Abstract
Pathology archives harbor large amounts of formalin-fixed, paraffin-embedded tissue samples, used mainly in clinical diagnostics but also for research purposes. Introduction of heat-induced antigen retrieval has enabled the use of tissue samples for extensive immunohistochemical analysis, despite the fact that antigen retrieval may not recover all epitopes, owing to alterations of the native protein structure induced by formalin. The aim of this study was to investigate how different fixatives influence protein recognition by immunodetection methods in tissues, cell preparations, and protein lysates, as compared with formalin. Seventy-two affinity-purified polyclonal antibodies were used to evaluate seven different fixatives. The aldehyde-based fixative Glyo-fixx proved to be excellent for preservation of proteins in tissue detected by immunohistochemistry (IHC), similar to formalin. A non-aldehyde–based fixative, NEO-FIX was superior for fixation of cultured cells, in regard to morphology, and thereby also advantageous for IHC. Large variability in the amount of protein extracted from the differently fixed tissues was observed, and the HOPE fixative provided the overall highest yield of protein. In conclusion, morphological resolution and immunoreactivity were superior in tissues fixed with aldehyde-based fixatives, whereas the use of non-aldehyde–based fixatives can be advantageous in obtaining high protein yield for Western blot analysis. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 58:237–246, 2010)
Keywords: fixative, tissue microarray, immunohistochemistry, protein profiling, monospecific antibody, protein extraction, Western blot
Formalin-fixed, paraffin-embedded (FFPE) tissue samples, after initial morphological diagnosis, are archived at surgical pathology departments all over the world. The objective of fixation is to retain cellular components in their native compartments and to present cells with a distinct and detailed microscopical appearance. Neutral-buffered formalin (NBF) is a crosslinking fixative that fixes tissues by inducing methylene bridges between amino acids and also between amino acids and nucleotides (Leong and Gilham 1989; Helander 1994). Treatment of tissue by NBF is well known to provide a detailed depiction of cellular morphology proverbial to clinical pathologists analyzing the histological landscape. Furthermore, together with heat-induced antigen retrieval (HIAR), formalin generally enables high-quality immunostaining, widely used as an aid in clinical diagnostics (Shi et al. 1991).
Depending on the intra- or extracellular component to be studied, and the choice of analysis method, formalin may not always be the most-optimal fixative (Beckstead 1994; Wester et al. 2003). The deleterious effects on DNA and RNA are well known, and variable outcomes of PCR and RT-PCR using NBF-fixed cells have been shown in multiple studies (Williams et al. 1999). Proteins are altered by the crosslinking mechanism, mainly in the tertiary and quaternary structures, and although this denaturation is partly reversible through different strategies of HIAR, the extent to which formalin fixation renders epitopes inaccessible to antibody recognition is not known. An optimal fixative should allow for high-quality histology and also preserve sufficient material for analysis using other technical approaches, e.g., immunohistochemistry (IHC), Western blot, in situ hybridization, and PCR. Unfortunately, such a fixative does not exist, and it is therefore important to assess the advantages and drawbacks of the existing fixatives for each platform.
Proteomics is a rapidly expanding field, partly as a result of accelerating progress in technological development. Generated data are most often made accessible through publicly available databases. Antibody-based proteomics aims to generate specific antibodies to all gene-encoded proteins, with the aim of analyzing protein expression patterns for full proteomes in different species (Uhlen and Ponten 2005). One important component in such an effort is analysis of the tissue distribution patterns and relative abundance of proteins in normal and diseased tissues. The development of tissue microarrays (Kononen et al. 1998) and of high-throughput strategies to generate validated antibodies (Nilsson et al. 2005) has enabled protein profiling on a proteome-wide scale (Uhlen et al. 2005; Berglund et al. 2008). The reliability of protein profiles obtained through the use of archival FFPE material is in part dependent on the effects of formalin fixation, and thus it is important to investigate fixation-related features. In contrast to DNA, proteins are rapidly degraded, and reliable results require well-preserved biological material, which in turn necessitates appropriate handling of tissues and cells (Werner et al. 2000). The availability and quality of proteins in a tissue context is dependent on several factors that may influence the final result (Shi et al. 1996). Such factors include the time period before the specimen is fixed, type of fixative, time of fixation, and temperature during storage, as well as protocols for protein detection and extraction.
In this study, normal human tissues representing a wide spectrum of cell types were fixed with different fixatives: NBF, Glyo-fixx, Zink formalin, FineFIX, HOPE, NEO-FIX, and Zinc-based fixative. Morphological features, immunoreactivity, and amount of protein extracted for Western blot applications were tested using 72 antibodies. The overall findings confirmed formalin as an appropriate fixative, although other aldehyde-based fixatives sufficed as well for histology and IHC. The non-aldehyde–based fixatives appeared more suitable for extraction of proteins than for histology and IHC.
Materials and Methods
Antibodies
In this study, 72 antibodies (Table ST1), representing proteins with different subcellular distribution and biological functions, were used. Forty-four antibodies were selected as highly validated polyclonal affinity-purified monospecific antibodies (msAbs), with previous evaluation data regarding antibody target specificity for IHC. Twenty antibodies were selected to represent msAbs with supportive data from Western blots (bands of the correct size as predicted from molecular mass) that had previously failed to recognize the expected target proteins using IHC on tissue sections from formalin-fixed tissues, i.e., antibodies with diffuse IHC staining patterns without support from published data. Finally, eight antibodies were selected to represent clinically relevant antibodies purchased from commercial distributors. Seven out of these eight antibodies were of monoclonal origin.
Tissue Processing
Fresh surgical tissue samples from tonsil, kidney, liver, colon, placenta, thyroid, prostate, and stomach were collected from the Department of Pathology (Uppsala University Hospital, Uppsala, Sweden) and fixed in six or seven fixatives (Table 1) for 24 hr at room temperature, followed by further histoprocessing according to standard procedures. In brief, each fresh tissue sample was divided into pieces, and each piece was fixed in one of the fixatives presented in Table 1 (Beckstead 1994). Three tissue types (tonsil, kidney, and thyroid) were also fixed in Zn-formalin (ZnF) (Thermo Fisher Scientific, Inc.; Waltman, MA). After fixation, tissues were dehydrated (starting with 70% ethanol) and paraffin impregnated using a vacuum infiltration processor (Ventana Medical Systemes, Inc.; Tuscon, AZ). The tissue specimens were embedded in paraffin, and from each block, 4-μm-thick sections were cut, placed on glass slides (SuperFrost Plus, Menzel-Glaser; Braunschweig, Germany), and stained with hematoxylin and eosin.
Table 1.
Fixatives used and their properties
| Fixative | Distributor | Active chemical agents | Fixating properties (according to distributor) |
|---|---|---|---|
| FineFIX | Milestone Medicals; Kalamazoo, MI | Ethanol (70%) together with patent formula | Not provided |
| Glyo-fixx | Thermo Electron Corporation | Glyoxal, a two-carbon dialdehyde | Binds to methyl, amide, and hydroxyl groups. Some crosslinking may occur, but linkages are longer than with formaldehyde. |
| HOPE | DCS Innovative; Hamburg, Germany | Hepes glutamic acid buffer | Amino acids and glucose, together with acetone as dehydrating agent, provide a protection effect. |
| NBF | Histolab Products AB | Formaldehyde | Crosslinking mechanism |
| NEO-FIX | Merck Pharmaceuticals; Whitehouse Station, NJ | Alcohol | Coagulating mechanism |
| ZBF | No distributor; made in laboratory | Zinc salts | Unknown mechanism |
| ZnF | Thermo Fisher Scientific, Inc. | Zinc salts and formaldehyde | Same as for NBF, thus providing more-efficient fixation due to zinc |
The fixatives are ordered alphabetically. NBF, neutral-buffered formalin.
Preparation of Cell Lines
Three cancer cell lines were cultured and fixed as previously described (Andersson et al. 2006). In brief, RT-4 [human urinary bladder transitional cell carcinoma; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany], U-251 (Westermark et al. 1973), and PC-3 (human prostate carcinoma; DSMZ), were cultured at 37C in 5% CO2 according to the manufacturers' recommendations. Cells were trypsinated, resolved in medium, and centrifuged at 400 × g for 10 min. The pellets were resuspended in cold PBS, and the concentration was adjusted to 100 × 106 cells/ml. The cells were fixed for 75 min in the respective fixatives and dispersed into agarose cell gels for subsequent histoprocessing. The cell gels were further paraffin-embedded and used for tissue microarray (TMA) production.
TMA Design
A TMA was designed to represent tissues and cells with all modes of fixation. In short, 1-mm cores were taken from each donor block of paraffin-embedded tissue and agarose cell gel, and transferred into a recipient paraffin block using an automated tissue arrayer (ATA-27, Beecher Instruments; Sun Prairie, CA) (Kampf et al. 2004). Prior to TMA construction, sections of each donor block were stained with hematoxylin-eosin for selection of representative areas. The TMA was cut into 4-μm sections using a waterfall microtome (Microm GmbH; Walldorf, Germany); sections were placed on objective slides (SuperFrost Plus; Menzel-Glaser) and incubated at 50C overnight prior to immunohistochemical staining.
IHC
TMA sections were immunostained as previously described (Kampf et al. 2004). Briefly, slides were deparaffinized in xylene, hydrated in graded alcohols, and blocked for endogenous peroxidase in 0.3% hydrogen peroxide diluted in 95% ethanol. For antigen retrieval, a decloaking chamber (Biocare Medical; Walnut Creek, CA) was used. Slides were immersed and boiled in citrate buffer, pH 6 (Lab Vision; Freemont, CA) for 4 min at 125C and then allowed to cool to 90C. Automated IHC was performed using an Autostainer 480 instrument (Lab Vision). Primary antibodies and a dextran polymer visualization system (UltraVision LP HRP polymer; Lab Vision) were incubated for 30 min each at room temperature, and slides were developed for 10 min using diaminobenzidine (Lab Vision) as chromogen. All incubations were followed by a rinse in a wash buffer (Lab Vision). Slides were counterstained in Mayer's hematoxylin (Histolab; Gothenburg, Sweden) and coverslipped using Pertex (Histolab) as mounting medium. Incubation with PBS instead of primary antibody served as negative control. All primary antibodies were diluted identically for all tissues and cell preparations, independent of fixative used. The dilutions were initially determined by titration on NBF-fixed tissues.
Analysis of Immunostaining and Morphological Examination
Immunohistochemically stained TMA slides were scanned using an automated image scanner (ScanScope, Aperio Technologies; Vista, CA). High-resolution images of immunostained tissues and cells were annotated manually using ImageScope software (Aperio Technologies), comparing all modes of fixation for each tissue and cell preparation with the respective counterparts fixed in NBF. Morphology was examined on hematoxylin-eosin–stained sections from the original donor blocks. The outcome of immunohistochemical staining was analyzed with respect to distribution and intensity of immunoreactivity, and the intensity of immunostaining in defined cell populations was evaluated and compared with NBF-fixed counterparts. The intensity was evaluated and judged as similar (0), slightly higher (1), markedly higher (2), slightly lower (−1), or markedly lower (−2). When differences in subcellular localization of immunoreactivity were detected between fixatives, information from protein databases such as Uniprot, Ensembl, and PubMed was used to assess the expected protein distribution and, thus, the most-reliable staining pattern.
Protein Extraction From Paraffin-embedded Tissues
Protein lysates were prepared from liver, tonsil, intestine, and kidney fixed in formalin, NEO-FIX, ZBF, Glyo-fixx, HOPE, and FineFIX. Owing to limited amounts of intestinal tissue, ZBF fixative was excluded. Six 50-μm paraffin sections of each tissue were placed into glass tubes for deparaffinization and dehydration in xylene and graded alcohols and immersed in distilled water. The sections were transferred into Eppendorf tubes and 400 μl of RIPA buffer, pH 7.5 (1 M sodium dihydrogen phosphate, 10 mM disodium hydrogen phosphate, 154 mM sodium chloride, 1% Triton X-100, 12 mM sodium deoxycholate, 0.2% sodium azide, 0.95 mM fluoride, 2 mM phenylmethylsulfonyl fluoride, 50 mg/ml aprotinin) (Sigma Chemical; St. Louis, MO) containing 10 mM leupeptin (Sigma, or Apollo Scientific; Cheshire, UK), and 2% SDS, was added to each tube. The samples were incubated at 100C for 20 min in a decloaking chamber (Biocare Medical) and thereafter at 60C for 2 hr. After incubation, the samples were centrifuged at 15,000 × g for 20 min at 4C. Supernatants were collected, and protein concentrations were determined using the Non-Interfering Protein Assay Kit (Calbiochem; Darmstadt, Germany). Ten μl of each sample was mixed with 500 μl reagent (UPPA-1; Calbiochem), and absorbance was measured at 490 nm using an ELISA reader. Protein concentrations were established using a standard curve based on BSA concentrations.
SDS-PAGE and Western Blot Analysis
Protein preparations were diluted in 5 × Red Buffer (1 M Tris-HCl, pH 8.0, 0.5 M EDTA, SDS, bromophenol blue, β-mercaptoethanol) with 20% glycerol and subsequently boiled at 90C for 5 min. Samples were thereafter loaded onto a 10–20% Tris-HCl Criterion precast gel (Bio-Rad; Hercules, CA) along with marker (Fermentas GmbH; St Leon-Rot, Germany). The gel was run with Tris-glycine-SDS buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, methanol, pH 8.3) (Bio-Rad) for ∼70 min at 200 V at 4C. This was followed by blotting onto a polyvinylidene difluoride membrane (Bio-Rad) in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 20% methanol, pH 8.3) (Bio-Rad) for 90 min at 100 V at 4C. Finally, the membrane was dried and stored at room temperature.
For Western blot, membranes were equilibrated in methanol, washed in TBST (TBS, 0.05% Tween 20) and thereafter incubated in blocking buffer (TBST containing 0.5% Tween 20 and with the addition of 5% dried milk) for 45 min on a shaker. Primary antibodies were diluted 1:250 in 5 ml blocking buffer and incubated with membranes for 60 min on a shaker. Prior to incubation with secondary antibody, the membranes were washed four times for 5 min with TBST. The horseradish peroxidase (HRP)-conjugated secondary antibody (Dakocytomation; Glostrup, Denmark) was diluted 1:3000 in 5 ml of blocking buffer, and the membranes were incubated for 1 hr on a shaker, followed by washing four times for 5 min with TBST. Immobilon Western chemiluminescent HRP substrate (Millipore; Billerica, MA) was used for detection, and the membranes were developed in a ChemiDoc XRS with a CCD camera (Bio-Rad) detection system.
Results
Tissue and Cell Morphology
To analyze the effect of fixation on morphological features, tissues and cells were fixed using seven different fixatives and thereafter stained with hematoxylin-eosin. Microscopical evaluation showed individual morphological features that distinguished the tested fixatives from NBF. The non-aldehyde fixatives FineFIX, HOPE, NEO-FIX, and ZBF generally caused an increased shrinkage of cells, most evident in the cytoplasm, in tissue sections. In sections from tissues fixed in NEO-FIX and ZBF, individual cells or cell groups appeared disconnected from surrounding connective tissue. A more-similar morphology was found in sections from tissues fixed in the three aldehyde-based fixatives (NBF, Glyo-fixx, and ZnF). The tissues fixed with Glyo-fixx and ZnF showed a preserved histology and crisp morphology, although cells appeared slightly condensed, i.e., a reflection of tissue shrinkage leading to sharper contrast between cellular and extracellular structures. A subset of hepatocytes in liver tissues fixed in Glyo-fixx showed condensed and asymmetric cell nuclei. Figures 1A–1C illustrate tissue morphology in tonsil fixed with NBF, NEO-FIX, and FineFIX, in which shrinkage of cells was prominent with alcohol-based fixatives.
Figure 1.
Morphological detail was investigated using hematoxylin-eosin (H&E) staining. Superior morphology was observed with H&E in tissues fixed with aldehyde-based fixatives [neutral-buffered formalin (NBF) and Glyo-fixx], e.g., NBF in tonsil (A), whereas some shrinkage of cytoplasm is observed with the non-aldehyde–based fixatives, e.g., in tonsil with NEO-FIX (B) and FineFIX (C). In cell lines, NEO-FIX was considered superior, as presented in RT4 cells (D). Also, NBF demonstrated good morphology (E). Glyo-fixx, on the other hand, showed tendencies toward cell aggregation in, e.g., PC3 cells (F). Bar = 50 μm.
The cellular morphology in sections from cultured cells varied between fixatives and was not always in accordance with the morphology of cells in sections from tissues fixed in the corresponding fixative. In contrast to tissues, we found cellular morphology to be superior, with a sharp outlining of nucleus and cytoplasm, in cultured cells fixed in NEO-FIX (Figure 1D), as compared with the other fixatives. Fixation in NBF (Figure 1E), NEO-FIX, and HOPE also resulted in a homogeneous distribution of dispersed cells, but cytoplasmic shrinkage was occasionally observed. The use of Glyo-fixx (Figure 1F) and ZBF had a tendency to aggregate the cells.
Immunohistochemical Staining Patterns
To scrutinize the effect of different fixatives on epitopes for antibody recognition, 72 antibodies were selected for IHC. HIAR was performed for all fixed tissues prior to IHC staining. A subset of antibodies was studied on tissues fixed in aldehyde-based or non-aldehyde–based fixatives, to determine the effect of HIAR on protein expression. The results indicated that no apparent difference in protein expression was observed with and without use of HIAR (data not shown).
The target proteins for the 72 antibodies were selected to represent a broad spectrum of proteins belonging to different protein classes (Table ST1) and distributed in different subcellular compartments. Based on previous performance in IHC and Western blotting, compared with patterns of expression expected from bioinformatic and experimental data, 44 antibodies were considered highly reliable and 20 antibodies showed the expected result in Western blot analysis but were considered unreliable in the IHC application. Fixative-dependent variability was mainly found in the intensity of immunoreactivity, whereas the localization of protein expression in different cell populations showed only minor variations. A subset of antibodies demonstrated discrepancies in subcellular distribution in the differently fixed tissues and cell lines. In general, tissues fixed in aldehyde-based fixatives showed a higher level of immunoreactivity, i.e., more-intense immunostaining compared with the non-aldehyde–based fixatives (Figure 2). Among the aldehyde-based fixatives, Glyo-fixx showed the strongest degree of intensity, most evident in tissues from liver, kidney, and stomach. The three tissues fixed in ZnF showed immunoreactivity similar to that seen with NBF, except for 10 antibodies for which a slightly increased intensity was observed in the ZnF-fixed tissues.
Figure 2.
Comparison of staining intensity among the differently fixed tissues, compared with NBF. Mean staining intensity is presented for 72 antibodies in tissues fixed with aldehyde-based fixatives (Glyo-fixx and ZnF) and non-aldehyde–based fixatives (FineFIX, HOPE, NEO-FIX, and ZBF) compared with NBF (considered as baseline, i.e., zero).
Generally, when discrepancies were observed, NBF-fixed tissues presented results supported by bioinformatic or experimental data. Eleven antibodies demonstrated fixative-related discrepancies in IHC staining, exemplified in Figure 3. Immunostaining of intestinal tissue fixed in NBF (Figure 3A) was essentially negative for the κ-type opioid receptor (antibody HPA014111), compared with intestinal tissue fixed in ZBF (Figure 3B), which showed a granular staining pattern with strong intensity. Expression of κ-type opioid receptor protein in rat and rabbit intestinal epithelia has previously been documented, e.g., by IHC and Western blot (Fickel et al. 1997; Jimenez et al. 2006).
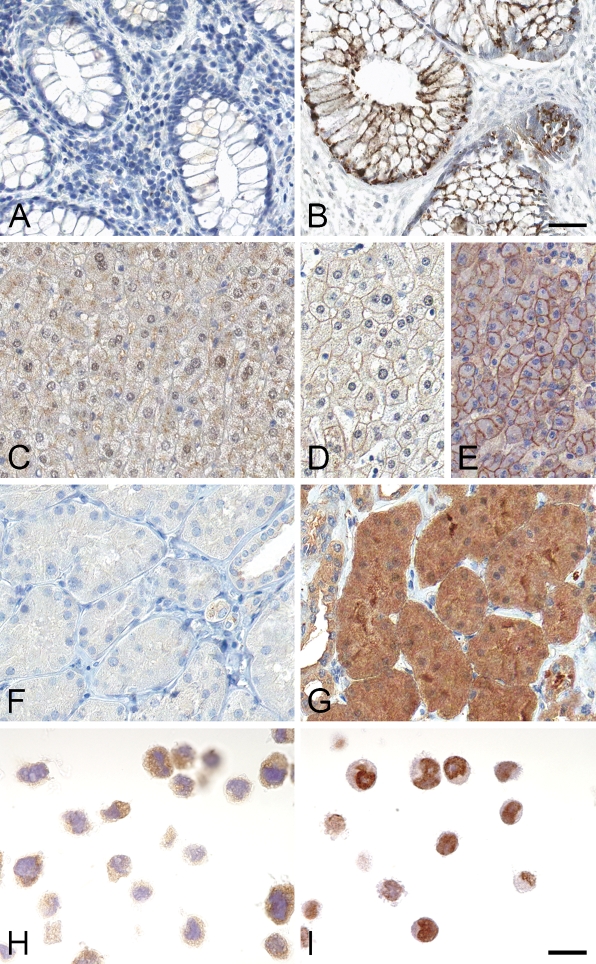
Figure 3.
Examples of discrepancies in immunohistochemistry staining between differently fixed tissues and cells. The left column represents NBF-fixed tissues or cells. (A) NBF-fixed intestinal tissue presented negative staining with an antibody to the opioid κ receptor, whereas ZBF-fixed intestinal tissue showed a dot-like granular protein expression (B). (C) A subcellular staining discrepancy in liver, in which NBF-fixed liver tissue showed a cytoplasmic pattern. Liver tissues fixed with NEO-FIX (D) and Glyo-fixx (E) showed a membranous pattern, in accordance with the literature. An antibody to β-galactosyltransferase demonstrated very weak or negative staining in NBF-fixed tubular cells of the kidney (F), whereas the same tissue type fixed in Glyo-fixx showed a strong granular-like cytoplasmic staining pattern (G). In cell lines, one example of a discrepancy was observed in U-251 cells with an antibody to selenoprotein S. NBF-fixed cells presented a cytoplasmic staining pattern (H), whereas cells fixed with HOPE demonstrated a nuclear-like distribution (I). Bars: A–G = 50 μm; H,I = 20 μm.
Moreover, the analysis of desmocollin-1 precursor protein expression (antibody HPA012891) showed a variable immunostaining pattern in liver tissue. Hepatocytes fixed with NBF (Figure 3C) mainly showed strong cytoplasmic immunoreactivity, with only weak membranous expression in a few cells. Hepatocytes fixed with Glyo-fixx and NEO-FIX (Figures 3D and 3E) displayed a membranous staining pattern, in accordance with the literature (Burdett 1998).
The antibody HPA014191, generated toward the β-galactosyltransferase T7 protein, showed strong immunoreactivity in tubular cells of the kidney fixed in Glyo-fixx (Figure 3G), whereas kidney fixed with NBF (Figure 3F) resulted in weak or negative immunostaining in kidney tubules. Tests with increased concentrations of the primary antibody did not result in any increase in immunostaining of NBF-fixed kidney tissue and thus verified a fixative-dependent discrepancy. The family of β-galactosyltransferases is widely expressed (Amado et al. 1999), and expression of β-GalT7 could be expected in kidney, although the tissue expression pattern for this particular family member has not previously been reported.
The three cancer cell lines generally demonstrated more-similar immunostaining patterns, with less variation in intensity than observed in tissues, independent of fixative. For a subset of antibodies, the subcellular localization pattern differed among cells treated with different fixatives. One example with a very different outcome is shown in Figures 3H and 3I. Immunostaining of U-251 cells fixed in NBF with the selenoprotein S antibody (HPA010025) showed a diffuse cytoplasmic staining pattern (Figure 3H). Interestingly, the same U-251 cells fixed in HOPE fixative demonstrated a nuclear-like immunoreactivity (Figure 3I). This pattern was evident only in the HOPE-fixed cells; the other fixatives also showed a more-diffuse and widespread cytoplasmic staining pattern. The selenoprotein S protein has previously been shown to be expressed in endoplasmic reticulum (ER) compartments, e.g., in brain tissue (Ye et al. 2004; Hoppe et al. 2008). In the tissue context, both NBF and HOPE fixation showed strong cytoplasmic immunoreactivity, with a pattern of ER positivity in lymphocytes of the tonsil (data not shown).
There was no clear impact of protein class, subcellular compartment of protein expression, or reliability of the antibody on the degree of variation in IHC staining patterns among the different fixatives.
Protein Extraction From Fixed Tissues
To determine the impact of fixation on the availability of extractable proteins from four tissue types, protein concentration was determined from six 50-μm-thick paraffin sections of each tissue. The quantity of proteins extracted with each fixative is presented in Table 2 and illustrated in Figure 4. The results showed that NBF-fixed tonsil generated the highest amount of protein (60 μg protein/mm3 tissue), followed by HOPE-fixed tonsil (58 μg protein/mm3). Extractions from liver generally resulted in a lower number of proteins, with a mean concentration of 23 μg/mm3 (ranging from 13 μg/mm3 to 53 μg/mm3), compared with tonsil, in which the mean concentration of protein was 38 μg/mm3 (range 23–60 μg/mm3). Fixation of liver tissue in HOPE fixative did, however, result in a higher protein concentration (53 μg protein/mm3). For intestinal tissue and kidney, generally lower yields of proteins were obtained for each fixative. For intestinal tissue and kidney, the mean yield was 21 μg/mm3 (ranging from 11 μg/mm3 to 33 μg/mm3) and 12 μg/mm3 (ranging from 4 μg/mm3 to 20 μg/mm3), respectively. Although there were large variations among different tissue types, fixation of tissues in HOPE resulted, in general, in the highest protein yields, compared with other fixatives used in the corresponding tissue types.
Table 2.
Protein extraction yields from differently fixed tissue types
| Fixative | Tonsil | Liver | Kidney | Intestine |
|---|---|---|---|---|
| NBF | 60 | 17 | 9 | 11 |
| NEO-FIX | 27 | 13 | 20 | 23 |
| ZBF | 23 | 28 | 4 | * |
| Glyo-fixx | 38 | 13 | 5 | 20 |
| HOPE | 58 | 53 | 20 | 33 |
| FineFIX | 20 | 14 | 15 | 15 |
Results are presented as μg/mm3. *Not available for this tissue.
Figure 4.
Protein extraction results from differently fixed tissue types. The tissues (liver, tonsil, intestine, and kidney) were fixed with NBF, NEO-FIX, ZBF, Glyo-fixx, HOPE, and FineFIX, except for intestinal tissue, in which ZBF fixative was absent. Protein concentrations from each fixed tissue are presented as μg protein/mm3 tissue.
Western Blot Analysis
To assess the effect of fixation on the quality of extracted proteins, Western blotting was performed on the protein lysates from liver, tonsil, intestine, and kidney tissues using 13 different antibodies. All lysates were analyzed, and protein concentration was adjusted to 15 μg/ml. As control, lysates with the same protein concentration prepared from frozen tissues were used. Results indicated that only cytokeratin 19 (KRT-1) was detected in all four lysates treated with all different fixatives. Detection of most proteins of the expected size was evident in lysates from liver and tonsil.
Out of 13 antibodies, 4 showed bands of the predicted size in all frozen lysates. The effects of the different fixatives are summarized in Tables 3–6. Generally, more proteins were detected in liver and tonsil lysates, compared with intestine and kidney. The best result was obtained in HOPE-fixed liver tissue, where 9 out of 13 Western blots showed the predicted results. For the immediate early response 3-interacting protein (IER3IP1), bands of the predicted size were detected in lysates from all fixed liver tissues, except for ZBF, but not in corresponding lysates from frozen tissue (Table 3). In lysates from fixed kidney (Table 5) and intestine (Table 6), only a few proteins were detected at the expected size. Generally, in lysates from tissues fixed with non-aldehyde–based fixatives, more proteins were detected compared with aldehyde-based–fixed tissues.
Table 3.
Western blot results from differently fixed liver lysates
| Protein name | Frozen tissue | NBF | Glyo-fixx | FineFIX | HOPE | NEO-FIX | ZBF |
|---|---|---|---|---|---|---|---|
| B4GALT7 | * | — | — | — | — | — | — |
| Ck17 | x | x | x | x | x | x | x |
| DSC1 | — | * | — | * | * | * | * |
| GOLGA5 | x* | — | — | — | x* | — | x* |
| IER3IP1 | — | x | — | x* | x* | x* | — |
| KI-67 | x* | x* | — | x* | x | x* | x |
| KRT1 | x* | x* | x* | x* | x* | x* | x* |
| LCA | * | * | — | * | * | * | * |
| PDK2 | x* | * | — | x | x* | — | x* |
| SERPINA4 | * | — | — | — | — | — | — |
| TFP1 | x* | * | — | * | x* | * | * |
| UBTF | x* | — | — | * | x* | x* | x* |
| WNT11 | x* | * | — | — | x | x | — |
Thirteen antibodies were used in Western blot on liver tissues fixed with NBF, NEO-FIX, ZBF, Glyo-fixx, HOPE, and FineFIX. The proteins are alphabetically ordered. The results were judged as follows: x, band of predicted size present; *, additional band(s) of wrong size present; —, no bands present.
Table 4.
Western blot results from differently fixed tonsil tissue
| Protein name | Frozen tissue | NBF | Glyo-fixx | FineFIX | HOPE | NEO-FIX | ZBF |
|---|---|---|---|---|---|---|---|
| B4GALT7 | * | — | — | — | — | — | — |
| Ck17 | x* | x* | x* | x* | x* | x* | x* |
| DSC1 | x* | * | — | * | * | * | * |
| GOLGA5 | x* | * | — | * | * | x* | x* |
| IER3IP1 | x | x | — | x | x | x | x |
| KI-67 | x* | x* | * | x* | x* | x* | x* |
| KRT1 | x* | x* | x* | x* | x* | x* | x* |
| LCA | * | * | * | * | * | * | * |
| PDK2 | x* | * | — | * | * | * | * |
| SERPINA4 | * | — | — | — | — | — | — |
| TFP1 | * | * | * | * | * | * | * |
| UBTF | x* | x* | — | x* | x* | x* | x* |
| WNT11 | x | x | — | x | — | x | x |
Western blot was performed for 13 antibodies on tonsil lysates fixed with NBF, NEO-FIX, ZBF, Glyo-fixx, HOPE, and FineFIX. The proteins are presented in alphabetical order. The results were judged as follows: x, band of predicted size present; *, additional band(s) of wrong size present; —, no bands present.
Table 5.
Western blot results from differently fixed kidney lysates
| Protein name | Frozen tissue | NBF | Glyo-fixx | FineFIX | HOPE | NEO-FIX | ZBF |
|---|---|---|---|---|---|---|---|
| B4GALT7 | n/a | — | — | — | — | — | — |
| Ck17 | — | — | — | — | — | — | — |
| DSC1 | x* | — | — | — | — | * | — |
| GOLGA5 | x* | — | — | — | — | x | x |
| IER3IP1 | x* | — | — | — | — | — | — |
| KI-67 | — | — | — | — | — | — | — |
| KRT1 | x | x | x | x | x | x | x |
| LCA | * | — | — | — | — | — | — |
| PDK2 | x* | * | — | — | — | — | — |
| SERPINA4 | x* | x* | — | x* | x* | x* | x* |
| TFP1 | * | x | — | — | — | x* | x* |
| UBTF | x* | — | — | — | x* | x* | * |
| WNT11 | x* | — | — | — | — | — | — |
Western blot was performed for 13 antibodies on kidney lysates fixed with NBF, NEO-FIX, ZBF, Glyo-fixx, HOPE, and FineFIX. The proteins are presented in alphabetical order. The results were judged as follows: x, band of predicted size present; *, additional band(s) of wrong size present; —, no bands present. n/a, not available due to technical error.
Table 6.
Western blot results from differently fixed lysates of intestinal tissue
| Protein name | Frozen tissue | NBF | Glyo-fixx | FineFIX | HOPE | NEO-FIX |
|---|---|---|---|---|---|---|
| B4GALT7 | n/a | — | — | — | — | — |
| Ck17 | — | — | — | — | — | — |
| DSC1 | x* | * | — | * | * | * |
| GOLGA5 | x* | — | — | x | — | — |
| IER3IP1 | x | — | — | — | — | — |
| KI-67 | — | — | — | — | — | — |
| KRT1 | x | x | x | x | x | x |
| LCA | * | — | — | — | — | — |
| PDK2 | * | — | — | — | — | — |
| SERPINA4 | x* | x | — | x* | — | x |
| TFP1 | * | — | — | — | — | — |
| UBTF | x* | — | — | x* | x* | x* |
| WNT11 | x* | — | — | — | — | — |
Western blot was performed for 13 antibodies on intestinal lysates fixed with NBF, NEO-FIX, Glyo-fixx, HOPE, and FineFIX. The proteins are presented in alphabetical order. The results were judged as follows: x, band of predicted size present; *, additional band(s) of wrong size present; —, no bands present. n/a, not available due to technical error.
Discussion
Defining the protein expression patterns of tissues in the human body is critical to understanding the unique characteristics of each cell type. IHC is a powerful method for analyzing distribution patterns of proteins in a tissue context and provides an excellent tool for pathology and biomarker discovery efforts (Ponten et al. 2008). Although IHC is per se not quantitative, immunostaining allows for a relative quantification of each antibody used. A more widespread use of IHC has been hampered by the lack of antibodies to the vast majority of our human proteins. However, there are ongoing large-scale efforts both to generate antibodies on a proteome-wide scale and to use these antibodies for the mapping of protein profiles in human normal and cancerous tissues (www.proteinatlas.org). The absence of data supporting expression of a previously undetected protein in a given cell type presents a problem when using antibodies for IHC. Owing to the lack of defined positive controls for immunohistochemical analysis for such antibodies, circumstantial evidence, including bioinformatic predictions, preabsorption with the corresponding antigen, and performance in other applications, i.e., Western blot, are often used to show that the antibody is reliable for IHC. The performance of an antibody in a Western blot is often considered proof of correct functionality and is extrapolated to the IHC setting, although the target protein may be altered due to fixation, rendering masking of epitopes available for antibody recognition. Knowledge about how different fixatives influence the behavior of the proteins in different applications is therefore of great importance.
In surgical pathology, NBF has been the “gold standard” fixative for decades. NBF enables long-term storage of surgical material and preserves tissue and cells with the detailed and crisp morphology that forms a fundament for microscopical diagnostics. Because NBF has been the standard fixative, antibodies used for diagnostic IHC have been developed to detect protein epitopes in their NBF-fixed form. The discovery of antigen retrieval strategies, in particular HIAR, has further advanced the use of IHC, allowing for a wider range of different antibodies to be used in routine diagnostics. However, there are also disadvantages to using NBF as a fixative, including the handling of formalin, inasmuch as formaldehyde is considered a carcinogen. Although HIAR uncovers most epitopes altered by NBF fixation, there probably exist a multitude of potential epitopes that remain inaccessible or truncated after HIAR treatment of NBF-fixed tissues. An alternative histoprocessing method often used in surgical pathology and research applications is snap freezing of tissue, followed by cryosectioning. Cryopreservation is non-chemical preservation in which the proteins are presented as less altered compared with their native composition. However, the microscopical appearance of tissues and cells is often compromised by freezing, rendering diagnostics more challenging. There is thus a need for a fixative that fulfills the requirements of morphological diagnostics and the protection of proteins in tissues, permitting further exploitation of the expanding repertoire of antibodies being generated in various efforts.
The focus of this study was to assess how different modes of tissue preservation influence subsequent protein detection by IHC on tissue sections and Western blot using tissue lysates. Our findings show that the aldehyde-based fixatives resulted in a superior microscopical appearance and a higher intensity of immunostaining compared with the non-aldehyde–based fixatives. Among the aldehyde-based fixatives, Glyo-fixx generated the most-intense immunoreactivity, also introducing difficulties in distinguishing accurate detection from background staining for antibodies raised against poorly characterized proteins. Previous work has also indicated that antibodies may be further diluted when used on tissues fixed in Glyo-fixx, as compared with corresponding NBF-fixed tissues (Umlas and Tulecke 2004). In the present study, it is important to note that all antibody dilutions were initially titrated for optimal performance in NBF-fixed tissues, and that individual titration of each primary antibody for each respective fixative is necessary to reach the best possible result. It cannot be excluded that intensity differences can reflect a certain loss of proteins when fixed in alternative fixatives.
For a subset of antibodies, discrepancies in staining patterns were observed among different fixatives. For certain proteins, the distribution of expression is well characterized and documented in the scientific literature. For a subset of proteins with expression patterns deviating from the expected distribution, this behavior could be explained by the presence of specific splice variants, exclusively appearing in defined subcellular compartments. However, for a large number of proteins, published data are lacking or different studies show conflicting results. For several proteins, available data are based on immunostaining patterns from experiments in which only one antibody has been used, rendering conclusions that may be questionable. In our study, a diffuse granular staining pattern in the cytoplasm was observed in certain tissues, e.g., stomach, kidney, and liver, fixed in NBF, although there was no bioinformatic or other available data to support such an expression pattern. To investigate whether this reccurring diffuse granular staining pattern in fact could be an artifact in NBF-fixed material, further stainings were performed. Similar granular patterns of immunoreactivity were also found in the same cellular compartments in tissues fixed in other aldehyde-based fixatives, and immunostainings with more-concentrated primary antibodies demonstrated that the granular staining pattern actually was also preserved in the non-aldehyde–based fixatives, although with a very weak intensity. Our findings suggest that these immunoreactivity patterns are artifactual and thus should be interpreted with caution.
Protein extraction for subsequent Western blot analysis resulted in variation of protein yield between different tissue types, regardless of fixation mode. One explanation for this result could be the difference in tissue composition and density. In Western blot, the non-aldehyde–based fixed lysates (although varying protein yields for each tissue type) detected more proteins than aldehyde-based fixed lysates. It is tempting to speculate that non-aldehyde–based fixatives, especially HOPE, preserve proteins sufficiently to permit both preparation of lysates and detection in Western blot, as compared with aldehyde-based fixatives. To confirm this conclusion, further research regarding fixation mechanisms needs to be done. Although fixation mechanisms of non-aldehyde–based fixatives can result in truncated or lost antigens, this study indicates that both yield and quality of protein can have an effect on Western blot results because antibodies recognized proteins to a higher extent and/or with a higher intensity in, e.g., lysates fixed with HOPE and NEO-FIX, compared with aldehyde-fixed lysates, using quantity adjusted amounts.
In summary, the most commonly used fixative, NBF, performs well as a fixative for tissues, although alternative fixatives may be more advantageous for detection and visualization of certain proteins using different applications. Although a universal fixative would be desirable, it is not currently available, and would require further development of new fixatives. Alternative fixation, in addition to routine NBF fixation, should be considered when collecting tissues for prospective studies of protein expression patterns, especially in the analysis of unknown or previously uncharacterized proteins. For future studies, the use of TMAs containing differently fixed tissues and cells provides an attractive method to analyze the effects of various fixatives on a multitude of tissues using large numbers of antibodies.
Acknowledgments
This work was funded by the Knut and Alice Wallenberg Foundation.
We thank Eva Wahlund for excellent technical support and advice.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Amado M, Almeida R, Schwientek T, Clausen H (1999) Identification and characterization of large galactosyltransferase gene families: galactosyltransferases for all functions. Biochim Biophys Acta 1473:35–53 [DOI] [PubMed] [Google Scholar]
- Andersson AC, Stromberg S, Backvall H, Kampf C, Uhlen M, Wester K, Ponten F (2006) Analysis of protein expression in cell microarrays: a tool for antibody-based proteomics. J Histochem Cytochem 54:1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead JH (1994) A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 42:1127–1134 [DOI] [PubMed] [Google Scholar]
- Berglund L, Bjorling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, Persson A, et al. (2008) A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics 7:2019–2027 [DOI] [PubMed] [Google Scholar]
- Burdett ID (1998) Aspects of the structure and assembly of desmosomes. Micron 29:309–328 [DOI] [PubMed] [Google Scholar]
- Fickel J, Bagnol D, Watson SJ, Akil H (1997) Opioid receptor expression in the rat gastrointestinal tract: a quantitative study with comparison to the brain. Brain Res Mol Brain Res 46:1–8 [DOI] [PubMed] [Google Scholar]
- Helander KG (1994) Kinetic studies of formaldehyde binding in tissue. Biotech Histochem 69:177–179 [DOI] [PubMed] [Google Scholar]
- Hoppe B, Brauer AU, Kuhbacher M, Savaskan NE, Behne D, Kyriakopoulos A (2008) Biochemical analysis of selenoprotein expression in brain cell lines and in distinct brain regions. Cell Tissue Res 332:403–414 [DOI] [PubMed] [Google Scholar]
- Jimenez N, Puig MM, Pol O (2006) Antiexudative effects of opioids and expression of kappa- and delta-opioid receptors during intestinal inflammation in mice: involvement of nitric oxide. J Pharmacol Exp Ther 316:261–270 [DOI] [PubMed] [Google Scholar]
- Kampf C, Andersson A-C, Wester K, Björling E, Uhlen M, Ponten F (2004) Antibody-based tissue profiling as a tool for clinical proteomics. Clin Proteomics 1:285–299 [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, et al. (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847 [DOI] [PubMed] [Google Scholar]
- Leong AS, Gilham PN (1989) The effects of progressive formaldehyde fixation on the preservation of tissue antigens. Pathology 21:266–268 [DOI] [PubMed] [Google Scholar]
- Nilsson P, Paavilainen L, Larsson K, Odling J, Sundberg M, Andersson AC, Kampf C, et al. (2005) Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics 5:4327–4337 [DOI] [PubMed] [Google Scholar]
- Ponten F, Jirstrom K, Uhlen M (2008) The Human Protein Atlas–a tool for pathology. J Pathol 216:387–393 [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Yang C, Chen C, Xu HJ, Benedict WF, Taylor CR (1996) Development of an optimal protocol for antigen retrieval: a ‘test battery’ approach exemplified with reference to the staining of retinoblastoma protein (pRB) in formalin-fixed paraffin sections. J Pathol 179:347–352 [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748 [DOI] [PubMed] [Google Scholar]
- Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, et al. (2005) A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics 4:1920–1932 [DOI] [PubMed] [Google Scholar]
- Uhlen M, Ponten F (2005) Antibody-based proteomics for human tissue profiling. Mol Cell Proteomics 4:384–393 [DOI] [PubMed] [Google Scholar]
- Umlas J, Tulecke M (2004) The effects of glyoxal fixation on the histological evaluation of breast specimens. Hum Pathol 35:1058–1062 [DOI] [PubMed] [Google Scholar]
- Werner M, Chott A, Fabiano A, Battifora H (2000) Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol 24:1016–1019 [DOI] [PubMed] [Google Scholar]
- Wester K, Asplund A, Backvall H, Micke P, Derveniece A, Hartmane I, Malmstrom PU, et al. (2003) Zinc-based fixative improves preservation of genomic DNA and proteins in histoprocessing of human tissues. Lab Invest 83:889–899 [DOI] [PubMed] [Google Scholar]
- Westermark B, Ponten J, Hugosson R (1973) Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Pathol Microbiol Scand [A] 81:791–805 [DOI] [PubMed] [Google Scholar]
- Williams C, Ponten F, Moberg C, Soderkvist P, Uhlen M, Ponten J, Sitbon G, et al. (1999) A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol 155:1467–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429:841–847 [DOI] [PubMed] [Google Scholar]