Abstract
Epithelial cell tight junctions (TJs) consist of a narrow belt-like structure in the apical region of the lateral plasma membrane that circumferentially binds each cell to its neighbor. TJs are found in tissues that are involved in polarized secretions, absorption functions, and maintaining barriers between blood and interstitial fluids. The morphology, permeability, and ion selectivity of TJ vary among different types of tissues and species. TJs are very dynamic structures that assemble, grow, reorganize, and disassemble during physiological or pathological events. Several studies have indicated the active role of TJ in intestinal, renal, and airway epithelial function; however, the functional significance of TJ in salivary gland epithelium is poorly understood. Interactions between different combinations of the TJ family (each with their own unique regulatory proteins) define tissue specificity and functions during physiopathological processes; however, these interaction patterns have not been studied in salivary glands. The purpose of this review is to analyze some of the current data regarding the regulatory components of the TJ that could potentially affect cellular functions of the salivary epithelium.
1. Introduction
The intercellular junctional complex in epithelial cellular sheets consists of four major components: (1) tight junctions (TJs) [1], (2) adherens junctions [2] and desmosomes [3], (3) gap junctions [4], and (4) focal adhesions [5, 6]. Adherens junctions and desmosomes are responsible for the mechanical adhesion between adjacent cells. Of particular interest in the adherens junctions of the salivary glands are the members of cadherin family, which play a role in salivary gland development, tissue organization, and cell differentiation [7]. In early morphogenesis, E-cadherin and β-catenin are likely to participate in salivary gland remodeling [8], whereas during cytodifferentiation, they form stable cell-cell contacts and may collaborate with Rho GTPases in the establishment and maintenance of salivary cell polarity [9]. Gap junction channels, which link the cytoplasm of adjacent cells, are made up of membrane-spanning proteins, the connexins [10]. The integrity of connexins is necessary for normal glandular secretory function [11]. Previous studies have shown that connexins become uncoupled during stimulation of saliva secretion by cholinergic agonists [12]. However, the molecular mechanisms by which connexins uncouple during salivary cholinergic stimulation remain to be determined. Focal adhesion molecules interact with the extracellular matrix and play critical roles in the differentiation of many tissues [13, 14]. In salivary glands, integrins play crucial roles in embryonic and adult cell adhesion, migration, morphogenesis, growth, and differentiation [14].
TJs are essential for the tight sealing of the cellular sheets [15]. Epithelial cell TJs consist of a narrow belt-like structure in the apical region of the lateral plasma membrane that circumferentially binds each cell to its neighbor [16]. In epithelial cells, TJs are thought to be the principal structures that contribute to cell polarity by acting as an intermembrane barrier that prevents the lateral movement of membrane proteins between the apical and basolateral membranes [17, 18]. TJs also form the primary barrier against the diffusion of solutes through the paracellular cleft [19], thus maintaining selective transepithelial ion gradients. Using freeze-fracture analysis of salivary epithelium cell membranes, TJs appear as aggregates of particles that form continuous anastomosing strands [20]. The particles are composed of transmembrane proteins, embedded in plasma membranes of neighboring cells, in which extracellular domains of these TJ proteins interact to seal the intercellular junction [19]. The major TJ proteins are the transmembrane proteins claudins, occludin, and junctional adhesion molecules (JAMs) [21–23]. TJ proteins associate with intracellular scaffold proteins, among which is the family of zonula occludins (ZOs) proteins [24]. The ZO proteins anchor TJ transmembrane proteins to the actin cytoskeleton [25].
TJs are found in tissues that are involved in polarized secretions, absorption functions, and maintaining barriers between blood and interstitial fluids [26]. The morphology, permeability, and ion selectivity of TJ vary among different types of tissues and species [27–32]. TJs are very dynamic structures that assemble, grow, reorganize, and disassemble during physiological or pathological events [33]. Several studies have indicated the active role of TJ in intestinal, renal, mammary, and airway epithelial function [27–32, 34]; however, the functional significance of TJ in salivary gland epithelium is poorly understood [35]. The purpose of this review is to analyze some of the current data regarding the TJ and its regulatory components. TJs in the salivary epithelium are necessary for salivary gland function and could potentially serve as indicators of salivary epithelial dysfunction.
2. Morphological and Functional Differences between the Salivary, Acinar, and Ductal Cells
Salivary glands consist of multiple secretory units connected to the oral cavity by a system of ducts [36]. Each secretory unit is a cluster of cells organized in secretory acini [37]. The salivary glands consist of three pairs of major salivary glands (parotid, submandibular, and sublingual), and minor salivary glands located throughout the oral cavity within the lamina propria of the oral mucosa [37]. The major salivary glands are encapsulated by a connective capsule, a feature that is absent in minor salivary glands [38]. The salivary glands are also classified according to their function in serous glands (i.e., the parotid gland) that produce almost exclusively protein [39], mucous glands that produce only a small amount of protein but a large amount of glycoprotein (e.g., the sublingual and minor salivary glands) [40], and mixed serous/mucous glands that secrete both protein and glycoprotein (i.e., the submandibular gland). In humans 90% of saliva is produced by the major salivary glands and 10% is produced by the minor salivary glands [41].
2.1. Parotid Gland
The parotid gland is composed of the following: (1) serous acinar secretory end pieces [39], (2) intercalated ducts within the parotid glands, which are long and branched [36], and (3) well-developed striated ducts [36]. The secretion in the parotid gland is watery and rich in amylase, prolin-rich proteins, and peroxidase [42].
2.2. Submandibular Gland
The submandibular gland is a mixed gland composed of the following: (1) serous cells, which are attached to secretory end pieces to form a demilune, with serous acini predominating over the mucous elements [40], (2) intercalated ducts [36], and (3) long and well-defined striated ducts [36]. The secretion of the submandibular gland contains more mucus than that of the parotid gland; therefore, it is more viscous [40].
2.3. Sublingual Gland
The sublingual gland is composed of the following: (1) acinar mucus-secreting cells, some of which are capped by serous demilunes [40], (2) short to nonexistent intercalated ducts [36], and (3) striated ducts that are less developed than the submandibular and parotid glands [36]. The secretion of the sublingual gland is predominantly mucous [40].
2.4. Minor Salivary Gland
Minor salivary glands are divided into three groups as follows: (1) anterior-lingual glands that are mucous secreting glands with serous demilunes [43], (2) Von Ebner, or posterior lingual glands, which are composed of lipase-rich serous acini [44], and (3) Weber's glands, which are lingual, posterior glands that secrete mucus [45].
2.5. Saliva Secretion
The main function of the salivary gland is the production of saliva. Primary saliva secretion is elaborated by the acinar cells then it is modified as it passes through a series of progressively larger ducts [46–49]. The glandular secretion consists mostly of ions and electrolytes, as well as proteins and glycoproteins [46, 50]. Because primary acinar secretion and its modification in the ducts vary depending on the gland type, it is clear that TJ structure and function must be different between serous, mucous, and mixed acini, as well as between intercalated, striated, and excretory ducts. However, how TJ structure and function are modulated among different salivary glands during saliva secretion is little understood.
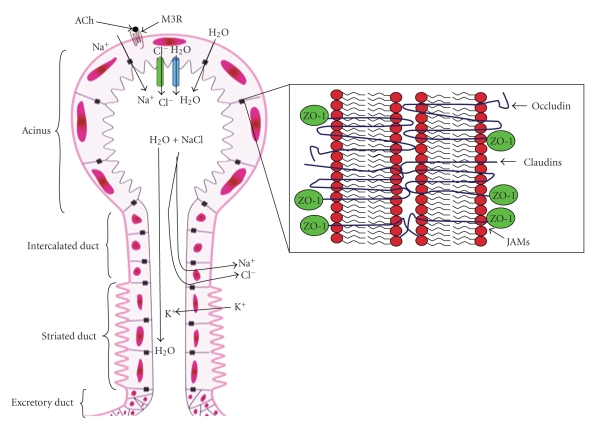
In currently accepted models of saliva secretion, the transepithelial movement of Cl− is the primary driving force for fluid and electrolyte secretion by salivary acinar cells (Figure 1) [51]. Agonist-stimulated secretion in acinar cells is initiated by concomitant activation of Ca2+-dependent apical Cl− channels and basolateral K+ channels [52]. The stimulated efflux of K+ and Cl− down their electrochemical gradients produces a transepithelial potential difference that is followed by Na+ and water diffusion across the epithelial TJ [53] (Figure 1). The secretion from the acinar cell, in addition to fluid, contains proteins such as amylase and mucins in a solution of ions similar to the other extracellular fluids [54]. Water diffusion appears to occur by paracellular pathways and transcellular transport via water channels [51]. As the primary saliva goes through the ducts, Na+ and Cl− reabsorption and secretion of K+ and HCO3− occur, because salivary ducts have low permeability to water, which results in a hypotonic saliva (Figure 1) [46].
Figure 1.
Diagram representing acinar salivary secretion and TJ proteins. Activation of basolateral M3 muscarinic receptors by neurotransmitters (e.g., acetylcholine) initiates signaling cascades that stimulate apical Ca2+-dependent Cl− channels. The stimulated efflux of Cl− produces a transepithelial potential difference that drives Na+ and H2O transport across the TJ. Alternatively, H2O can reach the lumen by water channels. These events create a plasma-like primary secretion in the lumen. As the primary saliva passes through the ducts, Na+ and Cl− are reabsorbed and K+ is secreted into the lumen. Inset (adapted from [55]) indicates the TJ proteins occludin, claudin, and JAM linked to the cytoskeleton via cytoplasmic ZO proteins. Clearly TJ structure varies depending on the cell function; the question is how combinations of TJ proteins define function in acinar and ductal cells.
TJs in salivary epithelia provide a barrier between the extracellular compartments and the lumen that is critical to normal acini functions, including the maintenance of cell polarity and normal transepithelial ion gradients [51]. A recent study indicated that apical electrolyte concentration modulates barrier function and TJ protein localization in bovine mammary epithelium [34]. Therefore, TJ protein interactions are likely to change in response to agonist-induced ion secretion in salivary glands. Proinflammatory cytokines also modulate TJ in several tissues [27, 28, 34, 56–60] including salivary epithelium [35]. Examining how TJs are modulated in response to agonists or inflammatory mediators is a significant step in defining the signaling pathways that regulate TJ integrity in salivary glands.
3. Transmembrane TJ Proteins
3.1. Claudins
Claudins are members of a multigene family, with < 24 members in humans/rodents [61], presenting a unique tissue expression pattern [62]. Claudins are integral transmembrane proteins that range from 22 kDa to 27 kDa [63]. Claudins span the cellular membrane 4 times, with both N-terminal and C-terminal ends located in the cytoplasm [21, 63, 64]. The C-terminal end diverges among different claudin subtypes, has potential phosphorylation sites, and has a putative PDZ binding domain of ZO proteins [64–66]. Claudins have two extracellular loops, which show a high degree of conservation [67]. The first loop is larger than the second, and it is involved in homophilic and heterophilic interactions [63, 64].
When claudin-1 or -2 is expressed in L-fibroblasts, which lack the endogenous claudins, they are able to reconstitute a well-developed network of strands (similar to TJ strand networks) [68, 69]. Conversely, occludin by itself cannot develop TJ strands [69], indicating that claudins are the backbone of TJ. Alterations of claudin expression strongly affect epithelial paracellular permeability (for a review see [67]). Analysis by freeze-fracture electron microscopy revealed an increase in number, depth, and complexity of TJ fibrils when claudins were overexpressed in epithelial cells [70, 71]. Claudin-null L-cells, transfected to express different claudins, have been used to demonstrate that claudin-1 heterotypically binds to claudin-3, but not to claudin-2 or claudin-5 [72]. Conversely, claudin-2 and claudin-5 heterotypically bind to claudin-3, but not to claudin-1 [72]. Thus, the compatibility of claudins for head-to-head binding is not easily predicted. The claudin-null HeLa cells stably expressing single or multiple claudins have been used to examine the ability of claudin-1, claudin-3, claudin-4, and claudin-5 to interact with each other [73]. Although the extracellular loop domains of claudin-3 and claudin-4 are highly conserved, claudins that interact with claudin-3 do not heterotypically bind to claudin-4 [73]. However, claudin-3 and claudin-4 do form heteromeric complexes [73]. To date, all of the known heterotypic claudin-claudin interactions appear to involve claudin-3 [72, 73]. However, since a limited subset of the claudins has been examined, future studies are necessary to determine other potential heterotypic claudin-claudin interactions. In contrast, homotypic claudin-claudin interactions appear to be universal [72]. Claudin-claudin interactions in salivary glands have not yet been defined; however, different claudin combinations may give functional specificity in acinar and ductal cells (and may help to better understand salivary gland functions). Kinetic analysis of GFP-claudin-1-containing strands in renal epithelial cells indicated that, although claudins are not mobile within paired strands, claudin-1-containing strands are dynamic: strands occasionally break and anneal, dynamically associating with each other in both an end-to-side and side-to-side manner [74]. Analysis by freeze-fracture electron microscopy revealed an increase in number, depth, and complexity of TJ fibrils when claudins were overexpressed in Madin-Darby canine kidney (MDCK) cells [70, 71]. In salivary glands, claudins are likely to regulate salivary gland functions by allowing cell polarity and by maintaining the transepithelial gradient necessary to establish unidirectional secretion [35]. To date, from the 24 known claudins, only claudins-1, -8, -10, -11, and -16 have been detected in salivary glands. These claudins (i.e., those that express in salivary glands) will be discussed in this section and are summarized in Table 1.
Table 1.
Localization of TJ proteins in salivary glands. This table summarizes TJ detected to date in acinar and ductal cells. * indicates that this protein is present only in serous acini. ∆ denotes an unusual basolateral or cytoplasmic localization where TJs do not exist.
| Species/Cell Type | Acinar | Ductal | References |
|---|---|---|---|
| Human Major Salivary Glands | Claudin-1* Claudin-2 Claudin-3 Claudin-4 Claudin-16∆ Occludin JAM-A ZO-1 | Claudin-1 Claudin-2 Claudin-3 Claudin-4 Claudin-16 Occludin JAM-A ZO-1 | [79, 109] |
| Human Minor Salivary Glands | Claudin-3 Claudin-4 | Claudin-1 Claudin-3 Claudin-4 Claudin-7 Claudin-11∆ | [77] |
| Rat Major Salivary Glands | Claudin-3 Claudin-10∆ | Claudin-1 Claudin-3 Claudin-4 | [78] |
| Mouse Major Salivary Glands | Claudin-4 Claudin-3 | Claudin-6 Claudin-8 Claudin-10 | [89] |
3.1.1. Claudin-1
Although the precise physiological role of claudin-1 is unclear, newborn claudin-1 deficient mice develop severe dehydration and die within 1 day of birth [75], indicating that claudin-1 plays a fundamental role within TJ. Additionally, overexpression of claudin-1 increased transepithelial electrical resistance (TER) and decreased paracellular permeability to 4–40 kDa FITC dextran in MDCK cells [76], further indicating the important role of claudin-1 in TJ formation.
Claudin-1 seems to be present only in ducts from human minor salivary gland [78] and rats (see Table 1) [79]. However, in human major salivary glands, claudin-1 was also found in serous acini (see Table 1) [80]. These studies indicate that claudin-1 expression varies among species and cell type. In polarized rat parotid gland Par-C10 cell monolayers [81] that endogenously express claudin-1 [35] treatment with proinflammatory cytokines TNFα and/or IFNγ caused a reduction of claudin-1 expression. The observed claudin-1 downregulation was associated with disruption of TJ structure and function [35]. These findings indicate that claudin-1 may contribute to TJ integrity in salivary epithelium, a condition that is necessary for cell polarity and unidirectional ion secretion.
3.1.2. Claudin-2
The commonly used experimental MDCK strains (i.e., types I and II) differ in TER when they form monolayers [68]. MDCK I cell monolayers do not express claudin-2 and have a very high TER [68, 82]. However, MDCK II cell monolayers have a 10- to 100-fold lower TER than MDCK I cell monolayers do, and they express claudin-2 in the intercellular space [68, 82]. Both cell strains express the TJ proteins claudin-1, -3, and -4, as well as ZO-1 and occludin [68]. These studies revealed that incorporation of claudin-2 converts the tight TJ strands to leaky strands in MDCK I cell monolayers. Other studies indicate that exogenous claudin-2 expression in MDCK-C7 cells (a twin to MDCK strain I cells) induces cation-selective channels in the TJ [77, 83]. Additionally, in the kidney, claudin-2 expression is restricted to leaky epithelium in the proximal tubule and thin descending limb of Henle [84]. Furthermore, claudin-2 is absent in the remaining distal nephron, which is considered to be a tight epithelium [84]. These studies indicate that claudin-2 causes leakiness within the TJ.
Claudin-2 has been detected in both acinar and ductal cells from human major salivary glands (see Table 1) [80]. However, claudin-2 was not detected in human minor salivary glands and rodents (see Table 1) [78, 79]. As for claudin-1, discrepancies in claudin-2 detection may exist due to the cell type and species. Although the role of claudin-2 in salivary gland is not known, high levels of claudin-2 in adult salivary acinar cells could contribute to the typical leakiness of salivary acinar cells (e.g., high permeability to water and Na+).
3.1.3. Claudin-3
Claudin-3 is found in equal amounts in two strains of MDCK cell monolayers (MDCK-C7 and MDCK-C11); however, these strains show different levels of transepithelial resistance [83]. In addition, transfection experiments showed no relationship between electrical transepithelial resistance and claudin-3 expression in MDCK I cell monolayers [68]. In contrast, claudin-3 knockdown by siRNA in the gastric polarized epithelial cell line (MKN28) caused a significant decrease in TER and increased dextran permeability [85]. The studies indicate that claudin-3 function varies depending on the cell type.
Claudin-3 has been detected in both acinar and ductal cells from human major and minor salivary glands and in the rat parotid gland (see Table 1) [78–80] as well as in Par-C10 cell monolayers [35]. The specific function of claudin-3 in epithelial cells of the acini and ducts in the salivary glands has not been elucidated. However, a recent study showed that claudin-3 expression decreased in the parotid glands of female mice lacking Aquaporin 5 (AQP5−/−), the major transcellular water transporter in salivary acinar cells [86]. This study suggests a possible link between claudin-3 and paracellular water secretion in salivary glands.
3.1.4. Claudin-4
The overexpression of claudin-4 in MDCK cells decreases transepithelial conductance by decreasing paracellular Na+ permeability, without affecting permeability to Cl− or flux for a noncharged solute [71]. In cultured pig kidney epithelial cells (LLC-PK1), knockdown of claudin-4 expression decreased Cl− permeability and caused the TJ to lose the anion selectivity [87]. When claudin-4 in MDCK I cells is removed, there is a decrease in the number of TJ strands and a reduction of TER [88]. These studies suggest that claudin-4 may be responsible for both conductance and ionic selectivity.
Claudin-4 has been detected in both acinar and ductal cells from human major and minor salivary glands [78, 80] (see Table 1). Claudin-4 has also been detected by Western blot analysis in Par-C10 polarized cell monolayers [35]. However, claudin-4 was detected only in ductal cells from rat parotid glands and mouse submandibular glands when using immunostaining in frozen sections (see Table 1) [79, 89]. Overexpression of claudin-4 increased TER and decreased epithelial permeability (to 70-kDa dextran, as compared to untransfected controls) in SMIE cell monolayers [90]. These results indicate that claudin-4 may function as a regulator of TJ barrier function in rat submandibular glands.
3.1.5. Claudin-5
Claudin-5 has been reported to be primarily present in TJ of endothelia, suggesting a role in the control of the endothelial barrier [91]. Indeed, mice deficient in claudin-5 show barrier failure, which is size selective and limited to the endothelium of the blood-brain barrier [92]. Claudin-5 stable transfection to human epithelial colorectal adenocarcinoma cells (Caco-2) increased TER and decreased paracellular permeability to mannitol (as compared to untransfected controls that lack claudin-5 and normally display low TER) [93]. However, when claudin-5 null cells displaying high TER (MDCK-C7) were transfected with claudin-5, no changes of barrier function were detected [93]. These findings suggest that claudin-5 contributes to the TJ sealing.
In human and rats major salivary glands, the expression of claudin-5 seems to be restricted to endothelial cells that surround acinar and ductal cells [79, 80]. Since previous studies showed that claudin-5 controls paracellular solute and water movement across endothelial monolayers [91], and because of its location in endothelial cell surrounding salivary epithelium [79, 80], it is tempting to speculate that claudin-5 could be involved in controlling nutrients supply from blood to salivary glands. However, studies are needed to elucidate the functions of claudin-5 in salivary glands.
3.1.6. Claudin-6
Claudin-6 was first identified through searching expressed sequence tag (EST) databases from embryos [61]. However, claudin-6 expression has not been detected in adult tissues [61, 94], indicating that claudin-6 may be regulated developmentally. Consistent with these studies, claudin-6 is expressed and concentrated at TJ only in the ducts at E16 (in mice submandibular glands) [89]. Conversely, it is almost completely absent after birth [89] (see Table 1), suggesting that claudin-6 is developmentally regulated in salivary glands.
3.1.7. Claudin-7
Claudin-7 is localized in the distal and collecting tubules, as well as in the thick ascending limb of Henle in porcine and rat kidneys [95]. Overexpression of claudin-7 in LLC-PK1 cells resulted in increased TER and a dramatic reduction in dilution potentials [95] due to a concurrent decrease in the paracellular conductance to Cl− and an increase in the paracellular conductance to Na+ [95]. These results indicate that claudin-7 may form a paracellular barrier to Cl− while acting as a paracellular channel to Na+.
Claudin-7 is expressed in ductal cells from early developmental stages through adulthood in human minor salivary glands (see Table 1) [78]. Similar to claudin-3, claudin-7 protein expression also decreased in parotid glands from female AQP5−/− mice, further indicating a relationship between claudins and water transport during saliva secretion [86].
3.1.8. Claudin-8
Claudin-8 is expressed along the aldosterone-sensitive distal nephron, including the entire collecting duct [59]. Induction of claudin-8 expression in MDCK II cells reduced permeability, not only to protons, but also to ammonium and bicarbonate [96, 97]. These studies suggest that claudin-8 probably limits the passive leak of these three ions via paracellular routes, thereby playing a permissive role in urinary net acid excretion.
Claudin-8 has been detected in the ducts of mouse submandibular glands, during both the pre- and post-natal stages (see Table 1) [89]. However, further research is needed to determine the role of claudin-8 in salivary glands.
3.1.9. Claudin-10
Although the exact function of claudin-10 is unknown, its expression has been associated with hepatocellular carcinoma recurrence [98] and papillary thyroid carcinoma [99], suggesting that claudin-10 contributes to cancer progression. Claudin-10 is expressed in the terminal tubules developing mouse submandibular glands, where this claudin-10 is colocalized with ZO-1 [89]. However, studies in rat major salivary glands indicated that claudin-10 is also present at the basolateral region of acinar cells, showing an ectopic subcellular localization where TJ strands do not exist (see Table 1) [76]. The role of claudin-10 in salivary glands remains to be determined.
3.1.10. Claudin-11
Previous studies determined that claudin-11 is present in the central nervous system and is involved in nerve conduction [100]. Claudin-11 is also present in Sertoli cells and apparently is involved in spermatogenesis [101]. Claudin-11 typically forms “pure” TJs, in the sense that other claudins are not present in these junctions [100]. In claudin-11 null mice, compartmentalization (established by claudin-11-based TJ in stria vascularis) is required for hearing through generation of endocochlear potential [102]. Overexpression of claudin-11 induces proliferation and enhances migration in an oligodendrocyte cell line [103].
In human minor salivary glands, claudin-11 has been detected in the cytoplasm of ductal cells unlike other claudins [78]. Claudin-11 is not expressed in acinar cells (see Table 1) [78]. The reason why claudin is expressed in a place where TJs do not exist (e.g., membrane-cytoplasmic) is unknown. The function of claudin-11 and its expression pattern in human major salivary glands remain to be determined. Claudin-11 is expressed in the terminal tubules and ducts of the mouse developing submandibular gland where it is colocalized with ZO-1 [89].
3.1.11. Claudin-16
Claudin-16 is expressed in the kidney of several mammalian species (e.g., in rodents, cattle, and humans) [104–107] and in mammary glands from mice [108]. When claudin-16 is missing, magnesium does not return from the renal tubule to the blood. Consequently, it is lost in the urine, which leads to hypomagnesemia [104]. These studies indicate that claudin-16 (a.k.a., paracellin-1) provides a cation-selective channel in the renal tubule [104].
Claudin-16 has been detected in the ducts of major human salivary glands (where claudin-16 colocalizes with the scaffold protein ZO-1 or with occludin) [109]. However, in acinar cells, claudin-16 was detected at the basolateral side of the cells (between cells of the same acinus and/or between cells of neighboring acini) (see Table 1) [109]. Consequently, the significance of claudin-16 expression pattern (in acinar cells) has yet to be determined.
3.2. Occludin
Occludin is a transmembrane protein that forms part of the TJ [22] which contributes to TJ barrier function and to formation of aqueous pores within TJ strands [15]. Occludin has a molecular mass of 60–65 kDa, two extracellular loops [110], and four transmembrane domains. Both the N- and C-terminal ends of occludin are located in the cytoplasm [110]. The N-terminal region is involved in sealing and barrier properties [111]. The C-terminal domain is rich in charged amino acids and binds specifically to a complex of ZO-1 and ZO-2 [110]. The extracellular loops have a high content of tyrosine and glycine residues that are thought to be involved in the regulation of paracellular permeability and cell adhesion [110].
Occludin-deficient embryonic stem cells are able to differentiate into polarized epithelial cells with functional TJ [112]. L-fibroblasts exhibited no cell-cell adhesion as a result of induced occludin expression [69], suggesting that occludin is not necessary for TJ formation. Expression of occludin in MDCK II cells increased TER and paracellular flux of a small molecular dextran tracer [113], suggesting a role for occludin in the formation of selective pores (despite high electrical resistance).
Four differentially spliced occludin-specific mRNA transcripts have been identified, which are the result of post-transcriptional events [114]. Expression of the translated proteins altered subcellular distribution of occludin and loss of colocalization with ZO-1 for two of the four splice variants [114]. Two splice variants (i.e., occludin types II and III) lack the fourth transmembrane domain [114]. It was observed that occludin types II and III did not co-localize with ZO-1, which highlights the significance of the fourth transmembrane domain in directing occludin to the TJ [114]. An occludin isoform lacking the fourth transmembrane domain (close to the C-terminal domain) was discovered in human embryo tissues [115]. Occludin 1B has been identified as a transcript encoding a longer form of occludin with a unique N-terminal sequence of 56 amino acids [116]. Immunostaining for occludin 1B shows that its distribution pattern in MDCKs is identical to that of occludin. The detection of occludin 1B in a range of epithelial tissues (and the preservation across species) implies that occludin 1B may be a significant player in the modulation of TJ barrier properties [116]. These findings also suggest that occludin and its isoforms may be a multigene family.
In human major salivary glands, occludin has been detected in ductal and acinar cells (see Table 1) and in endothelial cells surrounding the salivary epithelium [80]. Occludin has also been detected in cell lines of salivary gland origin such as the polarized Par-C10 and SMIE cell monolayers [35, 90]. Mice lacking occludin showed loss of cytoplasmic granules in striated ducts from salivary glands [117]; however, the significance of this observation remains to be determined.
The functional role of occludin in salivary gland TJ has been demonstrated through studies involving polarized cell lines. In a rat parotid gland epithelial cell line (Pa-4, similar to Par-C10 but different clone), transfection of an oncogenic Raf-1 resulted in a complete loss of TJ function (and the acquisition of a stratified phenotype that lacked cell-cell contact growth control) [118]. The expression of occludin and claudin-1 was downregulated, and the distribution patterns of ZO-1 and E-cadherin were altered. Introduction of the human occludin gene into Raf-1-activated Pa-4 cells resulted in reacquisition of a monolayer phenotype and the formation of functionally intact TJ [118]. These studies indicate that not only occludin is a critical component of functional TJ in salivary epithelium, but that it also controls the phenotypic changes associated with epithelium oncogenesis.
In murine submandibular gland carcinoma cells (CSG), the expression of an N-terminally truncated occludin construct decreased TER and paracellular permeability to 4–42 kDa tracers [111]. These studies suggest that occludin may be a regulator of the paracellular pathway, rather than a structural or functional component of the TJ, given that TJs form and appear functionally normal in the absence of occludin [69, 112].
3.3. Junctional Adhesion Molecules (JAMs)
JAMs are members of the immunoglobulin superfamily of proteins and are expressed in epithelial cells [119]. JAMs are subdivided into a group consisting of JAM proteins (JAM-A, JAM-B, JAM-C) and another group consisting of Coxsackievirus-adenovirus receptor (CAR), Coxsackie- and adenovirus receptor-Like Membrane Protein (CLMP), Endothelial cell adhesion molecule (ESAM), and JAM-4 [69, 112, 120, 121]. In epithelia, JAM-A and JAM-C localize to the TJ, whereas JAM-B exists along the lateral membrane [122]. Unlike occludin and claudins, JAM protein family members have a single transmembrane domain, an extracellular domain containing two Ig-like motifs, and a cytoplasmic tail [123]. The extracellular domains of JAM-A, JAM-B, and JAM-C contain dimerization motifs that play a role in their interactions [123, 124]. The C-terminal end has a putative PDZ binding domain, which interacts with the PDZ domains of accessory proteins (e.g., ZO-1) [125]. These studies indicate that JAM-A might be involved in the propagation of signal cascades, resulting from homophilic and heterophilic TJ protein interactions. JAM-A apparently plays a role in the adhesion and transmigration of monocytes through endothelial cells [23]. JAM-A functional significance in epithelial cells is less clear; however, inhibition of JAM-A with a monoclonal antibody caused a decrease in TER, and defects in TJ assembly, in intestinal epithelial T84 cells [126]. These studies indicate that JAM-A is important for TJ integrity in these cells.
Among the JAM protein family, JAM-A is the only member that has been detected in acinar and ductal cells from human major salivary glands (see Table 1) [80]. Further studies are needed, both to characterize its overall function and to determine the process by which it is regulated in salivary epithelium.
4. Multiprotein Complexes at the TJ
Three major protein complexes involve one or more scaffolding proteins: (1) the ZO protein complex [127], (2) the protein associated with Lin Seven (Pals1)-ALS1-associated TJ protein (PATJ) complex [128], and (3) the partitioning defective-3 (PAR)-3-atypical protein kinase C (aPKC)-PAR-6 complex [129]. To date, only the ZO protein complex has been described in mammalian salivary gland epithelium; the details of which will be described below.
ZO-1 is a classical scaffolding protein of the membrane-associated guanylate kinases (MAGUKs) family. It has three PDZ domains, one SH3 domain, and one guanylate kinase (GuK) domain [127]. Unlike other TJ proteins, ZO-1 is not a transmembrane protein; rather, it is a large cytosolic phosphoprotein [130]. This role is critically important for interaction with integral membrane proteins at TJ [131]. ZO-1 also interacts with other cytoplasmic proteins (e.g., ZO-2 and ZO-3 homologs of ZO-1) and with the actin cytoskeleton [120]. ZO-1 forms complexes with ZO-2 and ZO-3 [132]. Moreover, both ZO-2 and ZO-3 interact with F-actin (and also with occludin and claudins) [65, 132, 133]. Collectively, the ZO complex is the major link to the actin cytoskeleton at the TJ. The absence of ZO-1 results in a slight delay in TJ formation but does not impair the formation of functional TJ in MDCK cells [134] or in the mouse mammary epithelial cell line Eph4 [135]. This indicates redundancy in the roles of ZO family members, as each can accomplish the role of other members. However, if all ZO family members are lost in mammary epithelial cells, TJ formation is blocked (i.e., TJ strands are lost and barrier function is disrupted) [131]. Together, these findings indicate a critical role for the ZO protein family in the development of TJ strands, probably by forming the physical scaffold for the strand-forming proteins (e.g., claudins and occludin).
In human major salivary glands, ZO-1 is present in acini, ducts, and interglandular endothelial cells [80, 109]. Additionally, ZO-1 is colocalized with claudin-16 at the excretory duct of human major salivary glands [109]. ZO-1 has been widely used as a marker to achieve salivary gland differentiation [136]; however, studies are needed to determine the molecular mechanisms by which ZO-1 modulates TJ in salivary glands.
5. Functional Approaches Available to Study TJ in Salivary Glands
Epithelial cell lines from rat salivary glands (e.g., Par-C10, SMIE, and CSG), exhibiting a high degree of differentiation when plated on permeable supports or on Matrigel, are currently available. The rat parotid Par-C10 cells are able to form polarized monolayers when cultured on permeable supports (i.e., in a two-dimensional culture) [35]. Par-C10 cells also form acinar spheres when single cells are grown on Matrigel (i.e., in a three-dimensional culture) [137] (Figure 2). These models have proven useful to study TJ morphology by freeze-fracture analysis [35], TJ organization through confocal microscopy (Figure 2), and TJ protein expression through Western blot analysis [35]. Par-C10 polarized monolayers allow for the study of TJ function by TER and agonist-induced short circuit current measurements [35]. Par-C10 acinar spheres make possible the study of TJ integrity (by measuring intrasphere changes in potential difference in response to relevant secretory agonists) [137].
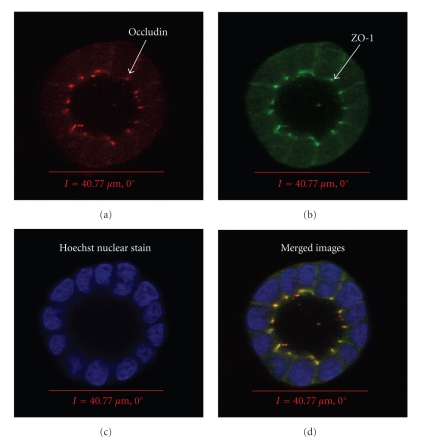
Figure 2.
Three-dimensional Par-C10 acinar-like spheres express TJ as markers of acinar differentiation. Protein expression was detected using immunofluorescence microscopy with goat antimouse occludin (a and d: red), rabbit anti-ZO-1 (b and d: green), followed by Hoechst nuclear stain (c and d: blue). Images were obtained and analyzed using a Carl Zeiss 510 confocal microscope. Sphere diameter taken at the widest point of the xy plane (I) is shown in μm. This model has been published [137].
The polarized rat submandibular SMIE cells are useful to study TJ structure and function [90] and allow the monitoring of transepithelial fluid movement in vitro [138]. The murine submandibular gland carcinoma cell line CSG is another polarized cell line that has been utilized to determine a role for occludin on paracellular permeability and the cytoplasmic plaque of TJ associated proteins in salivary epithelium [111]. To our knowledge, SMIE or CSG cells have not been yet grown on Matrigel; however, further study is warranted to determine whether they are able to form acinar spheres.
Previous reports described methods for culturing human primary submandibular cells that formed functional TJs [139, 140]. These in vitro cell systems could be used to study TJ function and to understand TJ formation during salivary gland regeneration. Other studies have been able to reconstitute three-dimensional human salivary gland tissues that exhibit TJ and secrete α-amylase [136]; however, due to the difficulty of obtaining human tissue samples, these approaches are limited.
The identification of molecular components of TJ has enabled researchers to analyze TJ functions by generating knockout mice of the several TJ proteins, a review of which can be found in reference number [141] of the current review. In addition, positional cloning has identified mutations in the genes of several TJ components in hereditary human and cattle diseases, further demonstrating critical roles for TJ in various organs [141]. TJ proteins in salivary glands (e.g., for claudin-1, -5, -16, occluding, and ZO-1) [141] may be studied in knockout mouse models. However, because salivary gland structure and function have not been yet studied in mice models, future studies involving in vivo models are warranted.
6. Concluding Remarks
Interactions between different combinations of the TJ family (each with their own unique regulatory proteins) define tissue specificity and functions during physiopathological processes; however, these interaction patterns have not been studied in salivary glands. Therefore, further research should determine how external signals modulate TJ structure and function in salivary glands.
At sites of epithelial cell damage, loss of TJ integrity represents a signal for cells to begin a repair program involving proliferation and migration activities at the wound edge, ending with the reassembly of TJ to reform an intact epithelial layer [142]. In Sjögren's syndrome (an autoimmune secretory disorder), lymphocytic infiltrates cause damage in ductal and acinar epithelial cells, which leads to salivary gland dysfunction [143]. Furthermore, Sjögren's syndrome-related proinflammatory cytokines compromised TJ integrity and resulted in salivary epithelial dysfunction [35]. Therefore, dysregulation cell-cell contacts by TJ alteration must occur in Sjögren's syndrome.
Additional studies are required to understand TJ regulation, both in healthy and diseased salivary glands. Such research could result in improved detection (i.e., early markers for salivary epithelial dysfunction) and better treatments (e.g., modulation of TJ to improve secretion).
Acknowledgment
Part of this work was supported by the NIH-NIDCR Grant no. K08 DE017633-01 and a Sjögren's Syndrome Foundation Research grant.
References
- 1.Sasaki H. Freeze-fracture analysis of renal-epithelial tight junctions. Methods in Molecular Medicine. 2003;86:155–166. doi: 10.1385/1-59259-392-5:155. [DOI] [PubMed] [Google Scholar]
- 2.Nagafuchi A. Molecular architecture of adherens junctions. Current Opinion in Cell Biology. 2001;13(5):600–603. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 3.Green KJ, Jones JCR. Desmosomes and hemidesmosomes: structure and function of molecular components. The FASEB Journal. 1996;10(8):871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- 4.Yeager M, Harris AL. Gap junction channel structure in the early 21st century: facts and fantasies. Current Opinion in Cell Biology. 2007;19(5):521–528. doi: 10.1016/j.ceb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lourenço SV, Kapas S. Integrin expression in developing human salivary glands. Histochemistry and Cell Biology. 2005;124(5):391–399. doi: 10.1007/s00418-005-0784-3. [DOI] [PubMed] [Google Scholar]
- 6.Lourenço SV, Kapas S, Williams DM, Leite K, Araújo VC. Expression patterns of integrins on pleomorphic adenoma and adenoid cystic carcinoma: study on specimens and in vitro investigation of the effects of extracellular matrix on the expression of these adhesion molecules. Journal of Oral Pathology and Medicine. 2004;33(9):574–580. doi: 10.1111/j.1600-0714.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 7.Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Developmental Cell. 2006;10(1):21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Walker JL, Menko AS, Khalil S, et al. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Developmental Dynamics. 2008;237(11):3128–3141. doi: 10.1002/dvdy.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menko AS, Zhang L, Schiano F, Kreidberg JA, Kukuruzinska MA. Regulation of cadherin junctions during mouse submandibular gland development. Developmental Dynamics. 2002;224(3):321–333. doi: 10.1002/dvdy.10111. [DOI] [PubMed] [Google Scholar]
- 10.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Communication and Signaling. 2009;7, article 4 doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serre-Beinier V, Mas C, Calabrese A, et al. Connexins and secretion. Biology of the Cell. 2002;94(7-8):477–492. doi: 10.1016/s0248-4900(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki Y, Shiba Y, Kanno Y. Delayed inhibition of gap-junctional intercellular communication in the acinar cells of rat submandibular glands induced by parasympathectomy and cholinergic agonists. Comparative Biochemistry and Physiology A. 1995;110(1):57–64. doi: 10.1016/0300-9629(94)00148-m. [DOI] [PubMed] [Google Scholar]
- 13.Danen EHJ, Lafrenie RM, Miyamoto M, Yamada KM. Integrin signaling: cytoskeletal complexes, MAP kinase activation, and regulation of gene expression. Cell Communication and Adhesion. 1998;6(2-3):217–224. doi: 10.3109/15419069809004477. [DOI] [PubMed] [Google Scholar]
- 14.Lafrenie RM, Yamada KM. Integrins and matrix molecules in salivary gland cell adhesion, signaling, and gene expression. Annals of the New York Academy of Sciences. 1998;842:42–48. doi: 10.1111/j.1749-6632.1998.tb09630.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nature Reviews Molecular Cell Biology. 2001;2(4):285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 16.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. The American Journal of Physiology. 2000;279(5):G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Advanced Drug Delivery Reviews. 2005;57(6):815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochimica et Biophysica Acta. 2008;1778(3):670–691. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News in Physiological Sciences. 2001;16(3):126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 20.Staehelin LA. Further observations on the fine structure of freeze cleaved tight junctions. Journal of Cell Science. 1973;13(3):763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 21.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. Journal of Cell Biology. 1998;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. Journal of Cell Biology. 1993;123(6):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martìn-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. Journal of Cell Biology. 1998;142(1):117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. Journal of Biological Chemistry. 1998;273(45):29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 25.Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(16):7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz KL, Siahaan TJ. Molecular structure of the apical junction complex and its contribution to the paracellular barrier. Journal of Pharmaceutical Sciences. 1997;86(9):977–984. doi: 10.1021/js970134j. [DOI] [PubMed] [Google Scholar]
- 27.Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. Journal of Immunology. 2003;171(11):6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 28.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Molecular Biology of the Cell. 2002;13(9):3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology. 2000;119(6):1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- 30.Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. Journal of Surgical Research. 2004;116(1):14–18. doi: 10.1016/s0022-4804(03)00311-1. [DOI] [PubMed] [Google Scholar]
- 31.Schulzke J-D, Schulzke I, Fromm M, Riecken E-O. Epithelial barrier and ion transport in coeliac sprue: electrical measurements on intestinal aspiration biopsy specimens. Gut. 1995;37(6):777–782. doi: 10.1136/gut.37.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. American Journal of Gastroenterology. 2002;97(8):2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 33.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. The American Journal of Physiology. 2006;290(4):G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 34.Quesnell RR, Erickson J, Schultz BD. Apical electrolyte concentration modulates barrier function and tight junction protein localization in bovine mammary epithelium. The American Journal of Physiology. 2007;292(1):C305–C318. doi: 10.1152/ajpcell.00567.2005. [DOI] [PubMed] [Google Scholar]
- 35.Baker OJ, Camden JM, Redman RS, et al. Proinflammatory cytokines tumor necrosis factor-α and interferon-γ alter tight junction structure and function in the rat parotid gland Par-C10 cell line. The American Journal of Physiology. 2008;295(5):C1191–C1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tandler B. Structure of the duct system in mammalian major salivary glands. Microscopy Research and Technique. 1993;26(1):57–74. doi: 10.1002/jemt.1070260107. [DOI] [PubMed] [Google Scholar]
- 37.Tandler B. Introduction to mammalian salivary glands. Microscopy Research and Technique. 1993;26(1):1–4. doi: 10.1002/jemt.1070260102. [DOI] [PubMed] [Google Scholar]
- 38.Hand AR, Pathmanathan D, Field RB. Morphological features of the minor salivary glands. Archives of Oral Biology. 1999;44(supplement 1):S3–S10. doi: 10.1016/s0003-9969(99)90002-x. [DOI] [PubMed] [Google Scholar]
- 39.Tandler B, Phillips CJ. Structure of serous cells in salivary glands. Microscopy Research and Technique. 1993;26(1):32–48. doi: 10.1002/jemt.1070260105. [DOI] [PubMed] [Google Scholar]
- 40.Tandler B. Structure of mucous cells in salivary glands. Microscopy Research and Technique. 1993;26(1):49–56. doi: 10.1002/jemt.1070260106. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Diseases. 2002;8(3):117–129. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 42.Gorr S-U, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. Journal of Dental Research. 2005;84(6):500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tandler B, Pinkstaff CA, Riva A. Ultrastructure and histochemistry of human anterior lingual salivary glands (glands of Blandin and Nuhn) Anatomical Record. 1994;240(2):167–177. doi: 10.1002/ar.1092400204. [DOI] [PubMed] [Google Scholar]
- 44.Hamosh M, Burns WA. Lipolytic activity of human lingual glands (Ebner) Laboratory Investigation. 1977;37(6):603–608. [PubMed] [Google Scholar]
- 45.Nagato T, Ren X-Z, Toh H, Tandler B. Ultrastructure of Weber’s salivary glands of the root of the tongue in the rat. Anatomical Record. 1997;249(4):435–440. doi: 10.1002/(SICI)1097-0185(199712)249:4<435::AID-AR2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Martinez JR. Ion transport and water movement. Journal of Dental Research. 1987;66:638–647. doi: 10.1177/00220345870660S206. [DOI] [PubMed] [Google Scholar]
- 47.Martinez JR. Cellular mechanisms underlying the production of primary secretory fluid in salivary glands. Critical Reviews in Oral Biology and Medicine. 1990;1(1):67–78. doi: 10.1177/10454411900010010601. [DOI] [PubMed] [Google Scholar]
- 48.Martinez JR, Cassity N. Salivary secretion induced from isolated, perfused rat submandibular glands by sympathomimetic agents. Archives of Oral Biology. 1983;28(12):1101–1108. doi: 10.1016/0003-9969(83)90165-6. [DOI] [PubMed] [Google Scholar]
- 49.Martinez JR, Holzgreve H, Frick A. Micropuncture study of submaxillary glands of adult rats. Pflügers Archiv für die Gesamte Physiologie des Menschen und der Tiere. 1966;290(2):124–133. doi: 10.1007/BF00363690. [DOI] [PubMed] [Google Scholar]
- 50.Anderson LC, Garrett JR, Zhang X, Proctor GB. Protein secretion from rat submandibular acini and granular ducts: effects of exogenous VIP and substance P during parasympathetic nerve stimulation. Comparative Biochemistry and Physiology A. 1998;119(1):327–331. doi: 10.1016/s1095-6433(97)00426-1. [DOI] [PubMed] [Google Scholar]
- 51.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annual Review of Physiology. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 52.Petersen OH. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. Journal of Physiology. 1992;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell DW. Barrier function of epithelia. The American Journal of Physiology. 1981;241(4):G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 54.Vreugdenhil AP, Nieuw Amerongen AV, de Lange GL, Roukema PA. Localization of amylase and mucins in the major salivary glands of the mouse. Histochemical Journal. 1982;14(5):767–780. doi: 10.1007/BF01033626. [DOI] [PubMed] [Google Scholar]
- 55.Aktories K, Barbieri JT. Bacterial cytotoxins: targeting eukaryotic switches. Nature Reviews Microbiology. 2005;3(5):397–410. doi: 10.1038/nrmicro1150. [DOI] [PubMed] [Google Scholar]
- 56.Adams RB, Planchon SM, Roche JK. IFN-γ modulation of epithelial barrier function: time course, reversibility, and site of cytokine binding. Journal of Immunology. 1993;150(6):2356–2363. [PubMed] [Google Scholar]
- 57.Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon γ and tumour necrosis factor α on T84 cell function. Gut. 1999;45(2):191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francoeur C, Escaffit F, Vachon PH, Beaulieu J-F. Proinflammatory cytokines TNF-α and IFN-γ alter laminin expression under an apoptosis-independent mechanism in human intestinal epithelial cells. The American Journal of Physiology. 2004;287(3):G592–G598. doi: 10.1152/ajpgi.00535.2003. [DOI] [PubMed] [Google Scholar]
- 59.Li WY, Huey CL, Yu ASL. Expression of claudin-7 and -8 along the mouse nephron. The American Journal of Physiology. 2004;286(6):F1063–F1071. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 60.Madara JL, Stafford J. Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. Journal of Clinical Investigation. 1989;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(2):511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120(2):411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 63.Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Annals of the New York Academy of Sciences. 2000;915:129–135. doi: 10.1111/j.1749-6632.2000.tb05235.x. [DOI] [PubMed] [Google Scholar]
- 64.Turksen K, Troy T-C. Barriers built on claudins. Journal of Cell Science. 2004;117(12):2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- 65.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. Journal of Cell Biology. 1999;147(6):1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rüffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. European Journal of Cell Biology. 2004;83(4):135–144. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- 67.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annual Review of Physiology. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 68.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. Journal of Cell Biology. 2001;153(2):263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. Journal of Cell Biology. 1998;143(2):391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarthy KM, Francis SA, McCormack JM, et al. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. Journal of Cell Science. 2000;113(19):3387–3398. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 71.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. Journal of Clinical Investigation. 2001;107(10):1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. Journal of Cell Biology. 1999;147(4):891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. Journal of Biological Chemistry. 2007;282(41):30005–30013. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 74.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3971–3976. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. Journal of Cell Biology. 2002;156(6):1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inai T, Sengoku A, Guan X, Hirose E, Iida H, Shibata Y. Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Archives of Histology and Cytology. 2005;68(5):349–360. doi: 10.1679/aohc.68.349. [DOI] [PubMed] [Google Scholar]
- 77.Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophysical Journal. 2003;84(3):1660–1673. doi: 10.1016/S0006-3495(03)74975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lourenço SV, Coutinho-Camillo CM, Buim MEC, Uyekita SH, Soares FA. Human salivary gland branching morphogenesis: morphological localization of claudins and its parallel relation with developmental stages revealed by expression of cytoskeleton and secretion markers. Histochemistry and Cell Biology. 2007;128(4):361–369. doi: 10.1007/s00418-007-0322-6. [DOI] [PubMed] [Google Scholar]
- 79.Peppi M, Ghabriel MN. Tissue-specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. Journal of Anatomy. 2004;205(4):257–266. doi: 10.1111/j.0021-8782.2004.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maria OM, Kim J-WM, Gerstenhaber JA, Baum BJ, Tran SD. Distribution of tight junction proteins in adult human salivary glands. Journal of Histochemistry and Cytochemistry. 2008;56(12):1093–1098. doi: 10.1369/jhc.2008.951780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quissell DO, Barzen KA, Redman RS, Camden JM, Turner JT. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cellular and Developmental Biology. Animal. 1998;34(1):58–67. doi: 10.1007/s11626-998-0054-5. [DOI] [PubMed] [Google Scholar]
- 82.Lipschutz JH, Li S, Arisco A, Balkovetz DF. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. Journal of Biological Chemistry. 2005;280(5):3780–3788. doi: 10.1074/jbc.M408122200. [DOI] [PubMed] [Google Scholar]
- 83.Amasheh S, Meiri N, Gitter AH, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. Journal of Cell Science. 2002;115(24):4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 84.Enck AH, Berger UV, Yu ASL. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. The American Journal of Physiology. 2001;281(5):F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 85.Hashimoto K, Oshima T, Tomita T, et al. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochemical and Biophysical Research Communications. 2008;376(1):154–157. doi: 10.1016/j.bbrc.2008.08.140. [DOI] [PubMed] [Google Scholar]
- 86.Kawedia JD, Nieman ML, Boivin GP, et al. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3621–3626. doi: 10.1073/pnas.0608384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. Journal of Biological Chemistry. 2006;281(47):36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 88.Sonoda N, Furuse M, Sasaki H, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. Journal of Cell Biology. 1999;147(1):195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hashizume A, Ueno T, Furuse M, Tsukita S, Nakanishi Y, Hieda Y. Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular gland. Developmental Dynamics. 2004;231(2):425–431. doi: 10.1002/dvdy.20142. [DOI] [PubMed] [Google Scholar]
- 90.Michikawa H, Fujita-Yoshigaki J, Sugiya H. Enhancement of barrier function by overexpression of claudin-4 in tight junctions of submandibular gland cells. Cell and Tissue Research. 2008;334(2):255–264. doi: 10.1007/s00441-008-0689-2. [DOI] [PubMed] [Google Scholar]
- 91.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. Journal of Cell Biology. 1999;147(1):185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. Journal of Cell Biology. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amasheh S, Schmidt T, Mahn M, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell and Tissue Research. 2005;321(1):89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 94.Morita K, Furuse M, Yoshida Y, et al. Molecular architecture of tight junctions of periderm differs from that of the maculae occludentes of epidermis. Journal of Investigative Dermatology. 2002;118(6):1073–1079. doi: 10.1046/j.1523-1747.2002.01774.x. [DOI] [PubMed] [Google Scholar]
- 95.Alexandre MD, Lu Q, Chen Y-H. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. Journal of Cell Science. 2005;118(12):2683–2693. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 96.Angelow S, Kim K-J, Yu ASL. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. Journal of Physiology. 2006;571(1):15–26. doi: 10.1113/jphysiol.2005.099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Angelow S, Schneeberger EE, Yu ASL. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. Journal of Membrane Biology. 2007;215(2-3):147–159. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 98.Cheung ST, Leung KL, Ip YC, et al. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clinical Cancer Research. 2005;11(2):551–556. [PubMed] [Google Scholar]
- 99.Aldred MA, Huang Y, Liyanarachchi S, et al. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. Journal of Clinical Oncology. 2004;22(17):3531–3539. doi: 10.1200/JCO.2004.08.127. [DOI] [PubMed] [Google Scholar]
- 100.Gow A, Southwood CM, Li JS, et al. CNS myelin and sertoli cell tight junction strands are absent in OSP/claudin-11 null mice. Cell. 1999;99(6):649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 101.Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. Journal of Cell Biology. 1999;145(3):579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gow A, Davies C, Southwood CM, et al. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. Journal of Neuroscience. 2004;24(32):7051–7062. doi: 10.1523/JNEUROSCI.1640-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tiwari-Woodruff SK, Buznikov AG, Vu TQ, et al. OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and β1 integrin and regulates proliferation and migration of oligodendrocytes. Journal of Cell Biology. 2001;153(2):295–305. doi: 10.1083/jcb.153.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285(5424):103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 105.Weber S, Schlingmann KP, Peters M, et al. Primary gene structure and expression studies of rodent paracellin-1. Journal of the American Society of Nephrology. 2001;12(12):2664–2672. doi: 10.1681/ASN.V12122664. [DOI] [PubMed] [Google Scholar]
- 106.Weber S, Schneider L, Peters M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Journal of the American Society of Nephrology. 2001;12(9):1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 107.Ohta H, Adachi H, Takiguchi M, Inaba M. Restricted localization of claudin-16 at the tight junction in the thick ascending limb of Henle’s loop together with claudins 3, 4, and 10 in bovine nephrons. Journal of Veterinary Medical Science. 2006;68(5):453–463. doi: 10.1292/jvms.68.453. [DOI] [PubMed] [Google Scholar]
- 108.Markov AG, Shadrin LV, Veshniakova AI, Amasheh S, Fromm M. The tight junction proteins claudin-2 and -16 expression in mammary epithelium of mice. Rossiĭskii fiziologicheskiĭ zhurnal imeni I.M. Sechenova. 2006;92(11):1382–1386. [PubMed] [Google Scholar]
- 109.Kriegs JO, Homann V, Kinne-Saffran E, Kinne RKH. Identification and subcellular localization of paracellin-1 (claudin-16) in human salivary glands. Histochemistry and Cell Biology. 2007;128(1):45–53. doi: 10.1007/s00418-007-0291-9. [DOI] [PubMed] [Google Scholar]
- 110.Furuse M, Itoh M, Hirase T, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. Journal of Cell Biology. 1994;127(6):1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. Journal of Cell Science. 1999;112(12):1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- 112.Saitou M, Fujimoto K, Doi Y, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. Journal of Cell Biology. 1998;141(2):397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. Journal of Cell Biology. 1996;134(4):1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mankertz J, Waller JS, Hillenbrand B, et al. Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochemical and Biophysical Research Communications. 2002;298(5):657–666. doi: 10.1016/s0006-291x(02)02487-7. [DOI] [PubMed] [Google Scholar]
- 115.Ghassemifar MR, Sheth B, Papenbrock T, Leese HJ, Houghton FD, Fleming TP. Occludin TM4−: an isoform of the tight junction protein present in primates lacking the fourth transmembrane domain. Journal of Cell Science. 2002;115(15):3171–3180. doi: 10.1242/jcs.115.15.3171. [DOI] [PubMed] [Google Scholar]
- 116.Muresan Z, Paul DL, Goodenough DA. Occludin 1B, a variant of the tight junction protein occludin. Molecular Biology of the Cell. 2000;11(2):627–634. doi: 10.1091/mbc.11.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Molecular Biology of the Cell. 2000;11(12):4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. Journal of Cell Biology. 2000;148(4):791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annual Review of Cell and Developmental Biology. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 120.Ebnet K. Organization of multiprotein complexes at cell-cell junctions. Histochemistry and Cell Biology. 2008;130(1):1–20. doi: 10.1007/s00418-008-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? Journal of Cell Science. 2004;117(1):19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 122.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98(13):3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 123.Kostrewa D, Brockhaus M, D’Arcy A, et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. The EMBO Journal. 2001;20(16):4391–4398. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on β1 integrins and Rap1 activity. Journal of Biological Chemistry. 2005;280(12):11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 125.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. Journal of Cell Biology. 2001;154(3):491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y, Nusrat A, Schnell FJ, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. Journal of Cell Science. 2000;113(13):2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 127.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annual Review of Biochemistry. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 128.Tepass U, Tanentzapf G. Epithelial cell polarity and cell junctions in Drosophila. Annual Review of Genetics. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 129.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Developmental Cell. 2007;13(5):609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. Journal of Cell Biology. 1986;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Umeda K, Ikenouchi J, Katahira-Tayama S, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126(4):741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 132.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. Journal of Biological Chemistry. 1999;274(49):35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 133.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. Journal of Cell Biology. 1997;138(1):181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Molecular Biology of the Cell. 2006;17(4):1922–1932. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Umeda K, Matsui T, Nakayama M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. Journal of Biological Chemistry. 2004;279(43):44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 136.Joraku A, Sullivan CA, Yoo J, Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007;75(4):318–324. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 137.Baker OJ, Schulz DJ, Camden JM. Rat Parotid Gland Cell Differentiation in 3D Culture. doi: 10.1089/ten.tec.2009.0438. Tissue Engineering Part C, [Epub ahead of print], 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.He X, Kuijpers GAJ, Goping G, et al. A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflugers Archiv European Journal of Physiology. 1998;435(3):375–381. doi: 10.1007/s004240050526. [DOI] [PubMed] [Google Scholar]
- 139.Szlávik V, Szabó B, Vicsek T, et al. Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Engineering Part A. 2008;14(11):1915–1926. doi: 10.1089/ten.tea.2007.0208. [DOI] [PubMed] [Google Scholar]
- 140.Tran SD, Wang J, Bandyopadhyay BC, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Engineering. 2005;11(1-2):172–181. doi: 10.1089/ten.2005.11.172. [DOI] [PubMed] [Google Scholar]
- 141.Furuse M. Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo. Biochimica et Biophysica Acta. 2009;1788(4):813–819. doi: 10.1016/j.bbamem.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 142.Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nature Cell Biology. 2001;3(5):E117–E123. doi: 10.1038/35074643. [DOI] [PubMed] [Google Scholar]
- 143.Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, Moutsopoulos HM. Modes of epithelial cell death and repair in Sjogren’s syndrome (SS) Clinical and Experimental Immunology. 1998;114(3):485–490. doi: 10.1046/j.1365-2249.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]