Abstract
Corrugator supercilii muscle activity is considered an objective measure of valence because it increases in response to negatively valenced facial expressions (angry) and decreases to positive expressions (happy). The authors sought to determine if corrugator activity could be used as an objective measure of positivity-negativity bias. The authors recorded corrugator responses as participants rated angry, happy, and surprised faces as “positive” or “negative.” The critical measure of bias was the percentage of positive versus negative ratings assigned to surprised faces by each participant. Reaction times for surprise expressions were longer than for happy and angry expressions, consistent with their ambiguous valence. Participants who tended to rate surprised faces as negative showed increased corrugator activity to surprised faces, whereas those who tended to rate surprise as positive showed decreased activity. Critically, corrugator responses reflected the participants’ bias (i.e., their tendency to rate surprise as positive or negative). These data show that surprised faces constitute a useful tool for assessing individual differences in positivity-negativity bias, and that corrugator activity can objectively reflect this bias.
Keywords: ambiguity, surprise, facial EMG, EDA, individual differences
The theoretical notion of “affect programs” (Tomkins, 1962) dictates that facial reactions are elicited spontaneously and automatically (Dimberg, 1996; Ekman, 1992). Facial electromyography (EMG) has a temporal resolution and sensitivity that can capture fast and subtle facial musculature changes (Cacioppo & Petty, 1981; Tassinary, Cacioppo, & Vanman, 2007). Specifically, previous research has documented that facial EMG is sensitive to the valence of a presented stimulus, in that activity in the corrugator supercilii muscle region is both potentiated by unpleasant pictures and inhibited by pleasant pictures (Cacioppo, Petty, Losch, & Kim, 1986; Lang, Greenwald, Bradley, & Hamm, 1993). This effect has been demonstrated for affective pictures and sounds (Larsen, Norris, & Cacioppo, 2003) as well as for faces, face-voice combinations, and bodily expressions (Magnee, Stekelenburg, Kemner, & de Gelder, 2007). Further, corrugator responses to emotional stimuli are rapidly and automatically evoked, being manifested within a few hundred milliseconds of exposure (Dimberg, 1991). Indeed, previous work has shown that corrugator responses to very short presentations of emotional stimuli that were not reliably identifiable were similar to those observed in response to longer presentations of stimuli that could be reliably identified (Dimberg, Thunberg, & Elmehed, 2000). These effects are particularly robust in response to stimuli that are considered “biologically relevant” (Dimberg, 1997). Of particular relevance to the present study, and consistent with the findings referenced above utilizing other types of affective stimuli, corrugator activity increases in response to angry facial expressions and is attenuated to happy facial expressions (Dimberg et al., 2000) and these responses adhere to the definition of automaticity outlined above (i.e., short durations do not differ from long; Dimberg et al., 2000).
Facial expressions provide information about the emotions and intentions of others. One basic signal communicated by facial expressions is the valence of the emotion being experienced by the expresser and, in turn, the valence of the outcome predicted for the perceiver. For example, an angry face is interpreted as negative because, in the past, angry faces have signaled unpleasant or confrontational social interactions. How an individual will interpret a given facial expression can be affected by their biases and personality characteristics (e.g., Canli, Sivers, Whitfield, Gotlib, & Gabrieli, 2002; Somerville, Kim, Johnstone, Alexander, & Whalen, 2004). An outstanding question that remains to be addressed is the degree to which these individual differences will interact with the clarity of valence of the perceived expression. For example, when the valence of a given expression is clear, as in the case of angry or happy expressions, individual differences on the part of perceivers might have less of an impact on their interpretations. However, some facial expressions send a more ambiguous message with respect to the valence of the outcomes that they predict. For instance, when surprised expressions are presented within an experimental context that provides no information to disambiguate the valence of this expression, they are interpreted negatively by some and positively by others (Kim, Somerville, Johnstone, Alexander, & Whalen, 2003; Kim et al., 2004). In this way, surprised expressions may offer a means to assess individual differences in positivity-negativity bias.
In the present study, we sought to examine corrugator responses to surprised faces in comparison to responses to expressions of clear valence (i.e., angry and happy faces). Specifically, we measured facial EMG while participants rated each face as either “positive” or “negative.” Based on previous results, we predicted that all participants would show increased corrugator activity to angry faces and attenuated activity to happy faces (Dimberg et al., 2000). The focus of this study was to then compare these corrugator responses to those elicited by surprised faces, as they might reflect a participant’s bias in their ratings of surprised faces as either negative or positive.
Our second aim was to offer data concerning the stability of these face ratings as a measure of bias, with the prediction that positivity-negativity bias is a stable individual difference. To this end, we invited participants to return 1 year later to perform the same experiment predicting that we would find a significant correlation in valence ratings from Time 1 to Time 2. Given that this prediction suggests that this bias measure is more trait-than state-like, participants were asked to complete several personality and trait measure scales. The particular scales are detailed in the Method section and were selected because previous studies have shown that interpretive positivity-negativity biases can be related to anxiety (Mathews & MacLeod, 1994), empathy (Surakkaa & Hietanenb, 1998), neuroticism (Nielsen & Petersen, 2008), and extraversion (Nielsen & Petersen, 2008).
Method
Participants
Thirty-five healthy, White Dartmouth undergraduates (22 women; ages 18 to 24 years old, mean age = 19.3) volunteered to participate. All participants had normal or corrected-to-normal vision, no psychoactive medication, and no significant neurological or psychiatric history. None were aware of the purpose of the experiment, and they were all compensated for their participation through monetary payment or course credit. Written informed consent was obtained from each participant before the session, and all procedures were approved by Dartmouth College Committee for the Protection of Human Subjects.
Procedure
Stimuli
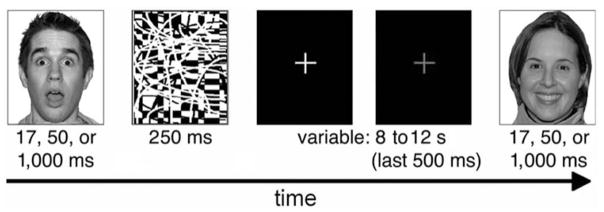
We selected images of 18 identities (9 female, 9 male) from the NimStim standardized facial expression stimulus set (Tottenham et al., 2009), each posing three emotional expressions: angry, happy, and surprised. The facial expressions in this stimulus set have been validated by a separate set of participants that labeled each expression; only faces over 60% correctly labeled were included. The stimuli were randomly presented in each of three runs of 54 trials (i.e., each face was presented once per run). An equal number of surprised, angry, and happy expressions were presented for 17 ms, 50 ms, and 1,000 ms, and each was followed by a black-and-white pattern that was presented for 250 ms, which served as a retinal wipe (see Figure 1).
Figure 1.
A depiction of the experimental design. Angry, happy, and surprised faces were presented for either 17, 50, or 1,000 ms. The task for each face was to decide whether the expression was positive or negative. The second fixation appeared in red. Reprinted with permission from MacArthur Network.
These stimulus presentations were selected to match those commonly employed in neuroimaging studies of face processing (e.g., Breiter et al., 1996; Whalen et al., 2004) as we hope to adapt this task to a subsequent imaging experiment. In addition, based on the results of Ogawa and Suzuki (1999) we included one condition we expected participants could not reliably identify (17 ms) for comparison with two others that we expected they could. We expected to be able to collapse across these presentation durations because Dimberg (1982) and Dimberg et al. (2000) showed that brief presentations (even those that participants cannot reliably identify) were just as effective as longer presentations for eliciting corrugator muscle responses in psychiatrically healthy participants, and that these corrugator responses do not differ for brief versus longer stimulus presentations. That said we routinely devise our paradigms in psychiatrically healthy participants for their eventual application to psychopathological groups (see, e.g., Rauch et al., 2000; Shin et al., 2005; Whalen et al., 2008). Because, for example, brief stimulus presentations of affective information have been shown to reveal pre-attentive biases in participants with pathological anxiety, while longer presentations have revealed differences in more elaborative processing in participants with major depression (see Mathews & MacLeod, 1994, for a review) we included variable stimulus durations in this initial study (i.e., it is possible in future studies that patient groups will show differential corrugator responses to differing stimulus durations).
Testing session
Participants were seated in a comfortable chair in a dimly lit room and viewed faces presented on a computer screen in front of them. During each testing session, a female experimenter attached electrodes for recording facial EMG and electrodermal activity (EDA) according to standard methods (for details, see Fowles et al., 1981; Fridlund & Cacioppo, 1986). To allow EDA to reach a stable baseline before beginning the experimental trials, participants viewed 27 images of neutral scenes (e.g., landscapes), during which they were asked to press a button corresponding to whether the scene was indoors or outdoors.
Faces were presented one at a time at the center of the screen (visual angle 7°), on a black background, with intertrial intervals varying between 8 and 12 s. During these intervals, a white fixation cross appeared on the screen. During the final 500 ms of this intertrial interval, the fixation cross turned red to ensure that participants were oriented for the next face presentation. For each face presented, participants were asked to make a two alternative forced-choice decision as quickly and accurately as possible according to their gut reaction as to whether the face had a positive or negative valence.
Following each recording session, participants also completed the following behavioral scales: Beck Depression Inventory (BDI; Beck, Ward, & Mendelson, 1961), State–Trait Anxiety Inventory (STAI–S, STAI–T; Spielberger, Gorsuch, & Lushene, 1988), the NEO Five-Factor Inventory (NEO–FFI; Costa & McCrae, 1991), and the Empathy Quotient (EQ; Baron-Cohen & Wheelwright, 2004).
Physiological Parameters and Data Reduction
EMG and EDA were sampled and recorded at 1000 Hz. Data were converted and amplified with an eight-channel amplifier (PowerLab 8/30; ADInstruments, New South Wales, Australia) and displayed, stored, reduced, and analyzed with the Chart 5.4.2 software package (ADInstruments, 2002).
Facial EMG
Facial EMG was measured using 4-mm standard Ag/Ag-Cl electrodes, filled with Signa Gel electrode paste, and attached bipolarly over the corrugator supercilii muscle region (Fridlund & Cacioppo, 1986). The skin was first cleaned with alcohol and rubbed with an abrasive gel. Electrode placement was counterbalanced such that half of the participants had electrodes placed on the right side of the face, and the other half on the left side of the face. To conceal the recording of facial muscle activity, we used a cover story, telling participants that we were measuring sweat gland activity. After the experiment, they were informed about the true purpose of the study.
Offline, EMG data were submitted to a DC Restore to center the signal at a zero point, a 60-Hz notch filter to reduce 60 Hz of noise present in the testing room, a 30-Hz high-pass filter to reduce movement and blink-related artifact, then full rectified. To correct for the positive skew found in our EMG data (and has been previously found to be inherent to EMG data), all data were then subjected to a square-root transformation (Fridlund & Cacioppo, 1986). Facial reactions were scored and averaged across a period of 1,000 ms after the stimulus onset, and a baseline of 500 ms before the red fixation appeared was subtracted for each trial, resulting in an overall EMG change score. We are using a period of 1,000 ms of activity as this time period has been frequently used to examine EMG activity (e.g., Dimberg et al., 2000), and though our stimulus presentation durations were often shorter than 1,000 ms, psychophysiological picture processing continues in the absence of a sensory stimulus (Codispoti, Bradley, & Lang, 2001).
EDA
EDA was obtained through two Ag/Ag-Cl electrodes (surface: 1 cm2), placed on the second phalanx of the index and the third digit of the nondominant hand, attached with a Velcro strap. After electrodes were attached and electrodermal activity reached a stable baseline, the baseline level of activity for each participant was brought to a zero point.
Offline, EDA data were submitted to a 0.1-Hz high-pass filter to remove drift. Due to the difference in temporal resolution between EDA and EMG (Dawson, Schell, & Filion, 2007), EDA activity was scored and averaged across a period of 6,000 ms, which allows for an estimate of the skin conductance response to each stimulus even in the absence of a sensory stimulus (Codispoti et al., 2001). A similar baseline of 500 ms before the red fixation appeared was subtracted for each trial, resulting in an overall EDA score (Dawson et al., 2007).
Standardization
As there are inherent extreme individual differences in the range of reactivity (Cacioppo et al., 1992; Lang et al., 1993; Norris, Larsen, & Cacioppo, 2007), and our goal was to compare EMG responses to face conditions according to groups of participants based on their explicit valence ratings in each condition of emotion (particularly in response to an ambiguous expression such as surprise), we standardized (i.e., z scores) responses for each participant to remove variability that would have existed between these conditions to directly compare them (Bensafi et al., 2003; Bush, Hess, & Wolford, 1993). Previous work has analyzed data in this manner (Pattyn, Neyt, Henderick, & Soetens, 2008), as such interindividual variability is a common issue in psychophysiology (Bush et al., 1993). As such, standardizing our data provides us with a relative EMG response to angry, happy, and surprised expressions for each individual, and it is this relative activity that we can then compare across participants. In other words, we were more interested in differences across groups in response to surprised faces than to different individual responses. These z scores were calculated based on the mean and standard deviation of activity across all trials for each participant, regardless of condition. We then averaged EMG and EDA activity across all trials within each condition (happy, angry, and surprised).
Valence ratings
Our goal was to use ratings of surprised faces to calculate each individual’s bias score. We used the facial expressions of clear valence (angry and happy) to first determine that participants could accurately rate the valence of these expressions. Recall that the 17-ms duration condition was selected as a comparison condition that participants would not be able to reliably identify (Ogawa & Suzuki, 1999). Consistent with this assertion, explicit behavioral responses for faces with a clear valence (i.e., angry, happy) in the 17-ms condition were not significantly different from chance, angry: t(29) = 1.93, p > .05, dz = .35 (M = 43.8%, SD = 18.6%); happy: t(29) = 1.00, p > .3, dz = .18 (M = 45.9%, SD = 18.0%). Conversely, ratings for the 50- and 1,000-ms presentation durations were accurate (i.e., different from chance) angry at 50 ms: t(29) = 21.19, p < .001, dz = 3.87 (M = 89.1%, SD = 9.6%); angry at 1,000 ms: t(29) = 44.06, p < .001, dz = 8.04 (M = 95.9%, SD = 5.5%); happy at 50 ms: t(29) = 20.61, p < .001, dz = 3.76 (M = 10.5%, SD = 10.1%); happy at 1,000 ms: t(29) = 41.69, p < .001, dz = 7.61 (M = 4.2%, SD = 5.8%). Because participants could not discriminate the clearly valenced expressions at 17 ms, we based calculation of participants’ bias scores for ratings of surprised faces on 50- and 1,000-ms presentations only. That said, once we determined each participants’ bias score we included all stimulus durations in the EMG analysis. This is consistent with our theoretical framework outlined in the Introduction suggesting that EMG responses are spontaneous and automatic in that they do not differ based on stimulus duration, even very short stimulus durations that produce differences in subjective report (i.e., 30 ms; Dimberg et al., 2000). Note that, inclusion of the 17-ms condition here is done within an omnibus analysis that will detect any main effects of duration and/or Duration × Expression interaction if this condition should turn out to systematically differ in the present study (see Results).
Results
Data Validation
We used ratings of angry and happy expressions as a manipulation check in case participants mistakenly switched the response buttons. As such, five participants were removed from the sample due to nonnormative ratings (e.g., happy faces were rated as negative/angry as positive). As a result, the final sample contained 30 participants (19 women).
Behavioral Results
Valence ratings
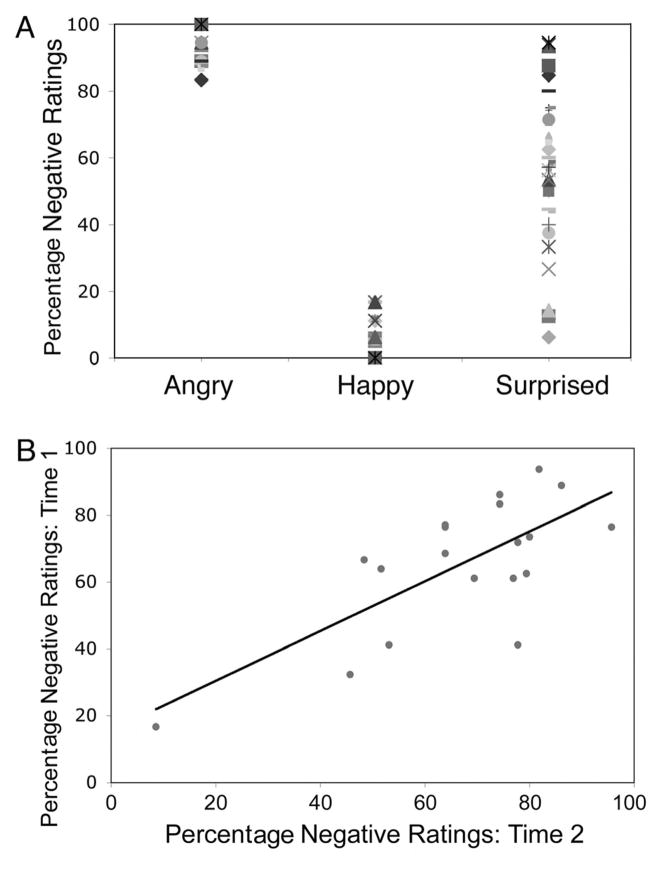
As expected, angry expressions were rated as negative more frequently than were happy expressions, t(29) = 45.20, p < .001, dz = 8.25. Angry and happy expressions were rated as consistently negative (92.67% of trials) and positive (92.71% of trials), respectively. Figure 2A shows that these same participants differed in their tendency to interpret surprised faces negatively versus positively. We used a median split to classify our participants into two groups based on their bias scores: 15 participants showing the greatest tendency to interpret surprised expressions as having a negative valence (Sneg group; 7 men, 8 women; M ± SE = 81.0% ± 1.96), and the other 15 participants showing a lesser tendency to interpret these expressions as having a negative valence (Spos group; 4 men, 11 women; M ± SE = 52.7% ± 4.03). An Expression (surprised, angry, happy) × Group (Sneg, Spos) repeated-measures analysis of variance (ANOVA) revealed a significant main effect of expression, F(2, 27) = 984.59, p < .001, η2 = .93; and pairwise comparisons (Bonferroni corrected) revealed that angry faces were rated as more negative than happy (p < .001), and surprised faces (p < .001), and that happy faces were rated as more positive than surprised faces (p < .001). There was also a significant Expression × Group interaction, F(2, 27) = 12.60, p < .001, η2 = .03; and pairwise comparisons (Bonferroni corrected) revealed that surprised faces were rated differently between the two groups (i.e., rated as negative by the Sneg group and positive by the Spos group), but angry and happy faces were rated the same across groups (p < .001). More important, participants were reliable in how they rated valence across different surprised faces (Cronbach’s α = .908). We found a similar effect for angry (Cronbach’s α = .699) and happy (Cronbach’s α = .697) expressions.
Figure 2.
Individual differences in rating valence of surprised expressions. (A) Angry faces are rated consistently negative, happy faces are consistently positive, and surprised ratings vary from negative to positive, revealing individual differences in how people interpret the valence of these expressions. (B) There was a significant positive correlation between percentage “negative” responses to surprised expressions from Time 1 to Time 2 (1 year later).
RT
We found a positive skew in our reaction time (RT) data that is not uncommon for this measure. Indeed, because RT has a lower boundary (i.e., individuals can only respond so fast physically), as RTs become longer, they often become more variable. In contrast, a mean RT that is short tends to have a smaller standard deviation. For this reason, RT data were standardized using z scores, as described above, because there was a positive skew in these data. An Expression (surprised, angry, happy) × Group (Sneg, Spos) ANOVA revealed a significant main effect of expression, F(2, 27) = 40.68, p < .001, η2 = .57; and corrected pairwise comparisons revealed that, as expected, participants took significantly longer to rate surprised expressions than angry (p < .001) and happy expressions (p < .001), but there was no difference between angry and happy expressions (p > .2). We also observed a significant Expression × Group interaction, F(2, 27) = 7.90, p = .002, η2 = .11. Corrected pairwise comparisons revealed that participants in the Spos group took even longer than the Sneg group to rate surprised expressions compared to angry (Spos: p < .001; Sneg: p = .01) and happy (Spos: p < .001; Sneg: p = .007) expressions, but there was no difference between angry and happy expressions (p > .1).
Questionnaire data
As noted in the Introduction, we administered personality and trait measure scales to document that these scores fell within a healthy psychiatric range on certain measures (i.e., depression, anxiety) across all participants, while also assessing whether they showed any systematic differences (i.e., anxiety, extraversion, neuroticism, and empathy). Results indicated that all scores for depression (BDI) and anxiety (STAI) were within normal limits. Moreover, independent samples t tests comparing scores for each survey between the Sneg and Spos groups revealed no significant differences in depression (BDI), anxiety (STAI), neuroticism or extraversion (NEO–FFI). On their scores for empathy (EQ), however, we observed a significant difference, such that participants in the Sneg group were less empathic than those in the Spos group, t(20) = −2.49, p = .02, d = 1.11; M ± SE: Sneg = 38.6 ± 1.81; Spos = 46.83 ± 2.61.
EMG Results
EMG responses were analyzed using an Expression (surprised, angry, happy) × Presentation Duration (17 ms, 50 ms, 1,000 ms) × Group (Sneg, Spos) repeated-measures ANOVA. This analysis revealed a significant main effect of expression, F(2, 27) = 8.15, p = .002, η2 = .07; and corrected pairwise comparisons (Bonferroni corrected) revealed that this was due to greater corrugator responses to angry expressions compared to happy expressions (p = .001) consistent with previous findings (Dimberg et al., 2000). There was no significant main effect of presentation duration (F(2, 27) = 2.75, p > .08, η2 = .01), and no significant Expression × Presentation Duration interaction, F(4, 25) = 2.68, p > .05, η2 = .07. The lack of a main effect for duration and lack of an interaction between duration and expression supports our decision to include 17-ms EMG responses in the overall analysis (see Method section). Moreover, there was a significant positive correlation between valence ratings and corrugator activity in response to surprised expressions, such that as valence ratings increased in negativity across participants, the corrugator activity from those participants increased (r = .36, p = .035, one-tailed). Put another way, the measure of the participants’ bias to rate faces as negative or positive (i.e., percentage negative ratings) was correlated with the absolute measure of corrugator activity in response to all surprised face trials.
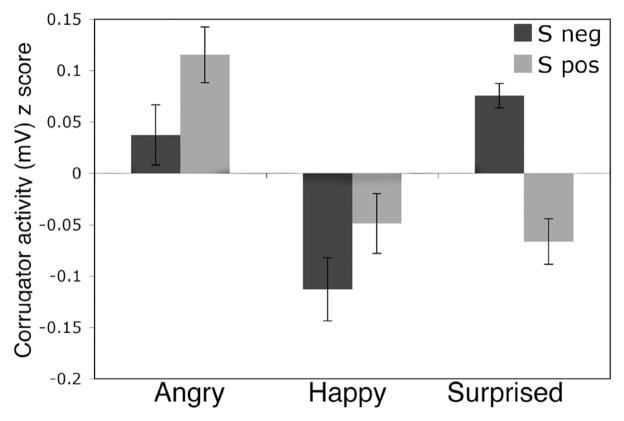
There was also a significant Expression × Group interaction, F(2, 27) = 3.75, p = .04, η2 = .02 (Figure 3A). Corrected pairwise comparisons revealed that the Sneg group showed more corrugator activity to surprised than to happy expressions (p = .003), and no significant difference between surprised and angry expressions. Conversely, the Spos group showed less corrugator activity to surprised expressions than to angry expressions (p = .02), and no significant difference between surprised and happy expressions. Note that we can only make meaningful inferences about the interaction of Group × Expression (i.e., the relative activity of surprise, angry, and happy expressions and how that relationship differs across groups) due to standardization (see Method section).
Figure 3.
Individual difference in corrugator supercilii activity in response to surprised facial expressions, as compared to those with clear valence. In the Sneg group, surprised expressions elicit significantly greater standardized corrugator activity than happy expressions that is not significantly different from angry expressions. For the Spos group, surprised expressions elicit activity that is significantly lower than angry expressions that is not significantly different from happy expressions.
Trial-by-trial analysis
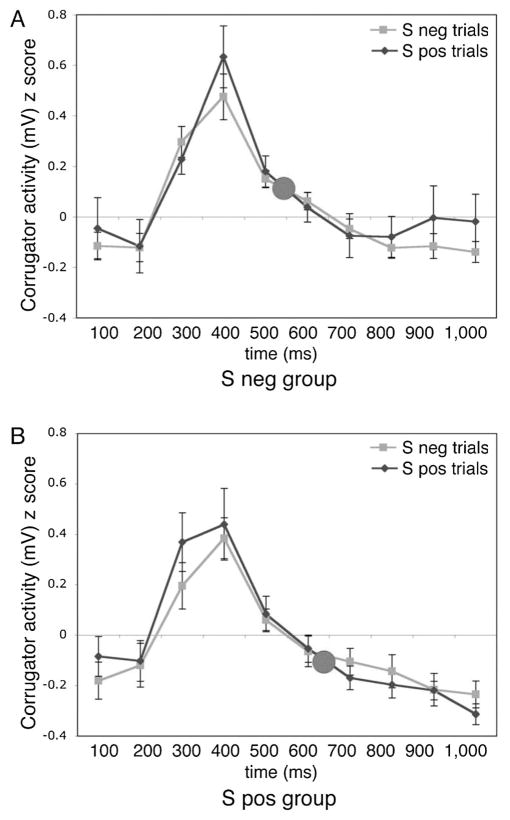
For theoretical reasons described in the Introduction and Method sections related to the automatic nature of EMG responses, we categorized participants based on their bias scores to examine their mean EMG responses to surprised faces. However, a more traditional analysis would have separated trials out according to their categorization as either negative or positive on a trail by trial basis. Indeed, perhaps our subject-based analysis somehow prejudiced the analyses to find that EMG responses reflected the bias of an individual, rather than the valence of the currently presented stimulus. To address this issue, we conducted an analysis comparing EMG responses for trials on which a presented surprised face was rated as negative compared to those trials rated as positive. There was no significant difference in corrugator activity for negative versus positive trials, t(29) = .32, p > .7, dz = .06. In other words, corrugator responses reflected participants’ rating bias, that is, their tendency to rate surprise as positive or negative, rather than their particular valence rating on a given trial. Figure 4 presents corrugator responses for the Sneg (Figure 4A) and Spos (Figure 4B) groups separately. Pictured here is the entire 1,000-ms response window that was analyzed (binned in 100-ms epochs). Though the mean response for the Sneg group is higher than the mean response for the Spos group (open circle represents mean of all 10 epochs for all trial types), the time courses for negative and positive trials within each group overlap. Thus, the observed difference in corrugator activity between groups reflected the group bias, rather than their interpretation of a given surprised face on a given trial.
Figure 4.
Corrugator supercilii activity reflects an individual’s bias more than their valence judgment on a given trial. (A) Time-course activity for participants who tend to rate surprise as negative reveals no difference in activity for trials in which they rate surprise as positive, as compared to trials in which they rate surprise as negative. (B) Time-course activity for participants who tend to rate surprise as positive reveals no difference in activity for trials in which they rate surprise as negative, as compared to trials in which they rate surprise as positive.
EDA Results
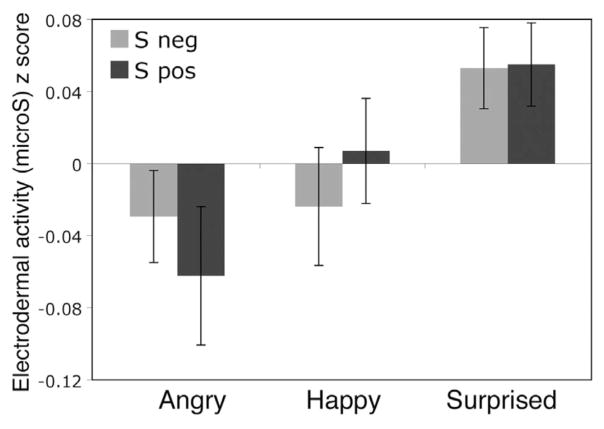
An Expression (surprised, angry, happy) × Presentation Duration (17 ms, 50 ms, 1,000 ms) × Group (Sneg, Spos) repeated-measures ANOVA conducted on EDA revealed a significant main effect of expression, F(2, 27) = 5.65, p = .009, η2 = .03; such that EDA responses were larger to surprised than to angry expressions (p = .02), but there was no difference in EDA for surprise and happy (p > .1) or angry and happy (p > .99; Figure 5) expressions. No other main effects or interactions were significant.
Figure 5.
Electrodermal activity to surprised facial expressions, as compared to those with clear valence. There was no significant difference in standardized electrodermal activity for angry and happy expressions, though surprised expressions elicited greater activity than expressions of clear valence.
Temporal Stability of Surprised Face Valence Ratings
We aimed to assess the stability of these behavioral responses over time by asking each of our 30 participants to return 1 year later to repeat the same experiment. Nearly two thirds of our participants (N = 19) returned (12 women). Our prediction was that valence ratings of surprised expressions would be consistent, even 1 year later. Indeed, we found that these ratings correlated positively with ratings at Time 1 (r = .72, p < .001; Figure 2B). The correlation is still significant when the extreme score is removed (r = .53, p = .02). We hoped to assess the stability of these EMG responses according to the difference in group bias but significant participant attrition made analysis of EMG data, perhaps due to its inherent low signal to noise ratio impossible. Indeed, an omnibus test of these data at Time 2 similar to that used at Time 1 showed no significant effects (Expression × Presentation Duration × Group, all main effect and interaction ps > .05). However, the fact that groups at Time 2 consisted of only 8 (Sneg) versus 11 (Spos) participants makes this outcome highly susceptible to Type II error. Another factor that may have affected our ability to assess EMG at Time 2 is the fact that EMG responses diminish over time. Specifically, a previous study has shown that EMG responses recorded a year later to the same stimuli were significantly diminished (Cohn, Schmidt, Gross, & Ekman, 2002). Future studies should aim to mitigate participant attrition at Time 2 to more carefully address whether such analyses are possible, perhaps utilizing novel stimuli, because our behavioral data suggest that this bias may be stable.
Discussion
The present study showed that when an ambiguous facial expression is encountered, corrugator activity reflects an individual’s general bias in interpreting the valence of these faces. Specifically, individuals who tended to interpret surprised faces as negative displayed higher corrugator muscle activity to all surprised faces, and those who tended to interpret surprised faces as positive displayed greater attenuation of corrugator activity to all surprised faces. This effect was not a result of between group differences in the experience of arousal, as electrodermal activity did not significantly differ across Sneg and Spos groups. Thus, when faced with a stimulus that can be interpreted either positively or negatively, corrugator responses reflected an individual’s bias rather than their actual rating on a given trial (i.e., even on trials when an individual with a negative bias rated a surprised expression as positive, their corrugator activity increased). This effect was observed in participants who otherwise showed corrugator responses to more clearly valenced stimuli that were consistent with previous reports—increases to clearly valenced negative stimuli and decreases to clearly valenced positive stimuli (Cacioppo et al., 1986; Dimberg, 1990; Dimberg et al., 2000; Larsen et al., 2003). Here we discuss the implications of this finding, considering the experimental context in which it was observed compared to previous studies.
Comparison of the Present Experimental Design With Previous Corrugator Studies of Valence Ratings
To date, much of the research employing facial EMG methods have examined emotional facial responses to clear positivity and negativity (e.g., facial expressions, affective pictures, scenes, and sounds, pleasant and unpleasant imagery; Dimberg, 1990; Cacioppo et al., 1986; Lang et al., 1993; Schwartz, Fair, Salt, Mandel, & Klerman, 1976). Other work revealed that EMG reflected personality differences (individuals with dysphoria do not show increased zygomatic activity in response to positive emotional stimuli; Sloan, Bradley, Dimoulas, & Lang, 2002), and emotional responses in a social context (individuals high in speech fear responded more negatively to emotional faces when primed with this fear; Vrana & Gross, 2004). In these cases, emotional displays express a sender’s emotional state, but they can also signal a listener’s understanding (e.g., Bavelas, Black, Lemery, & Mullett, 1986), a facial mimicry response (e.g., Dimberg, 1982; Kappas, Hess, & Banse, 1992), or shared affect between two individuals (Levenson & Ruef, 1992). These results converge on the finding that corrugator responses reflect reactions to clear positivity and negativity.
The purpose of the present study was to assess how this objective measure of affect (Harmon-Jones & Allen, 2001) would reflect affective responses during the interpretation of stimuli of ambiguous valence. Critically, in response to surprised faces, corrugator reflected bias (i.e., an individual’s tendency to rate surprise as positive or negative), rather than reflecting their vageneral valence ratings on any given trial. In addition, though participants could not reliably rate 17-ms presentations, their corrugator responses were similar to those observed for longer presentations that they could reliably rate. Taken together, these data suggest that corrugator responses may be more related to the way in which participants approach surprised faces, rather than their reactions to them. Future research is necessary to determine if this effect is specific to ambiguous surprised faces, or if it transfers to more classically employed stimuli in EMG studies (e.g., IAPS).
Though this study reports on clear individual differences in rating ambiguous facial expressions, we have little evidence for what may be causing these differences. In the present study we were offered a single clue that these differences are not related to differences in depression, anxiety, neuroticism, or extraversion, but could be related to differences in empathy (i.e., higher empathy being associated with a greater tendency to interpret surprised faces as positive). However, we also presented initial support that these ratings may be based on some sort of trait-like individual differences, as behavioral responses were reasonably consistent in two thirds of our participants 1 year later. Indeed, previous research examining the negativity bias has shown that such measures can be independent of traditional personality measures and yet can predict behavior on various tasks in a meaningful way (Norris, Larsen, Crawford, & Cacioppo, 2009). Taken together, such findings suggest that this paradigm could be used to study patient groups who show deficits in empathy and/or theory of mind (e.g., autism, Baron-Cohen, 2002; psychopaths, Blair et al., 1996), predicting an even greater tendency to interpret these faces as negative compared to a control participant group. Further research will be needed to determine the multiple sources of variability that might lead some individuals to have a negative bias in rating surprised expressions, whereas others have a positive bias in rating the same stimuli.
RT as a Measure of Ambiguity
RT data observed here support our assertion that surprised faces are ambiguous with respect to valence. That is, it could have been the case that surprised faces are not ambiguous at all. For example, an individual participant might believe this face to be immediately negative and not consider the positive interpretation at all. If this were true, we would have expected RTs for negative assessments of surprise to match negative assessments of anger, for example. This was not the case. RTs for surprised faces were longer than those for angry and happy faces, and RTs to clear expressions did not differ, consistent with the notion that surprised faces evoke a dual valence representation that must be resolved.
We predicted this effect based on a previous functional MRI (fMRI) study showing that surprised faces evoked involvement of a prefrontal—amygdala circuitry that was not evoked in response to expressions of clear negative valence (Kim et al., 2003). Specifically, differing interpretations of surprised faces were shown to correlate with distinct inverse reactivity patterns between the amygdala and a region of ventral medial prefrontal cortex (vmPFC; Kim et al., 2003). Participants who interpreted surprised faces as negative showed increased amygdala activity that correlated with attenuated vmPFC responses. Conversely, participants who interpreted these expressions as positive showed the opposite response pattern—increased vmPFC activity that correlated with attenuated amygdala responses. Based on these results, it was suggested that prefrontal cortex was necessary to resolve the predictive nature of surprised faces because of their ambiguity of valence (i.e., the fact that they have predicted both positive and negative outcomes in the past). That is, unlike faces of clear valence (i.e., angry or happy faces), surprised faces require an extra layer of processing as the viewer assesses their potential negative versus positive predictive nature.
As noted above, a simple prediction was made based on these data that is should take longer to provide a valence rating of a surprised expression compared to a clearly valenced expression. The present data support this prediction. Because these fMRI data were also consistent with the possibility that the prefrontal cortex had to send some sort of override message to the amygdala when a surprised expression was interpreted as positive, we further predicted that positive ratings of surprised faces should take even longer than negative ratings. We note that, in the present study, the RT difference between surprised faces and faces with clear expressions was larger for participants with a positive bias than the same difference for participants with a negative bias. These data are consistent with the proposition that a positivity bias requires an extra layer of regulation in the form of additional prefrontal—amygdala interaction (Kim et al., 2003; Sharot, Riccardi, Raio, & Phelps, 2007).
Caveats and Limitations
We used standardized data for EMG and EDA responses to compare responses across groups of participants. It is important to note that this is only one way of examining these data, and that this method does indeed obscure individual differences in measures along which sympathetic nervous system output varies (Cacioppo et al., 1992). In other words, individuals fall onto a wide spectrum of how physiologically reactive they are in response to emotional stimuli: Whereas some have large peaks and valleys in their activity, others have a smaller range in activity. With regards to our sample, some individuals may be inherently more responsive than others, and our standardized z scores were used to obscure such individual differences in the magnitude of their responses. We sought to control these inherent individual differences to directly compare responses to the same set of emotional stimuli (i.e., to evaluate activity elicited by surprised expressions as compared to angry and happy expressions) across groups of participants. However, when using standardized data, one can only make meaningful inferences about the interaction of Group × Expression (i.e., the relative activity of surprise, angry, and happy expressions and how that relationship differs across groups), as well as comparisons within group. The reason for this is that within-subject z transformation expresses each individual’s responses relative to his or her mean and standard deviation (e.g., Ben-Shakhar, 1985, 1987). Thus, differences between groups on a single condition are not meaningful. Future studies could be conducted to explicitly examine these individual differences in magnitude of reactivity.
Moreover, it is possible that our sample size, though both reasonable in size and providing sufficient effect sizes, may not have been large enough to capture individual differences within the personality characteristics examined here. Perhaps future research can focus on collecting participants that are preselected based on scores of these personality scales (e.g., high anxiety) to more directly examine these potential effects.
Conclusions
The present data raise the interesting possibility that a measure that has to date been shown to purely respond according to the valence of a presented stimulus or the valence of a current mood state when these stimuli or states are clear, can also be influenced by individual differences in one’s positivity-negativity bias when the valence of an encountered stimulus is more ambiguous. Given that a negativity bias has been shown to be characteristic of the emotional disorders (e.g., anxiety and depression) especially in the face of ambiguously valenced stimuli (see Mathews & MacLeod, 1994), the present paradigm could be amenable to the study of these populations.
Acknowledgments
This work was supported by National Institute of Mental Health Grants 069315 and 080716. We thank F. Ahs, F. C. Davis, T. Johnstone, and C. van Reekum for advice on experimental design and implementation. We also thank G. Wolford for statistical advice, and A. L. Duffy, A. R. Palmer, and E. J. Ruberry for help with survey data entry.
References
- ADInstruments. Chart (Version 5.4.2) [Computer software] Colorado Springs, CO: Author; 2002. [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends in Cognitive Sciences. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bavelas JB, Black A, Lemery CR, Mullett J. “I show how you feel”: Motor mimicry as a communicative act. Journal of Personality and Social Psychology. 1986;50:322–329. [Google Scholar]
- Beck AT, Ward C, Mendelson M. Beck Depression Inventory (BDI) Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Brown WM, Tsutsui T, Mainland JD, Johnson BN, Bremnder EA, et al. Sex-steroid derived compounds induce sec-specific effects on autonomic nervous system function in humans. Behavioral Neuroscience. 2003;117:1125–1134. doi: 10.1037/0735-7044.117.6.1125. [DOI] [PubMed] [Google Scholar]
- Ben-Shakhar G. Standardization within individuals: A simple method to neutralize individual differences in skin conductance. Psychophysiology. 1985;22:292–299. doi: 10.1111/j.1469-8986.1985.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shakhar G. The correction of psychophysiological measures for individual differences in responsivity should be based on typical response parameters: A reply to Stemmler. Psychophysiology. 1987;24:247–249. doi: 10.1111/j.1469-8986.1987.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Blair J, Sellars C, Strickland I, Clark F, Williams A, Smith M, et al. Theory of mind in the psychopath. Journal of Forensic Psychiatry & Psychology. 1996;7(1):15–25. [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bush LK, Hess U, Wolford G. Transformations for within-subject designs: A Monte Carlo investigation. Psychological Bulletin. 1993;113:566–579. doi: 10.1037/0033-2909.113.3.566. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE. Electromyograms as measures of extent and affectivity of information processing. American Psychologist. 1981;36:441–456. doi: 10.1037//0003-066x.36.5.441. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Losch ME, Kim HS. Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology. 1986;50:260–268. doi: 10.1037//0022-3514.50.2.260. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Crites SL, Jr, Snydersmith MA, Smith G, Berntson GG, et al. Relationship between facial expressiveness and sympathetic activation in emotion: A critical review, with emphasis on modeling underlying mechanisms and individual differences. Journal of Personality and Social Psychology. 1992;62:110–128. doi: 10.1037//0022-3514.62.1.110. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296(5576):2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Bradley MM, Lang PJ. Affective reactions to briefly presented pictures. Psychophysiology. 2001;38(3):474–478. [PubMed] [Google Scholar]
- Cohn JF, Schmidt K, Gross R, Ekman P. Individual differences in facial expressions: Stability over time, relation to self-reported emotion, and ability to inform personal identification. Proceedings of the International Conference on Multimodal User Interfaces (ICMI 2002); 2002. pp. 491–496. [Google Scholar]
- Costa PT, McCrae RR. NEO Five-Factor Inventory (NEOFFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 267–302. [Google Scholar]
- Dimberg U. Facial reactions to facial expressions. Psychophysiology. 1982;19:643–647. doi: 10.1111/j.1469-8986.1982.tb02516.x. [DOI] [PubMed] [Google Scholar]
- Dimberg U. Facial electromyography and emotional reactions. Psychophysiology. 1990;27:481–494. doi: 10.1111/j.1469-8986.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Dimberg U. Emotional reactions to facial expressions: A case of automatic responding? Psychophysiology. 1991;28:819. [Google Scholar]
- Dimberg U. Psychophysiological reactions to facial expressions. In: Segerstrale U, Molnar P, editors. Nonverbal communication: Where nature meets culture. Mahwah, NJ: Erlbaum; 1996. pp. 47–60. [Google Scholar]
- Dimberg U. Facial reactions: Rapidly evoked emotional responses. Psychophysiology. 1997;11:115–123. [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18(30):232–239. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. [Google Scholar]
- Harmon-Jones E, Allen JJB. The role of affect in the mere exposure effect: Evidence from psychophysiological and individual differences approaches. Personality and Social Psychology Bulletin. 2001;27:889–898. [Google Scholar]
- Kappas A, Hess U, Banse R. Skin conductance reactions to dynamic facial expressions revisited: Empathic responding or information processing? [Abstract] Psychophysiology. 1992;29:42. [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander A, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. NeuroReport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over the zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40:776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ruef AM. Empathy: A physiological substrate. Journal of Personality and Social Psychology. 1992;63:234–246. [PubMed] [Google Scholar]
- Magnee MJCM, Stekelenburg JJ, Kemner C, de Gelder B. Similar facial electromyographic responses to faces, voices, and body expressions. NeuroReport. 2007;18:369–372. doi: 10.1097/WNR.0b013e32801776e6. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Nielsen TC, Petersen KE. Electrodermal correlates of extraversion, trait anxiety and schizophrenism. Scandinavian Journal of Psychology. 2008;17(1):73–80. doi: 10.1111/j.1467-9450.1976.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, Cacioppo JT. Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology. 2007;44:823–826. doi: 10.1111/j.1469-8986.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, Crawford LE, Cacioppo JT. Better (or worse) for some than others: Individual differences in the positivity offset and negativity bias. 2009 Manuscript in preparation. [Google Scholar]
- Ogawa T, Suzuki N. Response differentiation to facial expression of emotion as increasing exposure duration. Perceptual and Motor Skills. 1999;89:557–563. [PubMed] [Google Scholar]
- Pattyn N, Neyt X, Henderick D, Soetens E. Psychophysiological investigation of vigilance decrement: Boredom or cognitive fatigue? Physiology & Behavior. 2008;93:369–378. doi: 10.1016/j.physbeh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Orr S, Lasklo N, et al. Exaggerated amygdala response to masked facial expressions in posttraumatic stress disorder. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Fair PL, Salt P, Mandel MR, Klerman GL. Facial expression and imagery in depression: An electromyographic study. Psychosomatic Medicine. 1976;38:337–347. doi: 10.1097/00006842-197609000-00006. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Bradley MM, Dimoulas E, Lang PJ. Looking at facial expressions: Dysphoria and facial EMG. Biological Psychology. 2002;60:79–90. doi: 10.1016/s0301-0511(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists; 1988. [Google Scholar]
- Surakkaa V, Hietanenb JK. Facial and emotional reactions to Duchenne and non-Duchenne smiles. International Journal of Psychophysiology. 1998;29(1):23–33. doi: 10.1016/s0167-8760(97)00088-3. [DOI] [PubMed] [Google Scholar]
- Tassinary LG, Cacioppo JT, Vanman EJ. The skeletomotor system: Surface electromyography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 267–302. [Google Scholar]
- Tomkins SS. Affect, imagery, and consciousness: The positive affects. New York: Springer-Verlag; 1962. [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009 doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Gross D. Reactions to facial expressions: Effects of social context and speech anxiety on responses to neutral, anger, and joy expressions. Biological Psychology. 2004;66:63–78. doi: 10.1016/j.biopsycho.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander A, et al. A functional MRI predictor of treatment response to venlafaxine in generalized anxiety disorder. Biological Psychiatry. 2008;63(9):858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]