Abstract
Head and neck tumors comprise a wide spectrum of heterogeneous neoplasms for which biomarkers are needed to aid in earlier diagnosis, risk assessment and therapy response. The search for biomarkers includes evaluation of tumor tissues and surrogate materials by molecular, genomic and phenotypic means. Ideal biomarkers should be accurate and easy to perform, highly specific, objective, quantitative, and cost effective. Because of the heterogeneity of head and neck tumors, the integration of multiple selected markers in association with the histopathologic features is advocated for risk assessment. For targeted therapy, however, a single key molecule must be identified. Key molecules and pathways for targeted therapy include growth factor receptors, MAPk/ERk pathway, angiogenesis, and epithelial to mesenchymal transition. Over-expression and mutations of genes in these pathways including EGFR, VEGF, HER2, BRAF and RET, contribute to tumorigenesis in head and neck cancers from squamous carcinomas, to salivary adenocarcinomas and thyroid carcinomas, both follicular and c-cell derived. Monoclonal antibodies to the EGFR receptor and oral tyrosine kinase inhibitors are currently being studied in multiple phase II and III clinical trials to determine their efficacy in head and neck cancers and correlative studies for biomarkers are on-going.
Keywords: Biomarkers, Squamous carcinoma, EGFR, Molecular targets, Head and neck cancer
Introduction
Head and neck cancers are markedly phenotypic and clinically heterogeneous neoplasms of different histogenesis. The most common is epithelial derived squamous carcinoma with over 500,000 new cases expected worldwide this year. The majority of patients will present with advanced disease and incur significant morbidity and mortality secondary to limited screening tools and markers for adjuvant therapy. Fortunately, progress has been achieved in the identification and validation of biomarkers as molecular targeted therapies and are advancing through clinical trials. To determine which patients will benefit from these treatments requires personalized medicine and the analysis of factors specific to the individual and their tumor. The twenty-first century is the age of biomarker discovery which holds the promise of augmenting cancer diagnosis and treatment expectations.
Biomarker Characteristics
Biomarkers are defined as biological molecules that (1) correlate with the presence or absence of a disease state, (2) are prognostic correlating with a disease course, or (3) predictive of a tumor’s response to a specific therapy. Biomarkers should be objective, independent and require validation by clinical testing and patient outcome. Ideally biomarkers should be easy to analyze, quantitative, affordable, and must be subject to quality control and assurance.
Specimens
Biomarkers may either be identified in tumor tissues or in surrogate materials (blood and saliva) obtained from patients with disease. These may include fresh/frozen or archival tissues from biopsy and excision specimens and even fine-needle aspiration (FNA) samples. All tissue-based samples require pathologic assessment for quality assessment and appropriate tumor specimen representation selection. Quality assurance for tissue handling and specimen storage for reproducibility of biomarkers is being defined at the national level (biospecimens.cancer.gov).
Methodologies
The identification of these markers is facilitated by advances in molecular genetics and bioinformatics fields. Common molecular techniques including polymerase chain reaction (PCR), in situ hybridization (for detecting gene amplification and chromosomal translocations), and DNA sequencing were instrumental in advancing the biomarker field. Applying PCR to determine microsatellite heterozygosity (LOH) was used in the molecular characterization of squamous tumorigenesis. Emerging fields in cell biology and tumorigenesis include epigenetics consisting of promoter methylation and histone acetylation as mechanisms to alter gene transcription. Additionally, microRNAs (miRNAs), small 18–24 bp non-coding segments, have been shown to play an important role in gene regulation at the translational level, and can be assessed by real time-PCR quantitative assessment. Viral factors may also serve as biomarkers for head and neck neoplasms and are routinely detected by in situ hybridization. Immunohistochemistry has remained a central methodology in biomarkers assessment, especially of those markers where a specific antibody has been identified (i.e. EGFR, HER2). As methodologies now allow for reproducible investigations of genomic, epigenomic, and proteomic alterations, the incorporation of potential biomarkers in clinical trials for head and neck cancers is required to advance our understanding of these diseases and advance patient care from diagnosis to more personalized targeted treatment.
Mucosal Tumorigenesis
Squamous carcinoma (SCC) is the most common head and neck malignancy. Approximately 45,000 new cases are seen in the USA per year. These tumors primarily develop in individuals with a protracted history of tobacco and alcohol abuse. Despite these known etiologic factors the specific cause of this disease remains to be identified.
Conventional Squamous Carcinoma Premalignancy Progression Model
SCC of the upper aerodigestive tract (HNSCC) is a multistep progression through dysplasia (mild, moderate, and severe) to ultimately invasive carcinoma with acquisition of specific chromosomal losses 3p, 9p, 17p and mutations in p53. (Table 1) An early and common genetic event in oral premalignancy includes LOH of 9p21 in dysplasia/hyperplasia (30%) and carcinoma (70–80%) [1]. p53 mutations begin to occur in severe dysplasia and are associated with decreased survival in invasive carcinomas (50% incidence) [2]. These markers may augment pathologic margin assessment identifying a group of patients at higher risk of local recurrence. Moreover, the detection of 3p and 9p loss by LOH analysis using comparative normal DNA (commonly peripheral blood lymphocytes) is currently being used in a NCI sponsored trial of Erlotinib in the prevention of oral cancer (EPOC trial) (Table 2).
Table 1.
Potential Biomarkers in Head and Neck Cancers and significance
| Biomarker/Target | Alteration | Incidence | Significance/Association |
|---|---|---|---|
| Upper aerodigestive tract | |||
| Nasopharyngeal carcinoma (non-keratinizing) | |||
| EBV | >75% | ||
| IgA-serum (VCA) | Diagnostic/screening | ||
| EBV nucleic acids-serum | Diagnostic/screening | ||
| Oropharyngeal SCC | |||
| HPV | 60% | Improved prognosis/local control | |
| Epithelial malignant progression to SCC | |||
| 3p, 9p21 | LOH | 30% dysplasia; 70–80% SCC | Increased risk for SCC |
| p53 | Mutation | 50% | Decreased overall survival |
| p16, RARβ2, MGMT | Promoter methylation | ||
| Pathways for molecular targeting of SCC | |||
| EGFR | Growth receptors | >90% | Nodal metastases; more rapid clinical course |
| VEGF | Angiogenesis | ||
| Src | (EMT) epithelial -mesenchymal transition | High-grade tumors, lymph node metastases, sarcomatous transformation | |
| Salivary gland | |||
| Salivary duct carcinoma | |||
| HER2 | Over-expression/amplification | 15–70% | Similar to breast tx with Herceptin |
| EGFR | Over-expression | 33–92% | Consideration for targeted therapy |
| AR | 60–90% | Consideration for targeted therapy | |
| Mucoepidermoid carcinoma | |||
| t(11;19) | Translocation | 50–80% | Unclear—prognostic? |
| Adenoid cystic carcinoma | |||
| t(6:9) | Translocation | To be defined | |
| 1p32-p36 | LOH | Aggressive behavior/basaloid(solid) phenotype | |
| Thyroid gland | |||
| Papillary thyroid carcinoma | |||
| Ret/PTC | Translocation | 20% | Younger age; radiation exposure |
| BRAF | Point mutation/inversion | 40–60% | High stage, extrathyroidal, older age |
| RAS | Point mutation | 10% | Follicular variant |
| Follicular thyroid carcinoma | |||
| RAS | Point mutation | 30–50% | Poor prognosis in poorly differentiated (n-Ras) |
| PAX8-PPARγ | Translocation | 30% | Unclear—less aggressive? |
| Medullary thyroid carcinoma | |||
| RET | Point mutation | 40–60%* | Predictive of therapy response? |
* sporadic; LOH Loss of heterozygosity; SCC Squamous carcinoma; tx Treatment
Table 2.
Molecular targeted agents and current clinical trials
| Class of drugs | Trade Name | Target | FDA approved | Head & Neck Cancers Clinical trials |
|---|---|---|---|---|
| TKI-small molecule | ||||
| E7080 | VEGFR2 | Phase II—Thyroid + MTC | ||
| Erlotinib | Tarceva | EGFR | Lung, Pancreas | Phase III—HNSCC |
| Gefitinib | Iressa | EGFR | Lung, Pancreas | |
| Imatinib | Gleevac | KIT, PDGFR, BCR-ABL | GIST | |
| Lapatinib | Tykerb | EGFR, HER2 | Breast | Phase III—HNSCC |
| Pazopanib | Votrient | VEGFR, PDGFR, KIT | Phase II—Thyroid | |
| Sorafenib | Nexavar | VEGFR, PDGFR, KIT, FLT-3 RAF | RCC, Hepatic | Phase II—Thyroid + MTC |
| Sunitinib | Sutin | VEGFR, PDGFR, KIT, FLT-3 RET | RCC, GIST | Phase II—Thyroid + MTC |
| Vandetanib | Zactima | VEGFR, RET,EGFR, | Phase II/III—MTC; Phase II —HNSCC | |
| XL-184 | MET, VEGFR, RET | Phase III—MTC | ||
| Monoclonal Ab | ||||
| Bevacizumab | Avastin | VEGF | Breast, Colon, Lung | Phase III—HNSCC |
| Cetuximab* | Erbitux | EGFR | HNSCC, Colon | Phase III—HNSCC** |
| Nimotuzumab | EGFR | Phase III—HNSCC | ||
| Panitumumab | Vetibix | EGFR | Colon | Phase II—HNSCC |
| Trastuzumab | Herceptin | HER2 | Breast | |
| Zalutumumab | EGFR | Phase III—HNSCC | ||
| Small molecule serine/threonine kinase inhibitor | ||||
| Temsirolimus | Torisel | mTOR (PI3k) | RCC | Phase II—HNSCC |
| Proteasome inhibitor | ||||
| Bortezomib | Valcade | 26s proteasome inhibitor | Phase II—Thyroid + MTC | |
| Histone deacetylase HDAC1 | ||||
| Valproic Acid | Epigenetic alterations | Phase II —Thyroid | ||
Ab Antibody; HNSCC Head & neck squamous carcinoma; MTC Medullary thyroid carcinoma; Thyroid Papillary, follicular and/or Hurthle carcinoma; TKI Tyrosine kinase inhibitor; GIST gastrointestinal stromal tumor; RCC renal cell carcinoma
* only agent approved for HN application—specifically SCC
** in various combinations (i.e. with chemotherapy)
Additional trial information is available through the NCI www.cancer.gov/clinicaltrials website
Viral Etiologies
Nasopharyngeal Carcinoma (EBV)
Nasopharyngeal carcinoma maybe the earliest tumor in the head and neck with a known biomarker. In 1970, Epstein Barr Virus (EBV) was identified in SCCs arising in the nasopharynx and can be detected in tumor tissue by in situ hybridization for EBV encoded RNAs (EBER) (Fig. 1-1) or by immunohistochemical evaluation though this is less sensitive (40–60%). In high-risk populations, predominantly in Southeast Asia, EBV associated proteins, antibodies (i.e. VCA IgA) and HPV nucleic acids in serum are now used in screening and surveillance for nasopharyngeal carcinoma [3]. Other emerging factors in the evaluation of nasopharyngeal carcinoma include gene promoter methylation of DAPK1, p16, and RASSF1A for assisting in screening patients and protein expression such as stathmin, annexin1, and cathepsin may have prognostic potential [3]. Procurement and assessment of tumor tissue for adjuvant studies will be essential for these correlative endeavors.
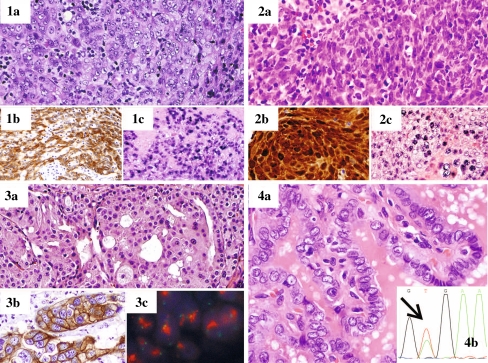
Fig. 1.
Head and neck cancers with pathologic and molecular assessment for biomarkers and targeted pathways. 1 Nasopharyngeal carcinoma showing a 1a hematoxylin and eosin (H&E) section, 1b strong membranous EGFR expression by immunohistochemistry, 1c Epstein Barr Virus encoded RNAs (EBER) nuclear expression by in situ hybridization. 2 Oropharyngeal squamous carcinoma with associated human papilloma virus (HPV) by 2a H&E section, 2b p16 immunohistochemical analysis showing nuclear and cytoplasmic over-expression, and 2c HPV detection in the nucleus by in situ hybridization. 3 Salivary duct carcinoma by 3a H&E evaluation with 3b strong membranous expression of HER2 and corresponding 3c amplification of the HER2 gene (red in clusters) by fluorescence in situ hybridization (FISH). 4 Papillary thyroid carcinoma with classic nuclear changes by 4a H&E evaluation, and 4b tumor DNA evaluated by polymerase chain reaction (PCR) followed by sequencing of the nucleotide base pairs allows for the detection of the change of T to A(arrow) signifying the presence of the V600E BRAF mutation which may be targeted for therapy
Oropharyngeal Squamous Carcinoma (HPV)
Approximately 30 years after EBV’s association with nasopharyngeal carcinoma, human papilloma virus (HPV), most often type 16, has emerged as both an etiologic factor and prognostic marker in oropharyngeal carcinomas [4]. Associated with a younger age of onset of HNSCC than in smokers, evidence supports HPV as a prognostic marker for improved local control and overall survival at 5 years of 79% for HPV + versus 20% for HPV negative patients [5].
Evaluation for HPV 16 maybe performed on archival sections of biopsy tissue or FNA (i.e. neck nodes) by in situ hybridization (Fig. 1-2) or PCR amplification of viral related genes (E6, E7). p16 over-expression by immunohistochemical evaluation maybe used as a surrogate marker of HPV as p16 over expression is not associated with SCC contributed to smoking exposure [5] (Fig. 1-2). HPV contributes to tumorigenesis through proteins E6 and E7 binding and inactivating p53 and Rb, respectively. HPV may be detected in saliva raising the possibility of a role in oropharyngeal cancer screening or monitoring, however further studies to improve sensitivity and applicability in asymptomatic patients, remain to be defined [6].
Cell Based Biomarkers
Epidermal Growth Factor Receptor (EGFR)
Epidermal growth factor receptor (EGFR) is a member of the tyrosine kinase family of receptors. Up regulation of this factor occurs early in the progression of dysplasia to HNSCC in the upper aerodigestive tract. High expression has been correlated with presence of nodal metastases and more rapid clinical course through its role in enhancing cell motility, altering cell adhesion and promoting angiogenesis [7, 8]. Mechanisms for EGFR over-expression in HNSCCs show EGFR gene mutations as in lung adenocarcinomas are uncommon and gene amplification is infrequent (<15%), however a truncated mutant activated EGFRvIII is prevalent (40%) [9]. Downstream signaling activated by EGFR includes STAT1, and 3, phophatidylinositol 3-kinase (PI3k) which activates Akt, the Ras-MAPk/ERk pathway and phospholipase c-γ (PLC-γ) which all contribute to cell survival, proliferation, and tumorigenesis in HNSCC [8].
Cetuximab, a monoclonal antibody to EGFR’s extra cellular domain, is an FDA approved molecular targeted therapy in HNSCC based on the Bonner et al. trial comparing treatment of newly diagnosed HNSCC with cetuximab plus radiation versus radiation alone[10]. The addition of cetuximab decreased local recurrence and increased 2 and 3 year survival from 55 to 62% and 44 to 57%, respectively. Trials comparing Cetuximab in conjunction with chemotherapy are on-going. Other EGFR targeting monoclonal antibodies (Table 2) including bevacizumab, and zalutumumab and small molecule oral tyrosine kinase inhibitors (TKIs) which target the intracellular domain, erlotinib and vandetanib, are currently under investigation in phase II/III trails [6]. (Table 2) Since only a subset of patients benefit from each of these therapies, predictive biomarkers are still needed in order to personalize treatment and enhance outcomes.
Angiogenic Factors
Neoangiogenesis, the formation of new blood vessels, is an important process for tumor growth and survival. Over-expression of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8), promoters of angiogenesis, occur in SCC[11]. EGFRvIII leads to autoactivation and up-regulated VEGF and tissue factor (TF) further augmenting this pathway [11]. Hypoxia is also a strong driving force in tumor angiogenesis and the associated transcription factor, hypoxia inducible factor-1 (HIF-1) is over-expressed and at the top of a cascade of inducible proangiogenic proteins contributing to angiogenesis in HNSCC. Agents targeting HIF-1 have been identified for further study. Another marker of tumor hypoxia, lysyl oxidase, is also a potential marker for distant metastasis.
Structural Related Biomarkers
Epithelial to Mesenchymal Transition (EMT)
Another mechanism contributing to cell migration and metastases in SCC is epithelial to mesenchymal transition (EMT), a process by which carcinoma cells loose adhesion factors (i.e. E-cadherin). This process is highlighted by the spectrum/progression of SCC from well differentiated tumors to poorly differentiated and ultimately sarcomatoid carcinoma with single cell invasion and morphologic features of mesenchymal cells [12]. Extra-cellullar matrix appears to orchestrate this process along with growth factors including EGFR, and TGF-beta, contributing to activated Src, Ras, and the PI3k and MAPk pathways. Src is a strong inducer of EMT abrogating loss of cell–cell adhesion and E-cadherin and associates with SCC progression and aggressive features [12]. Investigational agents with anti-src properties (i.e. Dasatinib, AZD-0530) are early in investigations, though, temsirolimus, a PI3k/Akt/mTOR inhibitor is actively being evaluated in phase II trails in HNSCC. The complex interconnecting pathways and growth factor receptors activated in SCC provide multiple targets for therapeutic consideration though may require combination therapy to block duel pathways of activation and feedback loops contributing to resistance.
Genome Wide Analyses
Global approaches for tumor assessment via DNA and gene expression microarrays have provided new information into altered molecules and pathways for further investigations in SCC, though remain limited in their benefit for individual tumor assessment as a biomarker.
Potential Saliva-Based Tumor Markers
Other sources for biomarkers, such as saliva and plasma, have been used to identify certain alterations, including epigenetic changes of hypermethylation specifically of promoters of tumor suppressor genes p16, MGMT, RARβ, E-cadherin, and DAPK in patients with HNSCC. As a potentially reversible process, demethylating agents have been identified and are early in clinical investigations in HNSCC.
Studies on saliva and exfoliated cells to predict disease or recurrence in the oral cavity have included detection of p53 mutations, LOH of 3p and 9p and HPV with differences in mRNA, miRNA, DNA methylation, and microsatellite instability profiles [6]. A recent proteomics study proposed a panel for identifying malignancy though confirmation studies are still necessary [13].
Salivary Gland Carcinomas
Biomarkers in salivary gland neoplasms are beginning to emerge as studies identify increased growth factor and hormonal receptors, and tumor specific chromosomal alterations which may serve as diagnostic markers and therapy targets [14]. Analysis of saliva is also an actively pursued area for characterization of potential biomarkers. Translating molecular pathways targeted for therapy in more prevalent tumors may provide key information regarding potential targets to be tested and validated in salivary gland tumors.
Mucoepidermoid Carcinoma
A chromosomal translocation t(11:19) between CRTC and MAML2 is present in the majority of mucoepidermoid carcinoma (50–80%) [14, 15]. Tumors without the translocation are associated with increase risk for distant metastases suggesting prognostic value. The detection of the fusion gene using FNA may have a diagnostic potential and may assist in patients’ prognostication. Investigations into the mechanisms by which this translocation contributes to tumorigenesis may provide insight into targeting possibilities.
Adenoid Cystic Carcinoma
A recently described translocation t(6;9) between MYB and NFIB in adenoid cystic carcinoma leads to over-expression of the MYB gene and downstream target genes including c-KIT which shows high-expression in these tumors [16]. Further characterization of this molecular alteration in a broad range of head and neck adenoid cystic carcinomas is needed to further define it’s role as a potential biomarker. Frequent loss of 1p32-p36 has also been identified in adenoid cystic carcinoma and correlated with solid/basaloid morphology and poorer prognosis [17]. These observations favor potential tumor suppressor genes in this region which are lost and may allow for directed targeted therapy in the future.
Salivary Duct Carcinoma
Salivary duct carcinoma, a high-grade adenocarcinoma that morphologically resembles breast adenocarcinoma also shares similar growth factor receptor expression: specifically, HER2 (at least 15%) and EGFR (50%) over-expression. Several studies have confirmed HER2 over-expression correlates with HER2 gene amplification which in breast adenocarcinoma is a biomarker for patients who may respond to trastuzumab therapy [14] (Fig. 1-3). Targeting of the EGF receptor and downstream pathways in salivary duct carcinomas as well as in other salivary gland carcinomas with high EGFR expression should be investigated further in advanced patients as a number of different agents are available targeting this pathway. Additionally, the presence of hormonal receptors in salivary duct carcinoma including androgen receptor (>60% of patients) and estrogen receptor-beta (75%) are prime pathways for therapeutic considerations.
Thyroid Gland: Well-Differentiated Carcinomas
Although most of the 35,000 patients to be diagnosed with thyroid cancer in the United States this year will be cured with conventional therapy (surgery ± I131), a subset (10–20%) will go onto develop advanced or metastatic disease for which conventional chemotherapy is non-effective. During the past 10–15 years molecular alterations specific to papillary thyroid carcinoma and follicular carcinoma point to potential predictive biomarkers for novel targeted therapy (Table 1). Although, genome-wide platforms for chromosomal and gene expression alterations in these tumors have not proven to be biomarkers, selective molecules were identified and are under investigation.
Follicular Thyroid Neoplasms
Molecular insight into the biology of follicular neoplasms include the translocation t(2;3)(q13;p25) of PAX8/PPARgamma and RAS mutations in a subset of follicular adenomas and carcinomas. These initial markers may be used to augment FNA cytologic assessment in follicular neoplasms, though a confounding factor is the lack of a progression model and the uncertain relationship of follicular adenomas to carcinomas. Alternatively, differentially expressed miRNA may allow distinction between benign and malignant entities on FNA samples; validation is still pending [18].
Papillary Thyroid Carcinoma
An activating point mutation (V600E) in BRAF, an oncogene upstream of the MAPk/ERk pathway, is present in 40–60% of PTCs and has not been identified in benign thyroid disease (Fig. 1-4). V600E associates with more aggressive disease (higher stage, extrathyroidal extension) and older patient age and may allow for a therapeutic target in patients with advanced progressive disease [18] (Table 2). TKIs are currently in phase II clinical trials with partial responses and stable disease occurring in up to 30 and 60% of patients respectively, and the BRAF mutation status suggests a predictive marker potential (Table 2). Interestingly, as TKIs target several receptors including VEGFR (angiogenesis pathway), their use has been reported to elicit response in patients with follicular, Hurthle and even medullary thyroid carcinomas.
Evaluation for the BRAF mutation requires DNA extraction from tumor tissue (archival or FNA), and sequencing via Sanger sequencing, pyrosequencing or other method for analysis. (Fig. 1-4) As tumoral heterogeneity in BRAF mutational status has been documented in multifocal PTCs and metastases, the source of tissue for analysis particularly in documenting factors of predictive response for clinical trials will require careful consideration and correlation.
Medullary Thyroid Carcinoma (MTC)
Activating mutations in the RET proto-oncogene occur in virtually all hereditary MTCs and in 40–60% of sporadic cases. As the RET gene is also targeted by TKIs, clinical trials for MTC are ongoing, with results from a large international trial of Vandetanib in advanced patients anxiously anticipated [19].
Summary and Perspectives
Robust biomarkers for disease detection and predicting therapeutic response in head and neck cancers are still needed. Current known factors contributing to tumorigenesis in head and neck cancers from SCC, to salivary adenocarcinoma and thyroid carcinoma comprise over-expressed growth factor receptors and factors contributing to angiogenesis including EGFR, VEGF, HER2, BRAF and RET. A number of agents including monoclonal antibodies to EGFR and oral TKIs are advancing through clinical trials with the urgent need for further biomarker evaluation to identify markers of response. Beyond known molecular alterations co-regulatory mechanisms including miRNA and epigenetic alterations are unfolding as major players in tumorigenesis and targets for future consideration. Ideally, a saliva or exfoliate cytologic based biomarker for oral SCC will aid in the identification of early disease with Lab-on-Chip technologies advancing to address this issue [20]. Throughout, the pathologist must play an active role in the assessment of tumor and surrogate tissues for biomarker evaluation including adaptation to small tissue samples, including FNA samples. Additionally, considering tumor heterogeneity in and between patients will also be required in selecting and interpreting biomarkers to optimize personalized cancer therapy for head and neck cancer patients.
References
- 1.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682–685. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 2.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol Cancer. 2007;6:1. doi: 10.1186/1476-4598-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 6.Chai RL, Grandis JR. Advances in molecular diagnostics and therapeutics in head and neck cancer. Curr Treat Options Oncol. 2006;7:3–11. doi: 10.1007/s11864-006-0027-4. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 8.Kim S, Grandis JR, Rinaldo A, Takes RP, Ferlito A. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck. 2008;30:667–674. doi: 10.1002/hed.20859. [DOI] [PubMed] [Google Scholar]
- 9.Sok JC, Coppelli FM, Thomas SM, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Howell GM, Grandis JR. Molecular mediators of metastasis in head and neck squamous cell carcinoma. Head Neck. 2005;27:710–717. doi: 10.1002/hed.20222. [DOI] [PubMed] [Google Scholar]
- 12.Mandal M, Myers JN, Lippman SM, Johnson FM, Williams MD, Rayala S, Ohshiro K, Rosenthal DI, Weber RS, Gallick GE, El-Naggar AK. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008;112:2088–2100. doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, Elashoff D, Wei R, Loo JA, Wong DT. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 2008;14:6246–6252. doi: 10.1158/1078-0432.CCR-07-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elledge R. Current concepts in research related to oncogenes implicated in salivary gland tumourigenesis: a review of the literature. Oral Dis. 2009;15:249–254. doi: 10.1111/j.1601-0825.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- 15.Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46:708–715. doi: 10.1002/gcc.20458. [DOI] [PubMed] [Google Scholar]
- 16.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–4. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao PH, Roberts D, Zhao YJ, Bell D, Harris CP, Weber RS, El-Naggar AK. Deletion of 1p32–p36 is the most frequent genetic change and poor prognostic marker in adenoid cystic carcinoma of the salivary glands. Clin Cancer Res. 2008;14:5181–5187. doi: 10.1158/1078-0432.CCR-08-0158. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 19.Morabito A, Piccirillo MC, Falasconi F, Feo G, Del Giudice A, Bryce J, Di Maio M, Maio E, Normanno N, Perrone F. Vandetanib (ZD6474), a dual inhibitor of vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) tyrosine kinases: current status and future directions. Oncologist. 2009;14:378–390. doi: 10.1634/theoncologist.2008-0261. [DOI] [PubMed] [Google Scholar]
- 20.Weigum SE, Floriano PN, Christodoulides N, McDevitt JT. Cell-based sensor for analysis of EGFR biomarker expression in oral cancer. Lab Chip. 2007;7:995–1003. doi: 10.1039/b703918b. [DOI] [PubMed] [Google Scholar]