Two interesting articles from Harvard University investigators in this issue of Biological Psychiatry challenge us to reconsider and re-think the complexities of the underlying neurobiology of posttraumatic stress disorder (PTSD). We will refer to these articles with a view to highlighting their unique contributions to the literature and the new directions they motivate for translational research in PTSD and other anxiety disorders.
Milad and colleagues (1) used an operant conditioning paradigm to show that patients with PTSD have deficient extinction retention relative to trauma-exposed controls who had not developed PTSD. They further demonstrate, using functional MRI (fMRI), that this reduced extinction retention is related to less activation in the hippocampus and bilateral ventromedial prefrontal cortex in patients with PTSD. This paper underscores our growing appreciation that PTSD is characterized by dysfunction of “unlearning” within fear circuitry, and the findings should motivate clinical research to enhance extinction retention through pharmacological and other means. Such approaches, in parallel with those aimed at the disruption of the consolidation of fear memories, are among the most promising translational leads for PTSD therapeutics (2).
In contrast to the aforementioned study which adds important information to the burgeoning literature on abnormal fear circuitry and deficient fear extinction mechanisms in PTSD, the paper by Elman and colleagues (3) delves into a much neglected area: the study of reward processing in PTSD. Consistent with a previous report of altered processing in the reward circuitry of patients with PTSD (4), these investigators found less striatal activation to monetary gains versus losses in PTSD in association with more self-reported motivational and social deficit. These observations can be considered complementary to those that find alterations in fear-motivated systems that underlie behavioral avoidance in PTSD (and, possibly, other anxiety disorders) in that they point to dysfunction in brain systems that underlie deficient approach behaviors. Reward circuitry has been largely neglected in the study of PTSD, and this paper (3) should be considered a call to action to intensify research on this topic. What can be gained by integrating investigation of reward systems in PTSD?
It has long been recognized that individuals balance the approach system, which is closely related to reward processes with the avoidance system, which focuses on fear-related processes (5). Nearly all research to date on PTSD has been focused on fear, therein ignoring the “ying-yang” of approach-avoidance that underlies all anxiety disorders, including PTSD. We would suggest that PTSD is a disorder characterized by imbalance of approach-avoidance systems. By considering both aspects when conceptualizing the dysfunctions observed in individuals with PTSD, one can generate new hypotheses and foster new insights with the potential to advance the field.
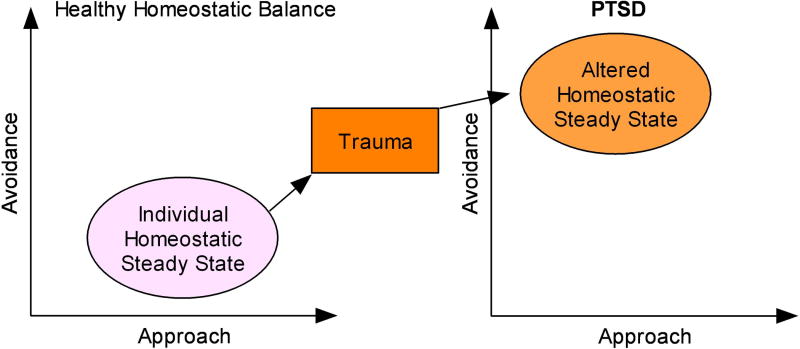
A critical assumption in this conceptualization is the notion that PTSD emerges as the individual's brain attempts to establish a new homeostatic steady state following the exposure to a trauma that has altered the approach/avoidance system. It is surprising that given the extensive literature on stress and homeostatic dysfunction (6), that homeostatic imbalance has not figured prominently in the conceptualization of PTSD. In comparison, its psychological analog, coping, has been widely used. Put differently, coping is the individual's attempt to reestablish homeostasis which, in large part, involves a decision to either approach or avoid.
Dopamine and norepinephrine have been implicated as key modulatory neurotransmitter systems in reward and fear-related processes, respectively. In particular, different personality characteristics have been related to these systems and to the degree to which individuals deploys actions to approach or avoid, respectively (7). If one assumes that an external trauma modulates the existing balance between approach and avoidance by up or down-regulating the sensitivity of neural substrates that process reward or fear, one can begin to conceptualize PTSD as an altered homeostatic balance between approach and avoidance (Figure 1). Moreover, the emerging symptoms can be understood as thoughts, feelings, or actions that result from the instability of this homeostatic balance.
Figure 1.
Conceptual representation of the impact of traumatic stress on altering homeostatic steady state in PTSD.
In particular, some of the key symptoms of PTSD that are readily associated with alterations in the approach/avoidance systems are:
emotional numbing and anhedonia - downregulation of approach
hyperarousal and irritability - upregulation of avoidance
associated substance use problems - upregulation of approach
behavioral avoidance - upregulation of avoidance
An obvious advantage of this conceptualization is that it permits – indeed, it requires that– PTSD be considered as an imbalance disorder involving failure to successfully adapt of several brain systems. Conversely, it discourages an approach to understanding (and treating) PTSD as a unitary, homogenous disorder. For example, this conceptualization:
Encourages linking of basic neurotransmitter systems to processing dysfunctions (similar to the approach proposed by Cloninger et al. for personality and temperament (7)). This connection can be used to generate testable hypotheses about the relationship between neurotransmitter systems dysfunction and specific symptoms, e.g., attenuated dopamine signaling and emotional numbing; sensitization of norepinephrine and hyperarousal. Furthermore, if PTSD involves a homeostatic imbalance of the approach-avoidance system then the same symptoms can be generated from different balance states, i.e., symptoms can emerge from both hyper and hypodopaminergic states based on the balance with the avoidance or norepinephrine system. Moreover, this balance need not be considered static; in fact, it should be expected that certain systems will predominate at different stages of illness (e.g., increased fear and avoidance early in the course of illness; increased anhedonia, isolation, and depression later).
Fosters translational links with animal research that has focused on approach-avoidance systems. In some instances, this linkage may bring together seemingly unrelated findings in human and animal research. For example, whereas it is well known that the incidence of PTSD is associated with an increase in cigarette smoking (8) the mechanism behind this association is not well understood. Rodent models of approach-avoidance conflict show that nicotine can be considered anxiolytic in some contexts (9). If PTSD can be understood as a disorder of dysfunctional approach-avoidance systems, then could smoking be an attempt to reestablish homeostasis (i.e., to cope) by making use of nicotine's properties to alter approach-avoidance conflict?
Provides a rationale (rather than an excuse) for the observation that pharmacotherapy of PTSD currently involves multiple pharmaceutical interventions (10). Consistent with the notion that approach-avoidance dysfunction in PTSD involves multiple neurotransmitter systems; it would be surprising to find that a single treatment works well for most patients. Rather, the expectation would be that whereas some treatments with pleiotropic effects on multiple neurotransmitter systems (e.g., selective serotonin reuptake inhibitors [SSRIs]) might be beneficial in some cases (2) no single treatment is likely to be salutary for all patients with PTSD. This admission of failure of existing treatments for PTSD does, by no means, suggest that a one-size-fits-all pharmacotherapy is impossible. But it does strongly suggest that it is highly improbable, opening the door widely for the development of therapies that can target particular aspects of this complex syndrome (e.g., anti-noradrenergic agents to restrain avoidance systems; dopamine modulating agents to enhance approach systems).
Motivates an approach to understanding and treating PTSD that acknowledges input from and reciprocity of distributed brain systems. Prevailing neurobiological models of personality have evolved from neurotransmitter specific views (7) to encompass the linking of personality characteristics to dissociable connectivity streams in the human brain (11). The latter research suggests, for example, that fiber tracts in a subcortical network underlie individual differences in novelty seeking (“avoidance”) whereas tracts between prefrontal cortex and the striatum underlie individual differences in reward dependence (“approach”). Certain symptom characteristics in PTSD may, analogously, reflect different underlying dysfunctions in cortical-limbic networks, contributing to imbalance in approach-avoidance behaviors. Thinking about connectivity of systems that differentially underlie approach and avoidance behaviors in PTSD can provide additional avenues for research and, potentially, additional leverage for novel therapeutics.
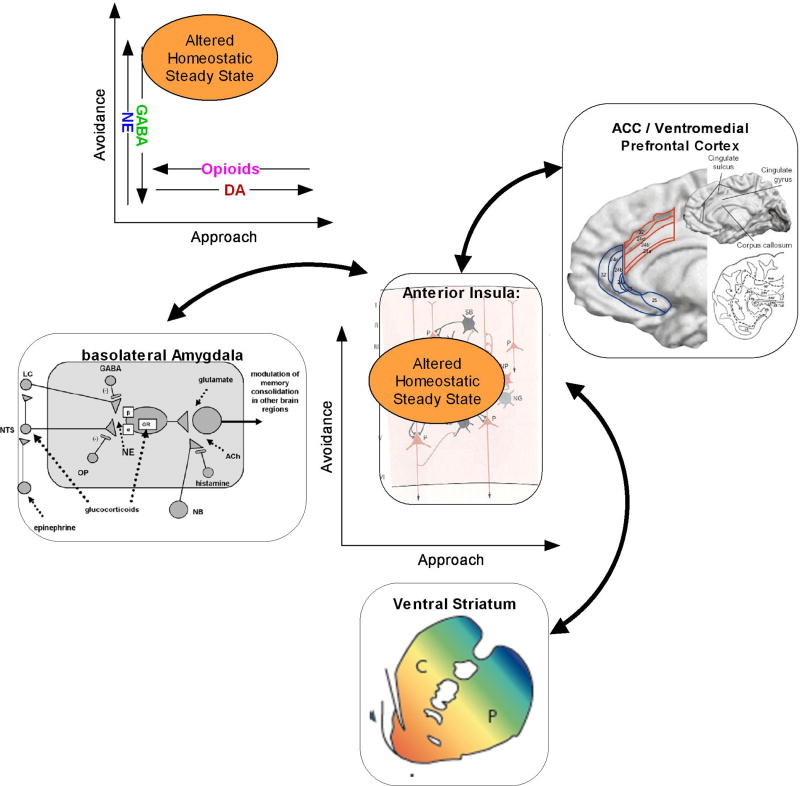
Taken together, these two articles bring us a step closer to viewing PTSD as an imbalance state involving the dynamical adjustment of both approach and avoidance systems – and the multiple neurotransmitter systems that modulate these systems – toward an altered homeostatic steady state (Figure 2). In short, individuals with PTSD face a multi-systems imbalance and will require integrated pharmacological and behavioral interventions targeted at up/down-regulating approach and avoidance processes.
Figure 2.
This figure attempts to summarize how one can begin to integrate (1) specific neural systems (2) behavioral processes, and (3) neurotransmitter systems in describing the pathology observed in PTSD. Specifically, given an altered imbalance of approach-avoidance systems, which could be related to modulating neurotransmitter systems, individuals find themselves processing approach avoidance situations differently using some of the key neural substrates, i.e., amygdala, ventral striatum, insula, and medial prefrontal cortex, to adjust to an altered homeostatic steady state.
Dr. Stein reports that in the past 3 years he has been a consultant to AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest, Hoffmann-La Roche, Jazz Pharmaceuticals, Johnson & Johnson, Pfizer, Sepracor, and Transcept Pharmaceuticals; and he has received research funding from Eli Lilly, GlaxoSmithKline, and Hoffmann-La Roche. Dr. Paulus reports research funding from GlaxoSmithKline, Hoffmann-La Roche, and Sepracor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Milad MR, Pitman RK, Ellies CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in Posttraumatic Stress Disorder. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.06.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravindran LN, Stein MB. Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res. doi: 10.1016/j.brainres.2009.03.037. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in Posttraumatic Stress Disorder. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.06.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sailer U, Robinson S, Fischmeister FP, Konig D, Oppenauer C, Lueger-Schuster B, Moser E, Kryspin-Exner I, Bauer H. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46(11):2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Berlyne DE. Conflict, arousal, and curiosity. New York: McGraw-Hill; 1960. [Google Scholar]
- 6.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N.Y.Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 7.Cloninger CR, Svrakic NM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 8.Koenen KC, Stellman SD, Sommer JF, Jr, Stellman JM. Persisting posttraumatic stress disorder symptoms and their relationship to functioning in Vietnam veterans: A 14-year follow-up. J Trauma Stress. 2008;21:49–57. doi: 10.1002/jts.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A. Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict. Psychopharmacology. 2009;204:541–549. doi: 10.1007/s00213-009-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed S, Rosenheck RA. Pharmacotherapy of PTSD in the U.S. Department of Veterans Affairs: diagnostic- and symptom-guided drug selection. J Clin Psychiatry. 2008;69:959–965. doi: 10.4088/jcp.v69n0611. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]