Abstract
The diagnostic classification of small round blue cell tumors of the sinonasal area to include diverse malignancies of epithelial, hematolymphoid, neuroectodermal, and mesenchymal origin is challenging to the surgical pathologist using conventional histopathologic approaches because the cytomorphologic features are often overlapping or indistinctive. Rare or occasional clinical presentations in atypical age groups or unusual locations, as well as small biopsy samples may further complicate the differential diagnosis. Immunohistochemistry represents an extensively investigated ancillary technique that may aid in the provision of a definitive diagnosis. In recent years, certain small round blue cell tumors have been shown by cytogenetic analysis to have specific and primary chromosomal alterations, providing clinicians with a valuable tool to enhance their diagnostic armamentarium. The addition of molecular cytogenetic [fluorescence in situ hybridization (FISH), comparative genomic hybridization (CGH)] and molecular pathologic [polymerase chain reaction (PCR) and reverse transcriptase (RT)-PCR] approaches has further enhanced the sensitivity and accuracy of detecting these genetic alterations including assessment in formalin-fixed, paraffin-embedded tissues. Establishing an accurate diagnosis of a small round blue cell tumor of the sinonasal tract frequently requires adjunctive studies including immunohistochemical and molecular analyses.
Keywords: Sinonasal undifferentiated carcinoma, Small round cell tumor, Olfactory neuroblastoma, Ewing’s sarcoma, Rhabdomyosarcoma
Introduction
Head and neck malignancies account for 3–5 percent of all cancers in the United States [1]. Those of the nasal cavity and paranasal sinuses comprise only 0.2–0.8 percent of all malignant neoplasms [2] and because of the close anatomic proximity of the paranasal sinuses with the orbits and skull base, most include disease extension into these structures. Sinonasal malignancies most commonly arise in the maxillary sinus (60%), followed by the nasal cavity (20–30%), the ethmoid sinus (10–15%), and the sphenoid and frontal sinuses (~1%) [2]. Although many different histopathological types of cancer occur in the sinonasal cavity, the most common is squamous cell carcinoma of the maxillary sinus [2]. A poorly differentiated, nonkeratinizing squamous cell carcinoma may be difficult to distinguish from olfactory neuroblastoma, neuroendocrine carcinoma, or other poorly differentiated, small round blue cell tumors of the sinonasal area.
The “small round blue cell tumors” (SRBCTs) constitute a heterogeneous group of malignant neoplasms characterized by a monotonous population of undifferentiated tumor cells with relatively small-sized nuclei and scant cytoplasm. An early and accurate diagnosis is imperative to allow a patient with a paranasal sinus or nasal cavity SRBCT to undergo the appropriate therapy. However, to affect a definitive diagnosis of a SRBCT based solely on the H & E light microscopic findings may be exceedingly difficult because of the frequent absence of distinguishing features. This diagnostic challenge may be further complicated if the pathologist is confronted with a biopsy of small or limited size. Ancillary studies to include immunohistochemical, ultrastructural, cytogenetic and molecular techniques may be used to aid in the differential diagnosis of SRBCTs.
In this review, emphasis was placed on a diagnostic approach to the differential diagnosis of small biopsy samples of SRBCTs of the sinonasal area. The group of sinonasal SRBCTs reviewed are listed in Table 1.
Table 1.
Clinicohistopathologic and genetic features of “small round cell tumors of the sinonasal area”
| Diagnosis | Age (years)/Location | Histopathology/Architecture | Mitotic activity/necrosis | Cytomorphology | Anaplasia or marked atypia | Cytogenetic/molecular | Immunohistochemistry |
|---|---|---|---|---|---|---|---|
| Malignant epithelial sinonasal small round blue cell tumors | |||||||
| Small cell carcinoma neuroendocrine type | 26–77/superior or posterior nasal cavity, maxillary, ethmoid sinuses | Sheets, ribbons, or nests of closely packed cells with nuclear molding | Frequent/common | Monotonous, small-sized cells with hyperchromatic nuclei, inconspicuous or absent nucleoli, and minimal cytoplasm | Variable | CD56 Cytokeratin (punctate perinuclear) Chromogranin (variable) NSE (variable) Synaptophysin (variable) TTF-1 (variable) |
|
| Sinonasal undifferentiated carcinoma | 20–80/nasal cavity, maxillary antrum, ethmoid sinuses, often with extension into adjacent sites | Tumor cells may be arranged in nests, lobules, trabeculae, or sheets | Frequent/common | Medium-sized nuclei with prominent nucleoli surrounded by scant eosinophilic cytoplasm | Common | No recurrent cytogenetic change No c-kit activating mutations or gene amplification |
Pankeratin CK7 CK8 CK19 Ki-67 (most cells, variable intensity) NSE (occasional) EMA (occasional) CD99 (rare) Synapthophysin (rare) S-100 protein (rare) Chromogranin (rare) |
| Squamous cell carcinoma (nonkeratinizing) | 55–65/maxillary sinus, nasal cavity, ethmoid sinus, sphenoid and frontal sinuses | Ribbons, nests, or strands; underlying tissue invasion often features well delineated border | Variable/limited | Poorly differentiated form most difficult to distinguish from other undifferentiated, small round cell tumors such as neuroendocrine carcinoma or olfactory neuroblastoma | Common | Pankeratin EMA CK5/6 CK8 CK13 CK14 CK19 |
|
| Neuroectodermal sinonasal small round blue cell tumors | |||||||
| Ewing sarcoma/primitive neuroectodermal tumor (PNET) | <30/maxillary sinus, nasal fossa | Sheets, lobules (less commonly cords or trabeculae may cause dx difficulty with carcinoid or undiff carcinoma) of uniformly round cells; ± Homer Wright rosettes | Variable/common | Small to intermediate sized cells with poorly defined, scant, or vacuolated cytoplasm and round nuclei with fine chromatin | Infrequent | t(11;22)(q24;q12) EWSR1-FLI1 (~95%) t(21;22)(q22;q12) EWSR1-ERG (~5%) Other EWSR1 or FUS variants (< 5%) |
CD99 (membranous pattern) Vimentin FLI1 NSE (variable) Synaptophysin (variable) AE1/AE3 and CAM5.2 (occasional) |
| Mucosal Malignant Melanoma | 40–70/nasal septum, paranasal sinuses (particularly maxillary) | Commonly deeply infiltrative with ulceration and frequent pseudopapillary architecture | Frequent/common | Amelanotic small round cell or larger melanotic epithelioid or spindle-shaped cells. Nuclear molding and/or prominent eosinophilic nucleoli may be present | Common |
CDKN2A/p16 (9p21) PTEN (10q23) 1q+,6p+,8q+ |
S-100 protein Vimentin HMB45 (usually) Melan-A (usually) Microphthalmia transcription factor (variable) Tyrosinase (variable) |
| Olfactory neuroblastoma | Broad age range (< 10 to > 80)/roof of nasal cavity, cribiform plate | Localized to submucosa; lobular to solid growth pattern in higher grade neoplasms. Rosettes (Homer Wright and Flexner-Wintersteiner) may be present | Variable/variable | Uniformly, small-sized cells with scant cytoplasm and round nuclei with fine to coarse granular chromatin and occasional small nucleoli (grade dependent) | Variable (more common in high-grade tumors) | Complex with aCGH studies demonstrating gain of 13q, 20q and loss of Xp as most frequent in high stage tumors | Neuron-specific enolase CD56 Synaptophysin (usually) S-100 protein (supporting sustentacular cells) CD57 (Leu7) (variable) Chromogranin (variable) GFAP (variable) Keratin (occasional) |
| Mesenchymal sinonasal small round blue cell tumors | |||||||
| Desmoplastic small round blue cell (DSRCT) | 15–35/sinonasal case reporta | Nests of undifferentiated cells embedded in a prominent desmoplastic stroma | Frequent/common | Small, round-oval cells with scant-moderate cytoplasm and hyperchromatic nuclei with inconspicuous nucleoli. Intracytoplasmic inclusions or vacuoles may be seen | Infrequent | t(11;22)(p13;q12) EWSR1-WT1 |
Desmin (perinuclear dot-like pattern) Pankeratin, EMA, AE1/AE3, CAM5.2 Vimentin WT1 NSE (usually) CD57 (usually) Synaptophysin (occasional) CD99 (occasional) |
| Rhabdomyosarcoma | <20 year/nasopharynx > sinonasal tract | Embryonal subtype (ERMS): alternating hyper- and hypocellular areas with myxoid or sparsely collagenized stroma Alveolar subtype (ARMS): collagenous fibrous septa separate nests of tumor cells with loss of central cohesion Solid ARMS: sheets of tumor cells without fibrous septa |
Variable/limited | Small round cells with scant cytoplasm ± scattered cells with eosinophilic cytoplasm and cross-striations | Common | ERMS: gain of all or portions of chromosomes 2, 7, 8, 11, 12, 13 and/or 20 with or without loss of 22 11p15 LOH ARMS: t(2;13)(q35;q14) PAX3-FOXO1 (50–60%) t(1;13)(p36;q14) PAX7-FOXO1 (~20%) Other PAX3 variants (<1%) Fusion neg. (20–30%) |
Desmin Myogenin (nuclear) myo-D1 (nuclear) Myoglobin (cytoplasmic) Vimentin (usually) CD56 (usually) Myosin (variable) |
| Synovial sarcoma (poorly differentiated) | <50/maxillary, sphenoid, ethmoid and frontal sinuses | Often solidly packed small round cells with richly vascular hemangiopericytoma like pattern; may be focal within a typical biphasic or monophasic synovial sarcoma or it may represent the predominant pattern. Other poorly differentiated forms include large (epithelioid) cell and high-grade spindle cell | Variable/variable | Poorly differentiated small cell pattern composed of small-sized cells with high nuclear to cytoplasmic ratios may be exceedingly difficult to distinguish from other small round cell tumors | Infrequent | t(X;18)(p11.2;q11.2) SYT-SSX1 or SYT-SSX2 (>99%) |
EMA BCL2 TLE1 Vimentin Cytokeratin (variable) CD99 (variable) S-100 (occasional) |
| Hematolymphoid sinonasal small round blue cell tumors | |||||||
| Extramedullary plasmacytoma | 35–75/nasal cavity, paranasal sinuses, nasal cavity | Diffuse infiltrate of uniform (well-differentiated) to pleomorphic (anaplastic) neoplastic plasma cells. Amyloid deposits (11–38%) | Variable/uncommon | Small to large (well to poorly differentiated) cells with fine to coarse nuclear chromatin and prominent nucleoli. Intracytoplasmic crystals, Dutcher bodies, and perinuclear hof may be present | Occasional | 14q32 (IGH) [although in contrast to multiple myeloma lacks the t(11;14)] −13 or 13q- |
Light chain restriction CD138 CD38 CD45 VS38 EMA (variable) CD79a (variable) CD31 (occasional) CD56 (occasional) |
| Extranodal NK/T cell lymphoma, nasal | 50–75/nasal cavity, paranasal sinuses, nasopharynx | Diffuse neoplastic lymphoid proliferation with angiocentric/angiodestructive growth pattern, mucosal ulceration, pseudoepitheliomatous hyperplasia and frequent associated inflammatory infiltrate | Frequent/common | Small or medium-sized cells to large transformed cells with round, oval. or irregular nuclei and azurophilic cytoplasmic granules | Common | del(6)(q21-25) i(6)(p10) EBV (ISH) 10% with T cell receptor gene rearrangement, no immunoglobulin light or heavy chain rearrangements |
CD2 CD3e (cytoplasmic) Granzyme B Perforin CD45 CD56 (cytoplasmic) (usually) TIA-1 (usually) |
aFinke et al. [18]
Epithelial SRBCTs of the Sinonasal Area
Poorly Differentiated, Nonkeratinizing Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) most frequently arises in the maxillary sinus, followed by the nasal cavity [2]. Analogous to other neoplasms originating in paranasal sinuses, the diagnosis is often delayed because the clinical symptoms are similar to those experienced with chronic sinusitis (nasal obstruction or congestion, facial pain, and swelling). In comparison, symptoms of nasal cavity SCC lead to earlier clinical recognition and detection of disease.
Histopathologically, SCC may be easily recognizable if it is of the well-differentiated and/or keratinizing form. Conversely, the poorly differentiated, nonkeratinizing variant may exhibit histopathologic features that overlap with other SRBCTs. Recognition of an in situ carcinoma component and/or direct continuity to the overlying surface epithelium of broad interconnecting or ramifying cords of neoplastic cells are features not typically identified in the other neoplastic entities presented in this review.
Immunohistochemically, cytokeratin immunoreactivity may be useful for distinguishing this neoplasm from olfactory neuroblastoma (ONB) and the lack of immunoreactivity for synaptophysin, chromogranin, and/or CD56 from small cell carcinoma neuroendocrine type (SCCNET). Franchi et al. [3] reported different cytokeratin staining patterns in keratinizing SCC, nonkeratizing SCC, and sinonasal undifferentiated carcinoma (SNUC). In contrast to both keratinizing and nonkeratinizing forms of SCC, SNUC was characterized by the exclusive expression of cytokeratins of simple epithelia, such as CK8, CK7, and CK19, Table 2. Moreover, SCC is almost always immunoreactive for EMA whereas less than 50% of SNUCs are EMA positive [4].
Table 2.
Cytokeratin Expression in Sinonasal Poorly Differentiated Squamous Cell Carcinoma (SCC), Nonkeratinizing Squamous Cell Carcinoma (NKSCC), and Sinonasal Undifferentiated Carcinoma (SNUC)
| CK4 | CK5/6 | CK7 | CK8 | CK10 | CK13 | CK14 | CK19 | |
|---|---|---|---|---|---|---|---|---|
| SCC | + (3/10) | + (9/10) | + (6/10) | + (9/10) | – | + (9/10) | + (8/10) | + (9/10) |
| NKSCC | – | + (9/10) | – | + (9/10) | – | + (8/10) | + (8/10) | + (9/10) |
| SNUC | – | – | + (3/6) | + (6/6) | – | – | – | + (3/6) |
From Franchi et al. [3]
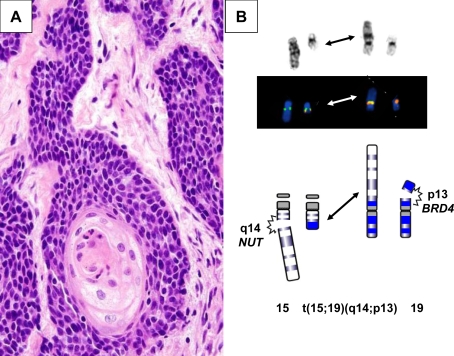
Recently, an undifferentiated carcinoma with focal squamous differentiation that may arise in the sinonasal area among other midline locations has been described in children and adults [5, 6]. This neoplasm, uniquely characterized by rearrangements of the NUT gene may show striking morphologic overlap with NUTwt SCC (Fig. 1a). The NUT (nuclear protein in testis) gene is localized to 15q14. In addition to molecular or cytogenetic identification of NUT or 15q14 rearrangements, respectively (Fig. 1b), nuclear expression of NUT can also be appreciated by immunohistochemistry in this neoplasm. Interestingly, the majority of the midline carcinomas with NUT rearrangements are associated with a t(15;19)(q14;p13) that gives rise to a BRD4-NUT chimeric oncogene. However, midline carcinomas fusing NUT with a gene partner other than BRD4 appear to have more prominent squamous differentiation, and these patients live fourfold longer when compared to patients with BDR4-NUT-positive tumors [5].
Fig. 1.
aNUT-rearranged midline carcinoma; monomorphic, poorly differentiated carcinoma with evidence of squamous differentiation. This neoplasm can mimic other undifferentiated neoplasms such as squamous cell carcinoma, Ewing’s sarcoma, and lymphoma. This particular patient initially received an erroneous diagnosis of squamous cell carcinoma. b Partial G-banded karyotype, FISH conducted with a custom-designed BRD4-NUT dual fusion probe set, and schematic illustrating the t(15;19)(q14;p13) identified in a subset of NUT-rearranged midline carcinomas
Sinonasal Undifferentiated Carcinoma (SNUC)
SNUC as defined by the World Health Organization is a rare, highly aggressive carcinoma that typically presents with locally extensive disease [2]. It is more common in men and the affected age range is broad (third to ninth decade). Although of uncertain histogenesis, SNUC does feature unique clinicopathologic characteristics permitting its segregation from other types of epithelial and nonepithelial neoplasms with small round cells.
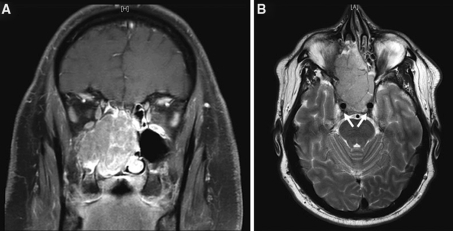
Clinically, SNUC patients characteristically develop symptoms of relatively short duration (weeks to months) and radiographically demonstrate large sinonasal lesions that are typically locally invasive with destruction of orbital and/or cranial bones, Fig. 2 [7]. Histopathologically, the tumor has a propensity to grow along the mucosal surface, extend into superficial mucosal glands, ulcerate, and invade lymphovascular spaces [2, 8]. Individual cells exhibit hyperchromatic to vesicular nuclei, poorly defined cell membranes, high nuclear-to-cytoplasmic ratios and often single, prominent nucleoli. Immunohistochemical stains are not particularly helpful in establishing a diagnosis of SNUC. The tumor cells are immunoreactive for pan-cytokeratins and simple keratins [2, 3]. SNUC lacks Epstein-Barr virus (EBV) RNA by in situ hybridization.
Fig. 2.
a and b Coronal and axial magnetic resonance images demonstrating a large SNUC mass filling both sides of the nasal cavity, bilateral ethmoid sinuses, and the right maxillary sinus in a 41-year-old man who presented with a 6-month history of nasal obstruction unresponsive to decongestants, steroids, or antibiotics. Arrows (part b) indicate carotid arteries with adjacent tumor involvement
Small Cell Carcinoma, Neuroendocrine Type (SCCNET)
SCCNET is a high-grade neoplasm that most frequently arises in the superior or posterior nasal cavity with frequent extension into the maxillary and/or ethmoid sinuses [2]. Sinonasal SCCNETs are composed of small to intermediate sized cells with oval or round hyperchromatic nuclei and absent or inconspicuous nucleoli. The tumor cells may be arranged in sheets, nests, and trabeculae and often exhibit extensive apoptosis, confluent necrosis, and hemorrhage. Crush artifact and a high mitotic rate are common [2, 9].
SCCNETs of the nasal cavity and paranasal sinuses display morphological and immunophenotypic features that are dissimilar to olfactory neuroblastoma and other sinonasal SRBCTs discussed in this review. Distinction is important because of the differences in prognosis and therapeutic approach. Most are positive for cytokeratin and may show the punctate perinuclear positivity typical of small cell carcinomas arising in other locations [9]. CD56 staining is common whereas staining for synaptophysin, chromogranin and NSE is variable [2]. EBV-RNA is negative by in situ hybridization.
Neuroectodermal SRBCTs of the Sinonasal Area
Olfactory Neuroblastoma
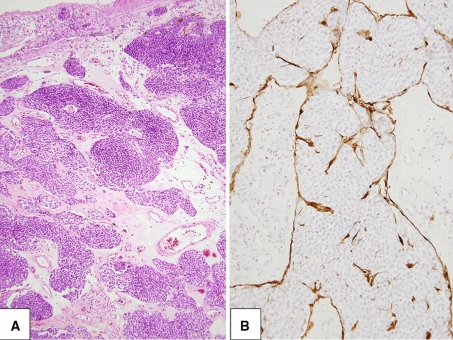
Olfactory neuroblastoma (ONB) is uncommon, accounting for only 1–5% of malignant nasal cavity neoplasms [10]. ONB is thought to originate from the olfactory portion of the mucous membrane lining the nasal fossa and is virtually confined to the upper nasal cavity in the region of the cribiform plate [11, 12]. Tumors such as SCCNET, melanoma, sinonasal lymphoma, and SNUC can develop in the same anatomical region as ONB and all can present with similar clinical, histological, and radiological features [10]. Even in the hands of an experienced pathologist, arriving at an accurate diagnosis may be challenging. Misdiagnoses are most commonly encountered in poorly sampled cases or with cases exhibiting extensive crush artifact or divergent differentiation [10, 13]. When present, features regarded as diagnostic of ONB and useful in distinguishing ONB from the aforementioned SRBCTs include the presence of fibrillary cell processes, Homer Wright rosettes, and S100-positive sustentacular cells, Fig. 3 [11, 13].
Fig. 3.
a Common to all grades of olfactory neuroblastoma is the lobular growth pattern. b Characteristic S100-positive sustentacular cells
Early cytogenetic studies that have subsequently been disproven suggested that ONB was a peripheral primitive neuroectodermal tumor family member. In contrast to pPNET, ONB is not immunoreactive for CD99. Most recently, array comparative genomic hybridization (aCGH) studies have shown complex gene copy number profiles with gain of 13q, 20q, and loss of Xp as most frequent in high stage tumors [14].
Sinonasal Mucosal Malignant Melanoma
The histologic appearance of sinonasal malignant melanomas may be variable; however, it is the amelanotic variant with small cell morphology that may be especially prone to misclassification, resulting in inappropriate clinical management [15]. Malignant melanoma occurs within a wide age range and arises more commonly in the nasal cavity than in the paranasal sinuses [2, 15]. Most are grossly polypoid and approximately half will demonstrate a brown or black pigmented surface [10]. Diffuse staining for S-100, HMB45, and vimentin as well as the light microscopic appearance of detectable melanin pigment (the latter observed in approximately 2/3 of cases) is useful in distinguishing this high-grade malignancy [2, 10, 15].
Extraskeletal Ewing’s sarcoma/Primitive Neuroectodermal Tumor
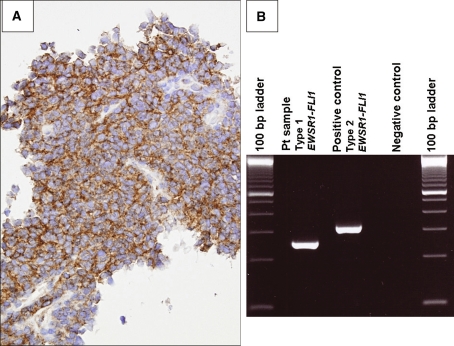
Similar to ONB, Ewing’s sarcoma/peripheral Primitive Neuroectodermal Tumor (ES/pPNET) usually affects a younger patient. The morphologic and immunophenotypic diversity of ES/pPNET may create diagnostic difficulties [16]. For example, some ES/pPNETs exhibit histological, immunohistochemical, and ultrastructural epithelial features [17]. Others may demonstrate staining for neuroendocrine markers. With the exception of the rare sinonasal desmoplastic small round cell tumor [18], synovial sarcoma [10], or lymphoma [19] CD99 immunoreactivity is useful in distinguishing ES/pPNET from most other sinonasal SRBCTs (Fig. 4a). Identification of the characteristic t(11;22)(q24;q12) or EWSR1-FLI1 fusion transcript or defined variant translocation can be invaluable in confirming this diagnostic entity (Fig. 4b).
Fig. 4.
a Characteristic membranous CD99 immunoreactivity in a maxillary sinus extraskeletal Ewing’s sarcoma. b The pathognomonic EWSR1-FLI1 fusion transcript identified by RT-PCR analysis
Mesenchymal SRBCTs of the Sinonasal Area
Desmoplastic Small Round Cell Tumor
Notably a single case of sinonasal desmoplastic small round cell tumor (DSRCT) has been reported [18]. The importance of this remarkably unusual presentation could come into play if the pathologist is presented with a biopsy of limited size as this entity may exhibit overlapping histological and immunohistological features with other sinonasal SRBCTs. Recognition of the multidirectional immunohistochemical differentiation pattern and the tumor-specific genetic findings [t(11;22)(p13;q12) and associated EWSR1-WT1 fusion transcript] are necessary for definitive diagnosis.
Rhabdomyosarcoma
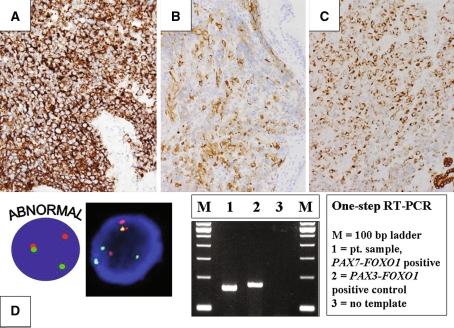
Both embryonal and alveolar rhabdomyosarcoma (ERMS and ARMS, respectively) frequently occur in the nasal cavity and sinuses [2]. Although these entities usually predominate in children and young adults, a recent study has illustrated ARMS involvement in older adults (median age, 61 years) [20]. In this study, establishing the diagnosis of ARMS was complicated by its rarity in this age group, lack of an alveolar pattern, and initially misleading immunoprofile (CD56, cytokeratin, and synaptophysin immunoreactivity with lack of inclusion of myogenic markers in the first set of immunohistochemical markers assessed), Fig. 5a and b. In addition to synaptophysin expression, some ARMSs also show keratin expression that may lead to a potential misdiagnosis of SCCNET, SNUC, or ONB [21]. Distinguishing ERMS and solid pattern ARMS may be exceedingly difficult without the use of genetic approaches for the identification of the 2;13 and 1;13 translocations or respective PAX3-FOXO1 and PAX7-FOXO1 fusion transcripts, Fig. 5c. Recently, PAX3 variant translocations that create a chimeric oncogene with one of the NCOA family members in place of FOXO1 have been described in RMS cases, including a skull-based lesion [22].
Fig. 5.
a CD56 immunostaining in a solid variant ARMS of the ethmoid sinus. b and c Synaptophysin and cytokeratin immunostaining, respectively in an ARMS of the nasopharynx. dSplit red and green signals indicative of a FOXO1 rearrangement characteristic of ARMS are identified by interphase FISH analysis with a FOXO1 breakapart probe (bottom left). PAX7-FOXO1 and PAX3-FOXO1 fusion transcripts identified by RT-PCR analysis (bottom right)
Poorly Differentiated Synovial Sarcoma
The head and neck region is the second most common site of involvement for synovial sarcoma, although cases arising in the sinonasal tract are rare [10, 23]. SS has previously been described in the frontal, maxillary, ethmoid, and sphenoid sinuses [23]. SS causes the greatest diagnostic difficulties with other sinonasal tumors when it is poorly differentiated with small cell morphology. In this setting, cytokeratin, EMA, BCL2, and TLE1 immunoreactivity coupled with cytogenetic, FISH, or molecular confirmation of the t(X;18)(p11.2;q11.2) or derived SYT-SSX chimeric transcripts facilitates an absolute diagnosis.
Hematolymphoid SRBCTs of the Sinonasal Area
Extramedullary Plasmacytoma
Extramedullary plasmacytoma (EMP) chiefly affects adults older than 65 years of age and involves the sinonasal/nasopharyngeal area in 75% of the cases [24]. Poorly differentiated tumors (immature plasma cells) are the most difficult to recognize and may result in diagnostic confusion with other sinonasal SRBCTs. For example, due to occasional EMA or cytokeratin positivity, EMP may be misdiagnosed as a carcinoma. Diagnostic confirmation can be achieved by immunohistochemistry or in situ hybridization for immunoglobulin mRNA with identification of light chain restriction [2].
Extranodal NK/T Cell Lymphoma (Nasal-Type)
The second most common malignancy of the sinonasal tract following SCC is malignant lymphoma; particularly extranodal NK/T cell lymphoma which frequently affects Asian and Latin American populations [13]. Necrosis, vascular invasion/destruction, and pseudoepitheliomatous hyperplasia are common. The latter may be mistaken for well-differentiated SCC. Because extranodal NK/T cell lymphoma may also be accompanied by a significant inflammatory infiltrate, it can also be confused Wegener’s granulomatosis and infectious disorders [25]. Demonstration of the EBV virus (present in nearly all cases) by in situ hybridization for EBV-encoded early RNAs (EBER) in addition to a NK-cell immunophenotype (CD2+, CD3−, CD3e+, CD56+, CD43+, perforin and granzyme B+) is indicative of this aggressive malignancy.
Conclusions
Malignancies arising in the nasal cavity and paranasal sinuses are heterogeneous. Accurate classification of sinonasal SRBCTs may be challenging due to overlapping clinical, radiographic and/or histopathologic features. Diagnosis may be further complicated if the biopsy material is limited in size or is of suboptimal quality. Advances in immunohistochemistry and molecular genetics have greatly assisted in these difficult differential diagnoses. Establishing a precise diagnosis is important not only in determining how aggressive the tumor might be, but is especially critical in identifying the type of treatment needed. Moreover, a careful review with the treating clinician is vital in further enhancing accuracy.
Acknowledgments
The authors would like to thank Cheryl Putnam and Kimberly Christian for their secretarial assistance. The authors would like to also thank Drs. Rodney McComb, Sonny Johansson, William West, Douglas Gnepp, and Dennis Weisenburger for their contributions and suggestions.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Barnes L, Everson JW, Reichart P, Sidransky D (eds) (2005) Pathology and genetics of head and neck tumours. Kleihues P, Sobin LH, series eds. World health organization classification of tumours. Lyon, France: IARC Press.
- 3.Franchi A, Moroni M, Massi D, et al. Sinonasal undifferentiated carcinoma, nasopharyngeal-type undifferentiated carcinoma, and keratinizing and nonkeratinizing squamous cell carcinoma express different cytokeratin patterns. Am J Surg Pathol. 2002;26:1597–1604. doi: 10.1097/00000478-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cerelli LA, Holst VA, Brandwein MS, et al. Sinonasal undifferentiated carcinoma: Immunohistochemical profile and lack of EBV association. Am J Surg Pathol. 2001;25:156–163. doi: 10.1097/00000478-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 5.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 6.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 7.Ejaz A, Wenig BM. Sinonasal undifferentiated carcinoma. Clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv Anat Pathol. 2005;12:134–143. doi: 10.1097/01.pap.0000163958.29032.56. [DOI] [PubMed] [Google Scholar]
- 8.Robinson RA, editor. Head and neck pathology. Atlas for histologic and cytologic diagnosis. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 9.Perez-Ordonez B, Caruana SM, Huvos AG, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol. 1998;29:826–832. doi: 10.1016/S0046-8177(98)90452-X. [DOI] [PubMed] [Google Scholar]
- 10.Iezzoni JC, Mills SE. “Undifferentiated” small round cell tumors of the sinonasal tract. Am J Clin Pathol. 2005;124:S110–S121. doi: 10.1309/59RBT2RK6LQE4YHB. [DOI] [PubMed] [Google Scholar]
- 11.Cohen ZR, Marmor E, Fuller GN, et al. Misdiagnosis of olfactory neuroblastoma. Neurosurg Focus. 2002;12:1–6. doi: 10.3171/foc.2002.12.5.4. [DOI] [PubMed] [Google Scholar]
- 12.Mills SE, Frierson HF., Jr Olfactory neuroblastoma. A clinicopathologic study of 21 cases. Am J Surg Pathol. 1985;9:317–327. doi: 10.1097/00000478-198505000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Wenig BM. Undifferentiated malignant neoplasms of the sinonasal tract. Arch Pathol Lab Med. 2009;133:699–712. doi: 10.5858/133.5.699. [DOI] [PubMed] [Google Scholar]
- 14.Faragalla H, Weinreb I. Olfactory neuroblastoma. A review and update. Adv Anat Pathol. 2009;16:322–331. doi: 10.1097/PAP.0b013e3181b544cf. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LDR, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas. A clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Folpe AL, Goldblum JR, Rubin BP, et al. Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol. 2005;29:1025–1033. [PubMed] [Google Scholar]
- 17.Bridge JA, Fidler ME, Neff JR, et al. Adamantinoma-like Ewing’s sarcoma: genomic confirmation, phenotypic drift. Am J Surg Pathol. 1999;23:159–165. doi: 10.1097/00000478-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Finke NM, Lae ME, Lloyd RV, et al. Sinonasal desmoplastic small round cell tumor. A case report. Am J Surg Pathol. 2002;26:799–803. doi: 10.1097/00000478-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Sung CO, Ko YH, Park S, et al. Immunoreactivity of CD99 in non-Hodgkin’s lymphoma: unexpected frequent expression in ALK-positive anaplastic large cell lymphoma. J Korean Med Sci. 2005;20:952–956. doi: 10.3346/jkms.2005.20.6.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuda T, Perry KD, Nelson M, et al. Alveolar rhabdomyosarcoma of the head and neck region in older adults: genetic characterization and a review of the literature. Hum Pathol. 2009;40:341–348. doi: 10.1016/j.humpath.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahrami A, Gown AM, Baird GS, et al. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21:795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 22.Sumegi J, Streblow R, Frayer RW, et al. Recurrent t(2;2)(p23;q35) and t(2;8)(q35;q13) translocations, in ARMS subset without the canonical PAX3-FKHR juxtaposition, fuse PAX3 to members of the nuclear receptor transcriptional co-activator (NCOA) family of genes. Genes Chromosomes Cancer. 2009; in press. [DOI] [PMC free article] [PubMed]
- 23.Gallia GL, Sciubba DM, Hann CL, et al. Synovial sarcoma of the frontal sinus. J Neurosurg. 2005;103:1077–1080. doi: 10.3171/jns.2005.103.6.1077. [DOI] [PubMed] [Google Scholar]
- 24.Wax MK, Yun KJ, Omar RA. Extramedullary plasmacytoma of the head and neck. Otolaryngol Head Neck Surg. 1993;109:877–885. doi: 10.1177/019459989310900517. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe ES. Lymphoid lesions of the head and neck: a model of lymphocyte homing and lymphomagenesis. Mod Pathol. 2002;15:255–263. doi: 10.1038/modpathol.3880521. [DOI] [PubMed] [Google Scholar]