Abstract
Dendritic cells (DCs) are potent antigen-presenting cells and play a central role in the initiation and regulation of primary immune responses. Therefore, their use for the active immunotherapy against cancers has been studied with considerable interest. The fusion of DCs with whole tumor cells represents in many ways an ideal approach to deliver, process, and subsequently present a broad array of tumor-associated antigens, including those yet to be unidentified, in the context of DCs-derived costimulatory molecules. DCs/tumor fusion vaccine stimulates potent antitumor immunity in the animal tumor models. In the human studies, T cells stimulated by DC/tumor fusion cells are effective in lysis of tumor cells that are used as the fusion partner. In the clinical trials, clinical and immunological responses were observed in patients with advanced stage of malignant tumors after being vaccinated with DC/tumor fusion cells, although the antitumor effect is not as vigorous as in the animal tumor models. This review summarizes recent advances in concepts and techniques that are providing new impulses to DCs/tumor fusions-based cancer vaccination.
1. Cancer Vaccine
Cancer vaccine is treatment that enhances the patient's own immune system. The antigen-presenting cells (APCs) most suitable for cancer vaccine are dendritic cells (DCs), which can be distinguished from B cells and macrophages by their abundant expression of costimulatory molecules and ability to initiate a strong primary immune response [1]. A major area of investigation in cancer vaccine involves the design of DC-based cancer vaccines [2]. DCs are specialized to capture and process tumor-associated antigens (TAAs), converting the proteins to peptides that are presented on major histocompatibility complex (MHC) class I and class II molecules. DCs then migrate to T-cell areas of secondary lymphoid organs and become competent to present antigens to T cells, thus initiating antigen-specific immune responses [1, 2]. Ex vivo generated, antigen-loaded DCs have been used as vaccines to improve immunity. But there is considerable controversy as to which forms of antigen loading are most effective. Different strategies have been developed to load DCs with TAAs, including synthetic peptides derived from the known antigens [3], tumor lysates [4], tumor RNA [5], and dying tumor cells [6] to induce antigen-specific immune responses. These DCs have been concomitantly treated with conditioning factors such as a standard mixture of cytokines (TNF-a, IL-1β, IL-6, and PGE2) [7] or Toll-like receptors (TLRs) [8] that induce DC maturation, thus converting them into potent APCs. The immunogenicity of antigens delivered by DCs has been shown in patients with cancer [4]. Although clinical trials have demonstrated immunological responses after vaccination with DCs loaded with tumor specific peptides, the efficacy of therapeutic vaccination against cancer has recently been questioned because of the undeniably limited rate of objective tumor regressions that has been observed in clinical trials [9]. However, several aspects of DC vaccination require optimization to improve clinical responses including the facilitation of innate and adaptive interactions and reduction of regulatory T cell (Treg) networks or suppressive tumor-microenvironments that inhibit the function of antitumor immune responses [10].
2. Antitumor Immunity by DCs/Tumor Fusions in Animal Model
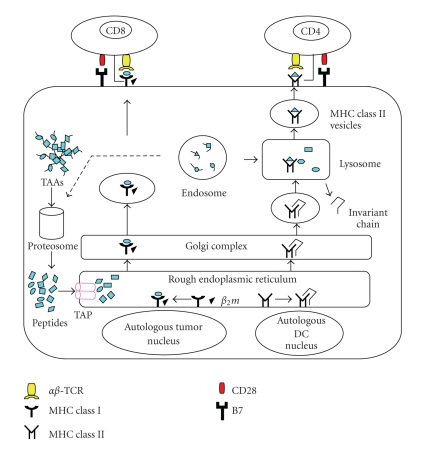
Effective delivery of antigens into DCs is an important aspect for clinical trials. Vaccination with DCs loaded with tumor specific peptides has been used [3, 4, 11]. However, a major drawback of this strategy comes from a limited number of known tumor peptides available in many HLA contexts and the potential evasion of immunological targeting through downregulation of their antigens. To solve this problem, an alternative approach has been developed by fusing DCs with tumor cells [12]. In this approach, a broad spectrum of TAAs, including those known and unidentified, can be fully presented on MHC class I and class II molecules in the context of costimulatory molecules (Figure 1) [13, 14].
Figure 1.
Fusions of autologous DCs and autologous tumor cells. The DCs/tumor fusion cells express MHC class I and class II and costimulatory molecules as well as tumor-associated antigens. The fusions are able to process tumor-derived peptides and MHC class I peptides derived from DCs. They form MHC class I-peptide complexes, in the endoplasmic reticulum, which are transported to the surface and presented to CD8+ T cells. Similarly, the fusion cells can also synthesize MHC class II peptides derived from DCs in the endoplasmic reticulum, which are transported to the cytoplasm where MHC class II-peptide complexes are assembled with tumor-derived peptides. These complexes are presented to CD4+ T cells, which are important for efficient CTL induction.
The fusion of syngeneic DCs and tumor cells creates a heterokaryon with both tumor-derived antigens and DCs-derived MHC class II costimulatory molecules (B7.1 and B7.2), intracellular adhesion molecule- (ICAM-) 1, lymphocyte function-associated antigen- (LFA-) 1 and -3, and CD40, all of which are efficient antigen-processing and presentation machinery [15, 16]. Ex vivo generated DCs can be fused with whole tumor cells and reinfused to the patients [17], or they can be used for ex vivo induction and expansion of cytotoxic T lymphocytes (CTLs) [18, 19]. Indeed, DCs/tumor fusion cell vaccines have been shown to possess the elements essential for processing and presenting tumor antigens to host immune cells for inducing effective antitumor immune response and for breaking T-cell tolerance to tumor-associated antigens in animal models [12, 20]. Many animal studies have demonstrated that the DCs/tumor fusion vaccine not only provided protection against challenge with tumor cells, but also regressed established tumors, including melanoma [21–27], colorectal [12–14, 18, 28–35], breast [36–39], esophageal [40], pancreatic [41], hepatocellular [42–46], lung [47, 48], laryngeal [49], renal cell carcinoma [50], sarcoma [51–53], myeloma [54–59], mastocytoma [60], and neuroblastoma [61].
In our initial study on DCs/tumor fusion cell vaccines, murine MC38 adenocarcinoma cells stably transfected with human MUC1 (MC38/MUC1) were fused to synergistic DCs derived from bone marrow in the presence of polyethylene glycol (PEG). MUC1 that is a high-molecular-weight glycoprotein that is overexpressed in breast, ovarian, and pancreatic adenocarcinomas [62] represents a potential target for active specific immunotherapy against certain human tumors [5]. We have used MUC1-transgenic (MUC1. Tg) mice that express MUC1 as a self-protein on normal ductal epithelial cells. Because MUC1.Tg mice that express MUC1 in a pattern and at a level similar to that found in humans are unresponsive to MUC1 antigen, these mice provide a potential model to assess the induction of anti-MUC1 immune responses [63]. Vaccination of wild-type mice with MUC1 RNA-transfected DCs (DCs/MUC1 RNA) induced anti-MUC1 immune responses against MUC1-positive MC38/MUC1, but not MUC1-negative tumor cells. In contrast, there is little if any anti-MUC1 immunity induced with the DCs/MUC1 RNA in MUC1.Tg mice [5]. Interestingly, vaccination with fusions of DCs and MC38/MUC1 tumor cells induced effective cellular and humoral immunity against MUC1 antigen in MUC1.Tg mice. The fusion vaccine provided protection against challenge with MUC1-positive tumor cells and mediated regression of established tumors in the mice. These findings indicate that vaccination with the fusion cells can induce CTLs against MUC1 and thereby reverse tolerance to human MUC1 antigen [20]. Therefore, DCs/tumor fusion-based vaccine may represent an effective strategy to induce antitumor immunity, including MUC1-positive tumor cells.
Although the transplantable tumor models have been contributed as the primary screening tools for cancer vaccine development, they do not fit this criterion since the tumor in these models grows very quickly, without the multiple stages of cancer development found in human cancers. On the other hand, genetically modified mice with spontaneous development of carcinoma provide a powerful tool to study the efficacy of tumor vaccines, since they mimic cancer development in humans. We have used a double transgenic mouse model expressing polyomavirus middle T oncogene and human MUC1 as self-antigen to determine the preventive effect of a DCs/tumor fusion cell vaccine. Even in the genetically altered model of spontaneous breast cancer, vaccination with DCs/tumor fusion cells conferred sufficient antitumor immunity to block or delay mammary tumor development [36, 37].
3. Antigen Presentation and Processing by DCs/Tumor Fusions
Immature DCs display a characteristic phenotype with high expression levels of MHC class I, class II, costimulatory molecules (CD80 and CD86), low levels of the maturation marker, CD83, but not TAAs. As compared with immature DCs, mature DCs expressed much higher levels of HLA-DR, CD80, CD86, and CD83. By contrast, almost all of tumors expressed an abundance of TAAs, MHC class I, but not MHC class II and costimulatory molecules. Fusion of DCs and tumor cells resulted in the formation of a heterokaryon that combined DC-derived MHC class I, class II, and costimulatory molecules, efficient antigen-processing and presentation machinery, and an abundance of tumor-derived MHC class I and antigens [12]. After fusion, the cytoplasm of DCs and tumor cells was integrated into one entry, whereas their nuclei remained separate entities [64]. Such a structure may make it possible to maintain the function of both original live cells (DCs and tumor cells), at least in part, including synthesis of TAAs, MHC class I, class II, and costimulatory molecules. Moreover, the DCs/tumor fusions also delivered not only proteins but also mRNA encoding the whole TAAs from tumor cells. The DCs/tumor fusions approach facilitates the entry of TAAs that are synthesized de novo in the fusions into the endogenous antigen-processing pathway of the DCs. Thus, the TAAs can be processed and presented through both MHC class I and class II pathways on the DCs in the context of costimulatory molecules [12]. The advantage of DCs/tumor fusion vaccine over pulsing DCs with tumor lysates is that endogenously synthesized antigens have better access to MHC class I pathway [65]. In animal studies, fusion vaccine was superior to DCs loaded with antigenic protein or peptide, tumor cell lysates, or irradiated tumor cells [25]. In human DCs/tumor fusions, it has been also demonstrated that the tumor antigens were processed through the endogenous pathway of DCs after fusion and T cells primed by the fusions were high quality antigen-specific cells, capable of mediating lysis of tumor targets [66]. In our reports, we have created hybrid cells by fusing autologous DCs and allogeneic tumor cell lines that did not express same MHC class I molecules as autologous DCs [67, 68]. These fusions expressed both MHC class I- and class II-restricted tumor-associated epitopes through the cross-priming.
4. Activation of Antigen-Specific CD4+ and CD8+ T Cells by DCs/Tumor Fusions
DCs reside at the port of entry, take up exogenous antigens, and migrate to draining lymph nodes, where the antigens are presented to CD4+ T cells through MHC class II pathways. In addition, DCs are capable of initiating CD8+ T cell response through a cross-presentation pathway [69, 70]. For cancer vaccination, the goal is to generate antigen-loaded DCs that efficiently stimulate robust and long-lasting CD4+ and CD8+ T cell responses in the patient with cancer, with the emphasis on “long-lasting” [71]. Importantly, vaccination with the fusion cells is associated with activation of antigen-specific CD4+ and CD8+ T cells [14, 19, 64]. To dissect the role of MHC class I- or class II-restricted antigen-specific T cell activation by the fusions, we have created various types of DCs/tumor fusion cells by alternating fusion cell partners using three kinds of knockout mice and wild type mice: (1) wild type fusions (WT-FCs), (2) MHC class I knockout fusions (IKO-FCs), (3) MHC class II knockout fusions (IIKO-FCs), and (4) MHC class I and class II double-knockout fusions (I/IIKO-FCs) [72]. In this study, immunization of MUC1.Tg mice with WT-FCs, IKO-FCs, IIKO-FCs, or I/IIKO-FCs provided 100%, 76.6%, 61.5%, and 15.4% protection, respectively, against tumor challenge with MC38/MUC1 tumor cells. This study has demonstrated that MHC class II antigen presentation targeting activation of CD4+ T cells was indispensable in antitumor immunity. The lower antitumor immunity by IIKO-FCs may be due to lack of help from MHC class II-restricted CD4+ T cells in the priming phase, whereas the induction of antitumor immunity by IKO-FCs is through cross-priming by the host DCs. The results suggest a novel mechanism of antitumor immunity mediated by CD4+ T cells [72]. Previously, much research has been devoted to the significance of CD8+ T cells, given the fact that most tumors express only MHC class I molecules and predominant effector cells are CD8+ CTLs. There is increasing evidence, however, that CD4+ T cells play a more direct role, beyond delivery of assistance in the generation of antitumor immunity [73]. In the priming phase, CD4+ T cells activate APCs through the interaction between CD40L and CD40, respectively, so that the educated APCs acquire the capacity to stimulate CD8+ T cells [74]. CD4+ T cells also function to maintain the numbers and cytotoxic capacity of CD8+ T cells and promote the infiltration of CD8+ T cells into tumors [75]. Through cross-priming, the fusion cells can activate antigen-specific CD4+ T cells that become multifunctional effectors producing IL-2, IFN-γ, IL-4, and IL-10 [14, 19, 64]. Moreover, the fusion cells also can function like APCs with the ability to migrate to draining lymph nodes, where they reside in the T cell area, interact with CD4+ and CD8+ T cells, and induce potent antitumor immunity [14, 27]. Both direct stimulation and cross-priming by host APCs participate in CD4+ and CD8+ T-cell activation by the fusion cells [14].
5. Antigen-Specific Polyclonal CTL Responses Induced by DCs/Tumor Fusions
The priming and expansion of polyclonal CTLs by vaccines has potential in vaccine applications for cancer. The goal of the vaccines is to prime the patient's own immune system to recognize and destroy the tumor without harming normal cells. Cancer vaccines that rely on induction of antitumor immunity against a single antigen are potentially subject to tumor-cell resistance mediated by downregulation of the single antigen. Therefore, antigen-specific polyclonal CTL responses have the potential to maximize the protection against various subsets of tumor cells with down regulation of certain tumor antigens, which may appear during the course of tumor progression. DCs/tumor fusions are potent inducers of antigen-specific polyclonal CD4+ T cells, which are essential for the induction of augmented polyclonal CTL responses against autologous tumor cells. Preclinical human studies have demonstrated that the fusions could induce antigens (CEA, MUC1, and WT1) specific CTLs simultaneously in HLA-A2- and/or -A24-restrictive elements in vitro [10, 19, 67, 76, 77]. Moreover, administration of the polyclonal CTLs could regress tumors in SCID mice and render mice free of disease up to the end of experiment [68]. In addition, DC/tumor fusion cells could be efficiently frozen without loss of either antigen presentation potency or T cell stimulatory capacity inducing polyclonal CTL responses [76]. The cryopreserved DC/tumor fusion cells have potential applicability in the field of antitumor immunotherapy and provide a platform for adoptive immunotherapy in the clinical setting.
6. Generation of Regulatory T Cells (Treg) by DCs/Tumor Fusions
Prevailing paradigms stipulate linear differentiation programs driving T cell lineage commitment, beginning with naive T cells that become Th1, Th2, Tregs, or Th17 depending on the cytokine milieu, where the T cells encounter at the time of antigenic stimulation. The presence of IL-12 causes naive T cells to differentiate into Th1 cells; IL-4 drives naive T cells to become Th2 cells; TGF-β drives them to become Tregs, and TGF-β, together with IL-6 and IL-21, promotes Th17 cell development [78, 79]. The cytokine milieu is associated with activated DCs, tumors, cancer-associated fibroblasts (CAF), or tumor associated macrophages (TAM). The control of immune-balance is essential for the cancer therapy. There is increasing evidence that DCs in situ induce antigen-specific unresponsiveness or tolerance in central lymphoid organs and in the periphery. The presentation of antigens to CD4+ or CD8+ T cells by immature or partially mature DCs results in tolerance [80] or induction of regulatory CD4+ and CD8+ T cells [81]. Tumors express or induce immunosuppressive cytokines such as TGF-β and IL-10. As a result, tumor-antigen cross-presentation by DCs induces T cell anergy or deletion and Treg instead of antitumor immunity [82]. Indeed, it has been reported that tumor progression correlated with an accumulation of immature DCs that induced the expansion of Tregs in lymphoid organs of tumor-bearing hosts [83]. Recently it has become possible to define Tregs on the basis of their expression of the transcription factor forkhead box protein 3 (Foxp3) [84]. Tregs have been shown to exert their effects through the activities of TGF-β [85], IL-10 [86], CTLA-4 [87], or through accumulation of IL-2 via expression of CD25 [88]. Tumor-derived TGF-β reduced the efficacy of DCs/tumor fusion vaccine via an in vivo mechanism [28]. The blockade of tumor-derived TGF-β reduced Tregs induction by the DCs/tumor fusions vaccine and enhanced antitumor immunity [38]. We have reported that the supernatant from human hepatocellular carcinoma (HCC) cells induced functional impairment of DCs as demonstrated by the downregulation of MHC class I and class II, CD80, CD86, and CD83 molecules [10]. Moreover, DCs exposed to the culture supernatants from HCC cells secreting TGF-β failed to undergo full maturation upon stimulation of TLR 4 agonist. Importantly, fusions of DCs and HCC cells generated in the presence of the culture supernatants from HCC cells promoted the generation of CD4+ CD25high Foxp3+ Treg and inhibited CTL induction. It has been demonstrated that CAF and TAM synthesized proteins, such as VEGF, TGF-β, and IL-10, all of which contributed to the local immunosuppressive environment [89, 90]. A major obstacle to the development of any active immunotherapeutic approach to cancer is the immunosuppressive environment by the growing tumor. Therefore, a combination of control of Treg and concomitant induction of efficient polyclonal CTLs may be a more effective immunotherapy to reduce recurrence and prolong survival.
7. Modified Fusions of DCs and Tumor Cells
While DCs/tumor fusions approach has been developed in animal studies, many adjuvants, including IL-2, IL-12, IL-18, and synthetic oligodeoxynucleotides (ODNs) containing specific bacterial unmethylated CpG motifs (CpG ODNs), have been used to enhance the ability of DC/tumor fusion vaccines to evoke antitumor immune responses [26, 29, 31, 54, 61]. These results suggest that the fusion vaccine needs to be modified to enhance antitumor immunity. The biggest advantage in DCs/tumor fusion strategy is that modifications of DCs as well as tumor cells are independently possible while their characters persist after the fusion. This is an important difference between the DCs/tumor fusion strategy and whole tumor lysates loading strategy. Therefore, the therapeutic efficacy of a vaccine requires the improved immunogenicity of both DCs and tumor cells. In the absence of proper costimulation, antigen presentation by DCs induces tolerance [1]. In particular, recent studies suggest that Toll-like receptor- (TLR-) agonist CpG ODNs or conserved pathogen-associated molecular patterns, such as penicillin-killed Streptococcus pyogenes (OK-432), start the DC maturation process, which is a critical event in the induction of full effector function in T cells. The DCs stimulated with the TLR agonist, OK-432 (OK-DCs), show higher expression levels of MHC class I and class II, CD80, CD86, CD83, IL-12, and heat shock proteins (HSPs) than do immature DCs [76]. On the other hand, the immunogenicity of tumor cells can be improved by heat-treatment [91]. Heat-treated autologous tumor cells display a characteristic phenotype with increased expression of HSPs, carcinoembryonic antigen (CEA), MUC1, and MHC class I [77]. Intracellular HSPs play an important role as molecular chaperones in cellular protein-folding pathways [92]. Moreover, cross-priming is based on the transfer of proteasome substrates that are transcriptionally upregulated by heat treatment in human tumor cells [91, 93]. In contrast, extracellular HSPs act as chaperon peptides and interact with DCs in a receptor-mediated manner, leading to maturation as well as proinflammatory responses [91, 94], all of which are likely to be key danger signals to the antitumor immune system. Therefore, we have created fusions of OK-DCs and heat-treated tumor cells to elicit potent antitumor responses. The modified fusions show to be active as demonstrated by (1) up-regulation of multiple HSPs, MHC class I and class II, CEA, CD80, CD86, CD83, and IL-12; (2) activation of CD4+ and CD8+ T cells able to produce IFN-γ at higher levels; (3) efficient induction of antigen-specific polyclonal CTL activity against tumor targets; and (4) superior abilities to induce CD107+ IFN-γ + CD8+ T cells and CD154+ IFN-γ + CD4+ T cells. These fusions may provide a promising means of inducing therapeutic antitumor immunity.
8. From Autologous to Allogeneic Tumor Cells for DCs/Tumor Fusions
As a fusion partner, the advantage of using autologous tumor cells is their possession of all the relevant TAAs required for mounting effective antitumor immunity. However, in the clinical setting of the patients with cancer, a major difficulty for the DCs/tumor fusion vaccine is the preparation of sufficient amounts of autologous tumor cells because of both the availability of limited tumor samples and the difficulty in culturing tumor cells. It has been reported that hybrid cells generated by fusing DC from healthy donor with allogeneic tumor cell line have induced CTL responses against the allogeneic tumor cells used for fusion [95, 96]. The basis for using allogeneic tumor cell lines instead of autologous tumor cells is that some antigens are shared by most of tumors. We have reported that fusions generated by autologous DCs and allogeneic tumor cell lines can induce antigen-specific polyclonal CTLs with cytotoxic activity against autologous tumor cells (Figure 2) [67, 68]. This strategy has numerous advantages. (a) Allogeneic tumor cell lines are well characterized as TAA source. (b) Allogeneic tumor cell lines, which shared with TAAs, can grow well in vitro; thus, there is no limiting factor for preparation of tumor cells. (c) It is not necessary to determine HLA typing of patients and allogeneic tumor cells as a partner of fusion cells, because autologous dendritic cells can process and present multiple TAAs from allogeneic tumor cells in the context of MHC class I and class II. Indeed, allogeneic tumor cells (melanoma and prostate cancer), transduced with granulocyte-macrophage colony-stimulating factor (GM-CSF), have been applied clinically and shown to induce antitumor immunity [97, 98]. In this trial, whole allogeneic tumor cells were genetically modified to secrete the immune stimulatory cytokine, GM-CSF, and then irradiated to prevent further cell division. After phase III trials evaluating an allogeneic GVAX immunotherapy in prostate cancer were finished, the trials have been suspended. While currently explored allogeneic approaches in whole tumor cell-based vaccination procedures represent an improvement in terms of standardization over their autologous counterparts, they nevertheless entail the culture of large batches of cells under good manufacturing practice (GMP) grade conditions [99]. Further optimization of these in vitro culture methodologies is required. A major challenge to develop an allogeneic tumor cell-based vaccine strategy is to overcome the potential hazards of fetal calf serum (FCS) that limit safety in clinical trials.
Figure 2.
Fusions of autologous DCs and allogeneic tumor cells (DCs/allo-tumor). The DCs/allo-tumor can stimulate both CD4+ and CD8+ T cells as same as fusions of autologous DCs and autologous DCs (DCs/auto-tumor). Moreover, the DCs/allo-tumor can also stimulate alloreactive T cells due to the presence of allogeneic HLA class I molecules from allogeneic tumor cells. Autologous MHC molecules present foreign peptide derived from allogeneic tumor cells to T cell selected to recognize self MHC-foreign peptide complexes. In addition, T cell also can recognize an allogeneic MHC molecule whose structure resembles the self MHC-foreign peptide complexes and structure formed by both the allogeneic MHC molecules and the bound peptide.
9. From Autologous to Allogeneic DCs for DCs/Tumor Fusions
The rationale for using allogeneic DCs as a fusion partner is based on the finding that a high frequency of unprimed T cells from an individual react against the foreign MHC antigens of another individual. Additional potential benefit of using allogeneic DCs is that DCs from healthy donors are readily available in unlimited amounts. DCs from cancer patients may be defective in APC function, owing to cancer treatment, such as chemotherapy and irradiation. It has been demonstrated that fusions of both autologous and allogeneic DCs are effective in inducing antitumor immunity in human and animal models [100, 101]. The allogeneic DCs/autologous tumor fusions express DCs-derived allogeneic HLA class II molecules and HLA class I molecules derived from both DCs and tumor cells [102–104]. There are mainly four cases using allogeneic DCs for fusions-based vaccine. (a) Where there is no sharing of any MHC molecules between allogeneic DCs and autologous tumor cells, autologous MHC-class I restricted presentation of tumor peptides by the DCs through cross-presentation is not possible. (b) Where there is sharing of MHC class I molecules, autologous MHC-class I restricted presentation of tumor peptides by the DCs through cross-presentation is possible. The direct CD4+ T cell response to the allogeneic MHC class II antigens on allogeneic DCs will provide potent T cell help for the generation of antigen-specific CD8+ CTL responses to autologous tumor peptides presented by the shared MHC class I molecules. (c) Where there is sharing of MHC class II molecules, autologous MHC-class IL restricted presentation of tumor peptides by the DCs through cross-presentation is possible. The direct CD4+ T cell response to the semi-allogeneic MHC class II antigens on semi-allogeneic DCs will provide potent T cell help for the generation of antigen-specific CD4+ T cell response to autologous tumor peptides presented by the shared MHC class II molecules. (d) Where there is some sharing of both MHC class I and class II molecules, the fusions express allogeneic MHC class I and class II molecules derived from semi-allogeneic DCs for direct stimulation of the patient's CD4+ T cells as well as all the patient's HLA class I and class II molecules for autologous MHC-restricted tumor peptide presentation. The alloreactive T cell response by semiallogeneic fusions might help for the initiation and expansion of antigen-specific responses to autologous tumors by autologous MHC-restricted elements. Therefore, where there is no sharing of MHC molecules between allogeneic DCs and autologous tumor cells, efficient antitumor immunity may not be induced in therapeutic experiment [51]. Semiallogeneic fusions may be effective to induce antigen-specific polyclonal CTL responses. Indeed, semiallogeneic fusions elicited a significantly stronger antitumor immunity than did by syngeneic fusions in animal studies [31, 34]. However, it has also been reported that expression of self MHC by semiallogeneic fusions could induce antigen-specific immunity; however, concurrently activated allogeneic bystander responses do not provide helper or adjuvant effects [105]. In clinical trials, both autologous and allogeneic DCs fused with autologous tumor cells have been more effective as vaccines in the induction of CTL responses and antitumor activities [106, 107].
10. HSP70-Peptide Complexes Derived from DCs/Tumor Fusions
Heat shock proteins (HSPs) play a primary role as intracellular molecular chaperones in the pathways of antigenic protein folding within the cell [108]. The HSP/peptide complexes can be taken by DCs through receptors and presented in MHC class I and class II molecules on DCs [109]. This phenomenon leads to activation of maturation and representation of peptide antigen cargo of HSPs by DCs and initiates antigen-specific polyclonal CTL responses [92]. In several clinical trials, autologous HSP/peptide complexes have induced CTLs against autologous targets [110]. To improve the potency of chaperone protein-based vaccine, we have produced an improved HSP70-based vaccine with the use of DCs/tumor fusions [111]. The HSP70/peptide complexes (HSP70.PC) derived from DCs/tumor fusions were especially different from those derived from tumor cells in enhanced association with immunologic peptides in animal models. The HSP70.PC derived from the fusions have increased their immunogenicity and therefore may constitute an improved formulation of chaperone protein-based tumor vaccine. Recently, it has been also reported that human DCs pulsed with HSP70.PC extracted from DCs/tumor fusions enhanced CTL responses significantly more than that obtained from DCs pulsed with HSP70.PC from DCs pulsed with tumor cell lysates [112]. Therefore, this is an alternative molecular chaperone-based cancer vaccine using DCs/tumor fusions. Future studies should be required to improve the field of the chaperone-based cancer vaccine.
11. Clinical Trials
Based on the unique features of fusion cell based vaccines and the observations of tumor eradication in animal studies, initial Phase I/II clinical trials with fusion vaccines have been conducted in a variety of tumors (Table 1). Fusions vaccination was first reported in patients with melanoma [113, 114]. The fusions of allogeneic DCs and autologous tumor were irradiated and injected subcutaneously as a vaccine. Seven of the 16 patients responded to the vaccination, one with complete response, one with partial response, and five with stable disease following to previous rapid progression. Similar results in patients with melanoma were reported from another group [116, 117]. In our initial clinical trials of fusions-based vaccination, eight patients with malignant glioma were treated with fusions of autologous DCs and autologous tumor cells. Vaccination with fusions resulted in immunological responses and two patients showed partial responses, indicating that limited success has occurred in clinical trials. To enhance clinical responses, we had conducted a Phase I/II clinical trials for the vaccination with low dose of recombinant human (rh) IL-12 in patients with malignant brain tumor, gastric, colorectal, ovarian carcinoma, and melanoma [115, 118]. Eleven out of 15 patients with malignant glioma achieved a stable response and 24 patients had a progressive disease after 8 weeks of the initial treatment [115]. No serious adverse effects were observed. In four patients, magnetic resonance imaging showed a greater than 50% reduction in tumor size. One patient had a mixed response. Therefore, administration of fusions and rhIL-12 can induce more effective antitumor effects than fusions alone in some patients with malignant glioma. These data are compatible with the results from mouse brain tumor model in which administration of fusions and rIL-12 markedly prolonged the survival of mice with brain tumors compared with fusions or rIL-12 alone [119]. Moreover, vaccination of autologous fusions alone in patients with breast, renal, colorectal, and gastric cancer resulted in immunological responses. Interestingly, two out of 10 patients with metastatic breast cancer exhibited disease regression, including a near complete response of a large chest wall mass [17]. Five out of 13 patients with renal carcinoma and one out of 10 patient with breast cancer had disease stabilization [17]. This group has also evaluated the effect of vaccination with fusions of allogeneic DCs and autologous tumor cells in patients with renal cell carcinoma [106]. Vaccination of patients with stage IV renal cell carcinoma with allogeneic DCs/autologous tumor fusions resulted in immunologic and clinical responses in a subset of patients. Two out of 21 patients demonstrated a partial clinical response and 8 patients with had stabilization of their disease. In clinical trials, only limited therapeutic results are obtained. One of the reasons is that all patients were in advanced stage and extremely small amounts of fusions are used. Fusions-based vaccine may work more effectively in patients in the early stage of the disease with low tumor burden. Moreover, patients with a still uncompromised immune system are expected to respond best to the vaccine. Fusions-based vaccine may be used in combination with conventional therapies, including surgery, chemotherapy, or irradiation.
Table 1.
Asessment of the fusions vaccine.
| Fusions | ||||||
|---|---|---|---|---|---|---|
| Tumor | Dendritic cell | Tumor | Adjuvant | Patients (n) | Clinical response | Ref. |
|
| ||||||
| Melanoma | Allogeneic | Autologous | 16 | 1 (CR) | [113, 114] | |
| 1 (PR) | ||||||
| 5 (SD) | ||||||
| 9 (PD) | ||||||
|
| ||||||
| Glioma | Autologous | Autologous | 8 | 2 (PR) | [115] | |
| 1 (SD) | ||||||
| 5 (PD) | ||||||
|
| ||||||
| Melanoma | Autologous | Autologous | 17 | 1 (PR) | [116] | |
| 1 (SD) | ||||||
| 15 (PD) | ||||||
|
| ||||||
| Melanoma | Allogeneic | Autologous | rh IL-2 | 11 | 1 (SD) | [117] |
| 10 (PD) | ||||||
|
| ||||||
| Glioma | Autologous | Autologous | rh IL-12 | 12 | 3 (PR) | [115, 118] |
| 2 (MR) | ||||||
| 4 (SD) | ||||||
| 3 (PD) | ||||||
|
| ||||||
| Breast cancer | Autologous | Autologous | rh IL-12 | 2 | 1 (SD) | [115, 118] |
| 1 (PD) | ||||||
|
| ||||||
| Gastric/Colorectal carcinoma | Autologous | Autologous | rh IL-12 | 3 | 1 (SD) | [115, 118] |
| 2 (PD) | ||||||
|
| ||||||
| Ovarian carcinoma | Autologous | Autologous | rh IL-12 | 3 | 2 (SD) | [115, 118] |
| 1 (PD) | ||||||
|
| ||||||
| Melanoma | Autologous | Autologous | rh IL-12 | 4 | 4 (PD) | [115, 118] |
|
| ||||||
| Breast cancer | Autologous | Autologous | 10 | 2 (PR) | [17] | |
| 1 (SD) | ||||||
| 7 (PD) | ||||||
|
| ||||||
| Renal cell carcinoma | Autologous | Autologous | 13 | 5 (SD) | [17] | |
| 8 (PD) | ||||||
|
| ||||||
| Renal cell carcinoma | Allogeneic | Autologous | 20 | 2 (PR) | [106] | |
| 8 (SD) | ||||||
| 10 (PD) | ||||||
|
| ||||||
| Hepatocellular carcinoma | Autologous | Autologous | 1 | 1 (PD) | [10] | |
|
| ||||||
| Renal cell carcinoma | Allogeneic | Autologous | 10 | 1 (PR) | [107] | |
| 6 (SD) | ||||||
| 3 (PD) | ||||||
CR: complete response; PR: partial response; MR: mixed response; SD: stable disease; PD: progressive disease.
12. Future View
The DCs/tumor fusions vaccine has been successfully used in mice models. Moreover, human DCs/tumor fusions have enormous potential activities to induce polyclonal CTL responses against autologous targets in vitro. However, the overall rate of clinical responses remains to be low. Fusions vaccine alone may be insufficient to have a significant contribution to treat advanced cancer patients. There are increasing evidences that tumor-derived soluble factors promote the induction of tolerance through the generation of CD4+ CD25highFoxp3+ Treg subset, which is linked to compromised immune responses in patients with advanced cancer [120]. Moreover, it has recently been reported that DCs are capable of inducing conversion of naive CD4+ T cells to adaptive CD4+ CD25+ Foxp3+ Treg in the presence of TGF-β or IL-10 derived from tumor cells [121]. We have also reported that soluble factors derived from tumor cells promoted the generation of CD4+ CD25high Foxp3+ Treg and inhibited CTL induction by fusions [10]. The elimination of immunosuppressive immune cells, including Tregs, myeloid derived suppressor cells (MDSCs), or tumor-associated macrophages (TAMs), may improve clinical responses. In animal study, enhancement of antitumor immunity can be induced with a combination of fusions and regulatory T cell depletion in pancreatic cancer bearing mice [41]. More importantly, in a Phase I/II clinical trial, partial removal of Tregs can further enhance DC vaccine-induced immune responses in cancer patients [122]. The combination of direct enhancement of CTL function and concomitant inhibition of Treg function through blockade of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) on both cell types is essential for mediating the full therapeutic effects of anti-CTLA-4 antibodies in cancer immunotherapy. Another approach would be to enhance T cell costimulation by administering agonistic antibodies specific for 4-1BB [123], OX40 [124], cytotoxic T lymphocyte-associated antigen 4 (CTLA4) [125], or programmed death 1 (PD-1) [126]. It has been reported that a common pathway of endogenous OX40 interaction is critical for the development of a therapeutic immune response by fusions vaccination [127]. The pathological interactions between cancer cells and host immune cells in the tumor microenvironment create an immunosuppressive network that promotes tumor growth, protects the tumor from immune attack, and attenuates immunotherapeutic efficacy. Therefore, it is also essential to develop interventions that counter the propensity of tumors to evade immune elimination, such as immunization against the tumor stroma cells [128].
Financial Disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature Reviews Immunology. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. The Journal of Experimental Medicine. 1996;183(1):283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature Medicine. 1998;4(3):328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 5.Koido S, Kashiwaba M, Chen D, Gendler S, Kufe D, Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. Journal of Immunology. 2000;165(10):5713–5719. doi: 10.4049/jimmunol.165.10.5713. [DOI] [PubMed] [Google Scholar]
- 6.Palucka AK, Ueno H, Connolly J, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. Journal of Immunotherapy. 2006;29(5):545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 7.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. Journal of Experimental Medicine. 1995;181(6):2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto M, Furuichi S, Nishioka Y, et al. Expression of Toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with active component of OK-432, a streptococcal preparation. Cancer Research. 2004;64(15):5461–5470. doi: 10.1158/0008-5472.CAN-03-4005. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Medicine. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koido S, Homma S, Hara E, et al. In vitro generation of cytotoxic and regulatory T cells by fusions of human dendritic cells and hepatocellular carcinoma cells. Journal of Translational Medicine. 2008;6(1):p. 51. doi: 10.1186/1479-5876-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nature Medicine. 1995;1(12):1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 12.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nature Medicine. 1997;3(5):558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 13.Gong J, Avigan D, Chen D, et al. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2715–2718. doi: 10.1073/pnas.050587197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koido S, Tanaka Y, Chen D, Kufe D, Gong J. The kinetics of in vivo priming of CD4 and CD8 T cells by dendritic/tumor fusion cells in MUC1-transgenic mice. Journal of Immunology. 2002;168(5):2111–2117. doi: 10.4049/jimmunol.168.5.2111. [DOI] [PubMed] [Google Scholar]
- 15.Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. Journal of Experimental Medicine. 1994;180(5):1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. Journal of Experimental Medicine. 1997;186(5):665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clinical Cancer Research. 2004;10(14):4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Apostolopoulos V, Chen D, et al. Selection and characterization of MUC1-specific CD8+ T cells from MUC1 transgenic mice immunized with dendritic-carcinoma fusion cells. Immunology. 2000;101(3):316–324. doi: 10.1046/j.1365-2567.2000.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koido S, Hara E, Torii A, et al. Induction of antigen-specific CD4- and CD8-mediated T cell responses by fusion of autologous dendritic cells and metastatic colorectal cancer cells. International Journal of Cancer. 2005;117(4):587–595. doi: 10.1002/ijc.21184. [DOI] [PubMed] [Google Scholar]
- 20.Gong J, Chen D, Kashiwaba M, et al. Reversal of tolerance to human MUC1 antigen in MUC1 transgenic mice immunized with fusions of dendritic and carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6279–6283. doi: 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. Journal of Immunology. 1998;161(10):5516–5524. [PubMed] [Google Scholar]
- 22.Cao X, Zhang W, Wang J, et al. Therapy of established tumour with a hybrid cellular vaccine generated by using granulocyte-macrophage colony-stimulating factor genetically modified dendritic cells. Immunology. 1999;97(4):616–625. doi: 10.1046/j.1365-2567.1999.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka H, Shimizu K, Hayashi T, Shu S. Therapeutic immune response induced by electrofusion of dendritic and tumor cells. Cellular Immunology. 2002;220(1):1–12. doi: 10.1016/s0008-8749(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Holmes LM, Franek KJ, Burgin KE, Wagner TE, Wei Y. Purified hybrid cells from dendritic cell and tumor cell fusions are superior activators of antitumor immunity. Cancer Immunology, Immunotherapy. 2001;50(9):456–462. doi: 10.1007/s002620100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu K, Kuriyama H, Kjaergaard J, Lee W, Tanaka H, Shu S. Comparative analysis of antigen loading strategies of dendritic cells for tumor immunotherapy. Journal of Immunotherapy. 2004;27(4):265–272. doi: 10.1097/00002371-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka K, Yamamoto S, Otsuru S, et al. Enhanced tumor-specific long-term immunity of hemagglutinating virus of Japan-mediated dendritic cell-tumor fused cell vaccination by coadministration with CpG oligodeoxynucleotides. The Journal of Immunology. 2004;173(7):4297–4307. doi: 10.4049/jimmunol.173.7.4297. [DOI] [PubMed] [Google Scholar]
- 27.Phan V, Errington F, Cheong SC, et al. A new genetic method to generate and isolate small, short-lived but highly potent dendritic cell-tumor cell hybrid vaccines. Nature Medicine. 2003;9(9):1215–1219. doi: 10.1038/nm923. [DOI] [PubMed] [Google Scholar]
- 28.Kao JY, Gong Y, Chen C-M, Zheng Q-D, Chen J-J. Tumor-derived TGF-β reduces the efficacy of dendritic cell/tumor fusion vaccine. Journal of Immunology. 2003;170(7):3806–3811. doi: 10.4049/jimmunol.170.7.3806. [DOI] [PubMed] [Google Scholar]
- 29.Iinuma T, Homma S, Noda T, Kufe D, Ohno T, Toda G. Prevention of gastrointestinal tumors based on adenomatous polyposis coli gene mutation by dendritic cell vaccine. Journal of Clinical Investigation. 2004;113(9):1307–1317. doi: 10.1172/JCI17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao JY, Zhang M, Chen C-M, Chen J-J. Superior efficacy of dendritic cell-tumor fusion vaccine compared with tumor lysate-pulsed dendritic cell vaccine in colon cancer. Immunology Letters. 2005;101(2):154–159. doi: 10.1016/j.imlet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T, Fukuhara T, Tanaka M, et al. Vaccination of dendritic cells loaded with interleukin-12 secreting cancer cells augments in vivo antitumor immunity: characteristics of syngeneic and allogeneic antigen-presenting cell cancer hybrid cells. Clinical Cancer Research. 2005;11(1):58–66. [PubMed] [Google Scholar]
- 32.Xu F, Ye Y-J, Cui Z-R, Wang S. Allogeneic dendritomas induce anti-tumour immunity against metastatic colon cancer. Scandinavian Journal of Immunology. 2005;61(4):364–369. doi: 10.1111/j.1365-3083.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda T, Kamigaki T, Nakamura T, et al. Dendritic cell-tumor cell hybrids enhance the induction of cytotoxic T lymphocytes against murine colon cancer: a comparative analysis of antigen loading methods for the vaccination of immunotherapeutic dendritic cells. Oncology Reports. 2006;16(6):1317–1324. [PubMed] [Google Scholar]
- 34.Yasuda T, Kamigaki T, Kawasaki K, et al. Superior anti-tumor protection and therapeutic efficacy of vaccination with allogeneic and semiallogeneic dendritic cell/tumor cell fusion hybrids for murine colon adenocarcinoma. Cancer Immunology, Immunotherapy. 2007;56(7):1025–1036. doi: 10.1007/s00262-006-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida A, Tanaka H, Hiura T, et al. Generation of anti-tumour effector T cells from naive T cells by stimulation with dendritic/tumour fusion cells. Scandinavian Journal of Immunology. 2007;66(5):546–554. doi: 10.1111/j.1365-3083.2007.02012.x. [DOI] [PubMed] [Google Scholar]
- 36.Xia J, Tanaka Y, Koido S, et al. Prevention of spontaneous breast carcinoma by prophylatic vaccination with dendritic/tumor fusion cells. The Journal of Immunology. 2003;170(4):1980–1986. doi: 10.4049/jimmunol.170.4.1980. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, Xia J, Tanaka Y, et al. Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells. Immunology. 2003;109(2):300–307. doi: 10.1046/j.1365-2567.2003.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M, Berndt BE, Chen J-J, Kao JY. Expression of a soluble TGF-β receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy. Journal of Immunology. 2008;181(5):3690–3697. doi: 10.4049/jimmunol.181.5.3690. [DOI] [PubMed] [Google Scholar]
- 39.Tamai H, Watanabe S, Zheng R, et al. Effective treatment of spontaneous metastases derived from a poorly immunogenic murine mammary carcinoma by combined dendritic-tumor hybrid vaccination and adoptive transfer of sensitized T cells. Clinical Immunology. 2008;127(1):66–77. doi: 10.1016/j.clim.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Guo GH, Chen SZ, Yu J, et al. In vivo anti-tumor effect of hybrid vaccine of dendritic cells and esophageal carcinoma cells on esophageal carcinoma cell line 109 in mice with severe combined immune deficiency. World Journal of Gastroenterology. 2008;14(8):1167–1174. doi: 10.3748/wjg.14.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto M, Kamigaki T, Yamashita K, et al. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncology Reports. 2009;22(2):337–343. [PubMed] [Google Scholar]
- 42.Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. Journal of Gastroenterology. 2001;36(11):764–771. doi: 10.1007/s005350170019. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J-K, Li J, Zhang J, Chen H-B, Chen S-B. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma in vivo. World Journal of Gastroenterology. 2003;9(3):479–484. doi: 10.3748/wjg.v9.i3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irie M, Homma S, Komita H, et al. Inhibition of spontaneous development of liver tumors by inoculation with dendritic cells loaded with hepatocellular carcinoma cells in C3H/HeNCRJ mice. International Journal of Cancer. 2004;111(2):238–345. doi: 10.1002/ijc.20247. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H-M, Zhang L-W, Liu W-C, Cheng J, Si X-M, Ren J. Comparative analysis of DC fused with tumor cells or transfected with tumor total RNA as potential cancer vaccines against hepatocellular carcinoma. Cytotherapy. 2006;8(6):580–588. doi: 10.1080/14653240600991353. [DOI] [PubMed] [Google Scholar]
- 46.Sheng XL, Zhang H. In-vitro activation of cytotoxic T lymphocytes by fusion of mouse hepatocellular carcinoma cells and lymphotactin gene-modified dendritic cells. World Journal of Gastroenterology. 2007;13(44):5944–5950. doi: 10.3748/wjg.v13.i44.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celluzzi CM, Falo LD., Jr. Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. Journal of Immunology. 1998;160(7):3081–3085. [PubMed] [Google Scholar]
- 48.Savai R, Schermuly RT, Schneider M, et al. Hybrid-primed lymphocytes and hybrid vaccination prevent tumor growth of lewis lung carcinoma in mice. Journal of Immunotherapy. 2006;29(2):175–187. doi: 10.1097/01.cji.0000197096.38476.fc. [DOI] [PubMed] [Google Scholar]
- 49.Weise JB, Maune S, Gorogh T, et al. A dendritic cell based hybrid cell vaccine generated by electrofusion for immunotherapy strategies in HNSCC. Auris Nasus Larynx. 2004;31(2):149–153. doi: 10.1016/j.anl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Siders WM, Vergilis KL, Johnson C, Shields J, Kaplan JM. Induction of specific antitumor immunity in the mouse with the electrofusion product of tumor cells and dendritic cells. Molecular Therapy. 2003;7(4):498–505. doi: 10.1016/s1525-0016(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 51.Kjaergaard J, Shimizu K, Shu S. Electrofusion of syngeneic dendritic cells and tumor generates potent therapeutic vaccine. Cellular Immunology. 2003;225(2):65–74. doi: 10.1016/j.cellimm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Guo Y, Tang S, Qu H, Zhao H. Dendritic cell-Ewing’s sarcoma cell hybrids enhance antitumor immunity. Clinical Orthopaedics and Related Research. 2008;466(9):2176–2183. doi: 10.1007/s11999-008-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsue H, Matsue K, Edelbaum D, Walters M, Morita A, Takashima A. New strategy for efficient selection of dendritic cell-tumor hybrids and clonal heterogeneity of resulting hybrids. Cancer Biology and Therapy. 2004;3(11):1145–1151. doi: 10.4161/cbt.3.11.1217. [DOI] [PubMed] [Google Scholar]
- 54.Gong J, Koido S, Chen D, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2000;99(7):2512–2517. doi: 10.1182/blood.v99.7.2512. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Zhang W, Chan T, Saxena A, Xiang J. Engineered fusion hybrid vaccine of IL-4 gene-modified myeloma and relative mature dendritic cells enhances antitumor immunity. Leukemia Research. 2002;26(8):757–763. doi: 10.1016/s0145-2126(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 56.Hao S, Bi X, Xu S, et al. Enhanced antitumor immunity derived from a novel vaccine of fusion hybrid between dendritic and engineered myeloma cells. Experimental Oncology. 2004;26(4):300–306. [PubMed] [Google Scholar]
- 57.Shi M, Su L, Hao S, Guo X, Xiang J. Fusion hybrid of dendritic cells and engineered tumor cells expressing interleukin-12 induces type 1 immune responses against tumor. Tumori. 2005;91(6):531–538. doi: 10.1177/030089160509100614. [DOI] [PubMed] [Google Scholar]
- 58.Queant S, Sarde C-O, Gobert M-G, Kadouche J, Roseto A. Antitumor response against myeloma cells by immunization with mouse syngenic dendritoma. Hybridoma. 2005;24(4):182–188. doi: 10.1089/hyb.2005.24.182. [DOI] [PubMed] [Google Scholar]
- 59.Xia D, Li F, Xiang J. Engineered fusion hybrid vaccine of IL-18 gene-modified tumor cells and dendritic cells induces enhanced antitumor immunity. Cancer Biotherapy and Radiopharmaceuticals. 2004;19(3):322–330. doi: 10.1089/1084978041424990. [DOI] [PubMed] [Google Scholar]
- 60.Lespagnard L, Mettens P, Verheyden AM, et al. Dendritic cells fused with mastocytoma cells elicit therapeutic antitumor immunity. International Journal of Cancer. 1998;76(2):250–258. doi: 10.1002/(sici)1097-0215(19980413)76:2<250::aid-ijc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 61.Iinuma H, Okinaga K, Fukushima R, et al. Superior protective and therapeutic effects of IL-12 and IL-18 gene-transduced dendritic neuroblastoma fusion cells on liver metastasis of murine neuroblastoma. The Journal of Immunology. 2006;176(6):3461–3469. doi: 10.4049/jimmunol.176.6.3461. [DOI] [PubMed] [Google Scholar]
- 62.Tang C-K, Katsara M, Apostolopoulos V. Strategies used for MUC1 immunotherapy: human clinical studies. Expert Review of Vaccines. 2008;7(7):963–975. doi: 10.1586/14760584.7.7.963. [DOI] [PubMed] [Google Scholar]
- 63.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Research. 1998;58(2):315–321. [PubMed] [Google Scholar]
- 64.Koido S, Ohana M, Liu C, et al. Dendritic cells fused with human cancer cells: morphology, antigen expression and T cell stimulation. Clinical Immunology. 2004;113(3):261–269. doi: 10.1016/j.clim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Benencia F, Courreges MC, Coukos G. Whole tumor antigen vaccination using dendritic cells: comparison of RNA electroporation and pulsing with UV-irradiated tumor cells. Journal of Translational Medicine. 2008;29(6):p. 21. doi: 10.1186/1479-5876-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parkhurst MR, DePan C, Riley JP, Rosenberg SA, Shu S. Hybrids of dendritic cells and tumor cells generated by electrofusion simultaneously present immunodominant epitopes from multiple human tumor-associated antigens in the context of MHC class I and class II molecules. Journal of Immunology. 2003;170(10):5317–5325. doi: 10.4049/jimmunol.170.10.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koido S, Hara E, Homma S, et al. Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer-specific antigens and induce antitumor immunity against autologous tumor cells. Clinical Cancer Research. 2005;11(21):7891–7900. doi: 10.1158/1078-0432.CCR-05-1330. [DOI] [PubMed] [Google Scholar]
- 68.Koido S, Tanaka Y, Tajiri H, Gong J. Generation and functional assessment of antigen-specific T cells stimulated by fusions of dendritic cells and allogeneic breast cancer cells. Vaccine. 2007;25(14):2610–2619. doi: 10.1016/j.vaccine.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann TK, Meidenbauer N, Dworacki G, Kanaya H, Whiteside TL. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Research. 2000;60(13):3542–3549. [PubMed] [Google Scholar]
- 70.Berard F, Blanco P, Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. The Journal of Experimental Medicine. 2000;192(11):1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilboa E. DC-based cancer vaccines. Journal of Clinical Investigation. 2007;117(5):1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka Y, Koido S, Ohana M, Liu C, Gong J. Induction of impaired antitumor immunity by fusion of MHC class II-deficient dendritic cells with tumor cells. Journal of Immunology. 2005;174(3):1274–1280. doi: 10.4049/jimmunol.174.3.1274. [DOI] [PubMed] [Google Scholar]
- 73.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. The Journal of Experimental Medicine. 1999;189(5):753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang AYC, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264(5161):961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 75.Marzo AL, Kinnear BF, Lake RA, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. The Journal of Immunology. 2000;165(11):6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 76.Koido S, Hara E, Homma S, et al. Streptococcal preparation OK-432 promotes fusion efficiency and enhances induction of antigen-specific CTL by fusions of dendritic cells and colorectal cancer cells. The Journal of Immunology. 2007;178(1):613–622. doi: 10.4049/jimmunol.178.1.613. [DOI] [PubMed] [Google Scholar]
- 77.Koido S, Hara E, Homma S, et al. Synergistic induction of antigen-specific CTL by fusions of TLR-stimulated dendritic cells and heat-stressed tumor cells. The Journal of Immunology. 2007;179(1):4874–4883. doi: 10.4049/jimmunol.179.7.4874. [DOI] [PubMed] [Google Scholar]
- 78.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the Th 17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 79.Qin H, Wang L, Feng T, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. The Journal of Immunology. 2009;183(1):97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual Review of Immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 81.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 82.Melief CJM. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. The Journal of Experimental Medicine. 2005;202(7):919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakaguchi S, Yamanoto T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-β-TGF-β receptor interactions in type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(19):10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. Journal of Experimental Medicine. 1999;190(7):995–1003. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(28):10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunology. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 89.Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clinical Cancer Research. 2007;13(16):4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 90.Ibe S, Qin Z, Schuler T, Preiss S, Blankenstein T. Tumor rejection by disturbing tumor stroma cell interactions. Journal of Experimental Medicine. 2001;194(11):1549–1559. doi: 10.1084/jem.194.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Callahan MK, Wohlfert EA, Menoret A, Srivastava PK. Heat shock up-regulates Imp2 and Imp7 and enhances presentation of immunoproteasome-dependent epitopes. Journal of Immunology. 2006;177(12):8393–8399. doi: 10.4049/jimmunol.177.12.8393. [DOI] [PubMed] [Google Scholar]
- 92.Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? International Journal of Hyperthermia. 2005;21(8):713–716. doi: 10.1080/02656730500340794. [DOI] [PubMed] [Google Scholar]
- 93.Norbury CC, Basta S, Donohue KB, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304(5675):1318–1321. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 94.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100(12):4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 95.Chan RC, Xie H, Zhao GP, Xie Y. Dendritomas formed by fusion of mature dendritic cells with allogenic human hepatocellular carcinoma cells activate autologous cytotoxic T lymphocytes. Immunology Letters. 2002;83(2):101–109. doi: 10.1016/s0165-2478(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 96.Trevor KT, Cover C, Ruiz YW, et al. Generation of dendritic cell-tumor cell hybrids by electrofusion for clinical vaccine application. Cancer Immunology, Immunotherapy. 2004;53(8):705–714. doi: 10.1007/s00262-004-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. International Reviews of Immunology. 2006;25(5-6):321–352. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- 98.Lassi K, Dawson NA. Emerging therapies in castrate-resistant prostate cancer. Current Opinion in Oncology. 2009;21(3):260–265. doi: 10.1097/CCO.0b013e32832a1868. [DOI] [PubMed] [Google Scholar]
- 99.de Gruijl TD, van den Eertwegh AJ, Pinedo HM, Scheper RJ. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunology, Immunotherapy. 2008;57(10):1569–1577. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gong J, Nikrui N, Chen D, et al. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. The Journal of Immunology. 2000;165(3):1705–1711. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka Y, Koido S, Chen D, Gendler SJ, Kufe D, Gong J. Vaccination with allogeneic dendritic cells fused to carcinoma cells induces antitumor immunity in MUC1 transgenic mice. Clinical Immunology. 2001;101(2):192–200. doi: 10.1006/clim.2001.5112. [DOI] [PubMed] [Google Scholar]
- 102.Kufe DW. Smallpox, polio and now a cancer vaccine? Nature Medicine. 2000;6(3):252–253. doi: 10.1038/73082. [DOI] [PubMed] [Google Scholar]
- 103.Fabre JW. The allogeneic response and tumor immunity. Nature Medicine. 2001;7(6):649–652. doi: 10.1038/89008. [DOI] [PubMed] [Google Scholar]
- 104.Koido S, Hara E, Homma S, Ohkusa T, Gong J, Tajiri H. Cancer immunotherapy by fusions of dendritic cells and tumor cells. Immunotherapy. 2009;1(1):49–62. doi: 10.2217/1750743X.1.1.49. [DOI] [PubMed] [Google Scholar]
- 105.Wells JW, Cowled CJ, Darling D, et al. Semi-allogeneic dendritic cells can induce antigen-specific T-cell activation, which is not enhanced by concurrent alloreactivity. Cancer Immunology, Immunotherapy. 2007;56(12):1861–1873. doi: 10.1007/s00262-007-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Avigan DE, Vasir B, George DJ, et al. Phase I/II study of vaccination with electrofused allogeneic dendritic cells/autologous tumor-derived cells in patients with stage IV renal cell carcinoma. Journal of Immunotherapy. 2007;30(7):749–761. doi: 10.1097/CJI.0b013e3180de4ce8. [DOI] [PubMed] [Google Scholar]
- 107.Zhou J, Weng D, Zhou F, et al. Patient-derived renal cell carcinoma cells fused with allogeneic dendritic cells elicit anti-tumor activity: in vitro results and clinical responses. Cancer Immunology, Immunotherapy. 2009;58(10):1587–1597. doi: 10.1007/s00262-009-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becker J, Craig EA. Heat-shock proteins as molecular chaperones. European Journal of Biochemistry. 1994;219(1-2):11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- 109.Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Review of Vaccines. 2008;7(7):1019–1030. doi: 10.1586/14760584.7.7.1019. [DOI] [PubMed] [Google Scholar]
- 110.Parmiani G, Testori A, Maio M, et al. Heat shock proteins and their use as anticancer vaccines. Clinical Cancer Research. 2004;10(24):8142–8146. doi: 10.1158/1078-0432.CCR-04-1194. [DOI] [PubMed] [Google Scholar]
- 111.Enomoto Y, Bharti A, Khaleque AA, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. The Journal of Immunology. 2006;177(9):5946–5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 112.Koide T, Iinuma H, Fukushima R. Efficient CTL productivity of modified fusion cells by increase of heat shock protein 70. Oncology Reports. 2009;21(3):737–746. [PubMed] [Google Scholar]
- 113.Trefzer U, Weingart G, Chen Y, et al. Hybrid cell vaccination for cancer immune therapy: first clinical trial with metastatic melanoma. International Journal of Cancer. 2000;85(5):618–626. doi: 10.1002/(sici)1097-0215(20000301)85:5<618::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 114.Trefzer U, Herberth G, Wohlan K, et al. Tumour-dendritic hybrid cell vaccination for the treatment of patients with malignant melanoma: immunological effects and clinical results. Vaccine. 2005;23(17-18):2367–2373. doi: 10.1016/j.vaccine.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 115.Kikuchi T, Akasaki Y, Abe T, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. Journal of Immunotherapy. 2004;27(6):452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 116.Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. Journal of Immunotherapy. 2002;25(5):421–428. doi: 10.1097/00002371-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 117.Haenssle HA, Krause SW, Emmert S, et al. Hybrid cell vaccination in metastatic melanoma: clinical and immunologic results of a phase I/II study. Journal of Immunotherapy. 2004;27(2):147–155. doi: 10.1097/00002371-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 118.Homma S, Sagawa Y, Ito M, Ohno T, Toda G. Cancer immunotherapy using dendritic/tumour-fusion vaccine induces elevation of serum anti-nuclear antibody with better clinical responses. Clinical and Experimental Immunology. 2006;144(1):41–47. doi: 10.1111/j.1365-2249.2006.03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akasaki Y, Kikuchi T, Homma S, Abe T, Kofe D, Ohno T. Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. Journal of Immunotherapy. 2001;24(2):106–113. [PubMed] [Google Scholar]
- 120.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 121.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(27):9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. The Journal of Clinical Investigation. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang H, Snyder KM, Suhoski MM, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. The Journal of Immunology. 2007;179(7):4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. Journal of Immunology. 2007;179(11):7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 125.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. Journal of Experimental Medicine. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fourcade J, Kudela P, Sun Z, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. The Journal of Immunology. 2009;182(9):5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuriyama H, Watanabe S, Kjaergaard J, et al. Mechanism of third signals provided by IL-12 and OX-40R ligation in eliciting therapeutic immunity following dendritic-tumor fusion vaccination. Cell Immunology. 2006;243(1):30–40. doi: 10.1016/j.cellimm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 128.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Reviews Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]