Abstract
Chromogranin A (CgA), a member of the granin family serves several important cell biological roles in (neuro)endocrine cells which are summarized in this review. CgA is a “prohormone” that is synthesized at the rough endoplasmic reticulum and transported into the cisternae of this organelle via its signal peptide. It is then trafficked to the Golgi complex and then to the trans-Golgi network (TGN) where CgA aggregates at low pH in the presence of calcium. The CgA aggregates provide the physical driving force to induce budding of the TGN membrane resulting in dense core granule (DCG) formation. Within the granule, a small amount of the CgA is processed to bioactive peptides, including a predicted C-terminal peptide, serpinin. Upon stimulation, DCGs undergo exocytosis and CgA and its derived peptides are released. Serpinin, acting extracellularly is able to signal the increase in transcription of a serine protease inhibitor, protease nexin-1 (PN-1) that protects DCG proteins against degradation in the Golgi complex, which then enhances DCG biogenesis to replenish those that were released. Thus CgA and its derived peptide, serpinin, plays a significant role in the formation and regulation, respectively, of granule biogenesis in (neuro)endocrine cells.
Keywords: Chromogranin A, granule biogenesis, AtT-20 cells, Protease Nexin-1, Secretogranin III
Introduction
Chromogranin A (CgA) is a genetically distinct member of the granin family of proteins which is stored together with hormones and neuropeptides in dense core secretory granules in endocrine cells and neurons [1]. CgA is released upon stimulation and in the adrenal medulla, it is co-released with catecholamines [1]. The CgA protein has a signal peptide and numerous paired basic residues, [2, 3] which are typical cleavage sites in prohormones to generate bioactive peptides (See Fig. 1) [2–4]. Thus CgA has been characterized as a “prohormone” and indeed various CgA-derived peptides e.g. vasostatin, have been isolated and their functions identified [5] (see also other chapters in this volume). However, the level of processing is generally low and varies with different tissues, and much of CgA remains intact upon release [6] (Cawley et al., unpublished data). For a long time, the function of intact CgA was unclear although it has been used as a diagnostic marker for tumors of endocrine origin and various inflammatory diseases such as arthritis [7]. At the cell biological level, CgA has been referred to as a “granulogenic protein” that plays an important role in dense-core granule (DCG) biogenesis and as a chaperone for prohormone sorting to the regulated secretory pathway in endocrine cell [8–10]. In this review, we will discuss the biosynthesis, processing and trafficking of CgA, as well as its cell biological function in granulogenesis. We will also discuss a potential new cleavage product, serpinin, involved in regulating DCG biogenesis in endocrine and neuroendocrine cells.
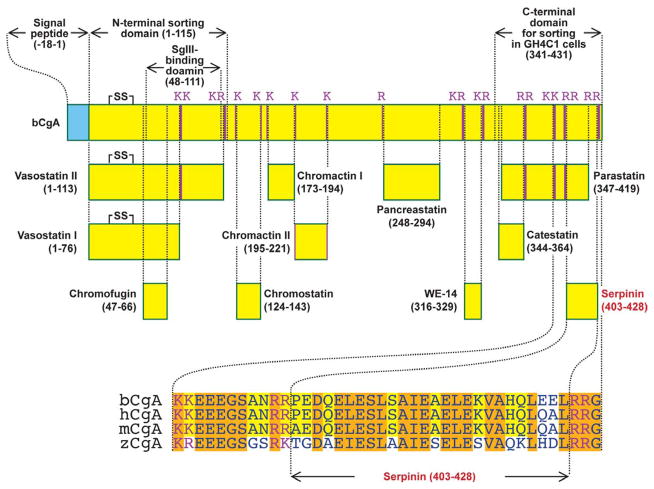
Fig. 1.
Schematic representation of the structure of CgA. Bovine CgA (bCgA; NCBI accession number P05059) showing functional domains and paired and single basic residue cleavage sites. N-terminal domain (1–115) and SgIII binding site (48–111) were identified in rat CgA but the location of these regions is identical with that of bCgA. The C-terminal domain which is required for sorting in GH4C1 cells (341–431) was identified in bCgA. Known CgA-derived peptides and a novel CgA-derived peptide, Serpinin (403–428) are displayed. Serpinin residues which are conserved in three mammalian species: bovine CgA (bCgA), human CgA (hCgA; accession number AAH09384) and mouse CgA (mCgA; NP031719), are highlighted in yellow. Residues conserved in the three mammalian species and zebra fish (zCgA; accession number NP001006059) are highlighted in orange. C-terminal domain and Serpinin have highly conserved sequences.
Biosynthesis, intracellular trafficking and processing of CgA
CgA is expressed in endocrine cells and peptidergic neurons which, in addition to having a constitutive secretory pathway contain a regulated secretory pathway unique to these cells [11]. It is secreted in a stimulus-dependent manner via the regulated secretory pathway. CgA is synthesized at the rough endoplasmic reticulum (RER) and inserted into the RER cisternae via the signal peptide (Figs. 1, 2). It is then transported to the Golgi apparatus and sorted away from other constitutively secreted and lysosomal proteins and packaged at the trans-Golgi network (TGN) into immature secretory granules. Subsequently, CgA unlike other prohormones, is only partially processed within the secretory granules to yield various bioactive peptides with different functions (Figs. 1, 2).
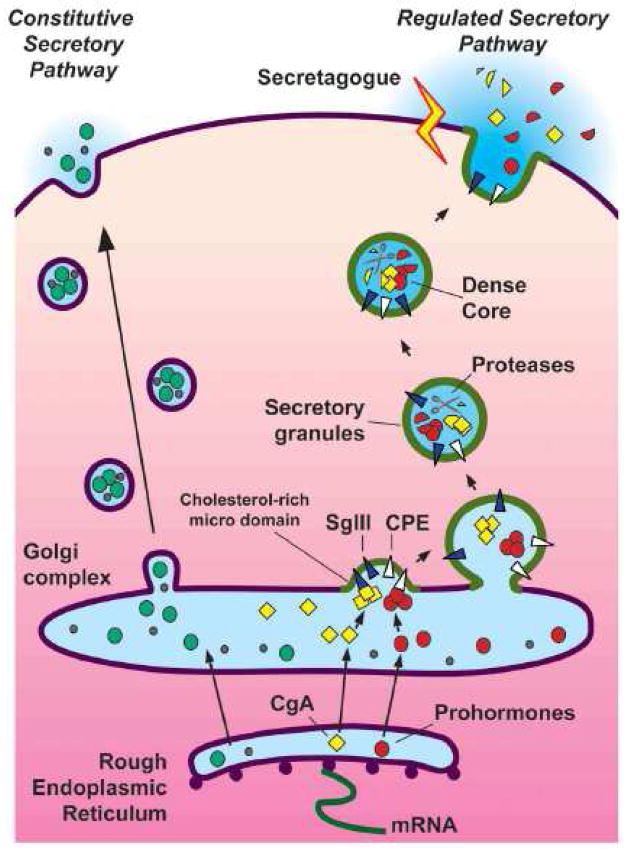
Fig. 2.
Secretory pathways and dense core granule biogenesis in (neuro)endocrine cells. CgA and prohormones which are synthesized in the rough endoplasmic reticulum (RER), are transported to the Golgi complex, aggregated and sorted into granules at cholesterol-rich membrane microdomains in the trans-Golgi network via sorting receptors, such as SgIII and CPE, respectively. Aggregated CgA and prohormones induce the budding of the TGN membrane to form the dense core granule (DCG) in the regulated secretory pathway. Prohormones and CgA (partially) are cleaved by proteases in DCGs to yield biologically active peptides. Stimulation of DCGs by secretagogues triggers exocytosis and secretion of hormones. In contrast, vesicles of the constitutive secretory pathway which is present in all cell types release their contents without stimulation.
How does CgA get sorted into regulated secretory pathway granules? Initial studies suggest that CgA which is highly acidic and forms large aggregates at an acidic pH and in the presence of Ca++ within the TGN, is sorted out from other proteins and enters the budding granule in a passive manner [12, 13]. More recently, a number of studies [8, 14, 15] have supported a sorting domain-receptor mediated model. Several groups have shown that the CgA N-terminal domain (residues 1–115) consisting of a disufide bonded loop structure (Cys17-Cys38) which is evolutionarily conserved is essential for targeting CgA to the regulated secretory pathway in PC12 cells (Fig. 1). However, this disulfide loop was not necessary for sorting CgA in GH4C1 cells. On the other hand, the C-terminus of CgA which is also highly conserved (Fig. 1) was critical for sorting in GH4C1 cells but does not play such a role in PC12 cells [14]. These results indicate that the N- and C-terminal highly conserved sorting domains function in a cell specific manner. Hosaka’s group has shown that the N-terminal domain (residues 48–111) of CgA strongly interacts with Secretogranin III (at SgIII 214–373 domain) in PC12 cells [16]. SgIII is a cholesterol binding protein and it has been shown that SgIII found in cholesterol-rich granule membranes functions as a sorting receptor at the TGN to target CgA aggregates to the regulated secretory pathway (Fig. 2) [15–17]. SgIII is also involved in the sorting of CgA to the regulated secretory pathway granules in pituitary AtT-20 cells and pancreatic β-cells [15]. Additionally, SgIII has been proposed to act as a prohormone sorting receptor since it also binds prohormones such pro-opiomelanocortin (POMC) albeit, more weakly than the membrane form of carboxypeptidase E (CPE), identified previously as a sorting receptor for prohormones [18, 19]. CgA-aggregates, upon entry into the newly formed secretory granule by budding of the TGN membrane, are stored within the cells and give the granules the dense core appearance when analyzed by electron microscopy [20].
Unlike other prohormones such as proinsulin, CgA is processed only to a small extent to yield biologically active peptides in granules from pituitary and chromaffin tissue (our unpublished data), perhaps because the aggregates are not very soluble in the acidic milieu of the secretory granule and remain as a dense core. Through cleavage of CgA at paired basic residues and single basic residues, a number of bioactive peptides are generated (see Fig. 1). More recently, a proteomic study suggests that CgA may be processed at more residues than was previously thought (Fig. 1), although the function of the “new” fragments remain unknown [3]. The paired basic residue processing sites are characteristic of those used by the endoproteases (proprotein convertases) PC1 and PC2 and more recently Cathepsin L, all of which have been shown to be present in chromaffin granules [21, 22]. After endoproteolytic cleavage, the exopeptidase, CPE, also present in dense core secretory granules removes the C-terminal extended basic residues to yield biologically active peptides or peptide fragments. These fragments may still require further processing by other endopeptidases, that are specific for single basic amino acids or other sites, as well as exopeptidases, e.g. aminopeptidases [23, 24], present in DCGs, to generate the final active peptide products shown in Fig. 1. While most of the processing likely occurs within the granule, further processing of CgA fragments may occur extracellularly after release, possibly by enzymes such as plasmin which is present in the circulation.
CgA functions as a granulogenic and chaperone protein
Members of the granin family of proteins which include CgA, CgB and secretogranin II–IV are expressed in abundance in endocrine and peptidergic neurons [25]. These proteins, in an acidic pH and high Ca++ environment, aggregate into large complexes and have been shown to physically induce the formation of dense core-like granules in fibroblasts [26–29]. Hence they are proposed to be the driving force that induces budding of the TGN membrane to form dense core granules and are therefore coined “granulogenic” proteins. Recently, domains in the CgA molecule have been identified to be essential for granulogenesis. A study by Stettler et al. using a series of CgA deletion constructs fused to GFP or other short epitope tags when expressed in fibroblast COS-7 cells revealed that the N-terminal 77 residues generated granule-like structures that co-localized with SgII when co-expressed in the same cells [28]. Additionally, Anouar’s group using frog CgA (fCgA) showed that deletion constructs missing the highly conserved N- or C-terminus when overexpressed in COS-7 cells was retained in the Golgi apparatus whereas full length fCgA induced formation of DCGs, the contents of which could be released in a regulated manner [30]. In that study, expression of full length but not N- or C-terminal truncated forms of fCgA in AtT-20 cells, a mouse pituitary corticotroph cell line resulted in the promotion of sorting of POMC into DCGs suggesting that CgA may facilitate the sorting of peptide hormones into the regulated secretory pathway [30]. However, while our studies indicate that CPE is the primary sorting receptor for directing POMC to regulated secretory granules, overexpression of CgA or CgB [31] in AtT-20 cells appears to enhance this process. In conditions of overexpression where CgA is in great excess, and since it is a highly charged molecule, other proteins such as POMC may well interact with it, resulting in enhanced numbers of prohormone molecules being targeted with CgA, in a “piggy back” manner, into the DCGs. In contrast, since overexpression of the N- and/or the C-terminal CgA deleted mutants reduced DCG formation causing retention of the molecules at the TGN, so too would POMC movement from the Golgi into DCGs be expected to be retarded due to lack of granule formation and perturbation of the TGN. Likewise, in the CgA-knockout mouse, lack of CgA diminished granule formation and hence the amount of releasable prohormones and their processed products being secreted into the circulation was decreased [32, 33]. While a CgA knock out mouse in another study [34] did not give the same phenotype, the cell biological [27–29] and two in vivo studies [32, 35] support the importance of CgA in inducing granule formation in a specific molecular domain-dependent manner. Perturbation of CgA-dependent granulogenesis, therefore has a profound negative effect on prohormone cargo sorting to the regulated secretory pathway.
CgA regulates granule biogenesis by modulating protease nexin-1 expression
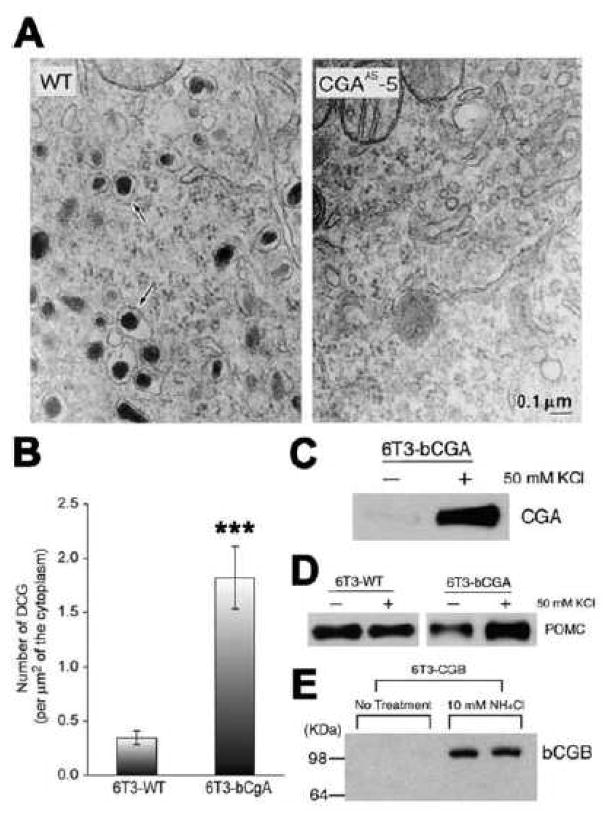
Distinct from its physical role as a granulogenic protein, CgA has been shown to upregulate granule biogenesis in endocrine and neuroendocrine cells. We demonstrated by quantitative electron microscopy (EM) that PC12 cells stably transfected with antisense to chromogranin A had a marked decrease in DCG biogenesis (Fig. 3A) [27]. Conversely, when 6T3 cells, a mutant AtT20 cell line lacking DCGs were transfected with bovine CgA (6T3-bCgA cells), there was a significant increase in the number of DCGs present, as revealed by quantitative EM (Fig. 3B) [36]. These newly formed DCGs were secretion competent since high K+-stimulation released the CgA (Fig. 3C) as well as an exogenous granule cargo protein, POMC, from these cells, but not from wild type 6T3 (6T3-WT) cells (Fig. 3D) [27]. The DCG induction effect was specific to CgA since CgB transfected into the 6T3 cells did not show an increase in DCG biogenesis. Instead transfected CgB was degraded as evidenced by its disappearance which was recovered by NH4Cl treatment to alkalinize the pH of the degradation compartment (Fig. 3E) [27]. The intracellular site of degradation of CgB was determined using cold temperature block, brefeldin A (BFA) and monensin treatment (Fig. 4A) [36]. When vesicular trafficking from the ER to the Golgi apparatus was blocked in these cells by BFA, which redistributes the Golgi apparatus to the ER [37], the cellular level of CgB was significantly increased [36]. When 6T3-WT cells expressing CgB were incubated at 20°C for 2 h, which prevented post Golgi trafficking, the level of CgB was not recovered, indicating that degradation of CgB was initiated in the Golgi complex. Monensin treatment for 2 h, which blocks trafficking of proteins from the medial-to the trans-Golgi cisternae [38] also did not recover the intracellular CgB level in 6T3-WT cells, suggesting that degradation is initiated in the cis-Golgi cisternae of the Golgi apparatus [36].
Fig. 3.
A. Effect of CgA antisense knock-out on granule biogenesis. Electron micrographs (EM) of wild-type PC12 cells (WT, left panel) and clone CGAAS-5 cells which stably express antisense constructs against CgA sense sequences (right panel). Dense-core secretory granules (arrows) are abundant in the wild-type PC12 cells but scarce in clone CGAAS-5 cells. B–E. Effect of CgA knock-in on granule biogenesis: B. 6T3-WT cells, a variant AtT-20 pituitary endocrine cell line lacking DCGs were stably transfected with bCgA. Bar graph shows EM morphometric analysis of the number of DCGs in 6T3-WT cells and 6T3-bCgA cells. DCGs were counted from 20 (6T3-WT) or 10 (6T3-bCgA) individual EM micrographs, and the number of DCGs per square micrometer was calculated by dividing the number of DCGs with the cytoplasmic area measured from each micrograph. 6T3-WT cells had 0.35 ± 0.06 DCG/μm2 (total of 112 μm2 of the cytoplasmic area measured), while 6T3-bCgA cells had 1.82 ± 0.29 DCG/μm2 (total of 48 μm2 of the cytoplasmic area measured) (***P < 0.0001). C. Western blot of release medium in the presence of 50 mM KCl/2 mM BaCl2 in 6T3 cells stably transfected with CgA (6T3-bCgA) shows that CgA secretion was significantly stimulated indicating that CgA expression in these mutant endocrine cells was able to restore the regulated secretory pathway. D. Western blots of release media from wild-type (6T3-WT) and CgA transfected 6T3 cells (6T3-bCgA). In 6T3-WT cells, transfected bovine POMC was secreted at a high basal level, and no stimulation was detected with 50 mM KCl/2 mM BaCl2. In 6T3-bCgA cells, stimulated secretion of exogenous POMC was restored. Thus, CgA alone was sufficient to rescue regulated secretion in a CgA-deficient mutant corticotroph endocrine cell line lacking the regulated secretory pathway. E. Degradation of transiently transfected CgB (6T3-CgB) was rescued by treatment with NH4Cl. 6T3 cells after transfection with bovine CgB construct did not show any detectable CgB immunoreactivity without NH4Cl treatment. Treatment with 10 mM NH4Cl (90 min) restored the level of expressed CgB. (Data taken from Figs. in Kim et al., 2001, Cell. [27], with permission and from Fig. in Kim et al., 2006, Mol. Biol. Cell. [36])
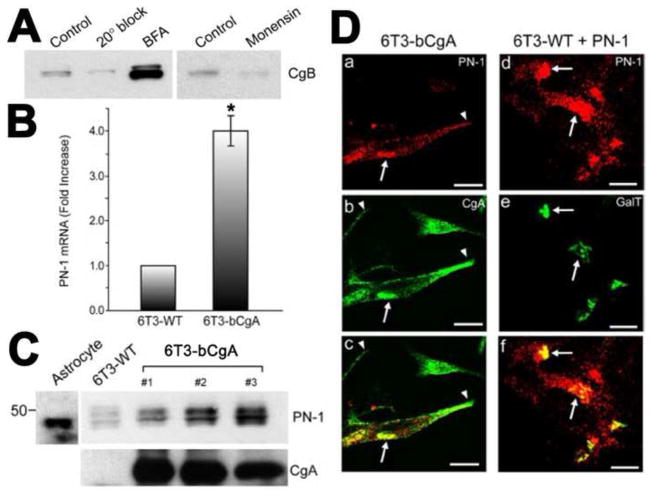
Fig. 4.
A. To identify the compartment responsible for degradation of DCG proteins in 6T3-WT cells, the degradation of exogenously expressed rat CgB as a representative DCG protein was tracked in CgB expressing 6T3-WT cells. When vesicular trafficking from the ER to the Golgi apparatus was blocked in these cells by BFA (5 μg/ml; 2 h) [37], the cellular level of CgB was significantly increased. When 6T3-WT cells expressing CgB were incubated at 20°C for 2 h, the level of CgB was not recovered, indicating that degradation of CgB was initiated in the Golgi complex. Monensin treatment (10 μg/ml; 2 h) [38, 42], did not recover the intracellular CgB level in 6T3-WT cells, further indicating that degradation is initiated in the early compartments of the Golgi apparatus. B-D. Up-regulation of PN-1 expression by CgA: B. Real-time RT-PCR analyses revealed that PN-1 mRNA levels in 6T3-bCgA cells were 4.01 ± 0.33-fold (± SEM; n = 3; *P < 0.05) higher than 6T3-WT cells. C. Western blot analysis showing the expression of PN-1 in mouse astrocytes (positive control), 6T3-WT, and three different clones of 6T3-bCgA cells (1~3). CgA expression in 6T3-bCgA clones is higher than that in 6T3-WT cells. D. Immunofluorescence microscopy for endogenous PN-1 [7] in 6T3-bCgA cells consistently showed a perinuclear, Golgi-like distribution (a and c, arrows), overlapping with CgA (b and c, arrows). Similarly, when coexpressed with CFP-tagged GalT, a Golgi marker, PN-1 (d and f) colocalized with GalT-CFP (GalT) in 6T3-WT cells (e and f, arrows), indicating that exogenously expressed PN-1 is also localized to the Golgi apparatus. However, PN-1 did not colocalize with CgA along and at the tips of cell processes, where DCGs reside (a–c, arrowheads). (Data taken from Figs. in Kim et al., 2006, Mol. Biol. Cell. [36])
To identify protease inhibitor genes that may be up-regulated to protect granule proteins from degradation, gene expression profiles from 6T3-bCgA cells and 6T3-WT cells using Affymetrix GeneChip microarray were compared. This analysis identified a serpin family serine protease inhibitor, protease nexin-1(PN-1) mRNA that was upregulated in 6T3-bCgA cells (Fig. 4B) [36]. Furthermore, PN-1 protein was also up-regulated in three clones of 6T3 cells stably transfected with CgA (Fig. 4C) [36]. Immunofluorescence microscopy of 6T3-bCgA cells showed co-localization of endogenous PN-1 with CgA in the perinuclear region, resembling a Golgi-like distribution, but no overlap was observed in the tips of the cells where DCGs reside (Fig. 4Da–c) [36]. Transfection of PN-1 and GalT, a Golgi marker, in 6T3-WT cells showed co-localization of these two molecules (Fig. 4Dd–f), substantiating the presence of PN-1 in the Golgi complex where CgB, a DCG protein is degraded [36].
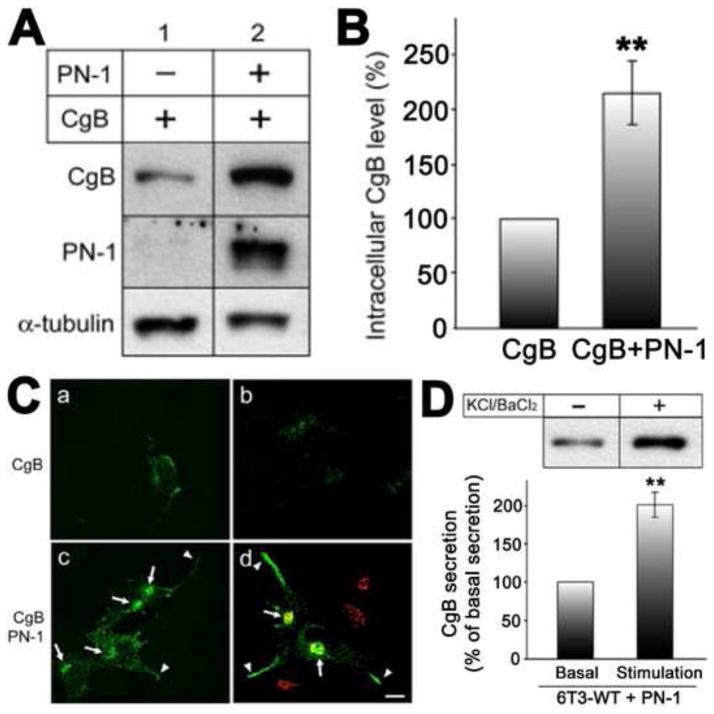
Biochemical studies showed that co-expression of CgB with PN-1 in 6T3-WT cells rescued the degradation of CgB, indicating that PN-1 is a bona fide protease inhibitor that can block DCG protein degradation in the Golgi complex in vivo (Fig. 5A and B) [36]. Moreover, immunofluorescence microscopy revealed that the CgB recovered by PN-1 expression had a Golgi-like localization and was also found in secretory granules at the tips of cells (Fig. 5C) [36]. Upon stimulation with KCl/BaCl2, the 6T3 cells transfected with PN-1 showed increased CgB release providing further evidence that the rescued CgB molecules were packaged in secretory granules of the regulated secretory pathway (Fig. 5D) [36]. Thus CgA up-regulates granule biogenesis by protecting granule proteins against degradation in the Golgi complex via an increase in PN-1 expression. PN-1 belongs to the family of serpins (serine protease inhibitors). It therefore specifically inhibits serine proteases. It has been demonstrated that serine proteases, such as furin, prohormone convertase-1 (PC-1), and subtilisin/kexin isozyme-1 (SKI-1), are active in the Golgi apparatus [39–41]. While the serine proteases inhibited by PN-1 in the Golgi have not been yet identified, these proteases are possible candidates.
Fig. 5.
A. Western blotting analysis of CgB and PN-1 from 6T3-WT cells infected with CgB expressing Tet-On adenovirus (lane 1), or with CgB and PN-1 adenovirus (lane 2). B. Bar graph shows CgB level in the cells expressing PN-1 (217 ± 29%, ± SEM; n = 7; **P < 0.01) compared with cells without PN-1 expression (100% as a control). α-tubulin was used as a loading control in A and used for normalization of CgB level for the graph in B. C. Immunofluorescence microscopy on CgB in 6T3-WT cells was performed after transfection of CgB alone (a and b) or both CgB and PN-1 adenoviruses (c and d). Arrows indicate the Golgi apparatus positive for a Golgi marker, GRASP65 (d; red). Arrowheads indicate CgB immunoreactivity in the processes. Bar, 10 μm. Immunoreactive CgB was distributed in a punctate manner along the processes and tips of these cells, characteristic of localization in DCGs. D. Western blotting analysis of CgB in conditioned medium from CgB- and PN-1-coexpressing 6T3-WT cells treated with (+) or without (−) 50mM KCl/2 mM BaCl2 (upper panel). CgB secretion was significantly increased to 201 ± 16% (± SEM; n = 4; **P < 0.01) with stimulation compared with basal secretion (100% as control) (lower panel). Taken together, these data indicate that CgB recovered by PN-1 expression was packaged into regulated secretion-competent DCGs in 6T3-WT cells. (Data taken from Figs. in Kim et al., 2006, Mol. Biol. Cell. [36])
Secreted CgA-derived peptide signals PN-1 expression and granule biogenesis
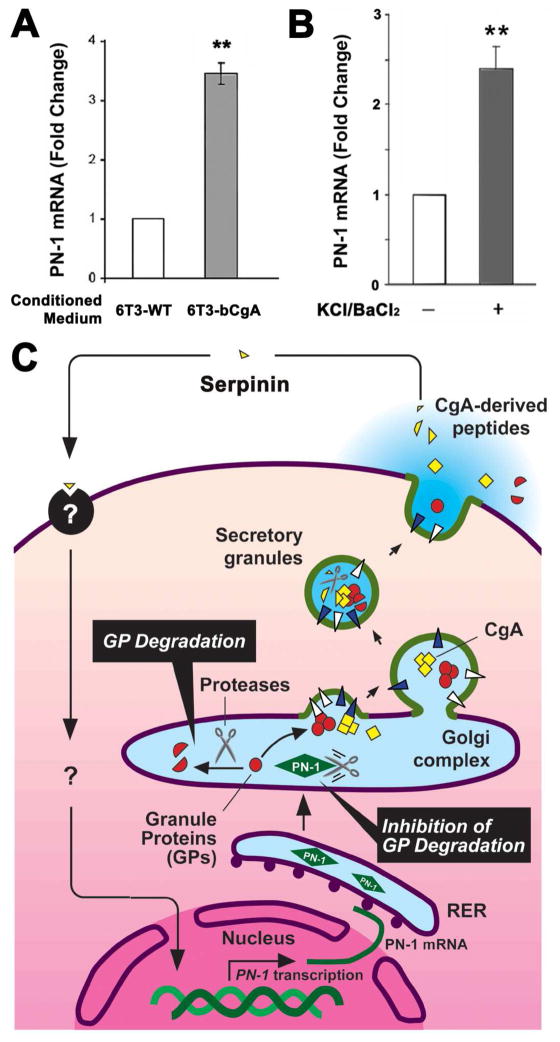
To begin to understand the mechanism by which CgA regulates PN-1 expression, 6T3-WT cells were treated with conditioned medium collected from 6T3-WT or 6T3-bCgA cells (Fig. 6A). Treatment with 6T3-bCgA conditioned medium increased PN-1 mRNA expression by ~3 fold compared to cells treated with 6T3-WT conditioned medium, suggesting that secreted CgA or its derived peptides can induce PN-1 expression via extracellular signal transduction. Moreover, stimulation of AtT-20 cells with KCl/BaCl2 induced PN-1 mRNA expression by ~2.5 fold compared to unstimulated cells (Fig. 6B), suggesting that the stimulation of the cells caused the release of CgA or a CgA derived peptide that could signal the cell to increase PN-1 expression. Our studies further showed that addition of intact CgA into the medium did not induce PN-1 mRNA expression in 6T3-WT cells, but a ≤3 kDa fraction of conditioned media from AtT-20 cells or 6T3-bCgA cells, but not 6T3-WT cells, did. This finding suggested that a small peptide cleaved from CgA likely mediated this effect. Finally, when we tested several synthetic peptides based on the paired basic residue cleavage sites that were ≤3 kDa in size and we found that serpinin (see Fig. 1), a CgA C-terminal peptide could significantly induce PN-1 expression in AtT-20 cells (Koshimizu et al. unpublished data).
Fig. 6.
A. CgA-dependent up-regulation of PN-1 mRNA expression in pituitary cell lines. Bar graphs show the effect of 20h treatment of 6T3-WT cells with conditioned medium from 6T3-WT cells, which lack CgA expression, or 6T3-bCgA cells, which express CgA, on PN-1 mRNA expression. Cells treated with 6T3-bCgA cell-conditioned medium showed a significant increase in PN-1 mRNA expression (3.30 ± 0.17 fold, ± SEM, **P < 0.01, N = 3) relative to cells treated with 6T3-WT cell-conditioned medium (1.00 fold as control, N =3). B. AtT-20 cells were stimulated with 50 mM KCl/2mM BaCl2. The bar graph shows that the fold change in PN-1 mRNA of stimulated cells was 2.40 ± 0.24 (± SEM, N = 3, *P < 0.05) relative to unstimulated cells (1.00 fold as control, N = 3). The PN-1 mRNA quantification was performed as previously described [36]. (Koshimizu et al., in preparation) C. Model for serpinin-inducing PN-1-dependent granule biogenesis in (neuro)endocrine cells. CgA is proteolytically cleaved to form serpinin which is secreted in an activity-dependent manner. Secreted serpinin binds to a cognate receptor and up-regulates PN-1 transcription. The increase in PN-1 protein stabilizes the secretory granule proteins at the Golgi apparatus to increase their levels which then promotes biogenesis of dense core granules.
From all our studies taken together, we propose the following model for the regulation of DCG biogenesis by CgA-derived peptide(s) (Fig. 6C). In this model, a (neuro)-endocrine cell, when stimulated, causes DCG exocytosis and discharge of their contents including hormones, bioactive peptides, CgA and its derived peptides into the extracellular space. Generally about 1–2% of the cell’s DCGs are released during stimulation and they need to be replaced. We propose that at least one CgA-derived peptide, serpinin located at the C-terminal of CgA, binds to a putative receptor/binding protein to signal the upregulation of PN-1 expression at the transcriptional level since we showed that it was actinomycin D inhibitable, and at the translational level. This leads to inhibition of proteolytic degradation in the Golgi complex of DCG proteins which has a constant turnover in (neuro)endocrine cells, resulting in greater accumulation of DCG proteins which then promotes formation of more granules. Thus our studies have provided a model that couples stimulus secretion with enhanced DCG biogenesis, with serpinin being a CgA-derived peptide that mediates extracellular signaling to a transcriptional event in the nucleus to increase PN-1 expression.
Conclusion
Once referred to as “a molecule without function” CgA plays several important cell biological roles within the (neuro)endocrine cell. First, it is a precursor molecule to a number of biologically active peptides that control multiple physiological functions. Secondly it is a granulogenic protein that mediates granule formation and may indirectly facilitate the targeting of prohormones to the regulated secretory pathway granules. Finally, we have recently shown that serpinin, a CgA-derived peptide plays a novel role as an extracellular signaling molecule to enhance granule biogenesis. Serpinin links DCG release with a transcriptional event of increasing the expression of a protease inhibitor PN-1, which protects granule proteins such as CgB against degradation in the Golgi complex, leading to promotion of granule formation.
Acknowledgments
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health intramural research programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Connor DT, Frigon RP. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984;259:3237–3247. [PubMed] [Google Scholar]
- 2.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JC, Hook V. Proteolytic fragments of chromogranins A and B represent major soluble components of chromaffin granules, illustrated by two-dimensional proteomics with NH(2)-terminal Edman peptide sequencing and MALDI-TOF MS. Biochemistry. 2009;48:5254–5262. doi: 10.1021/bi9002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 5.Aardal S, Helle KB. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept. 1992;41:9–18. doi: 10.1016/0167-0115(92)90509-s. [DOI] [PubMed] [Google Scholar]
- 6.Helle KB. The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp266. epublished. [DOI] [PubMed] [Google Scholar]
- 7.Di Comite G, Rossi CM, Marinosci A, Lolmede K, Baldissera E, Aiello P, Mueller RB, Herrmann M, Voll RE, Rovere-Querini P, et al. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-alpha-elicited endothelial activation. J Leukoc Biol. 2009;85:81–87. doi: 10.1189/jlb.0608358. [DOI] [PubMed] [Google Scholar]
- 8.Courel M, Rodemer C, Nguyen ST, Pance A, Jackson AP, O’connor DT, Taupenot L. Secretory granule biogenesis in sympathoadrenal cells: identification of a granulogenic determinant in the secretory prohormone chromogranin A. J Biol Chem. 2006;281:38038–38051. doi: 10.1074/jbc.M604037200. [DOI] [PubMed] [Google Scholar]
- 9.Iacangelo AL, Eiden LE. Chromogranin A: current status as a precursor for bioactive peptides and a granulogenic/sorting factor in the regulated secretory pathway. Regul Pept. 1995;58:65–88. doi: 10.1016/0167-0115(95)00069-n. [DOI] [PubMed] [Google Scholar]
- 10.Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology (Bethesda) 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- 11.Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. [PubMed] [Google Scholar]
- 12.Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain RK, Chang WT, Geetha C, Joyce PB, Gorr SU. In vitro aggregation of the regulated secretory protein chromogranin A. Biochem J. 2002;368:605–610. doi: 10.1042/BJ20021195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowley DJ, Moore YR, Darling DS, Joyce PB, Gorr SU. N- and C-terminal domains direct cell type-specific sorting of chromogranin A to secretory granules. J Biol Chem. 2000;275:7743–7748. doi: 10.1074/jbc.275.11.7743. [DOI] [PubMed] [Google Scholar]
- 15.Hosaka M, Watanabe T, Sakai Y, Uchiyama Y, Takeuchi T. Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic beta-cells. Mol Biol Cell. 2002;13:3388–3399. doi: 10.1091/mbc.02-03-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han L, Suda M, Tsuzuki K, Wang R, Ohe Y, Hirai H, Watanabe T, Takeuchi T, Hosaka M. A large form of secretogranin III functions as a sorting receptor for chromogranin A aggregates in PC12 cells. Mol Endocrinol. 2008;22:1935–1949. doi: 10.1210/me.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosaka M, Suda M, Sakai Y, Izumi T, Watanabe T, Takeuchi T. Secretogranin III binds to cholesterol in the secretory granule membrane as an adapter for chromogranin A. J Biol Chem. 2004;279:3627–3634. doi: 10.1074/jbc.M310104200. [DOI] [PubMed] [Google Scholar]
- 18.Cool DR, Normant E, Shen F-S, Chen H-C, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: Genetic obliteration leads to endocrine disorders in Cpefat mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 19.Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol. 2007;177:191–196. doi: 10.1083/jcb.200701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crivellato E, Nico B, Ribatti D. The chromaffin vesicle: advances in understanding the composition of a versatile, multifunctional secretory organelle. Anat Rec (Hoboken) 2008;291:1587–1602. doi: 10.1002/ar.20763. [DOI] [PubMed] [Google Scholar]
- 21.Biswas N, Rodriguez-Flores JL, Courel M, Gayen JR, Vaingankar SM, Mahata M, Torpey JW, Taupenot L, O’Connor DT, Mahata SK. Cathepsin L colocalizes with chromogranin a in chromaffin vesicles to generate active peptides. Endocrinology. 2009;150:3547–3557. doi: 10.1210/en.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci U S A. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 24.Yasothornsrikul S, Toneff T, Hwang SR, Hook VY. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J Neurochem. 1998;70:153–163. doi: 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]
- 25.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 26.Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem. 2004;279:20242–20249. doi: 10.1074/jbc.M310613200. [DOI] [PubMed] [Google Scholar]
- 27.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 28.Stettler H, Beuret N, Prescianotto-Baschong C, Fayard B, Taupenot L, Spiess M. Determinants for chromogranin A sorting into the regulated secretory pathway are also sufficient to generate granule-like structures in non-endocrine cells. Biochem J. 2009;418:81–91. doi: 10.1042/BJ20071382. [DOI] [PubMed] [Google Scholar]
- 29.Yoo SH. pH- and Ca(2+)-dependent aggregation property of secretory vesicle matrix proteins and the potential role of chromogranins A and B in secretory vesicle biogenesis. J Biol Chem. 1996;271:1558–1565. [PubMed] [Google Scholar]
- 30.Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natori S, Huttner WB. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc Natl Acad Sci U S A. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, Alvarez de la Rosa D, Carmona E, Castañeyra A, Viveros OH, O’Connor DT, et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 2008;28:3350–3358. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendy GN, Li T, Girard M, Feldstein RC, Mulay S, Desjardins R, Day R, Karaplis AC, Tremblay ML, Canaff L. Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol Endocrinol. 2006;20:1935–1947. doi: 10.1210/me.2005-0398. [DOI] [PubMed] [Google Scholar]
- 35.Kim T, Zhang CF, Sun Z, Wu H, Loh YP. Chromogranin A deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J Neurosci. 2005;25:6958–6961. doi: 10.1523/JNEUROSCI.1058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim T, Loh YP. Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol Biol Cell. 2006;17:789–798. doi: 10.1091/mbc.E05-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffiths G, Quinn P, Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983;96:835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidah NG, Mowla SJ, Hamelin J, Mamarbachi AM, Benjannet S, Touré BB, Basak A, Munzer JS, Marcinkiewicz J, Zhong M, et al. Mammalian subtilisin/kexin isozyme SKI-1: A widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci U S A. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vey M, Schäfer W, Berghöfer S, Klenk HD, Garten W. Maturation of the trans-Golgi network protease furin: compartmentalization of propeptide removal, substrate cleavage, and COOH-terminal truncation. J Cell Biol. 1994;127:1829–1842. doi: 10.1083/jcb.127.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]
- 42.Fernandez CJ, Haugwitz M, Eaton B, Moore HP. Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol Biol Cell. 1997;8:2171–2185. doi: 10.1091/mbc.8.11.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]