Abstract
A unique feature of the retina is the presence of photoreceptors, which require an enormous amount of oxygen for the conversion of light to an electrical signal. Hypoxia-inducible factor-1 alpha (HIF-1α) is a transcription factor that is the master regulator of cellular adaptation to low oxygen tension. Only in hypoxic conditions is HIF-1α protein stabilized and translocated to the nucleus, where it induces transcription of target genes involved in oxygen delivery and energy metabolism. We hypothesized that HIF-1α is constitutively stabilized and active in the normal human retina. We investigated the cellular distribution of HIF-1α and the expression of its downstream targets, vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT-1), and carbonic anhydrase IX (CAIX), by immunohistochemistry and immunoblotting in the retina of normal rats and human donor eyes. Both human and rat retinas displayed prominent staining of HIF-1α in nuclei of most cell types in inner and outer nuclear layers and the ganglion cell layer, a cellular distribution pattern which was confirmed in human retina by immunoblotting of nuclear extracts. A negative correlation was found between HIF-1α protein levels and postmortem times. In human retina, staining of VEGF, GLUT-1, and CAIX was found. Our observations indicate that active HIF-1 signaling occurs constitutively in the normal human and rat retina, suggesting that HIF-1 has a physiological role in the retina. (J Histochem Cytochem 58:247–254, 2010)
Keywords: hypoxia, retina, hypoxia-inducible factor, VEGF, glucose transporter, carbonic anhydrase
The retina is known to be the most metabolically active tissue in the human body, mainly owing to rod cells that renew their entire cytoplasmic volume in darkness every 30 sec. This process requires more oxygen than any process in any other cell (Arden et al. 2005). The huge demand for oxygen is seemingly juxtaposed with the relative avascularity of the human retina, leading to physiological rod-driven retinal hypoxia (Arden et al. 2005). Among other species, hypoxia has been demonstrated in the normal mouse inner retina, whereas in the retina of mice lacking rod cells, hypoxia was significantly reduced (Arden et al. 2005; De Gooyer et al. 2006).
The outer retina, consisting of photoreceptors, is completely avascular, receiving oxygen and other essential nutrients by free diffusion from the choroid. The choroid is the best-perfused tissue in the human body, but it is barely able to provide sufficient oxygen and nutrients to meet the metabolic needs of the photoreceptors. This is illustrated by loss of dark adaptation when oxygen levels in the blood are low, for example, in healthy individuals at altitudes above 1000 m and in a variety of pathological conditions involving systemic hypoxia, such as polycythaemia vera and partial carotid occlusion (Havelius et al. 1997a,b,2000).
It has been proposed that the peculiar feature of the retina to be hypoxic in physiological conditions is an important driving force in the pathogenesis of conditions such as diabetic retinopathy and age-related macular degeneration (Lahdenranta et al. 2001; Arden et al. 2005; De Gooyer et al. 2006).
Hypoxia-inducible factor-1 (HIF-1) is a crucial player in the regulation of cellular oxidative metabolism (Semenza 2003; Ziello et al. 2007). This heterodimeric nuclear transcription factor is composed of an α and a β subunit. Both subunits are constitutively expressed in all cells, with the exception of cells in the peripheral blood, but HIF-1α is immediately degraded by an oxygen-dependent mechanism. Under conditions of hypoxia, HIF-1α is stabilized, dimerizes with HIF-1β, and translocates to the nucleus, where, depending on the cell type, it induces the transcription of a wide variety of genes, including vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT-1), erythropoietin-1 (EPO-1), carbonic anhydrase IX (CAIX), and glyceraldehyde-3-phosphate dehydrogenase (Forsythe et al. 1996; Jiang et al. 1996; Gleadle and Ratcliffe 1997; Iyer et al. 1998).
HIF-1α has mainly been investigated in relation to cancer (Bos et al. 2001; Horrée et al. 2007a,b). Owing to its presumed specificity for tumor hypoxia, it is considered to be a potential therapeutic target in cancer patients (Williams et al. 2001; Semenza 2003; Ziello et al. 2007). For years, HIF-1α was assumed to be expressed only under normal conditions in the developing embryo (Wang and Semenza 1993; Iyer et al. 1998; Zelzer et al. 1998; Talks et al. 2000; Poulaki et al. 2002). Recently, HIF-1α was also found in the nuclei of cells in a number of normal human tissue types as well: epidermis, dermal glands and hair follicles, epithelium of esophagus and colon, and cartilage (Talks et al. 2000; Giles et al. 2006; Pfander and Gelse 2007; Rosenberger et al. 2007; Boutin et al. 2008).
In the retina, HIF-1α expression has been investigated in relation to ischemia (Tang et al. 2006; Zhu et al. 2007), diabetes (Poulaki et al. 2002; Calvert et al. 2004; Kalesnykas et al. 2008), and glaucoma (Tezel and Wax 2004). However, expression in normal adult human retina has not been reported. Given the tenuous nature of oxygen tension in the retina, its possible role in retinal disease (Arden et al. 2005), and the constitutive expression in the retina of VEGF (Witmer et al. 2003) and GLUT-1 (Mantych et al. 1993), we hypothesize that HIF-1α is constitutively stabilized in the normal retina. To this end, we investigated HIF-1α distribution and the expression of its downstream target genes in normal human and rat retina.
Materials and Methods
Subjects
Donor eyes were kindly provided by the Corneabank, Amsterdam, The Netherlands, after removal of corneal buttons for transplantation. Eyes were either frozen in liquid nitrogen and stored at −80C for Western blotting (n=15), or embedded in paraffin after overnight incubation in 4% paraformaldehyde for immunohistochemistry (n=6). Cause of death of the patients was heart failure, respiratory failure, or brain failure; the age of patients was 38–78 years (mean ± SD, 61.6 ± 10.6); eight patients were female; and postmortem time was 14–26 hr (mean ± SD, 19.9 ± 3.5) until freezing or fixation. The use of human material was in accordance with the Declaration of Helsinki on the use of human material for scientific research.
Perfusion Fixation of Rat Retina
Experiments were carried out on male Wistar rats weighing 300–350 g. The animals were anesthetized intraperitoneally with KMA mixture [ketamine (100 mg/ml), medetomidine (1 mg/ml), atropine (0.5 mg/ml) in saline]. Either the carotid arteries were briefly perfused in situ with heparin, immediately followed by 4% paraformaldehyde under physiological pressure, by opening the jugular vein to fix the retina as rapidly as possible (n=3), or eyes were dissected and kept postmortem for 4 hr (n=3) or 24 hr (n=3) in phosphate-buffered saline. After enucleation, all eyes were stored in 4% paraformaldehyde, embedded in paraffin, and prepared for histological and immunohistological examination. Animal handling and experimental procedures were reviewed and approved by the Ethical Committee for Animal Care and Use of the Royal Netherlands Academy of Sciences, acting in accordance with the European Community Council directive of 24 November 1986 (86/609/EEC) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Immunohistochemistry
Paraffin-embedded human and rat retinas were sectioned (section thickness, 5 μm). Staining, using monoclonal mouse anti-HIF-1α (clone 54, dilution, 1:50; BD Transduction Laboratories, San Diego, CA), was performed as described previously (Bos et al. 2003). Polyclonal goat anti-VEGF (dilution, 1:50; R and D Systems, Abingdon, UK), polyclonal rabbit anti-GLUT-1 (dilution, 1:200; Dako, Glostrup, Denmark), and polyclonal rabbit anti-CAIX (dilution, 1:1000; Novus, Littleton, CO) were used as decribed by Horrée et al. (2007a,b). Sections were counterstained with hematoxylin. Control incubations were performed in the absence of primary antibodies.
Immunoblotting
Protein was isolated from frozen human retinas with a nuclear extraction kit (Active Motif; Rixensart, Belgium) according to the manufacturer's instructions. Twenty μg of protein was subjected to 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blot analysis. Blots were incubated overnight with anti–HIF-1α (dilution, 1:250) and anti–HIF-1β (dilution, 1:1500; BD Transduction Laboratories) as loading control. Secondary antibodies were horseradish peroxidase-conjugated goat anti-mouse IgG + IgM (dilution 1:5000; Biosource, Camarillo, CA). Enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK) was used for visualization as described by the manufacturer. Each sample was analyzed twice in separate runs. Intensity of HIF-1α and HIF-1β bands was quantified by densitometry (AlphaEase; AlphaInnotech Corp., San Leandro, CA), and their ratio was calculated.
Results
Immunohistochemical Analysis of Human Tissue Samples
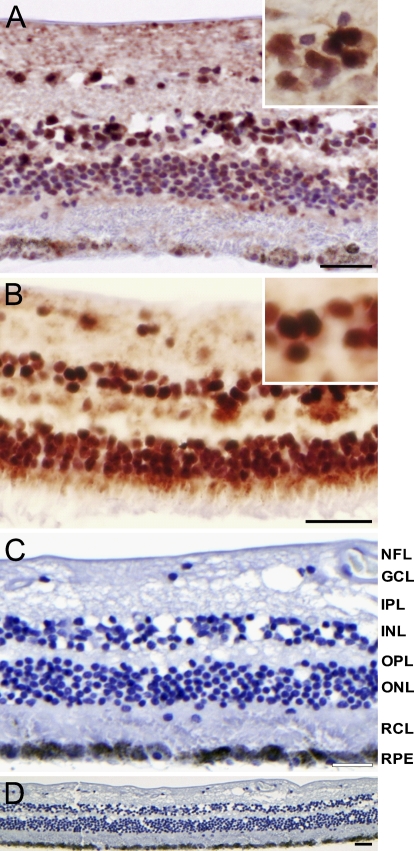
Nuclear staining of HIF-1α was observed in all human retinas (Figure 1). Nuclear staining intensity increased from central to peripheral retina. At least 75% of all nuclei of the ganglion cell layer (GCL), more than 50% of nuclei in the inner nuclear layer (INL), and less than 50% of nuclei in the outer nuclear layer (ONL) were stained. Retinal staining was absent after control incubations (Figures 1C and 1D).
Figure 1.
Immunohistochemical staining of hypoxia-inducible factor-1 alpha (HIF-1α) in human retina. Nuclear HIF-1α staining was present in ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL) in central retina (A) and particularly in peripheral retina (B) of normal donor eyes. Magnifications of nuclei of the INL are shown in insets. Lack of staining in the absence of primary antibody is shown at higher magnification (C) and lower magnification (D). NFL, nerve fiber layer; IPL, inner plexiform layer; OPL, outer plexiform layer; RCL, rod and cones layer; RPE, retinal pigment epithelium. Bar = 5 μm.
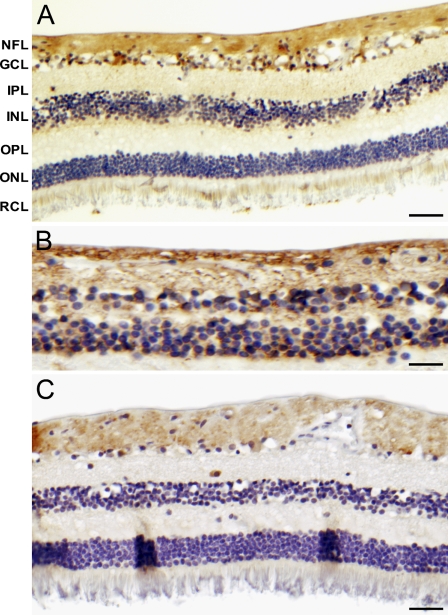
Downstream target proteins of HIF-1α were also expressed in normal retina (Figure 2). VEGF staining was present in cells of the GCL and in the nerve fiber layer (NFL). GLUT-1 staining was observed in the cytoplasm of cells from the outer retina toward the NFL. CAIX immunostaining was present in cells of the GCL and NFL.
Figure 2.
Immunohistochemical staining of vascular endothelial growth factor (VEGF) (A), glucose transporter 1 (GLUT-1) (B), and carbonic anhydrase IX (CAIX) (C) in normal human retina. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RCL, rod and cones layer. Bar = 5 μm.
Immunohistochemical Analysis of Rat Tissue Samples
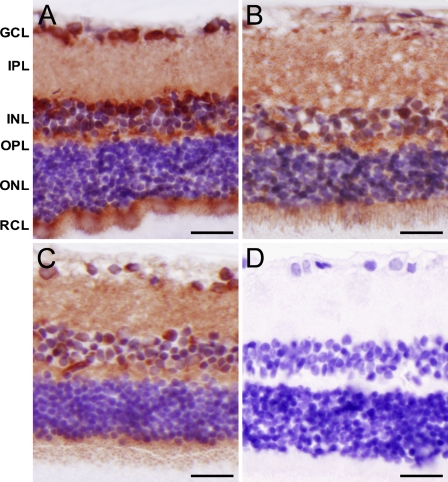
To exclude the possibility that the nuclear localization in the human tissue samples was the result of postmortem hypoxia, rats were perfused in vivo with paraformaldehyde through the carotid artery to immediately fix the retina. The jugular vein was opened to allow the outflow of the fixative. For comparison, eyes were kept for 4 or 24 hr postmortem at room temperature. Again nuclear staining in the GCL, the INL, and the ONL of HIF-1α was present in rat retina, whereas no staining was present after control incubation (Figure 3). Overall staining was more intense, as compared with human retina, probably because a higher antibody concentration was used. In the immediately perfusion-fixed eyes, the amount of nuclei stained in the three retinal nuclear layers was similar to that in human and relatively higher than in eyes that were kept for 4 or 24 hr postmortem in phosphate-buffered saline (Figure 3).
Figure 3.
Immunohistochemical staining of HIF-1α in rat retina that was immediately perfusion-fixed (A), fixed after 4-hr postmortem interval (B), or after 24-hr postmortem interval (C), and in the absence of primary antibody (D). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RCL, rod and cones layer. Bar = 5 μm.
Immunoblot Analysis of Human Retina Nuclear Extracts
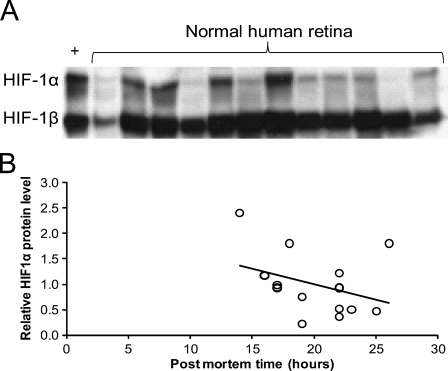
To confirm the presence of HIF-1α protein in human retina, nuclear extracts of total retina of 15 patients were analyzed by immunoblotting. An example of one of the blots is shown in Figure 4A. Stabilized HIF-1α was present in various amounts in all nuclear extracts, whereas HIF-1β was present in higher and more constant amounts. The positive control of cells incubated at low levels of oxygen showed strong HIF-1α expression. Nuclear HIF-1α protein levels were negatively correlated with the postmortem time (Figure 4B). Age, sex, and disease history of the patients did not correlate with HIF-1α protein levels.
Figure 4.
Retinal nuclear expression of HIF-1α protein. (A) Nuclear protein extracts of 15 normal human retinas were separated on SDS-PAGE and stained for HIF-1α and HIF-1β, respectively, on Western blots, of which 12 are shown here. HIF-1β is considered to be constitutively expressed and serves as loading control. As positive control, total lysate of U2OS cells exposed to 1% oxygen for 24 hr was used (+). (B) Relative optical density of the 15 HIF-1α bands on Western blots, normalized to that of the respective HIF-1β bands, in relation to the postmortem time, shows that duration of the postmortem time has a negative effect on the amount of nuclear HIF-1α in the human retina (p=0.03).
Discussion
Stabilized HIF-1α is assumed to be present only in cellular nuclei during embryogenesis, where its activity is involved in vascular development (Iyer et al. 1998; Talks et al. 2000). In adult tissues, stabilized HIF-1α has only been found under pathological conditions associated with tissue hypoxia (Wang and Semenza 1993) and diabetes (Poulaki et al. 2002). Recently, it was reported that specific cells in certain normal epithelial and cartilaginous tissues also express HIF-1α (Giles et al. 2006; Pfander and Gelse 2007; Rosenberger et al. 2007; Boutin et al. 2008). In this study, we demonstrate that stabilized HIF-1α is also present in normal human retina. Our findings in rats confirm this, and they are in line with earlier reports showing the presence of nuclear HIF-1α protein in non-diabetic rat retina (Poulaki et al. 2002; Calvert et al. 2004; Kalesnykas et al. 2008). The nuclear staining pattern and the immunoblotting data of nuclear extracts indicate that stabilized HIF-1α was located in the nuclei. This strongly suggests that in these retinal cells, HIF-1α is dimerized with HIF-1β and able to induce gene transcription (Jiang et al. 1996).
We considered the obvious possibility that stabilization of HIF-1α is a postmortem effect due to tissue hypoxia after circulatory arrest. Although this cannot be ruled out with complete certainty by our study, it is, however, unlikely. We have never observed nuclear staining in human postmortem or surgically obtained material of other organs, irrespective of time after death, cause of death, or time before fixation or freeze-fixation (Bos et al. 2003; Horrée et al. 2007a,b). Furthermore, a similar HIF-1α staining pattern was found in the rat retina that was immediately fixed and in which postmortem effects were virtually eliminated. Interestingly, increasing postmortem times led to a decrease in nuclear HIF-1α staining in the rat retina. and likewise, a significant negative correlation was found between HIF-1α protein expression and postmortem times in human retina. Therefore, it seems more likely that stabilized nuclear HIF-1α is degraded postmortem rather than increased due to postmortem hypoxia.
The retinal expression of VEGF, GLUT-1, and CAIX, three downstream targets of HIF-1, indicates the presence of nuclear HIF-1 transcriptional activity in normal retina (Kim et al. 1999). All three proteins have cell-protective properties, especially under hypoxic conditions (Grimm et al. 2004,2006; Potter and Harris 2004; Tang et al. 2006; Bernhardt et al. 2007; Zhu et al. 2007). These three proteins had differential but partly overlapping staining patterns, with the GCL and NFL layers staining most prominently. This staining pattern is in agreement with the observed HIF-1α staining, inasmuch as the percentage of nuclei stained increases from outer to inner retina. This is also in agreement with the pO2 gradient of the retina, which declines from the outer retina to the inner retina, with a relatively hypoxic inner retina with a pO2 of only ∼25 mm Hg (Wangsa-Wirawan and Linsenmeier 2003). Furthermore, these data are in agreement with those of De Gooyer et al. (2006), who demonstrated hypoxia by pimonidazole in the INL and GCL of normal mouse retina that was significantly reduced in the retina of rhodopsin knockout mice lacking rod cells.
Expression of stabilized HIF-1α in normal retina is probably due to the tenuous state of oxygen tension indigenous to the retina. The dark-adapted retina, in particular, is hypoxic because of the enormous oxygen demand of rods (Lahdenranta et al. 2001; Arden et al. 2005).
We have previously proposed that rod-driven hypoxia is an important factor in the pathogenesis of conditions such as diabetic retinopathy and age-related macular degeneration (Arden et al. 2005). Against the background of physiological hypoxia, the threshold toward pathological hypoxia is probably lowered. A small further increase of hypoxia caused by the diabetic milieu and/or a compromised vasculature is likely to have pathological consequences through upregulation of the expression of VEGF and other factors. Our present finding of stabilized HIF-1α in the retina provides evidence that even in the normal eye, the downstream biochemical effects of hypoxia are already activated, which supports the potential role of rod-driven hypoxia as a causal factor in eye disease.
We found a gradual increase in HIF-1α staining intensity from central to peripheral retina, in line with the previously reported observation that in the peripheral retina, the venous oxygen tension is lower (Alder et al. 1991; Wangsa-Wirawan and Linsenmeier 2003) and that the retinal microvasculature is more sparse than in the central retina (Toussaint et al. 1961). This suggests that physiological rod-driven hypoxia is even more profound in the peripheral retina. This may explain why in patients with diabetic retinopathy, retinal vascular occlusion and ischemia usually tend to develop first in the peripheral retina.
The large interindividual variation in nuclear HIF-1α levels in human retina can partly be explained by the variation in postmortem time (14–26 hr) and its effect on nuclear HIF-1α. Furthermore, HIF-1α is constitutively expressed in the cytoplasm and continuously degraded, unless it is stabilized by hypoxia, and it is translocated to the nucleus. Therefore, individual/local variation in oxygen levels in the human retina may affect the nuclear HIF-1α levels.
It is important to note that our findings indicate that caution should be taken when considering HIF-1α as a therapeutic target for ocular diseases such as diabetic retinopathy and age-related macular degeneration, because it appears to have a physiological role in the maintenance of retinal function. This also applies to anti-tumor therapies targeting HIF-1α.
Our findings suggest that HIF-1α not only plays a role in pathology, but also regulates cell survival, inasmuch as many of its downstream effector genes are neuroprotective. In the absence of hypoxia and subsequent HIF-1α signaling, neuroprotection may be insufficient and the retina may be more vulnerable. This may provide a new explanation for why in conditions with rod loss due to degeneration or to genetic defects such as retinitis pigmentosa, not only do the rods die, but eventually other neurons such as cones do so as well (Usui et al. 2009).
In summary, we provide evidence that HIF-1 signaling is constitutively active in the normal human retina. The retina is distinct from other tissues because of the presence of photoreceptors, which require enormous amounts of oxygen. The present study indicates that this causes not only physiological hypoxia, but also hypoxic molecular signaling via HIF-1α. These are unique features when compared with those of other normal tissues.
Acknowledgments
This work was supported by grants from the Dutch Diabetes Fund (grant number 1998.131); the Edward and Marianne Blaauw Foundation; the Landelijke Stichting voor Blinden en Slechtzienden, Utrecht; the Blindenpenning Foundation, Amsterdam; the Society for the Blind, Rotterdam; and the Society for the Blind, Gelderland, The Netherlands.
The authors kindly thank Adrie Maas for perfusion of rats.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Alder VA, Yu DY, Cringle SJ (1991) Vitreal oxygen tension measurements in the rat eye. Exp Eye Res 52:293–299 [DOI] [PubMed] [Google Scholar]
- Arden GB, Sidman RL, Arap W, Schlingemann RO (2005) Spare the rod and spoil the eye. Br J Ophthalmol 89:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt WM, Warnecke C, Willam C, Tanaka T, Wiesener MS, Eckardt KU (2007) Organ protection by hypoxia and hypoxia-inducible factors. Methods Enzymol 435:221–245 [DOI] [PubMed] [Google Scholar]
- Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, et al. (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97:1573–1581 [DOI] [PubMed] [Google Scholar]
- Bos R, Zhong H, Hanrahan CF, Mommers ECM, Semenza GL, Pinedo HM, Abeloff MD, et al. (2001) Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst 93:309–314 [DOI] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, et al. (2008) Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 133:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Zhou C, Zhang JH (2004) Transient exposure of rat pups to hyperoxia at normobaric and hyperbaric pressures does not cause retinopathy of prematurity. Exp Neurol 189:150–161 [DOI] [PubMed] [Google Scholar]
- De Gooyer TE, Stevenson KA, Humphries P, Simpson DAC, Curtis TM, Gardiner TA, Stitt AW (2006) Rod photoreceptor loss in Rho−/− mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest Ophthalmol Vis Sci 47:5553–5560 [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles RH, Lolkema MP, Snijckers CM, Belderbos M, van der Groep P, Mans DA, van Beest M, et al. (2006) Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene 25:3065–3070 [DOI] [PubMed] [Google Scholar]
- Gleadle JM, Ratcliffe PJ (1997) Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood 89:503–509 [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Acar N, Keller S, Seeliger M, Gassmann M (2006) Hypoxic preconditioning and erythropoietin protect retinal neurons from degeneration. Adv Exp Med Biol 588:119–131 [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, et al. (2004) Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci 24:5651–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelius U, Berglund S, Falke P, Hindfelt B, Krakau T (2000) Impaired dark adaptation in polycythemia. Improvement after treatment. Acta Ophthalmol Scand 78:53–57 [DOI] [PubMed] [Google Scholar]
- Havelius U, Bergqvist D, Falke P, Hindfelt B, Krakau T (1997a) I. Impaired dark adaptation in symptomatic carotid artery disease. Neurology 49:1353–1359 [DOI] [PubMed] [Google Scholar]
- Havelius U, Bergqvist D, Hindfelt B, Krakau T (1997b) II. Improved dark adaptation after carotid endarterectomy. Evidence of a long-term ischemic penumbra? Neurology 49:1360–1364 [DOI] [PubMed] [Google Scholar]
- Horrée N, van Diest PJ, Sie-Go DM, Heintz AP (2007a) The invasive front in endometrial carcinoma: higher proliferation and associated derailment of cell cycle regulators. Hum Pathol 38:1232–1238 [DOI] [PubMed] [Google Scholar]
- Horrée N, van Diest PJ, van der Groep P, Sie-Go DM, Heintz AP (2007b) Hypoxia and angiogenesis in endometrioid endometrial carcinogenesis. Cell Oncol 29:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12:149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Rue E, Wang GL, Roe R, Semenza GL (1996) Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271:17771–17778 [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J (2008) Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience 155:937–947 [DOI] [PubMed] [Google Scholar]
- Kim I, Ryan AM, Rohan R, Amano S, Agular S, Miller JW, Adamis AP (1999) Constitutive expression of VEGF, VEGFR-1, and VEGFR-2 in normal eyes. Invest Ophthalmol Vis Sci 40:2115–2121 [PubMed] [Google Scholar]
- Lahdenranta J, Pasqualini R, Schlingemann RO, Hagedorn M, Stallcup WB, Bucano CD, Sidman RL, et al. (2001) An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc Natl Acad Sci USA 98:10368–10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantych GJ, Hageman GS, Devaskar SU (1993) Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology 133:600–607 [DOI] [PubMed] [Google Scholar]
- Pfander D, Gelse K (2007) Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments. Curr Opin Rheumatol 19:457–462 [DOI] [PubMed] [Google Scholar]
- Potter C, Harris AL (2004) Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle 3:164–167 [PubMed] [Google Scholar]
- Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, et al. (2002) Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109:805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger C, Solovan C, Rosenberger AD, Jinping L, Treudler R, Frei U, Eckardt KU, et al. (2007) Upregulation of hypoxia-inducible factors in normal and psoriatic skin. J Invest Dermatol 127:2445–2452 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732 [DOI] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157:411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Pacary E, Fréret T, Divoux D, Petit E, Schumann-Bard P, Bernaudin M (2006) Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis 21:18–28 [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB (2004) Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol 122:1348–1356 [DOI] [PubMed] [Google Scholar]
- Toussaint D, Kuwabara T, Cogan DG (1961) Retinal vascular patterns. II. Human retinal vessels studied in three dimensions. Arch Ophthalmol 65:575–581 [DOI] [PubMed] [Google Scholar]
- Usui S, Komeima K, Lee SY, Jo YJ, Ueno S, Rogers BS, Wu Z, et al. (2009) Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther 17:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90:4304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA (2003) Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 121:547–557 [DOI] [PubMed] [Google Scholar]
- Williams KJ, Cowen RL, Stratford IJ (2001) Hypoxia and oxidative stress. Tumour hypoxia: therapeutic considerations. Breast Cancer Res 3:328–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29 [DOI] [PubMed] [Google Scholar]
- Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B (1998) Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17:5085–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang Y, Ojwang BA, Brantley MA Jr, Gidday JM (2007) Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci 48:1735–1743 [DOI] [PubMed] [Google Scholar]
- Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80:51–60 [PMC free article] [PubMed] [Google Scholar]