of the effectors. The difference in effector repertoire may influence pathogenicity as well as tissue tropism and host specificity of these pathogens. It is therefore important to characterize the secretome for each A/E pathogen.
We have previously identified and characterized several proteins and effectors secreted by the C. rodentium locus of enterocyte effacement (LEE)-encoded T3SS (15–19). The genome of C. rodentium has recently been sequenced. Despite recent progress, a comprehensive survey of type III-secreted proteins and effectors by proteomics has not been undertaken for C. rodentium. Here we applied stable isotope labeling with amino acids in cell culture (SILAC) (20), a sensitive and quantitative proteomics method, to analyze the type III secretome in C. rodentium. Our analysis confirmed the type III secretion of the translocators EspA, EspB, and EspD, and revealed that the needle protein EscF and the putative inner rod component rOrf8 are also secreted by the LEE-encoded T3SS. We also verified the type III secretion of 15 (Tir, EspZ, EspF, EspG, EspH, Map, EspJ, NleA, NleB, NleC, NleD, NleE, NleF, NleG, and NleH) effectors previously identified in C. rodentium by conventional proteomics, genetic screens, and bioinformatics (16–19, 21–25). In addition, we identified 5 new or additional effectors (NleK, EspM2, EspT, OspB, and EspX7) in C. rodentium. The type III secretion and translocation of these five effectors were confirmed by other assays, and their importance in C. rodentium virulence was assessed in mice. This SILAC-based quantitative proteomic approach can be easily adapted for identifying substrates for protein secretion systems in other pathogens.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
C. rodentium strain DBS100 (26) was used to generate all the C. rodentium derivatives and mutants described in this study. EPEC O127:H6 strain E2348/69 (27) and its ΔescN mutant (28) was used for the translocation assay. The bacteria were grown in Luria-Bertani (LB) broth or agar plates. MacConkey agar from OXOID (Hampshire, United Kingdom) was used for plating C. rodentium after in vivo mouse infections. DMEM from HyClone (Logan, UT) was used as the medium for inducing type III secretion. However, custom synthesized l-lysine- and l-arginine-deficient DMEM from Caisson Labs (Logan, UT) was used for analyzing the type III secretome of C. rodentium using SILAC.
Antibodies and Oligonucleotide Primers
The mouse monoclonal antibody against the hemagglutinin (HA.11) epitope was purchased from Covance (Emeryville, CA), and mouse antibody against N-terminal His-tagged TEM-1 β-lactamase was from QED Bioscience (San Diego, CA). Primers used in this study are listed in supplemental Table S1.
Generation of lysA and argH Deletion Mutants in C. rodentium
The sacB gene-based allelic exchange method was used to generate lysA and argH double in-frame deletion mutants in C. rodentium strain DBS100 using the suicide vector pRE118 (29) as previously described (17). We first deleted lysA to generate DBS100ΔlysA. PCR was used to generate two fragments (1.2 and 1.4 kb, respectively) using primer pairs CRlysA-1/CRlysA-DR as well as CRlysA-DF/CRlysA-2 (supplemental Table S1). The PCR products were digested with KpnI/NheI and NheI/SacI, respectively, and cloned into pRE118 digested with KpnI/SacI in a 3-way ligation and transformed into E. coli SY327λpir. The resulting plasmid pRE118-ΔCRlysA contained 1.2 to 1.4 kb of flanking regions on both sides of lysA and the lysA gene with an internal in-frame deletion of about 83%. An NheI site was introduced into the deletion site. Plasmid pRE-ΔCRlysA was introduced into DBS100 by electroporation. After sucrose selection, C. rodentium colonies resistant to sucrose and sensitive to kanamycin were screened and verified for deletion of lysA by multiple PCRs, thus creating DBS100ΔlysA.
Similarly, a suicide vector for deleting argH, pRE118-ΔCRargH, was generated using primer pairs CRargH-1/CRargH-DR and CRargH-DF/CRargH-2 (supplemental Table S1). About 85% of argH was deleted. This construct was introduced into DBS100ΔlysA to generate the double mutant ΔlysAΔargH using the same sucrose selection protocol. We subsequently introduced pRE118-ΔCRescN and pRE118-ΔCRsepD (17) into ΔlysAΔargH to generate ΔlysAΔargHΔescN and ΔlysAΔargHΔsepD, respectively. Finally, pRE118-ΔCRescN was introduced into ΔlysAΔargHΔsepD to create ΔlysAΔargHΔsepDΔescN.
SILAC Analysis
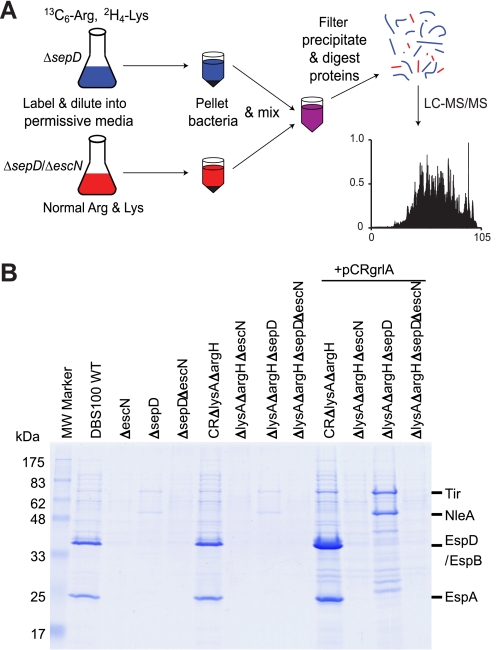
A schematic diagram of the procedure for analyzing type III-secreted proteins using SILAC (20) is shown in Fig. 1A. DBS100 ΔlysAΔargH and ΔlysAΔargHΔescN were paired to identify type III-secreted proteins by “wild-type” (WT) C. rodentium, whereas DBS100 ΔlysAΔargHΔsepD and ΔlysAΔargHΔsepDΔescN were contrasted to identify type III-secreted proteins by the ΔsepD mutant, with all strains carrying plasmid pCRgrlA (17). The strains were grown in l-lysine- and l-arginine-deficient DMEM supplemented with either normal isotopic abundance (“light”) l-lysine and l-arginine for strains ΔlysAΔargHΔescN and ΔlysAΔargHΔsepDΔescN, or “heavy” [2H4]lysine and [13C6]arginine (Cambridge Isotope Laboratories, Cambridge, MA) for strains ΔlysAΔargH and ΔlysAΔargHΔsepD. The strains were grown in 3 ml of the supplemented DMEM overnight at 37 °C in a shaker at 225 rpm, and then subcultured 1:20 into 12 ml (3 ml per well) of the same, pre-warmed DMEM in a 6-well cell culture plate (Corning Inc.). The plate was incubated in a tissue culture incubator with 5% CO2 at 37 °C without shaking to induce type III secretion. After 6 h, the cultures for each strain were pooled together, and their optical density at 600 nm was measured. The cultures were centrifuged at 16,100 × g for 10 min, and the supernatant from ΔlysAΔargH was combined with that from ΔlysAΔargHΔescN in a 1:1 ratio normalized according to their A600 readings. The supernatant from ΔlysAΔargHΔsepD and ΔlysAΔargHΔsepDΔescN was pooled similarly. The combined supernatant was filtered through a Millex-GV13 filter unit (0.22 μm, Millipore) to remove any residual bacteria. Trichloroacetic acid was added to a final concentration of 10% to the supernatant to precipitate the proteins. The proteins were pelleted by spinning at 16,100 × g for 30 min in 2-ml Eppendorf SafeLock tubes, washed once with cold acetone, and dried in air.
FIGURE 1.
SILAC analysis of the type III secretome by C. rodentium. A, overview of the SILAC protocol using C. rodentium ΔsepD and ΔsepDΔescN as examples. The ΔsepD (T3SS competent) and ΔsepDΔescN (T3SS deficient) strains were grown in DMEM to induce type III secretion. The ΔsepDΔescN strain was grown in DMEM containing normal l-arginine (Arg) and l-lysine (Lys), whereas the ΔsepD strain was labeled by heavy isotope-labeled [13C6]Arg and [2H4]Lys. Their secreted proteins were pooled before being subjected to filtration, protein precipitation, and digestion, and LC-MS/MS analysis. B, type III secretion profiles of C. rodentium ΔlysAΔargH mutants used for SILAC analysis. The C. rodentium strains were grown in DMEM to induce type III secretion. Secreted proteins in the supernatant were analyzed in SDS-13% PAGE and stained by Coomassie G-250. Secreted proteins from an equal amount of cultures for each strain (normalized by A600) were loaded in each lane. Plasmid pCRgrlA expresses the C. rodentium LEE-encoded positive regulator GrlA.
The protein pellet was solubilized in 6 m urea, 2 m thiourea in 10 mm HEPES (pH 8.0), and in-solution tryptic digestions were performed as described (30). The samples were next diluted 2-fold with a solution of 1% trifluoroacetic acid, 3% (v/v) acetonitrile, 0.5% (v/v) acetic acid, and desalted, concentrated, and filtered on C18 STop And Go Extraction tips (31), and then eluted directly into a 96-well plate. Peptide mixtures were analyzed on a 1100 series nanoflow high performance liquid chromatograph (Agilent) on-line coupled via a nanoelectrospray ion source (Proxeon, Odense, Denmark) to a linear trapping quadrupole-Orbitrap (LTQ-OrbitrapXL, ThermoFisher Scientific, Bremen, Germany) tandem mass spectrometer (32). Briefly, peptides were injected directly onto a reversed phase (3 μm-diameter ReproSil-Pur C18, Dr. Maisch, Ammerbuch-Entringen, Germany) column manually packed into a 15 cm-long, 75 μm-inner diameter fused silica emitter. Peptides were eluted directly into the LTQ-OrbitrapXL using a linear gradient from 4.8% acetonitrile in 0.5% acetic acid to 24% acetonitrile in 0.5% acetic acid over 60 min at a flow rate of 200 nl/min. The LTQ-OrbitrapXL was set to acquire a full-range scan in the Orbitrap, from which the 5 most abundant multiply charged ions were selected for fragmentation in the LTQ (32).
Mass Spectrometry Data Analysis
Peak lists of fragment ions were generated by Extract_MSN (version 3.2, ThermoFisher) using the default parameters. Monoisotopic peak and charge state assignments were checked by DTA Supercharge, part of the MSQuant suite of software (33). Fragment spectra were searched against a data base comprised of normal contaminants (e.g. keratins and albumin) and exogenous additives (e.g. trypsin and immunoglobulins), as well as translations of all open reading frames predicted in all six reading frames of the C. rodentium genome sequence (54,093 sequences, including reversed), using Mascot (version 2.2, Matrix Science) with the following parameters; trypsin specificity allowing up to one missed cleavage, cysteine carbamidomethylation as a fixed modification, [13C6]arginine and [2H4]lysine as appropriate for the particular experiment as variable modifications, ESI-trap fragmentation characteristics, 5-ppm mass tolerance for precursor ion masses, and 0.6 Da tolerance for fragment ion masses. Acceptance criteria for protein identifications were set so that only proteins identified by at least two unique peptides of seven or more amino acids with Mascot IonsScores >25 were accepted, criteria resulting in an estimated false discovery rate of less than 1%. Quantitative ratios were extracted from the raw data using MSQuant (version 1.4.3), which calculates an intensity weighted average of within spectra ratios from all spectra across the chromatographic peak of each peptide ion. For automatic quantification, only those proteins with a coefficient of variation less than 30% were accepted with no further verification. For proteins with high coefficient of variations or with only one quantified peptide in a particular experiment, the chromatographic peak assignment was manually verified or rejected. In the overall study the expression ratios for all proteins reported here were based on quantification of at least two peptides. Analytical variability of SILAC data in the types of experiments performed here is typically <20% in our hands and biological variability was addressed in these experiments by performing at least three independent replicates of each experiment. All peptide and protein information acquired in this study can be found in supplemental Tables S2–S4.
Generation of Fusion Proteins with a 2HA Epitope
C-terminal 2HA fusions were generated using the vector pTOPO-2HA (17). The coding regions with their immediate upstream regions, but without the stop codon, of C. rodentium nleK, ospB, espM2, espT, espX7, and EPEC rorf8 were amplified by PCR using primer pairs CRnleK-HAF/CRnleK-HAR, CRospB-HAF/CRospB-HAR, CRespM2-HAF/CRespM2-HAR, CRespT-HAF/CRespT-HAR, CRespX-HAF/CRespX-HAR, and EPr8-HAF/EPr8-HAR (supplemental Table S1), respectively, and then cloned as either a SacI/XhoI (for nleK, ospB, espM2, espT, and EPEC rorf8) or a KpnI/SalI (for espX7) fragment into SacI/XhoI or KpnI/XhoI-digested pTOPO-2HA. These constructs express fusion proteins with a double HA tag at their C termini.
Construction of β-Lactamase TEM-1 Fusions
N-terminal translational fusions to TEM-1 β-lactamase were generated in pCX341 (a generous gift from I. Rosenshine, Hebrew University, Israel), a derivative of pCX340 (34, 35). The coding regions, without the stop codon, of C. rodentium nleK, ospB, espM2, espT, and espX7, and EPEC espZ and rorf8 were amplified by PCR using primer pairs TEM-CRnleKF/TEM-CRnleKR, TEM-CRospBF/TEM-CRospBR, TEM-CRespM2F/TEM-CRespM2R, TEM-CRespTF/TEM-CRespTR, CRespX-HAF/TEM-CRespXR, TEM-EPespZF/TEM-EPespZR, and TEM-EPr8F/TEM-EPr8R (supplemental Table S1), respectively, and then cloned as either an NdeI/EcoRI (for nleK, ospB, espT, espZ, and rorf8) or a KpnI/EcoRI (for espM2 and espX7) fragment into NdeI/EcoRI- or KpnI/EcoRI-digested pCX341 to generate pCRnleK-TEM, pCRospB-TEM, pCRespM2-TEM, pCRespT-TEM, pCRespX7-TEM, pEPespZ-TEM, and pEPr8-TEM. These constructs were introduced by electroporation into EPEC strain E2348/69 and its ΔescN mutant for translocation assays.
Type III Secretion and Translocation Assays
Type III secretion assays for C. rodentium proteins were performed essentially as described before for EPEC (36). Translocation of effectors into host cells was assayed using the TEM-1 β-lactamase translocation assay (34). EPEC wild-type and its ΔescN strains carrying N-terminal translational fusions to TEM-1 β-lactamase were used to infect HeLa cells (CCL2, American Type Culture Collection), translocation was detected by fluorescence microscopy using the fluorescent substrate CCF2-AM (Invitrogen), and fluorescence was quantified as previously described (34).
Construction of nleK, espM2, espT, espX7, and ospB In-frame Deletion Mutants in C. rodentium
Using the same vector and sucrose selection procedure as described above for generating lysA and argH mutants, we constructed deletion constructs pRE118-ΔCRnleK, pRE118-ΔCRespM2, pRE118-ΔCRespT, pRE118-ΔCRespX7, and pRE118-ΔCRospB by cloning into pRE118 the PCR fragments generated using primer pairs CRnleK-1/DCRnleK-R and DCRnleK-F/CRnleK-2, CRespM2–1/DCRespM2-R and DCRespM2-F/CRespM2–2, CRespT-1/DCRespT-R and DCRespT-F/CRespT-2, CRespX-1/DCRespX-R and DCRespX-F/ CRespX-2, as well as CRospB-1/DCRospB-R and DCRospB-F/CRospB-2 (supplemental Table S1), respectively. About 87% of the coding region of nleK, 92% of espM2, 92% of espT, 93% of espX7, and 89% of ospB were deleted. These constructs were introduced into C. rodentium DBS100 by electroporation for allelic exchange and sucrose selection, and the respective deletion mutants, DBS100 ΔnleK, ΔespM2, ΔespT, ΔespX7, and ΔospB, were obtained and verified by multiple PCRs.
Mouse Infections by C. rodentium Strains
Five to six-week-old female C57BL/6 and C3H/HeJ mice were purchased from Jackson Laboratories (Bar Harbor, ME), and housed in the animal facilities at the University of British Columbia in direct accordance with guidelines established by the Canadian Council on the Use of Laboratory Animals. All mouse experiments described here were approved by the University of British Columbia Animal Care Committee. Mice were infected by oral gavage with 100 μl of overnight C. rodentium cultures in LB broth (225 rpm at 37 °C) containing ∼3–5 × 108 bacteria. The C57BL/6 mice were sacrificed at 6 or 10 days post-infection. The whole colon, including luminal contents, was placed into 1 ml of sterile phosphate-buffered saline and homogenized with a MixerMill 301 (Retsch, Newtown, PA). Serial dilutions of the homogenate were plated on MacConkey (OXOID) agar plates. After incubation overnight at 37 °C, the C. rodentium colonies that display the distinctive morphology of a pink center with a white rim were enumerated. Tissues were fixed in 10% neutral buffered formalin overnight and then placed into 70% ethanol. Fixed tissues were embedded in paraffin and cut into 5-μm sections. Tissues were stained with hematoxylin and eosin by Wax-it Histology Services (Vancouver, BC), and subjected to pathological scoring (37).
RESULTS
SILAC Analysis of Type III-secreted Proteins by C. rodentium: Rationale
Our aim was to use SILAC to identify proteins specifically secreted by the LEE-encoded T3SS in C. rodentium, i.e. the type III secretome. We have previously shown that, when grown statically in DMEM at 37 °C in a 5% CO2-containing environment, WT C. rodentium, EHEC, and EPEC preferentially secrete translocators EspA, EspB, and EspD, with only very small amounts of effectors secreted (38). In contrast, the sepD mutants of these pathogens predominately secrete effectors (both LEE- and non-LEE-encoded), with the translocators hardly detectable by Western blot (17, 38). We have also shown that overexpression of the LEE-encoded positive regulator GrlA on a plasmid (pCRgrlA) can greatly enhance LEE gene expression and type III secretion in C. rodentium (17). In this report, we intended to identify the C. rodentium type III secretome, including both translocators and effectors, using SILAC to specifically distinguish type III-secreted proteins from background, non-type III-secreted proteins of WT C. rodentium and its ΔsepD mutant. As shown in Fig. 1A, we compared the supernatant proteins from cultures of the C. rodentium WT strain versus its ΔescN mutant (T3SS-deficient), and a ΔsepD strain (effector hyper-secreting) versus a ΔsepDΔescN mutant, all carrying the plasmid pCRgrlA. The escN gene encodes an ATPase essential for type III secretion (11, 36). The culture supernatants of the paired strains were combined, and the proteins were precipitated, digested, and analyzed by LC-MS/MS. In each binary comparison (WT/ΔescN or ΔsepD/ΔsepDΔescN), those proteins showing a high differential SILAC ratio (heavy isotopes-labeled versus normal/light isotopes-labeled) should represent proteins specifically secreted through the T3SS, whereas proteins not secreted by the T3SS should have a SILAC ratio around 1.
Using SILAC to analyze the secreted proteins involves differential labeling of bacterial proteins with isotopic amino acids (Lys and Arg in this case). Proteins from bacteria with lysA and argH mutations that block the de novo biosynthesis of Lys and Arg were labeled more efficiently with the isotopic Lys and Arg when grown in medium containing these amino acids (data not shown). We therefore generated deletion mutants of lysA and argH in C. rodentium WT, ΔescN, ΔsepD, and ΔsepDΔescN strains to create DBS100 ΔlysAΔargH, ΔlysAΔargHΔescN, ΔlysAΔargHΔsepD, and ΔlysAΔargHΔsepDΔescN. As shown in Fig. 1B, mutations in lysA and argH did not affect the type III secretion profiles of the respective C. rodentium strains grown in DMEM when compared with their parental strains.
SILAC Analysis of Type III-secreted Proteins by WT C. rodentium
We first analyzed the secreted proteins from DBS100 ΔlysAΔargH (C. rodentium “WT”) and ΔlysAΔargHΔescN (“ΔescN,” T3SS deficient) by SILAC. The proteins secreted by the WT, but not or poorly secreted by the escN mutant, based on their SILAC ratios higher than at least 3.0, are listed in Table 1. As expected, the 3 translocators, EspA, EspD, and EspB, showed both high overall abundance and high ratios in the SILAC analysis (Table 1), confirming their type III secreted status. In addition, secretion of EscF and rOrf8 was found to be T3SS-dependent, as well as secretion of several known effectors including Tir, NleE, and EspF. Tir is one of the most abundant and earliest effectors secreted (35, 39), and accordingly, it had a high SILAC ratio. We also identified one novel type III secreted protein that was later shown to be a new non-LEE-encoded effector and designated as NleK.
TABLE 1.
Proteins secreted by the LEE-encoded T3SS in C. rodentium identified using SILAC-based mass spectrometry and validated by other means
| Protein name | Averaged ratioa | S.D.b | Category of secretion substrates |
|---|---|---|---|
| Proteins identified from the DBS100 WT vs. ΔescN samples | |||
| EspB | High | NAc | Translocator; effector as well? |
| EspA | High | NA | Translocator |
| Tir | High | NA | LEE-encoded effector |
| EspD | High | NA | Translocator |
| NleE | High | NA | Non-LEE-encoded effector |
| EscF | 6.2 | 1.0 | T3SS needle component |
| EspF | 5.3 | 0.9 | LEE-encoded effector |
| NleK | 3.5 | Non-LEE encoded effector | |
| rOrf8 | 3.0 | 0.6 | Component of the T3SS inner rod? |
| Proteins identified from the DBS100 ΔsepD vs ΔsepDΔescN samples | |||
| Tir | High | NA | LEE-encoded effector |
| NleA | High | NA | Non-LEE-encoded effector |
| NleE | High | NA | Non-LEE-encoded effector |
| NleH | High | NA | Non-LEE-encoded effector |
| NleC | High | NA | Non-LEE-encoded effector |
| EspG | High | NA | LEE-encoded effector |
| EspH | High | NA | LEE-encoded effector |
| EspB | High | NA | Translocator; effector as well? |
| NleB | 9.0 | 5.6 | Non-LEE-encoded effector |
| EspF | 8.9 | 2.3 | LEE-encoded effector |
| SepZ/EspZ | 8.3 | 11.4 | LEE-encoded effector |
| EspD | 8.3 | 1.7 | Translocator |
| NleG | 7.6 | 7.6 | Non-LEE-encoded effector |
| EscF | 7.6 | 0.7 | T3SS needle component |
| NleF | 6.5 | 7.4 | Non-LEE-encoded effector |
| EspX7 | 6.3 | 2.5 | Non-LEE-encoded effector |
| OspB/Ibe | 5.7 | 2.9 | Non-LEE-encoded effector |
| Map | 5.3 | 0.8 | LEE-encoded effector |
| EspM2 | 5.1 | 4.8 | Non-LEE-encoded effector |
| EspA | 4.5 | 4.2 | Translocator |
| EspT | 3.7 | Non-LEE-encoded effector | |
| NleD | 3.3 | Non-LEE-encoded effector | |
| rOrf8 | 3.2 | 0.2 | Component of the T3SS inner rod? |
a Averaged SILAC ratio (heavy isotope/light isotope) measured. “High” was given as the ratio for the type III secreted proteins that showed only heavy isotope labeled peptides in the samples.
b S.D., standard deviation as determined from quantification of several peptides when available.
c NA, not applicable.
SILAC Analysis of Type III-secreted Proteins by the sepD Mutant of C. rodentium
To identify additional C. rodentium effectors, we next analyzed the secreted proteins from DBS100 ΔlysAΔargHΔsepD and ΔlysAΔargHΔsepDΔescN. Proteins with SILAC ratios higher than at least 3.0, thus secreted by the effector hypersecreting ΔsepD strain in a T3SS-dependent manner, are listed in Table 1 and Fig. 2A. The two most abundant effectors analyzed by MS that showed high SILAC ratios were Tir and NleA, which have been previously shown to be the most abundant proteins secreted by the sepD mutant (17, 35, 40). The translocators EspA, EspD, and EspB also displayed high SILAC ratios. This was unexpected because the trace amounts of translocators secreted by the sepD mutant can hardly be detected by Western blot (38), thus demonstrating the high sensitivity of SILAC. Secretion of EscF and rOrf8 by the sepD mutant further confirmed their type III secretion status, because they were found to be type III secreted during the SILAC analysis of the secreted proteins from WT versus ΔescN (Table 1). All the effectors previously identified in C. rodentium by gel electrophoresis and proteomics (17, 19), including both LEE-encoded (Tir, Map, SepZ/EspZ, EspH, EspG, and EspF) and non-LEE-encoded (NleA, NleB, NleC, NleD, NleE, NleF, NleG, and NleH), were identified and confirmed by our SILAC analysis. In addition, 4 new secreted proteins (OspB, EspM2, EspX7, and EspT) were also identified (see Table 1, Figs. 2 and 3). These most likely represent additional non-LEE-encoded effectors, as they have homology to known effector proteins from EHEC and other enteric pathogens (8–10). EspM2 and EspT have been identified as potential effectors based on their homology to the WXXXE family of effectors in EHEC, Salmonella and Shigella, and have recently been shown to have effector functions when expressed in EPEC (24, 41, 42). Our analysis demonstrated that these proteins are indeed expressed in C. rodentium and are type III secreted. One known C. rodentium effector, EspJ (43), was shown to have a relatively low SILAC ratio of 2.1, suggesting that there might be some EspJ secreted independently of the T3SS. Another putative effector, EspO (25), was shown to have a low SILAC ratio of 1.3, suggesting that it may not be type III secreted. We did not detect EspK (23) and EspM3 (42), which were hypothesized to be C. rodentium effectors because they have significant homology to effectors present in EHEC. Our results suggested that some of these proteins may not be highly expressed or type III secreted under the growth conditions tested here.
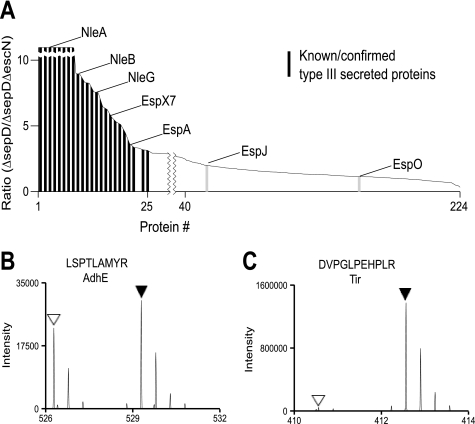
FIGURE 2.
Summary and sample spectra of SILAC analysis of the C. rodentium type III secretome. A, SILAC ratios for proteins from C. rodentium ΔsepD versus ΔsepDΔescN were plotted in decreasing order. The black bars represent previously known or confirmed type III-secreted proteins by this study, and the light gray bars indicate suspected effectors with a relative low SILAC ratio. B and C, MS spectra of sample peptides of AdhE (B) and Tir (C). Normal Lys- and Arg-labeled peptides are indicated by open triangles, and [2H4]Lys and/or [13C6]Arg-labeled peptides by filled triangles. Proteins specifically secreted by the T3SS, such as Tir, are identified by primarily [2H4]Lys and/or [13C6]Arg-labeled peptides, whereas nonspecific secreted proteins, such as AdhE, should have a close to equal ratio (1:1) of normal, unlabeled peptides, and labeled peptides.
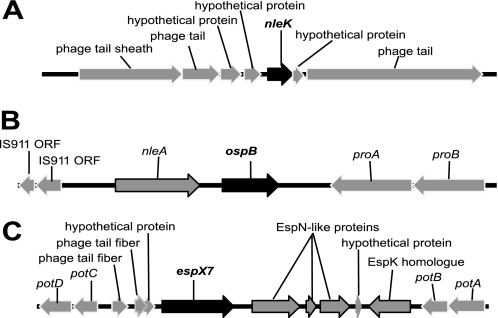
FIGURE 3.
Schematic diagrams of the C. rodentium chromosomal regions containing the nleK (A), ospB (B), and espX7 (C) genes. The nucleotide sequences of the C. rodentium genomic regions containing these genes were retrieved from the Sanger Institute website, and analyzed using Vector NTI (Invitrogen). The genes encoding the newly identified effectors NleK, OspBCR, and EspX7 are highlighted with black arrowed bars.
Type III Secretome of C. rodentium
To summarize the SILAC data on the secretome of WT C. rodentium and its ΔsepD mutant, a sum of 1924 peptides were analyzed and quantified (supplemental Table S2). A total of 266 proteins, with at least two unique peptides each, were identified in the culture supernatant of WT C. rodentium and its ΔsepD mutant (supplemental Table S3). Among these, at least 24 proteins (see Table 1 and supplemental Table S4) were secreted in a LEE-encoded T3SS-dependent manner as judged by their high SILAC ratios. These type III-secreted proteins include the translocators EspA, EspB, and EspD, the needle protein EscF, and the putative inner rod component rOrf8, and possibly 20 effectors (EspB included). Shown in Fig. 2 are the MS/MS spectra for AdhE (Fig. 2B), a protein present in the supernatant but whose SILAC ratio was close to 1, suggesting that it is not secreted by the T3SS; and Tir (Fig. 2C), a known effector.
Our SILAC analysis identified 5 potential additional effectors (NleK, EspM2, EspT, OspB, and EspX7) in C. rodentium. NleK does not show similarity to any known effectors, possibly representing a new class of type III effectors (see next), whereas OspB, EspX7, EspM2, and EspT have homology to known effectors in EHEC, EPEC, or other enteric pathogens (6–10). EspM2 and EspT are encoded by the same pathogenicity island in C. rodentium, as recently reported (42). Schematic gene maps of the genomic regions encoding NleK, OspB, and EspX7 are presented in Fig. 3. The genes encoding these effectors are associated with prophages or remnants of mobile genetic elements and insertion sequences.
NleK shows 70% identity, 83% similarity to a conserved hypothetical protein in EPEC strain E22, as well as to STY1628 of Salmonella enterica serovar Typhi. The nleK gene is associated with a prophage in C. rodentium (Fig. 3A) in a similar fashion to that in Salmonella Typhi. The sequences of the prophages, belonging to the bacteriophage P2 family, are highly conserved between C. rodentium and Salmonella Typhi, with some of their phage tail proteins sharing 95% identity and 98% similarity.
OspBCR shows 34% identity to the Shigella effector OspB, and more than 60% identity to various homologues of OspB in EPEC and EHEC, as well as the effector Ibe in an atypical EPEC strain (44), but its gene is located in a completely different genomic context (Fig. 3B). The gene for Ibe is located in a region immediately after the LEE in the atypical EPEC strain 3431–4/86 and EHEC O103:H2 strain RW1374 (44). An ibe homologue is also found in EPEC strain B171-8 located next to the LEE, but is truncated. Interestingly, EPEC B171-8 carries another ospB homologue in a different chromosomal location (8). In C. rodentium, ospBCR is located downstream of nleA (Fig. 3B), whereas nleA in EHEC O157 strains EDL933 and Sakai is located in the pathogenicity island OI-71 and is associated with a prophage and several effector genes, but not ospB (45, 46). This suggests that ospB-like effector genes have been acquired by the A/E pathogens multiple times.
Finally, the location of espX7 in C. rodentium (Fig. 3C) is similar to that in EHEC O157. In EHEC strain EDL933, espX7 is associated with prophage CP-933N (46) inserted in the potABCD operon. The effector genes espK and espN are also located in the same island (23). In C. rodentium, the gene organization around espX7 is the same as that in EDL933, suggesting the same evolutionary origin. However, several mutations have accumulated in the C. rodentium espN-like gene, splitting its coding region into 3 smaller open reading frames (Fig. 3C).
Confirmation of rOrf8, NleK, OspB, EspM2, EspX7, and EspT as Type III-secreted Proteins
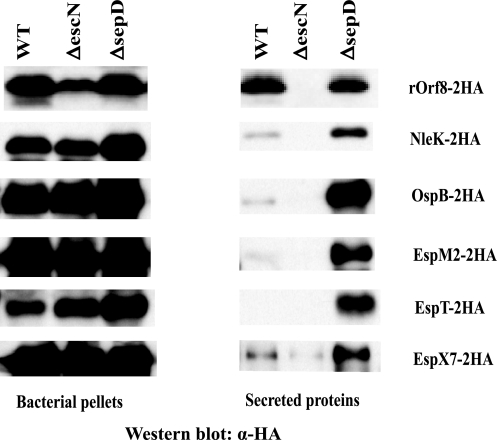
The majority of the type III-secreted proteins identified by our SILAC analysis (see Table 1 and Fig. 2A) has been validated as type III-secreted proteins or effectors by other means (6, 11, 17). EscF is the needle component based on its homology to other T3SS needle proteins, and it is well established that the needle protein is secreted by the T3SS in other pathogens (1). We therefore chose to only confirm the type III secretion of rOrf8, NleK, OspB, EspM2, EspX7, and EspT by using an alternative method, because the type III secretion of these proteins has not been previously demonstrated in C. rodentium.
We generated constructs expressing C. rodentium NleK, OspB, EspM2, EspT, and EspX7, and EPEC rOrf8 with double HA epitope fusions at the C terminus. These constructs were introduced into C. rodentium DBS100 or EPEC WT, ΔescN, and ΔsepD strains, and assayed for type III secretion of the 2HA-tagged proteins by Western blot. Although all the fusions were expressed well in all the C. rodentium or EPEC strains, they were only secreted by the WT and the sepD mutant strains, not by the escN mutant (Fig. 4), confirming that their secretion is dependent on the LEE-encoded T3SS. Although rOrf8–2HA was secreted at similar levels by the WT and sepD mutant strains, the other 5 proteins (NleK, EspM2, EspT, OspB, and EspX7) were secreted by the WT strain at very low levels, but were hyper-secreted by the sepD mutant, similar to the secretion patterns of known effectors (38).
FIGURE 4.
Confirmation of rOrf8, NleK, OspB, EspM2, EspT, and EspX7 as type III secreted proteins in EPEC and C. rodentium. C-terminal double HA-tagged EPEC rOrf8 and C. rodentium NleK, OspB, EspM2, EspT, and EspX7 were expressed in EPEC or C. rodentium WT, ΔescN, and ΔsepD strains, and assayed for type III secretion. All constructs were tested in C. rodentium strains, except the rOrf8–2HA construct, which was tested in EPEC. Proteins from bacterial pellets and secreted proteins in DMEM were separated in SDS-PAGE, blotted onto nitrocellulose membranes, and probed with monoclonal antibodies against HA by Western blot.
Translocation of NleK, EspM2, EspT, OspB, and EspX7 into Host Cells
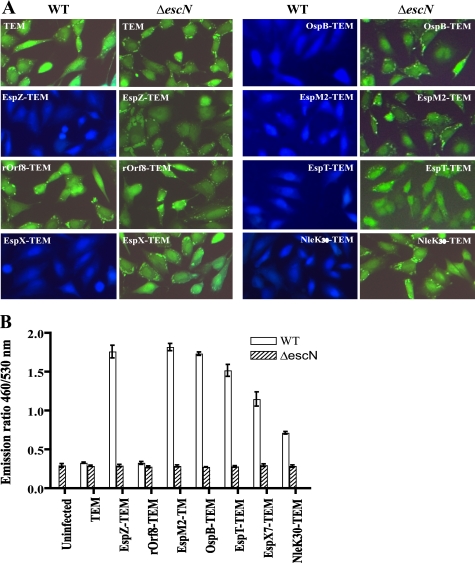
To determine whether any of these new secreted proteins are actual effectors, which should not only be secreted in a T3SS-dependent manner but also translocated into host cells, we generated fusion constructs of rOrf8, NleK, OspB, EspM2, EspT, and EspX7 to β-lactamase TEM-1 (34, 35). These constructs were introduced into C. rodentium and EPEC strain E2348/69 WT and their ΔescN mutants. All these fusions were expressed similarly under control of the same promoter in the vector, and they were type III secreted in both C. rodentium and EPEC as expected (data not shown). The EPEC strains were then used to infect cultured HeLa cells to assess type III translocation using fluorescence microscopy. EPEC, instead of C. rodentium, strains were used because EPEC infects cultured cells much more efficiently than C. rodentium. HeLa cells were loaded with the fluorescent β-lactamase substrate CCF2/AM. Uninfected HeLa cells or cells infected with EPEC strains expressing untranslocated TEM-1 fusions appear green when excited at 409 nm, whereas translocated TEM-1 cleaves the CCF2 β-lactam ring resulting in blue fluorescence. As shown in Fig. 5A, a known effector-TEM fusion, EspZ-TEM, was translocated in an EscN-dependent manner as expected, whereas the TEM control was not. There was no translocation of rOrf8-TEM, indicating that rOrf8 is not an effector. This is consistent with our previous observation that rOrf8 is essential for type III secretion (17), suggesting that rOrf8 likely exerts its function in the bacteria. On the other hand, OspB, EspM2, and EspT were all translocated into HeLa cells. EspX7 was translocated into HeLa cells as well, albeit less efficiently (Fig. 5B), probably due to its large size. Interestingly, NleK-TEM inhibited type III secretion when overexpressed in WT EPEC and C. rodentium, as well as their ΔsepD mutants (data not shown), so its translocation could not be assessed. However, when the first 30 amino acid residues of NleK were fused to TEM-1, the construct did not have any inhibitory effect on type III secretion (data not shown), and the NleK30-TEM fusion was translocated into HeLa cells (Fig. 5). This indicated that the first 30 amino acid residues of NleK contain the type III secretion and translocation signals, similar to other type III effectors (34). Collectively, these results demonstrated that NleK, OspB, EspM2, EspT, and EspX7 can be translocated into host cells, and are very likely effectors for C. rodentium.
FIGURE 5.
Translocation of NleK, EspM2, EspT, OspB, and EspX7 into HeLa cells. A, HeLa cells were infected with EPEC WT and its ΔescN mutant carrying constructs expressing fusion proteins of EPEC EspZ and rOrf8, as well as C. rodentium EspX7, OspB, EspM2, EspT, and the N-terminal 30 amino acid residues of NleK, to β-lactamase TEM-1. The infected cells were loaded with CCF2/AM, and assessed for protein translocation using fluorescence microscopy with excitation at 409 nm. Blue fluorescence indicates positive type III translocation, and green fluorescence shows negative translocation. B, fluorescence quantification of HeLa cells infected by the same EPEC strains using a fluorescence plate reader. The data are averages with standard deviations of triplicate values of the results from one of two experiments, and are presented as the emission ratio between blue fluorescence (460 nm) and green fluorescence (530 nm).
Role of NleK, EspM2, EspT, OspB, and EspX7 in C. rodentium Infection of Mice
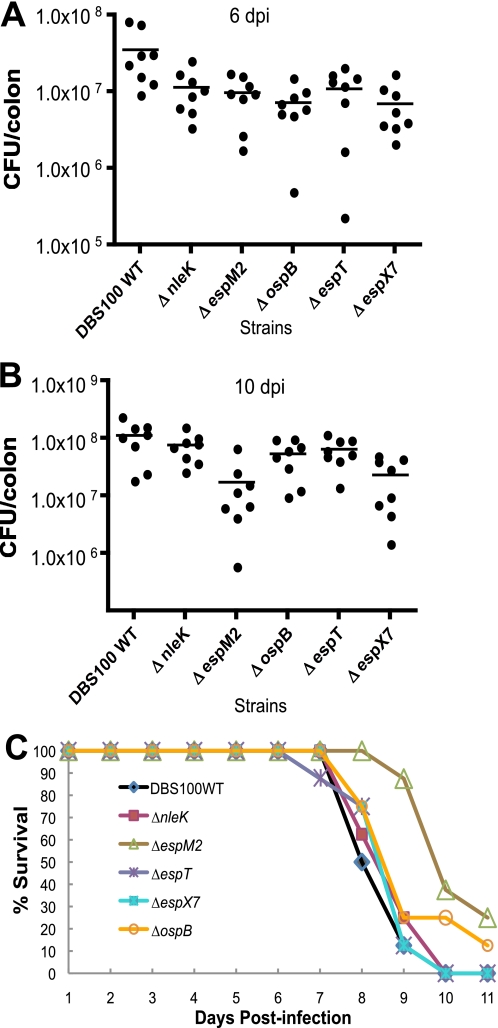
We have previously shown that a C. rodentium Δrorf8 mutant was severely attenuated in virulence in mice, due to its defect in type III secretion (17). However, the role for nleK, espM2, espT, ospB, and espX7 in C. rodentium virulence has never been tested. To assess whether any of these effectors influences C. rodentium virulence in mice, we generated in-frame deletion mutants in C. rodentium for nleK, espM2, espT, ospB, and espX7. Mutations in these genes did not affect C. rodentium type III secretion as expected (data not shown). C57BL/6 mice were infected with DBS100 WT and its deletion mutants of nleK, espM2, espT, ospB, and espX7, and the mice were sacrificed at 6 and 10 days post infection (dpi) to assess bacterial colonization in the mouse colon. As shown in Fig. 6A, ΔnleK, ΔespM2, ΔespT, ΔospB, and ΔespX7 mutants showed varying degrees of attenuation in colonization at 6 dpi when compared with DBS100 WT, with the difference for ΔnleK, ΔespM2, ΔospB, and ΔespX7 reaching statistic significance (p < 0.05). Even at 10 dpi (Fig. 6B), ΔespM2 and ΔespX7 mutants displayed significantly less colonization than the WT strain (p < 0.05), whereas ΔnleK, ΔespT, and ΔospB mutants showed no significant difference.
FIGURE 6.
Colonization and mortality in C57BL/6 or C3H/HeJ mice infected by C. rodentium DBS100 WT, ΔnleK, ΔespM2, ΔespT, ΔospB, and ΔespX7 mutants. C57BL/6J mice were infected with the C. rodentium strains, and the mice were sacrificed at 6 and 10 dpi to assess bacterial colonization. A, colonization data for 6 dpi. B, colonization data for 10 dpi. C, survival data of C3H/HeJ mice infected by WT C. rodentium and its ΔnleK, ΔespM2, ΔespT, ΔospB, and ΔespX7 mutants. Eight mice were used for each group. The data were plotted using the software Prism, and statistical analysis was carried out using two-tailed paired t test.
The data in C57BL/6 mice were supported by our infection studies with the ΔnleK, ΔespM2, ΔespT, ΔospB, and ΔespX7 mutants in the highly susceptible mouse strain C3H/HeJ, in which WT C. rodentium causes a fatal infection between days 6 and 11 (47). Although all the mice infected by the C. rodentium ΔnleK, ΔespM2, ΔespT, ΔospB, and ΔespX7 mutants eventually succumbed to the infection, mice infected by ΔespM2 and ΔospB survived longer than the WT-infected mice, although only ΔespM2-infected mice reached statistical significance (p < 0.05; Fig. 6C). These results indicated that, although none of the new effectors are essential for C. rodentium colonization and pathogenesis in mice, they, especially EspM2, contribute to full C. rodentium virulence.
DISCUSSION
Proteins secreted by T3SSs are important virulence factors and play critical roles in pathogenesis at the interface of pathogen-host interactions. They perform functions ranging from type III secretion/translocation and regulation of secretion, to modulation and subversion of host cell pathways and functions by the effectors (1). Many biochemical, genetic, and bioinformatic screens have been used to identify type III-secreted proteins (48), and the recent rapid advances in genomic research have greatly aided these efforts. Although these approaches are complimentary to each other, some of them lack sensitivity and throughput, and are labor intensive and prone to false positives.
In this study, we characterized the type III secretome of C. rodentium, a model organism for the clinically significant A/E pathogens EHEC and EPEC, using the quantitative proteomic tool SILAC (20). There are several advantages in using SILAC to search for type III-secreted proteins (Fig. 1A): 1) by avoiding gel-based methods, it minimizes sample handling and increases sensitivity; 2) the sampling procedure involves simultaneously processing the target sample and its negative control, providing an internal standard for relative quantification and thus dramatically reducing nonspecific background and false positives; and 3) by measuring proteins directly, it is invaluable for discovering new classes of effectors, such as NleK, which cannot be deduced by bioinformatics based on homology searches to known secreted proteins and effectors.
Our SILAC analysis revealed that there are at least 266 proteins present in the culture supernatant of C. rodentium, including 24 that are type III-secreted based on their SILAC ratios. We confirmed that the C. rodentium type III secretome includes the 3 translocators (EspA, EspD, and EspB), in addition to the 6 LEE-encoded effectors (Tir, EspF, Map, EspG, EspH, and EspZ) as well as the 9 non-LEE-encoded effectors (NleA/EspI, -B, -C, -D, -E, -F, -G, and -H, and EspJ) that have been identified previously in C. rodentium using biochemical, bioinformatical, and classical proteomic approaches (6, 11). The only effectors that were not verified by our screen are EspK, EspM3, and EspO, which were postulated to be effectors in C. rodentium based on their homology to known effectors in EHEC and EPEC (23–25), but their expression and type III secretion status have yet to be demonstrated in C. rodentium. Furthermore, we have added the T3SS needle protein EscF and the probable inner rod protein rOrf8, and 5 additional effectors to the growing list of type III-secreted proteins in C. rodentium.
The function of EscF has been studied in the A/E pathogens, but little is known about rOrf8. Although EscF has been shown to be essential for secretion of translocators and effectors (17, 49, 50), our studies here showed for the first time that EscF is itself a type III-secreted protein. Type III-dependent secretion of EscF is expected, because the needle components of other T3SSs are similarly secreted (1, 25). Our previous results indicated that rOrf8 is an essential part of the T3SS (17), and similar to EscF, it is also a type III-secreted protein. It has been proposed that rOrf8 may be an inner rod component because it has some limited similarity to known inner rod proteins of other T3SSs, such as PrgJ of the Salmonella pathogenicity island 1-encoded T3SS and YscI of the Yersinia T3SS (51). Indeed, both PrgJ and YscI have been shown to be secreted in a type III-dependent manner (52, 53), consistent with our finding that rOrf8 is type III secreted.
Functional redundancy, and multiple and overlapping functions are common themes of effector roles in pathogenesis (6). In A/E pathogens, the effectors that play critical roles in virulence include Tir, EspZ, NleA, and NleB (6, 11, 16–18, 21, 22, 54). All the other effectors collectively contribute to full virulence, yet mutations in each individual effector gene have only minor effects on virulence, partially because many effectors have closely related homologues in the same pathogen. For example, there are two nleH genes in certain EHEC strains, and some EPEC strains express both EspG and its close homologue EspG2 (6). NleG has as many as 14 homologues in EHEC, although not all of them are translocated (9, 10). Many of the LEE-encoded effectors, including Tir, Map, EspH, and EspG, modulate the host cytoskeleton by various mechanisms, and some effectors perform multiple functions. We have found that among the 5 additional effectors, EspM2 appears to contribute the most to C. rodentium virulence, whereas NleK, OspB, and EspX7 are also needed for full C. rodentium virulence (Fig. 6). EspM2 and EspT, as well as the LEE-encoded effector Map, belong to a family of bacterial type III effectors with a conserved WXXXE motif that subvert the host cell cytoskeleton by modulating the functions of small GTPases (24, 41, 42, 55). This family of effectors also includes Shigella IpgB1 and IpgB2 and Salmonella SifA and SifB (41). Although all these effectors modulate actin dynamics, they appear to target distinct Rho family GTPases and signaling pathways (24, 41, 42). It is therefore not surprising that individual mutants of some of these effectors, i.e. Map (17, 21) and EspM2 (this study), in C. rodentium have obviously discernable virulence attenuation, suggesting that they may contribute to different aspects of the disease, and thus underlining the importance of knowing the full effector repertoire of these pathogens.
Our results have now put the total number of C. rodentium type III-secreted proteins at 24. Counting the 4 putative effectors (EspJ, EspK, EspM3, and EspO) in C. rodentium based on their homology to known effectors in EHEC, the total number of type III-secreted proteins in C. rodentium stands at 28, among which 24 are effectors. This places the total number of C. rodentium effectors between that of EHEC (31 to 41 effectors depending on the serotypes) (9) and EPEC (21 effectors) (7). However, this will likely prove to be an underestimate of the number of C. rodentium effectors for several reasons: 1) our methods may not distinguish between closely related effector homologues. For example, there are two highly similar copies of nleD (nleD and nleD2) (22), and 3 other nleG homologues in C. rodentium (data not shown). 2) We only tested one set of growth conditions and the secretome of the WT and ΔsepD strains. It is possible that some effectors, such as EspK and EspO, are not expressed well, or are not stable when secreted, in these genetic backgrounds under these growth conditions. In addition, the culture conditions used may not fully mimic the host infection process, which may induce the expression of additional effectors. 3) There are proteins that showed a SILAC ratio around 3 that we did not have confidence calling them type III secreted due to their low abundance or the small number of peptides sampled. Because EspJ is a proven effector (43) and only had a ratio of 2.1 in our SILAC analysis, it is possible that some of the proteins showing a SILAC ratio around 3 may be effectors, and we are currently pursuing this possibility. 4) A few proteins are highly prevalent in the secreted proteins, and their presence may obscure the sampling of the less abundant proteins. This may explain why NleK was not observed in the SILAC screen of the secretome of the effector-hypersecreting sepD mutant, because NleK has a smaller size and lower abundance compared with other effectors. Resolving the proteins first by gel electrophoresis or other prefractionation methods and dividing the gels into slices before LC-MS/MS analysis may circumvent the problem. Taking these possibilities into consideration, the actual type III effector repertoire of C. rodentium will undoubtedly be higher than our estimation. Indeed, just before the submission of this manuscript, the C. rodentium genome was published (56), and a survey by bioinformatics and homology searches in this article indicated that C. rodentium may also encode homologues of EHEC effectors EspL, EspV, and EspN, and that the total number of potential effectors can reach 29 in C. rodentium. Therefore, C. rodentium possesses at least 30 effector genes when the new class of effectors, represented by NleK discovered by our proteomic analysis in this study, is included.
In summary, we have reported here an expanded repertoire of type III-secreted proteins by C. rodentium. Our analyses have provided new insight into the function of rOrf8, and identified several effectors that contribute to full virulence of C. rodentium in mice. Our results demonstrate that SILAC is a simple, quantitative, high-throughput, and sensitive tool for identifying secreted protein substrates, and it has the potential to be readily applied in identifying substrates of other protein secretion systems in many bacterial pathogens.
Supplementary Material
Acknowledgments
We thank Nikolay Stoynov for technical assistance, and Kristie Keeney and Stephanie Shames for critical reading of the manuscript. Infrastructure used in this project was funded by the Canadian Foundation for Innovation, the British Columbia (BC) Knowledge Development Fund, and the Michael Smith Foundation through the BC Proteomics Network.
This work was supported in part by Canadian Institutes of Health Research grants, a Genome Canada/Genome BC through the PRoteomics for Emerging PAthogen REsponse project (to L. J. F. and B. B. F.), and a Howard Hughes Medical Institute operating grant (to B. B. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S4.
- T3SS
- type III secretion system
- EPEC
- enteropathogenic E. coli
- EHEC
- enterohemorrhagic E. coli
- A/E
- attaching effacing
- LEE
- locus of enterocyte effacement
- Nle
- non-LEE-encoded effectors
- SILAC
- stable isotope labeling with amino acids in cell culture
- LC-MS/MS
- liquid chromatography/tandem mass spectrometry
- HA
- hemagglutinin
- WT
- wild-type
- dpi
- days post-infection
- DMEM
- Dulbecco's modified Eagle's medium.
REFERENCES
- 1.Cornelis G. R. (2006) Nat. Rev. Microbiol. 4, 811–825 [DOI] [PubMed] [Google Scholar]
- 2.Blocker A. J., Deane J. E., Veenendaal A. K., Roversi P., Hodgkinson J. L., Johnson S., Lea S. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6507–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marlovits T. C., Kubori T., Lara-Tejero M., Thomas D., Unger V. M., Galán J. E. (2006) Nature 441, 637–640 [DOI] [PubMed] [Google Scholar]
- 4.Kimbrough T. G., Miller S. I. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nataro J. P., Kaper J. B. (1998) Clin. Microbiol. Rev. 11, 142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean P., Kenny B. (2009) Curr. Opin. Microbiol. 12, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iguchi A., Thomson N. R., Ogura Y., Saunders D., Ooka T., Henderson I. R., Harris D., Asadulghani M., Kurokawa K., Dean P., Kenny B., Quail M. A., Thurston S., Dougan G., Hayashi T., Parkhill J., Frankel G. (2009) J. Bacteriol. 191, 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogura Y., Abe H., Katsura K., Kurokawa K., Asadulghani M., Iguchi A., Ooka T., Nakayama K., Yamashita A., Hattori M., Tobe T., Hayashi T. (2008) J. Bacteriol. 190, 6948–6960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogura Y., Ooka T., Iguchi A., Toh H., Asadulghani M., Oshima K., Kodama T., Abe H., Nakayama K., Kurokawa K., Tobe T., Hattori M., Hayashi T. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17939–17944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobe T., Beatson S. A., Taniguchi H., Abe H., Bailey C. M., Fivian A., Younis R., Matthews S., Marches O., Frankel G., Hayashi T., Pallen M. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14941–14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundy R., MacDonald T. T., Dougan G., Frankel G., Wiles S. (2005) Cell. Microbiol. 7, 1697–1706 [DOI] [PubMed] [Google Scholar]
- 12.Campellone K. G., Robbins D., Leong J. M. (2004) Dev. Cell 7, 217–228 [DOI] [PubMed] [Google Scholar]
- 13.Garmendia J., Ren Z., Tennant S., Midolli Viera M. A., Chong Y., Whale A., Azzopardi K., Dahan S., Sircili M. P., Franzolin M. R., Trabulsi L. R., Phillips A., Gomes T. A., Xu J., Robins-Browne R., Frankel G. (2005) J. Clin. Microbiol. 43, 5715–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loukiadis E., Nobe R., Herold S., Tramuta C., Ogura Y., Ooka T., Morabito S., Kérourédan M., Brugère H., Schmidt H., Hayashi T., Oswald E. (2008) J. Bacteriol. 190, 275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng W., Li Y., Vallance B. A., Finlay B. B. (2001) Infect. Immun. 69, 6323–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng W., Vallance B. A., Li Y., Puente J. L., Finlay B. B. (2003) Mol. Microbiol. 48, 95–115 [DOI] [PubMed] [Google Scholar]
- 17.Deng W., Puente J. L., Gruenheid S., Li Y., Vallance B. A., Vázquez A., Barba J., Ibarra J. A., O'Donnell P., Metalnikov P., Ashman K., Lee S., Goode D., Pawson T., Finlay B. B. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruenheid S., Sekirov I., Thomas N. A., Deng W., O'Donnell P., Goode D., Li Y., Frey E. A., Brown N. F., Metalnikov P., Pawson T., Ashman K., Finlay B. B. (2004) Mol. Microbiol. 51, 1233–1249 [DOI] [PubMed] [Google Scholar]
- 19.García-Angulo V. A., Deng W., Thomas N. A., Finlay B. B., Puente J. L. (2008) J. Bacteriol. 190, 2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong S. E., Mann M. (2006) Nat. Protocols 1, 2650–2660 [DOI] [PubMed] [Google Scholar]
- 21.Mundy R., Petrovska L., Smollett K., Simpson N., Wilson R. K., Yu J., Tu X., Rosenshine I., Clare S., Dougan G., Frankel G. (2004) Infect. Immun. 72, 2288–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly M., Hart E., Mundy R., Marchès O., Wiles S., Badea L., Luck S., Tauschek M., Frankel G., Robins-Browne R. M., Hartland E. L. (2006) Infect. Immun. 74, 2328–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlisidou I., Marchés O., Dziva F., Mundy R., Frankel G., Stevens M. P. (2006) FEMS Microbiol. Lett. 263, 32–40 [DOI] [PubMed] [Google Scholar]
- 24.Arbeloa A., Bulgin R. R., MacKenzie G., Shaw R. K., Pallen M. J., Crepin V. F., Berger C. N., Frankel G. (2008) Cell. Microbiol. 10, 1429–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M., Ogawa M., Fujita Y., Yoshikawa Y., Nagai T., Koyama T., Nagai S., Lange A., Fässler R., Sasakawa C. (2009) Nature 459, 578–582 [DOI] [PubMed] [Google Scholar]
- 26.Schauer D. B., Zabel B. A., Pedraza I. F., O'Hara C. M., Steigerwalt A. G., Brenner D. J. (1995) J. Clin. Microbiol. 33, 2064–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine M. M., Bergquist E. J., Nalin D. R., Waterman D. H., Hornick R. B., Young C. R., Sotman S. (1978) Lancet 1, 1119–1122 [DOI] [PubMed] [Google Scholar]
- 28.Gauthier A., Puente J. L., Finlay B. B. (2003) Infect. Immun. 71, 3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards R. A., Keller L. H., Schifferli D. M. (1998) Gene 207, 149–157 [DOI] [PubMed] [Google Scholar]
- 30.Foster L. J., De Hoog C. L., Mann M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rappsilber J., Ishihama Y., Mann M. (2003) Anal. Chem. 75, 663–670 [DOI] [PubMed] [Google Scholar]
- 32.Chan Q. W., Howes C. G., Foster L. J. (2006) Mol. Cell. Proteomics 5, 2252–2262 [DOI] [PubMed] [Google Scholar]
- 33.Mortensen P., Gouw J. W., Olsen J. V., Ong S. E., Rigbolt K. T., Bunkenborg J., Cox J., Foster L. J., Heck A. J., Blagoev B., Andersen J. S., Mann M. (2010) J. Proteome Res. 9, 393–403 [DOI] [PubMed] [Google Scholar]
- 34.Charpentier X., Oswald E. (2004) J. Bacteriol. 186, 5486–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills E., Baruch K., Charpentier X., Kobi S., Rosenshine I. (2008) Cell Host Microbe. 3, 104–113 [DOI] [PubMed] [Google Scholar]
- 36.Zarivach R., Vuckovic M., Deng W., Finlay B. B., Strynadka N. C. (2007) Nat. Struct. Mol. Biol. 14, 131–137 [DOI] [PubMed] [Google Scholar]
- 37.Coburn B., Li Y., Owen D., Vallance B. A., Finlay B. B. (2005) Infect. Immun. 73, 3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng W., Li Y., Hardwidge P. R., Frey E. A., Pfuetzner R. A., Lee S., Gruenheid S., Strynakda N. C., Puente J. L., Finlay B. B. (2005) Infect. Immun. 73, 2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas N. A., Deng W., Baker N., Puente J., Finlay B. B. (2007) J. Biol. Chem. 282, 29634–29645 [DOI] [PubMed] [Google Scholar]
- 40.Thomas N. A., Deng W., Puente J. L., Frey E. A., Yip C. K., Strynadka N. C., Finlay B. B. (2005) Mol. Microbiol. 57, 1762–1779 [DOI] [PubMed] [Google Scholar]
- 41.Alto N. M., Shao F., Lazar C. S., Brost R. L., Chua G., Mattoo S., McMahon S. A., Ghosh P., Hughes T. R., Boone C., Dixon J. E. (2006) Cell 124, 133–145 [DOI] [PubMed] [Google Scholar]
- 42.Bulgin R. R., Arbeloa A., Chung J. C., Frankel G. (2009) Cell. Microbiol. 11, 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahan S., Wiles S., La Ragione R. M., Best A., Woodward M. J., Stevens M. P., Shaw R. K., Chong Y., Knutton S., Phillips A., Frankel G. (2005) Infect. Immun. 73, 679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buss C., Müller D., Rüter C., Heusipp G., Schmidt M. A. (2009) Cell. Microbiol. 11, 661–677 [DOI] [PubMed] [Google Scholar]
- 45.Hayashi T., Makino K., Ohnishi M., Kurokawa K., Ishii K., Yokoyama K., Han C. G., Ohtsubo E., Nakayama K., Murata T., Tanaka M., Tobe T., Iida T., Takami H., Honda T., Sasakawa C., Ogasawara N., Yasunaga T., Kuhara S., Shiba T., Hattori M., Shinagawa H. (2001) DNA Res. 8, 11–22 [DOI] [PubMed] [Google Scholar]
- 46.Perna N. T., Plunkett G., 3rd, Burland V., Mau B., Glasner J. D., Rose D. J., Mayhew G. F., Evans P. S., Gregor J., Kirkpatrick H. A., Pósfai G., Hackett J., Klink S., Boutin A., Shao Y., Miller L., Grotbeck E. J., Davis N. W., Lim A., Dimalanta E. T., Potamousis K. D., Apodaca J., Anantharaman T. S., Lin J., Yen G., Schwartz D. C., Welch R. A., Blattner F. R. (2001) Nature 409, 529–533 [DOI] [PubMed] [Google Scholar]
- 47.Vallance B. A., Deng W., Jacobson K., Finlay B. B. (2003) Infect. Immun. 71, 3443–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alfano J. R. (2009) Mol. Plant Pathol. 10, 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekiya K., Ohishi M., Ogino T., Tamano K., Sasakawa C., Abe A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11638–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson R. K., Shaw R. K., Daniell S., Knutton S., Frankel G. (2001) Cell. Microbiol. 3, 753–762 [DOI] [PubMed] [Google Scholar]
- 51.Pallen M. J., Beatson S. A., Bailey C. M. (2005) BMC Microbiol. 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukhan A., Kubori T., Galán J. E. (2003) J. Bacteriol. 185, 3480–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood S. E., Jin J., Lloyd S. A. (2008) J. Bacteriol. 190, 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wickham M. E., Lupp C., Mascarenhas M., Vazquez A., Coombes B. K., Brown N. F., Coburn B. A., Deng W., Puente J. L., Karmali M. A., Finlay B. B. (2006) J. Infect. Dis. 194, 819–827 [DOI] [PubMed] [Google Scholar]
- 55.Kenny B., Jepson M. (2000) Cell. Microbiol. 2, 579–590 [DOI] [PubMed] [Google Scholar]
- 56.Petty N. K., Bulgin R., Crepin V. F., Cerdeño-Tárraga A. M., Schroeder G. N., Quail M. A., Lennard N., Corton C., Barron A., Clark L., Toribio A. L., Parkhill J., Dougan G., Frankel G., Thomson N. R. (2010) J. Bacteriol. 192, 525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.