Abstract
Purpose
Hypertension and left ventricular (LV) hypertrophy often precede diastolic dysfunction and are risk factors for diastolic heart failure. Although pharmacologic inhibition of the renin angiotensin system (RAS) improves diastolic function and functional capacity in hypertensive patients with LV hypertrophy, the effects of combination therapy with an angiotensin converting enzyme inhibitor (ACEi) and an angiotensin receptor blocker (ARB) are unclear.
Methods
We assessed the effects of the combined 10-week administration of lisinopril (10 mg/kg/day, p.o.) and losartan (10 mg/kg/day, p.o.) (LIS/LOS) on diastolic function and LV structure in seven young (5 wks), prehypertensive congenic mRen2.Lewis male rat, a model of tissue renin overexpression and angiotensin II (Ang II)-dependent hypertension compared to vehicle (VEH) treated (n=7), age-matched rats.
Results
Systolic blood pressures were 64% lower with the combination therapy (P<0.001), but there were no differences in heart rate or systolic function between groups. RAS inhibition increased myocardial relaxation, defined by tissue Doppler mitral annular descent (e’), by 2.2 fold (P< 0.001). The preserved lusitropy in the LIS/LOS-treated rats was accompanied by a reduction in phospholamban-to-SERCA2 ratio (P<0.001). Despite lower relative wall thicknesses (VEH: 1.56 ± 0.17 vs. LIS/LOS: 0.78 ± 0.05) and filling pressures, defined by the transmitral Doppler-to-mitral annular descent ratio (E/e’, VEH: 28.7 ± 1.9 vs. LIS/LOS: 17.96 ± 1.5), no differences in cardiac collagen were observed.
Conclusion
We conclude that the lusitropic benefit of early dual RAS blockade may be due to improved vascular hemodynamics and/or cardiac calcium handling rather than effects on extracellular matrix reduction.
Keywords: diastolic function, fibrosis, hypertension, myocardial relaxation, renin-angiotensin system (RAS)
Introduction
Recent treatment guidelines emphasize the use of combination therapy to control blood pressure thereby decreasing the risk of hypertension-related events such as diastolic dysfunction (Chobanian et al., 2003; Mancia et al., 2007; Williams et al., 2004). Among the potential therapeutic combinations, dual renin angiotensin system (RAS) blockade using angiotensin converting enzyme (ACE) inhibitors (ACEi) and angiotensin II (Ang II) type 1 receptor (AT1) blockers (ARBs), has been proposed to achieve more complete RAS suppression, better blood pressure control, and incremental reno- and cardioprotective effects when compared to either drug alone (Kolesnyk et al., 2007; Werner et al., 2008). The additional rationale for this type of combination therapy is based on mechanistic evidence regarding incomplete suppression of Ang II formation by ACE inhibitors due in part to increased renin activity and higher Ang I. This “escape phenomenon” ultimately results in the plasma levels of Ang II returning to their pretreatment values (Roig et al., 2000; van den Meiracker et al., 1992). However, clinical experience with the combination of ACE-inhibitors and ARBs has produced conflicting results. Most studies show a modest reduction in systolic and diastolic pressure when an ARB is combined with an ACE inhibitor and visa versa (Doulton et al., 2005). In addition, a meta-analysis involving more than 6,000 hypertensive patients found that dual RAS blockade reduced proteinuria to a greater extent (~ 25%) than either drug alone that cannot be attributed to differences in blood pressure (Kunz et al., 2008). In spite of this, findings from the ONTARGET (Renal Outcomes With Telmisartan, Ramipril, or Both, in People at High Vascular Risk) study of more doubling of creatinine and need for dialysis in the combination arm of telmisartan and ramipril when compared to monotherapy have cast doubts on the potential synergistic advantage of dual RAS blockade (Mann et al., 2008). Likewise, in systolic heart failure patients, addition of candesartan to an ACE inhibitor in the CHARM-Added trial (Effects of Candesartan in Patients With Chronic Heart Failure and Reduced Left-Ventricular Systolic Function Taking Angiotensin-Converting-Enzyme Inhibitors), reduced heart failure hospitalizations, but more patients discontinued study medications in the combination arm due to adverse side effects, including increases in plasma creatinine and potassium, than in the placebo/ACEi arm (McMurray et al., 2003). Hypotension, worsening of renal function, and hyperkalemia were also more common with combination therapy than with ACE inhibitors alone in over 18,000 patients with LV dysfunction (Lakhdar et al., 2008). While these outcome and safety data have evoked skepticism with regard to dual RAS blockade for hypertension, renoprotection and heart failure, the therapeutic potential of combination RAS blockade for preclinical diastolic dysfunction remains unclear.
Several experimental and clinical studies have suggested the beneficial effects of RAS blockade, using either ACE inhibitors or ARBs in both hypertensive and hypertensive-prone animals and patients to improve diastolic function, via reductions in LV hypertrophy, myocardial fibrosis, and vascular stiffness. Our laboratory and others have demonstrated that ACEi and ARBs normalize blood pressure, improve left ventricular (LV) function, reverse cardiac hypertrophy and proteinuria in experimental rodent models of hypertension; changes that are often associated with a compensatory activation of the angiotensin converting enzyme 2 (ACE2)/angiotensin-(1-7) [Ang-(1-7)]/mas receptor axis (Chappell, 2007; Ferrario et al., 2005a; Ferrario et al., 2005b; Ferrario et al., 2005c; Jessup et al., 2006; Varo et al., 1999; Varo et al., 2000). In addition, sub depressor doses of ARBs attenuated diastolic impairment and LV remodeling in the Dahl, salt-sensitive rat, in part, through their anti-inflammatory effects (Nishio et al., 2007). Interestingly, the cardio-, vascular-, and renoprotective effects of ACEi and ARBs persisted through adulthood when treatment was initiated in young, prehypertensive rats (Baumann et al., 2007a; Baumann et al., 2007b; Baumann et al., 2007c; Berecek et al., 2005). Long-lasting effects of the AT1 antagonist, olmesartan, were observed following cessation of treatment in the female hypertensive mRen2.Lewis strain (Chappell et al., 2003). Preemptive therapeutic RAS blockade with either an ACEi or an ARB in prehypertensive humans, characterized by “high normal” blood pressures, also delayed the development of hypertension (Julius et al., 2006; Luders et al., 2008). Likewise, the Vascular Improvement with Olmesartan medoxomil Study (VIOS) showed that AT1 blockade using olmesartan normalized blood pressure and reversed vascular hypertrophy in prehypertensive subjects who otherwise would not have been treated by current clinical guidelines (Smith et al., 2008). This attenuation in vascular remodeling was a pressure-independent effect of olmesartan as similar pressure reductions using atenolol had no effect on arterial morphology (Smith et al., 2008).
Therefore, we investigated the effect of combined early administration of ACEi and ARB in a model of experimental hypertension that over-expresses the mouse Ren2 gene. The mRen2.Lewis, which we have extensively characterized elsewhere (Groban et al., 2008; Jessup et al., 2006; Pendergrass et al., 2006; Pendergrass et al., 2008; Yamaleyeva et al., 2007a; Yamaleyeva et al., 2007b) expresses higher circulating Ang II, but similar cardiac levels of the peptide (Pendergrass et al., 2006; Pendergrass et al. 2008) The congenic strain is derived from the original transgenic [mRen2]27 rat developed by Mullins et al (Mullins et al., 1990; Mullins and Ganten, 1990) and backcrossed in the inbred Lewis. The transgenic model exhibits accelerated hypertension accompanied by hypertension-induced cardiac and vascular hypertrophy (Bachmann et al., 1992; Bohm et al., 1996; Ohta et al., 1996) as well as disturbances in body fluid regulation and renal function (Gross et al., 1996; Springate et al., 1994); key factors leading to diastolic dysfunction. Accordingly, we hypothesized that early administration of chronic antagonism of Ang II synthesis and AT1 signal transduction prevents the development of hypertension and preserves diastolic function in the mRen2.Lewis strain.
Methods
Animals
Male mRen2.Lewis rats were obtained from the Hypertension and Vascular Research Center Congenic Colony of Wake Forest University School of Medicine. Rats were housed in an Association for Assessment and Accreditation of Laboratory Animal-approved facility individual cages (12-hr light/dark cycle) with ad libitum access to rat chow and tap water.
Experimental protocol
Rats (5 wks of age) were randomly assigned to drink either tap water (vehicle, n = 4) or tap-water to which lisinopril and losartan (combination, 10 mg/kg/day of each, n = 7) were added for 10 consecutive weeks. Doses were chosen based on efficacy in other rodent models using blood pressure, neurohormonal, and renal endpoints (Ferrario et al. 2005a, Ferrario et al. 2005b, Jessup et al. 2006). Systolic blood pressure was determined by tail-cuff plethysmography at the conclusion of treatment. Immediately prior to decapitation, an experienced echocardiographer (L. Groban) who was masked to the treatment protocol performed transthoracic echocardiography on the anesthetized rats [ketamine HCl (60 mg/kg) and xylazine HCl (5 mg/kg)]. Once the echocardiograms were completed, the rats were decapitated. The hearts were removed and weighed with one half being submerged in 10% formalin and the other frozen on dry ice for biochemical analyses.
Echocardiographic studies
The echocardiograms were obtained in sedated animals as previously described using a Philips Envisor echocardiograph (Philips Medical Systems, Andover, MA) and a 12 MHz phased array probe (Groban et al., 2008). Left ventricular end-diastolic and end-systolic diameters (LVEDD and LVESD, respectively), LV posterior wall thickness (PWT), and anterior wall thickness (AWT) were measured from midpapillary, short-axis images obtained by M-mode echocardiography. The percentage of LV fractional shortening (%FS), an index of contractile function, was calculated as FS (%) = [(LVEDD – LVESD)/LVEDD] × 100. LV mass was calculated using a standard cube formula, which assumes a spherical LV geometry according to the formula: LV mass (LVmass) = 1.04 × [[LVEDD + PWT + AWT]3 – LVEDD], where 1.04 is the specific gravity of muscle. Relative wall thickness (RWT) was calculated as: 2 x PWT/ LVEDD. Mitral inflow measurements of early and late filling velocities (Emax and Amax, respectively), deceleration slope (Edec slope), isovolumic relaxation time (IVRT), and early deceleration time (Edec time) were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Doppler tissue imaging (DTI) to assess early mitral valve annular velocity (e’) was measured from the apical four-chamber view with the pulse-wave Doppler sample volume placed at the base of the left ventricle, in the region of the septal mitral annulus. All measurements were performed with an off-line analysis system (Cardiac Workstation, Freeland Systems) by one observer who was blinded to previous results. An average of at least five consecutive cardiac cycles to minimize beat-to-beat variability was used for all represented measured and calculated systolic and diastolic indices.
Histopathology
Vertical, long-axis sections of the formalin-fixed heart were taken through the left ventricle. Specimens were dehydrated with ethanol and embedded into paraffin blocks. Following microtome sectioning, the 4 μm tissue sections were stained with Verhoeff-van Gieson (VVG) and picrosirius red (PSR) for assessment of collagen and elastin fibers. The sections were examined under both bright field and polarized light using a Leica DM4000B microscope system (Bannockburn, IL) and an Olympus polarizing microscope system (Center Valley, PA), respectively. Bright field photomicrographs were captured with a Leica DFC digital camera and processed using Leica Application Suite software, while polarized images were taken using Diagnostic Instruments Inc. Digital SPOT RT, 3-pass capture, thermoelectrically cooled charge-coupled camera (Sterling Heights, MI) and processed using the SPOT® Advanced software. A blinded observer took 2 images from each of 4 randomized quadrant fields totalling 8 images per section under bright field and with polarization magnified 200-times. The digitized images of equal pixel composition were analyzed using Adobe Photoshop Creative Suite 3. The quantified collagen content was determined as a ratio of VVG-stained or PSR-stained pixels divided by total pixels.
Western blot hybridization
Sarcoplasmic reticulum (SR) membranes were prepared as previously reported (Groban et al., 2006). Immunoblots were ran and probed for sarcoplasmic endoplasmic reticulum ATPase (SERCA2) antibody (1:1000 dilution; Abcam, Cambridge, MA) and phospholamban (PLB) (1:5000 dilution; Abcam, Cambridge, MA) as previously detailed (Groban et al., 2006). The PLB-to-SERCA2 ratio was used as a measure of SERCA2 inhibition. To normalize the variability of protein loading, the antibody to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5000 dilution; Cell Signaling, Danvers, MA) was probed onto the SERCA2 and PLB-stripped membranes (Western Blot Recycling Kit; Alpha Diagnostic International, Inc., San Antonio, TX). The bands corresponding to SERCA2, PLB, and GAPDH were scanned and digitized (MCDI image analysis software; Imaging Research Inc., Ontario, Canada). Each SERCA2 and PLB bands were normalized to its own GAPDH band.
Statistical analysis
All values are expressed as mean ± SEM. Two-tailed Student’s t-tests were used for comparing differences in the vehicle vs. combination groups at a p value < 0.05.
Results
Ten weeks of dual RAS blockade in the mRen2.Lewis rat, significantly reduced body weights compared to vehicle treatment (LIS/LOS: 357 ± 6 g vs. VEH: 426 ± 8 g, respectively), but did not affect urine output (LIS/LOS: 22.7 ± 1.1 mL/24 h vs 25.0 ± 1.7 mL/24 h). The tail-cuff systolic arterial pressure in congenic rats medicated with the combination therapy was 64% less than the rats that were maintained on vehicle treatment (210 ± 2 mmHg vs. 76 ± 4 mmHg, respectively). The treatment had no effect on cardiac rate (Table 1).
Table 1. M-mode-derived measures of Left Ventricular Dimension, Wall Thickness, and Systolic Function.
| Variables | Vehicle | Combination |
|---|---|---|
| Heart Rate, bpm P = |
243 ± 11 | 260 ± 2 0.18 |
| LVEDD, cm P = |
0.68 ± 0.02 | 0.79 ± 0.02 0.004 |
| LVESD, cm P = |
0.37 ± 0.02 | 0.44 ± 0.02 0.04 |
| RWTed, cm P < |
0.78 ± 0.05 | 0.37 ± 0.02 0.0001 |
| PWTed, cm P < |
0.27 ± 0.02 | 0.15 ± 0.01 0.0001 |
| AWTed, cm P < |
0.23 ± 0.01 | 0.14 ± 0.01 0.0001 |
|
LV Mass, g P < |
1.56 ± .17 |
0.81 ± .07 0.001 |
| %FS P = |
46 ± 3 | 45 ± 2 0.77 |
Data are expressed as mean ± SEM. LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; RWTed = relative wall thickness; PWTed = posterior wall thickness at end diastole; AWTed = anterior wall thickness at end diastole; LV Mass = left ventricular mass; FS = fractional shortening.
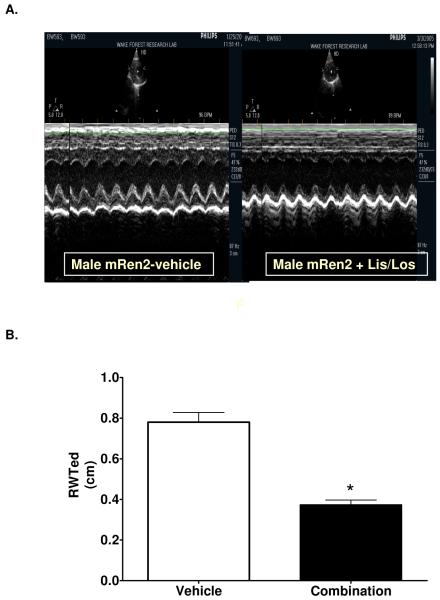
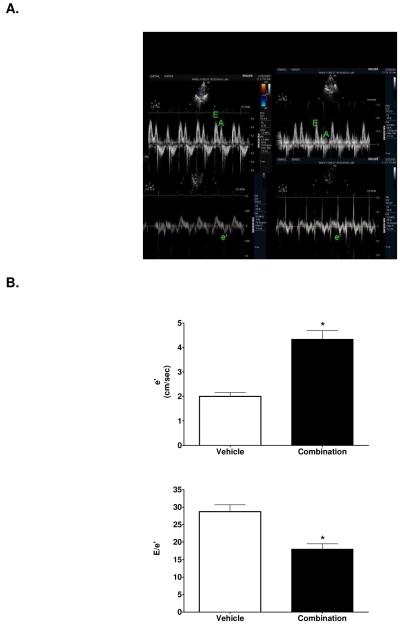
Table 1 summarizes the M-mode measurements of LV dimensions, wall thicknesses, LV mass and systolic function. LV end-diastolic and end-systolic dimensions were higher in treated rats, which was accompanied by a lower relative wall thickness (Figure 1). Since body weights were significantly lower in treated rats, LV mass was normalized to body weight. LV mass normalized to body weight, also known as LV mass index, was significantly lower in LIS/LOS-treated rats compared to saline-treated control rats (.0023 ± .0002 vs .0036 ± .0004 mg/gram body weight). There was no effect of the treatment on percent fractional shortening. Using Doppler flow and Doppler tissue imaging, assessment of diastolic function revealed a significantly lower isovolumic relaxation time and a higher mitral annular descent (e’) in treated rats (Table 2 and Figure 2). RAS blockade resulted in a greater E wave to A wave ratio, a function most likely due to the 1.4-fold higher maximum early filling velocity of the left ventricle through the mitral valve. The preserved myocardial relaxation elicited by inhibiting Ang II synthesis as well as the activity at its receptor resulted in a 37% lower filling pressure, as determined by the ratio of early transmitral filling velocity to early mitral annular velocity (E/e’) (P < 0.001) (Figure 2).
Figure 1.
A. Representative M-mode echocardiogram of a mRen2.Lewis receiving either vehicle (left panel) or combination (right panel). B. Relative wall thickness (RWT). Data represent mean ± SEM. *, P < 0.001 compared to vehicle.
Table 2. Echocardiographic Indices of Diastolic Function.
| Variables | Vehicle | Combination |
|---|---|---|
| Emax, cm/sec P < |
54 ± 2 | 74 ± 1 0.0001 |
| Amax, cm/sec P = |
43 ± 5 | 39 ± 2 0.54 |
| E/A P = |
1.4 ± 0.2 | 1.9 ± 0.1 0.02 |
| Edectime, sec P = |
0.058 ± 0.003 | 0.058 ± 0.001 0.87 |
| e’, cm/sec P < |
2.0 ± 0.2 | 4.3 ± 0.4 0.0001 |
| E/e’ P < |
28.7 ± 1.9 | 18.0 ± 1.5 0.0001 |
| IVRT, sec P < |
0.037 ± 0.003 | 0.021 ± 0.0001 0.0001 |
Data are expressed as mean ± SEM. Emax = maximum early transmitral filling velocity; Amax = maximum late transmitral filling velocity; E/A = early-to-late transmitral filling ratio; Edectime = early-filling deceleration time; e’ = early mitral annular velocity; E/e’ = early transmitral filling velocity-to-mitral annular velocity; late mitral annular velocity; IVRT = isovolumic relaxation time.
Figure 2.
A. Representative tissue Doppler image of a mRen2.Lewis receiving either vehicle (left panel) or combination (right panel). E = early transmitral filling velocity; A = late transmitral filling velocity; e’ = early mitral annular descent (septal) velocity. B. Early mitral annular velocity (e’) and early transmitral filling velocity – to – mitral annular velocity ratio (E/e’). Data represent mean ± SEM. *, P < 0.0001 compared to vehicle.
Systolic blood pressure and relative wall thickness in the mRen2.Lewis rats were linked to TDI measures of septal mitral annular descent. Specifically, tissue Doppler imaging of myocardial motion, or e’, showed significant inverse correlations with blood pressure and relative wall thickness (r = −0.83, P = 0.002 and r = −0. −0.72, P < 0.01, respectively). As expected, systolic blood pressure was significantly correlated with RWT (r = 0.95, P < 0.0001).
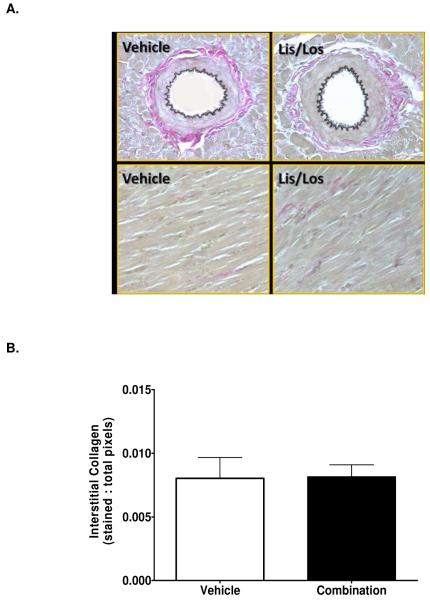
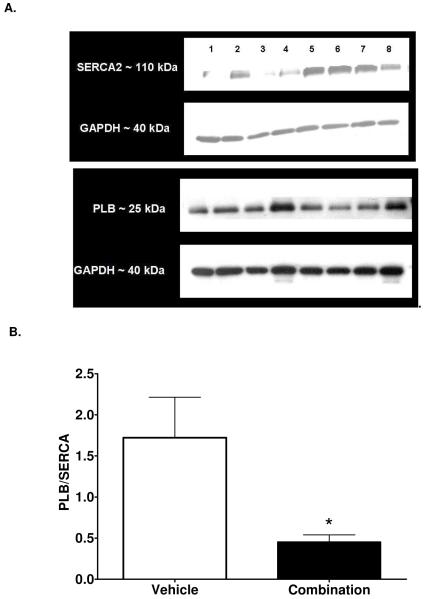
Interstitial (Figures 3 and 4) and perivascular (0.4 ± 0.1 % vs. 0.5 ± 0.2 % total area, n=6 each group, P = 0.86) collagen content following RAS blockade was not different compared to vehicle treatment (Figures 3 and 4). Displayed in Figure 5, the resulting immunoblots probed for SERCA2 and Phospholamban (PLB) and their respective GAPDH blots revealed single bands of molecular weight 110 kDa, 25 kDa, and 40 kDa, respectively. The ratio of PLB- to -SERCA2 levels normalized to their respective GAPDH decreased 74% in the mRen2.Lewis medicated with the ACEi and ARB compared to VEH treatment (Figure 5).
Figure 3.
Representative VVG staining showing perivascular and interstitial collagen content in vehicle- and combination-treated rats (upper panel). Quantification of interstitial collagen shown in lower panel. Data represent mean ± SEM. *, P < 0.05 compared to vehicle, n = 6. Magnification = 200x.
Figure 4.
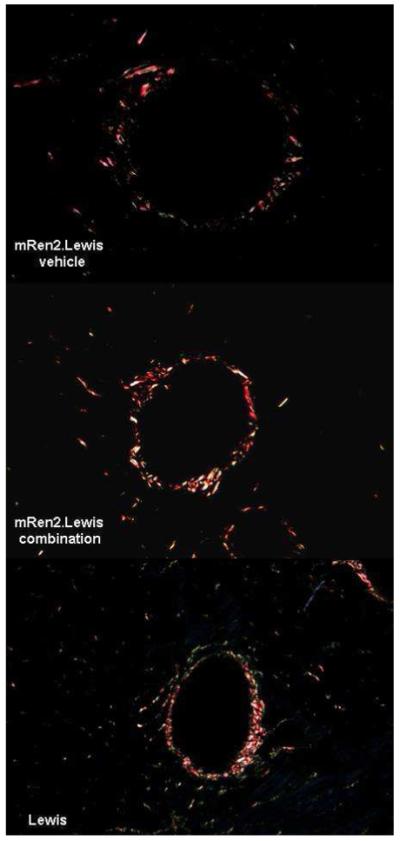
Representative picrosirius red staining showing perivascular collagen in vehicle- and combination-treated rats mRen2.Lewis rats compared to a normotensive Lewis rat. Magnification = 200x.
Figure 5.
A. Representative immunoblots of SERCA and PLB with their respective GAPDH loading controls from vehicle and combination treated rats. B. Protein concentration of phospholamban to sarco/endoplasmic reticulum ATPase (SERCA) 2. Data represent mean ± SEM. *, P < 0.05 compared to vehicle.
Discussion
In the current study we examined the effect of early dual RAS blockade on cardiac structure and diastolic function in young, prehypertensive male mRen2.Lewis rats. Chronic inhibition of Ang II synthesis combined with blockade of Ang II’s actions at the AT1 receptor effectively prevented the development of LV hypertrophy and the functional alterations of the LV relaxation phase. While these effects were associated with reduced LV filling pressures, indicated by a relatively lower E/e’ ratio compared to vehicle-treated mRen2.Lewis rats, markers of collagen deposition were comparable in both treated and vehicle rats. The preserved LV lusitropy with dual RAS blockade was coupled by decreases in phospholamban-to-SERCA2 ratios and low, normal systemic blood pressures compared to untreated rats. Taken together, our data show that early commencement of dual RAS blockade attenuated the development of hypertension and early diastolic dysfunction in the 15 week old mRen2.Lewis male rat.
Arterial hypertension is a common etiologic factor in the development of diastolic dysfunction, even with the presence of normal systolic function. The transmitral Doppler filling and time interval abnormalities specific to our 15 week old untreated hypertensive mRen2.Lewis rats include decreases in E velocity and E/A ratio, and prolongation in IVRT compared to the RAS blockade-treated cohort. Although the echocardiographic changes are similar to that which has been reported in patients with hypertension and delayed relaxation (Gardin et al., 1987; Pearson et al., 1987) the lack of an increase in late filling (A velocity) or a prolongation in deceleration time or reversal in E/A suggests a transitioning to a pseudonormal pattern. It is well known that diastolic filling is determined by the atrial-ventricular pressure gradient and is, therefore, regulated not only by relaxation but the driving pressure and compliance of the LV (Appleton et al., 1988; Thomas and Weyman, 1991). Accordingly, we used pulsed-wave tissue Doppler imaging of mitral annular descent, or e’, as a preload independent index of LV relaxation to differentiate pseudonormal from normal Doppler inflow patterns (Nagueh et al., 1997). Indeed, the untreated mRen2.Lewis rats exhibited a reduced e’ and increased E/e’ ratio, compared to treated mRen2.Lewis, suggesting that the increased afterload in the hypertensive mRen2.Lewis rats may have partially contributed to the impaired relaxation and increased filling pressure.
In the presence of LVH, lower mitral annular velocities in diastole have been reported and reduced early filling velocities have been correlated with the time constant of relaxation or tau (Kasner et al., 2007). Moreover, reductions in e’ have been noted in patients with LVH in whom mitral inflow velocities have been “normalized” due to elevations in left atrial pressure (Di, V et al., 2004; Wachtell et al., 2000). Although we cannot discount changes in chamber stiffness or compliance, increases in wall thickness were observed in our untreated, mRen2.Lewis cohort in the absence of myocardial fibrosis. These finding are in keeping with the observation that in 13 week old Dahl salt-sensitive hypertensive rats, increases in tau and LV mass were observed without a change in myocardial fibrosis (Masuyama et al., 2000). Indeed, the mechanisms by which LVH alters diastolic function are associated with changes in the extracellular matrix such as collagen infiltration (Weber et al., 1987), but also with increases in LV mass, myocyte diameter, altered calcium currents, subendocardial ischemia, and LV dyssynchrony (Gradman and Alfayoumi, 2006; Periasamy and Janssen, 2008; Tan et al., 2008).
Given that LVH is independently an important risk factor in hypertension, preventing its development and enforcing its regression are indisputable necessities. LVH regression leads to beneficial alterations in diastolic filling, limits the propensity for ventricular arrhythmias, improves systolic midwall performance, autonomic function, and coronary reserve (Agabiti-Rosei et al., 2006; Cuspidi et al., 2008; Muiesan et al., 2000; Shimizu et al., 2000; Teniente-Valente et al., 2008; Tsuyuki et al., 1997). Numerous studies have demonstrated that the use of ARBs facilitates the regression of LVH. Previous documentation has shown that an ARB (olmesartan 0.6 mg/kg/day) given to 17 week old Dahl salt-sensitive rats for three weeks reversed diastolic dysfunction, which has been, in part, attributed to the anti-inflammatory effects of the drug as assessed by measurements of NADPH oxidase activity, transforming growth factor-ß1 (TGF-ß1), and monocyte chemoattractant protein-1 (MCP-1) (Nishio et al., 2007). Rothermund et al.(Rothermund et al., 2001) reported that administration of eprosartan prevented LV diastolic functional impairment in [mRen2]27, independent of its anti-hypertensive action. Submaximal doses of eprosartan (6 mg/kg given for 20 wks from age 10 wk - 30 wk) elicited profound decreases in LV end-diastolic pressure and increases in LV relaxation (Rothermund et al., 2001), suggestive of improvements in LV compliance and lusitropy, respectively. It has been shown in canine experiments that acute exposure to the AT1 antagonist, L-158,809, significantly decreases LV end-diastolic pressure and chamber-as well as myocardial stiffness constants after saline loading in dogs with LVH (Hayashida et al., 1997). Clinically, ARB therapy improves diastolic function in diabetic patients with LV diastolic dysfunction (Kawasaki et al., 2007). The authors attributed the improvement in cardiac diastolic function to increased collagen turnover since the ratio of synthesis versus degradation decreased (Kawasaki et al., 2007). On an outcomes level, the CHARM-Preserved clinical trial found that candesartan reduced hospitalizations in the sub-group of heart failure patients with preserved ejection fraction (Yusuf et al., 2003).
Blocking ACE activity, similar to blocking AT1 receptors, results in beneficial outcomes when it comes to cardiac diastolic function, however, the mechanisms appear to be different. Satoh et al. (Satoh et al., 2003) demonstrated that ACEi restored sarcoplasmic reticulum Ca2+ uptake in 17 week old Dahl rats that had been treated for seven weeks with temocapril (10 mg/kg/day). Similarly, restoration of Ca2+ handling and correction of the overall Ca2+ homeostasis through the use of ACEi or even an ARB, was mechanistically deemed responsible for reducing LV systolic and end-diastolic pressures in 14 week old [mRen2]27 rats, while simultaneously increasing the ventricle’s relaxation velocity (Flesch et al., 1997). Furthermore, in a publication detailing the impact of ARB add-on therapy to ACEi there was even more attenuation of diastolic dysfunction in that LV end-diastolic pressure and the percentage of fibrosis were significantly less in ACEi and ARB (temocapril 0.2 mg/kg/day and olmesartan 0.3 mg/kg/day, respectively) treated Dahl rats compared to animals receiving only ACEi (Yoshida et al., 2004).
The role of dual RAS blockade in collagen synthesis and degradation remains unclear as in the mRen2.Lewis model treated for 10 consecutive weeks there were no measurable differences in interstitial or perivascular collagen content. However, Ferrario et al. (Ferrario et al., 2005a) showed that up-regulation of the cardiac ACE2/Ang-(1-7) axis induced by a 12-day administration of either lisinopril or losartan in Lewis rats was abrogated when both drugs were given in combination. Other factors may contribute as well. The age at which treatment is initiated may play a role since the collagen content of the mRen2.Lewis compared to a Lewis of the same age was not different (Figure 4). In keeping with this interpretation, the reduction in collagen volume fraction reported by Varo et al. (Varo et al., 1999; Varo et al., 2000) in SHR occurred in 30 week-old rats in which losartan was given at doses of 20 mg/kg/day for 14 weeks. Comparative results reported by Lambert’s group were found in SHR in which ACEi treatment was initiated at age 4 weeks and continued for 12 to 20 weeks (Gagnon et al., 2004). Thus far, no studies have evaluated the effect of combining an ACE-inhibitor and ARB on cardiac collagen deposition in hypertensive rats.
N-acetyl-Ser-Asp-Lys-Pro, a tetrapeptide specifically degraded by ACE, has potent anti-fibrotic actions and its novel anti-inflammatory mechanisms in hypertension-induced target organ damage may in part be related to the inhibition of the inflammatory cytokine tumor necrosis factor alpha (TNF-α) (Liu et al., 2004; Sharma et al., 2008). Likewise, the effects of AT1 blockade on cardiac fibrosis may be more complex than previously anticipated as the net action may depend not only on the tissue concentration of Ang II, but also on interactions among kinins, AT1, and AT2 receptors (Liu et al., 2004).
A limitation of the current study is that that the analysis of cardiac function was based on noninvasive evaluation of hemodynamics and myocardial performance. Therefore, the direct filling pressure data as well as the decrease in LV isovolumetric pressure or its time constant are not available. Regardless, we used transmitral and tissue Doppler echocardiography, both of which are the methods of choice for routine noninvasive evaluation of diastolic function in humans as previously discussed (Groban et al., 2008). Additionally, whether the changes in diastolic function following early initiation of combined RAS blockade are merely reflective of a reduced afterload is unclear. Indeed, vascular load is an important determinant of ventricular function as it comprises both resistive load (arising primarily at the arteriolar resistance vessels) and pulsatile load (determined by aortic stiffness and early return to the heart of reflected peripheral waves). Since other cellular factors have been commonly associated with maladaptive cardiac hypertrophy (Izumo et al. 1987, Izumo et al. 1988), such as the ratio of α- and β-myosin heavy chain mRNA and myocyte hypertrophy, future studies will investigate whether combination RAS blockade in the mRen2 will limit these markers of LV remodeling.
In summary, we show that the beneficial lusitropic effects of combined RAS blockade by angiotensin converting enzyme inhibition and angiotensin II receptor blockade in young, prehypertensive rats are more likely due to improvements in cardiac calcium handling and/or vascular hemodynamics rather than its effects on myocardial fibrosis.
Acknowledgments
The work described here was supported in part by grants from the National Institute of Health grants KO8-AG026764-04 Paul Beeson award (LG), HL-56973 (MCC), and HL-51952 (PI-C.M. Ferrario).
Footnotes
The authors have nothing to disclose.
References
- Agabiti-Rosei E, Muiesan ML, Salvetti M. Evaluation of subclinical target organ damage for risk assessment and treatment in the hypertensive patients: left ventricular hypertrophy. J Am Soc Nephrol. 2006;17:S104–S108. doi: 10.1681/ASN.2005121336. [DOI] [PubMed] [Google Scholar]
- Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426–440. doi: 10.1016/0735-1097(88)90416-0. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Peters J, Engler E, Ganten D, Mullins J. Transgenic rats carrying the mouse renin gene--morphological characterization of a low-renin hypertension model. Kidney Int. 1992;41:24–36. doi: 10.1038/ki.1992.4. [DOI] [PubMed] [Google Scholar]
- Baumann M, Hermans JJ, Janssen BJ, Peutz-Kootstra C, Witzke O, Heemann U, et al. Transient prehypertensive treatment in spontaneously hypertensive rats: a comparison of spironolactone and losartan regarding long-term blood pressure and target organ damage. J Hypertens. 2007a;25:2504–2511. doi: 10.1097/HJH.0b013e3282ef84f8. [DOI] [PubMed] [Google Scholar]
- Baumann M, Janssen BJ, Hermans JJ, Peutz-Kootstra C, Witzke O, Smits JF, et al. Transient AT1 receptor-inhibition in prehypertensive spontaneously hypertensive rats results in maintained cardiac protection until advanced age. J Hypertens. 2007b;25:207–215. doi: 10.1097/HJH.0b013e3280102bff. [DOI] [PubMed] [Google Scholar]
- Baumann M, Megens R, Bartholome R, Dolff S, van Zandvoort MA, Smits JF, et al. Prehypertensive renin-angiotensin-aldosterone system blockade in spontaneously hypertensive rats ameliorates the loss of long-term vascular function. Hypertens Res. 2007c;30:853–861. doi: 10.1291/hypres.30.853. [DOI] [PubMed] [Google Scholar]
- Berecek KH, Reaves P, Raizada M. Effects of early perturbation of the renin-angiotensin system on cardiovascular remodeling in spontaneously hypertensive rats. Vascul Pharmacol. 2005;42:93–98. doi: 10.1016/j.vph.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Bohm M, Lippoldt A, Wienen W, Ganten D, Bader M. Reduction of cardiac hypertrophy in TGR(mREN2)27 by angiotensin II receptor blockade. Mol Cell Biochem. 1996;163-164:217–221. doi: 10.1007/BF00408661. [DOI] [PubMed] [Google Scholar]
- Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2.Lewis rat. Hypertension. 2003;42:781–786. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cuspidi C, Negri F, Zanchetti A. Angiotensin II receptor blockers and cardiovascular protection: focus on left ventricular hypertrophy regression and atrial fibrillation prevention. Vasc Health Risk Manag. 2008;4:67–73. doi: 10.2147/vhrm.2008.04.01.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di B,V, Giorgi D, Pedrinelli R, Talini E, Palagi C, le Donne MG, et al. Left ventricular hypertrophy and its regression in essential arterial hypertension. A tissue Doppler imaging study. Am J Hypertens. 2004;17:882–890. doi: 10.1016/j.amjhyper.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Doulton TW, He FJ, MacGregor GA. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension. 2005;45:880–886. doi: 10.1161/01.HYP.0000161880.59963.da. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005a;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005b;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005c;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch M, Schiffer F, Zolk O, Pinto Y, Stasch JP, Knorr A, et al. Angiotensin receptor antagonism and angiotensin converting enzyme inhibition improve diastolic dysfunction and Ca(2+)-ATPase expression in the sarcoplasmic reticulum in hypertensive cardiomyopathy. J Hypertens. 1997;15:1001–1009. doi: 10.1097/00004872-199715090-00011. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Legault F, Geraldes P, Tanguay JF, Lambert C. Diverse effects of Ace inhibitors and angiotensin II receptor antagonists on prevention of cardiac hypertrophy and collagen distribution in spontaneously hypertensive rats. Int J Cardiol. 2004;97:373–381. doi: 10.1016/j.ijcard.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Drayer JI, Weber M, Rohan MK, Knoll M, Shu VW, et al. Doppler echocardiographic assessment of left ventricular systolic and diastolic function in mild hypertension. Hypertension. 1987;9:II90–II96. doi: 10.1161/01.hyp.9.2_pt_2.ii90. [DOI] [PubMed] [Google Scholar]
- Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006;48:326–341. doi: 10.1016/j.pcad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- Groban L, Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, et al. Progressive diastolic dysfunction in the female mRen(2).Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- Gross V, Lippoldt A, Bohlender J, Ganten D, Ganten U, Luft FC. The renin-angiotensin system and renal function in transgenic (mRen2)27 rats. Exp Nephrol. 1996;4(Suppl 1):20–26. [PubMed] [Google Scholar]
- Hayashida W, Donckier J, Van MH, Charlier AA, Pouleur H. Diastolic properties in canine hypertensive left ventricular hypertrophy: effects of angiotensin converting enzyme inhibition and angiotensin II type-1 receptor blockade. Cardiovasc Res. 1997;33:54–62. doi: 10.1016/s0008-6363(96)00194-0. [DOI] [PubMed] [Google Scholar]
- Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- Kawasaki D, Kosugi K, Waki H, Yamamoto K, Tsujino T, Masuyama T. Role of activated renin-angiotensin system in myocardial fibrosis and left ventricular diastolic dysfunction in diabetic patients--reversal by chronic angiotensin II type 1A receptor blockade. Circ J. 2007;71:524–529. doi: 10.1253/circj.71.524. [DOI] [PubMed] [Google Scholar]
- Kolesnyk I, Dekker FW, Noordzij M, le CS, Struijk DG, Krediet RT. Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit Dial Int. 2007;27:446–453. [PubMed] [Google Scholar]
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. J Card Fail. 2008;14:181–188. doi: 10.1016/j.cardfail.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Liu YH, Yang XP, Shesely EG, Sankey SS, Carretero OA. Role of angiotensin II type 2 receptors and kinins in the cardioprotective effect of angiotensin II type 1 receptor antagonists in rats with heart failure. J Am Coll Cardiol. 2004;43:1473–1480. doi: 10.1016/j.jacc.2003.11.044. [DOI] [PubMed] [Google Scholar]
- Luders S, Schrader J, Berger J, Unger T, Zidek W, Bohm M, et al. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure - a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26:1487–1496. doi: 10.1097/HJH.0b013e3282ff8864. [DOI] [PubMed] [Google Scholar]
- Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- Masuyama T, Yamamoto K, Sakata Y, Doi R, Nishikawa N, Kondo H, et al. Evolving changes in Doppler mitral flow velocity pattern in rats with hypertensive hypertrophy. J Am Coll Cardiol. 2000;36:2333–2338. doi: 10.1016/s0735-1097(00)01000-7. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- Muiesan ML, Salvetti M, Monteduro C, Rizzoni D, Corbellini C, Castellano M, et al. Changes in midwall systolic performance and cardiac hypertrophy reduction in hypertensive patients. J Hypertens. 2000;18:1651–1656. doi: 10.1097/00004872-200018110-00017. [DOI] [PubMed] [Google Scholar]
- Mullins JJ, Ganten D. Transgenic animals: new approaches to hypertension research. J Hypertens Suppl. 1990;8:S35–S37. [PubMed] [Google Scholar]
- Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- Nishio M, Sakata Y, Mano T, Yoshida J, Ohtani T, Takeda Y, et al. Therapeutic effects of angiotensin II type 1 receptor blocker at an advanced stage of hypertensive diastolic heart failure. J Hypertens. 2007;25:455–461. doi: 10.1097/HJH.0b013e328010d635. [DOI] [PubMed] [Google Scholar]
- Ohta K, Kim S, Wanibuchi H, Ganten D, Iwao H. Contribution of local renin-angiotensin system to cardiac hypertrophy, phenotypic modulation, and remodeling in TGR (mRen2)27 transgenic rats. Circulation. 1996;94:785–791. doi: 10.1161/01.cir.94.4.785. [DOI] [PubMed] [Google Scholar]
- Pearson AC, Labovitz AJ, Mrosek D, Williams GA, Kennedy HL. Assessment of diastolic function in normal and hypertrophied hearts: comparison of Doppler echocardiography and M-mode echocardiography. Am Heart J. 1987;113:1417–1425. doi: 10.1016/0002-8703(87)90657-0. [DOI] [PubMed] [Google Scholar]
- Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex Differences in Circulating and Renal Angiotensins of Hypertensive mRen(2).Lewis but not Normotensive Lewis Rats. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M, Janssen PM. Molecular basis of diastolic dysfunction. Heart Fail Clin. 2008;4:13–21. doi: 10.1016/j.hfc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- Rothermund L, Pinto YM, Vetter R, Herfort N, Kossmehl P, Neumayer HH, et al. Effects of angiotensin II subtype 1 receptor blockade on cardiac fibrosis and sarcoplasmic reticulum Ca2+ handling in hypertensive transgenic rats overexpressing the Ren2 gene. J Hypertens. 2001;19:1465–1472. doi: 10.1097/00004872-200108000-00015. [DOI] [PubMed] [Google Scholar]
- Satoh S, Ueda Y, Suematsu N, Oyama J, Kadokami T, Sugano M, et al. Beneficial effects of angiotensin-converting enzyme inhibition on sarcoplasmic reticulum function in the failing heart of the Dahl rat. Circ J. 2003;67:705–711. doi: 10.1253/circj.67.705. [DOI] [PubMed] [Google Scholar]
- Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, et al. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol. 2008;294:H1226–H1232. doi: 10.1152/ajpheart.00305.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Ino H, Okeie K, Emoto Y, Yamaguchi M, Yasuda T, et al. Cardiac sympathetic activity in the asymmetrically hypertrophied septum in patients with hypertension or hypertrophic cardiomyopathy. Clin Cardiol. 2000;23:365–370. doi: 10.1002/clc.4960230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Yokoyama H, Averill DB, Schiffrin EL, Ferrario CM. Reversal of vascular hypertrophy in hypertensive patients through blockade of angiotensin II receptors. J Am Soc Hypertension. 2008;2:165–172. doi: 10.1016/j.jash.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Springate JE, Feld LG, Ganten D. Renal function in hypertensive rats transgenic for mouse renin gene. Am J Physiol. 1994;266:F731–F737. doi: 10.1152/ajprenal.1994.266.5.F731. [DOI] [PubMed] [Google Scholar]
- Tan HW, Zheng GL, Li L, Wang ZH, Gong HP, Zhang Y, et al. Impaired left ventricular synchronicity in hypertensive patients with ventricular hypertrophy. J Hypertens. 2008;26:553–559. doi: 10.1097/HJH.0b013e3282f2b91f. [DOI] [PubMed] [Google Scholar]
- Teniente-Valente R, Solorio S, Vargas-Salado E, Aguirre-Vazquez C, Hernandez-Gonzalez MA, Olvera-Lopez JA, et al. Improvement of diastolic function after regression of left ventricular hypertrophy. Arch Cardiol Mex. 2008;78:392–399. [PubMed] [Google Scholar]
- Thomas JD, Weyman AE. Echocardiographic Doppler evaluation of left ventricular diastolic function. Physics and physiology. Circulation. 1991;84:977–990. doi: 10.1161/01.cir.84.3.977. [DOI] [PubMed] [Google Scholar]
- Tsuyuki RT, Yusuf S, Rouleau JL, Maggioni AP, McKelvie RS, Wiecek EM, et al. Combination neurohormonal blockade with ACE inhibitors, angiotensin II antagonists and beta-blockers in patients with congestive heart failure: design of the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) Pilot Study. Can J Cardiol. 1997;13:1166–1174. [PubMed] [Google Scholar]
- van den Meiracker AH, t Veld AJ, Admiraal PJ, Ritsema van Eck HJ, Boomsma F, Derkx FH, et al. Partial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: does it exist and does it affect the antihypertensive response? J Hypertens. 1992;10:803–812. Man in. [PubMed] [Google Scholar]
- Varo N, Etayo JC, Zalba G, Beaumont J, Iraburu MJ, Montiel C, et al. Losartan inhibits the post-transcriptional synthesis of collagen type I and reverses left ventricular fibrosis in spontaneously hypertensive rats. J Hypertens. 1999;17:107–114. doi: 10.1097/00004872-199917010-00016. [DOI] [PubMed] [Google Scholar]
- Varo N, Iraburu MJ, Varela M, Lopez B, Etayo JC, Diez J. Chronic AT(1) blockade stimulates extracellular collagen type I degradation and reverses myocardial fibrosis in spontaneously hypertensive rats. Hypertension. 2000;35:1197–1202. doi: 10.1161/01.hyp.35.6.1197. [DOI] [PubMed] [Google Scholar]
- Wachtell K, Smith G, Gerdts E, Dahlof B, Nieminen MS, Papademetriou V, et al. Left ventricular filling patterns in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Losartan Intervention For Endpoint. Am J Cardiol. 2000;85:466–472. doi: 10.1016/s0002-9149(99)00773-0. [DOI] [PubMed] [Google Scholar]
- Weber KT, Janicki JS, Pick R, Abrahams C, Shroff SG, Bashey RI, et al. Collagen in the hypertrophied, pressure-overloaded myocardium. Circulation. 1987;75:I40–I47. [PubMed] [Google Scholar]
- Werner C, Baumhakel M, Teo KK, Schmieder R, Mann J, Unger T, et al. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol. 2008;97:418–431. doi: 10.1007/s00392-008-0668-3. [DOI] [PubMed] [Google Scholar]
- Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18:139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- Yamaleyeva LM, Gallagher PE, Vinsant S, Chappell MC. Discoordinate regulation of renal nitric oxide synthase isoforms in ovariectomized mRen2.Lewis rats. Am J Physiol Regul Integr Comp Physiol. 2007a;292:R819–R826. doi: 10.1152/ajpregu.00389.2006. [DOI] [PubMed] [Google Scholar]
- Yamaleyeva LM, Pendergrass KD, Pirro NT, Gallagher PE, Groban L, Chappell MC. Ovariectomy is protective against renal injury in the high-salt-fed older mRen2.Lewis rat. Am J Physiol Heart Circ Physiol. 2007b;293:H2064–H2071. doi: 10.1152/ajpheart.00427.2007. [DOI] [PubMed] [Google Scholar]
- Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, et al. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension. 2004;43:686–691. doi: 10.1161/01.HYP.0000118017.02160.fa. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]