Abstract
The spontaneous colonic migrating motor complex (CMMC) is a cyclical contractile and electrical event that is the primary motor pattern underlying fecal pellet propulsion along the murine colon. We have combined Ca2+ imaging with immunohistochemistry to determine the role of different classes of myenteric neurons during the CMMC. Between CMMCs, myenteric neurons usually displayed ongoing but uncoordinated activity. Stroking the mucosa at the oral or anal end of the colon resulted in a CMMC (latency: ∼6 to 10 s; duration: ∼28 s) that consisted of prolonged increases in activity in many myenteric neurons that was correlated to Ca2+ transients in and displacement of the muscle. These neurons were likely excitatory motor neurons. Activity in individual neurons during the CMMC was similar regardless of whether the CMMC occurred spontaneously or was evoked by anal or oral mucosal stimulation. This suggests that convergent interneuronal pathways exist which generate CMMCs. Interestingly, Ca2+ transients in a subset of NOS +ve neurons were substantially reduced during the CMMC. These neurons are likely to be inhibitory motor neurons that reduce their activity during a complex (disinhibition) to allow full excitation of the muscle. Local stimulation of the mucosa evoked synchronized Ca2+ transients in Dogiel Type II (mitotracker/calbindin-positive) neurons after a short delay (∼1–2 s), indicating they were the sensory neurons underlying the CMMC. These local responses were observed in hexamethonium, but were blocked by ondansetron (5-HT3 antagonist), suggesting Dogiel Type II neurons were activated by 5-HT release from enterochromaffin cells in the mucosa. In fact, removal of the mucosa yielded no spontaneous CMMCs, although many neurons (NOS +ve and NOS −ve) exhibited ongoing activity, including Dogiel Type II neurons. These results suggest that spontaneous or evoked 5-HT release from the mucosa is necessary for the activation of Dogiel Type II neurons that generate CMMCs.
Introduction
Neurons in networks can exhibit emergent patterns of behaviour that cannot be predicted by the study of a single neuron. Experiments using single/dual electrical recordings in invertebrates have demonstrated many important features of how neurons communicate and develop patterns of motor activity (Levitan & Kaczmarek, 2002). Such studies were feasible due to the low number of neurons and their topographical arrangement in these organisms; for example, each segmental ganglion controlling swimming/crawling movements in the leech has only 30 neurons, and their positions and function within the ganglion are conserved (see Nicolls et al. 2001). Using such techniques in vertebrates has been less productive due to the large number of neurons and their interconnectedness resulting in high degrees of integration.
The mammalian gut, nonetheless, is an ideal preparation in which to begin to piece together how neuronal activity in interconnected networks is organized to produce motor behaviour. The enteric nerve plexuses are arranged in almost two-dimensional ganglionated networks, and most classes of neurons and their projections are known (Lomax & Furness, 2000). Moreover, stereotypical motor patterns occur spontaneously and polarized reflexes can be activated by mucosal stimulation and circumferential or longitudinal stretch (Bywater et al. 1989; Spencer & Smith, 2001; Smith et al. 2007; Spencer et al. 2007; Dickson et al. 2009b; Heredia et al. 2009). When opened as a flat sheet, activities in myenteric neurons and apposed muscle can be visualized using Ca2+ imaging techniques, and the motor output of the myenteric plexus can be assessed by visualizing smooth muscle activity (Stevens et al. 1999). However, in the mammalian gastrointestinal tract there appears to be little topographical organization of different functional classes of enteric neurons within ganglia. Moreover, the number of ganglia is very large compared to those in invertebrates; in a 1 cm length of murine colon there are approximately 1600 (40 × 40) myenteric ganglia (Spencer et al. 2007), each ganglion containing between 40 and 120 neurons.
In this paper we have taken the first steps to identify how the pattern of activity in enteric neurons initiates and propagates the colonic migrating motor complex (CMMC) that is a predominant motor pattern in the large bowel. This remarkable, neurally mediated event consists of a rhythmically occurring series of long-duration contractions that migrate over the whole or partial lengths of colon, propagating in either an oral or an anal direction (Wood, 1973; Bywater et al. 1989; Heredia et al. 2009). While the physiological role of the CMMC is unclear in large mammals (Sarna, 1985), including humans (Dining et al. 2008), it is necessary for faecal pellet propulsion down the murine colon (Heredia et al. 2009). The CMMC is not dependent upon the CNS, since it occurs in isolated colons, and is generated by the enteric nervous system or ‘little brain’ within the colonic wall (Wood, 1973; Bywater et al. 1989; Bush et al. 2000).
Most of the information regarding the CMMC has been obtained through either intracellular/extracellular electrophysiological recordings or from tension recordings from the muscle. In the current study we attempted to identify relationships among myenteric neurons and the smooth muscle syncytium to further advance our understanding of the CMMC using calcium imaging and immunohistochemistry.
Methods
Tissue preparation
Adult C57/BL6 mice of either sex (4–6 weeks of age) were killed by isoflurane inhalation and cervical dislocation, in accordance with the requirements of the Animal Ethics Committee at the University of Nevada, Reno. A ventral midline incision was made and the entire colon was removed and placed in a Sylgard (Dow-Corning, Midland, MI, USA)-lined dish containing Krebs–Ringer buffer solution (KRB; composition (mm): NaCl: 120.35; KCl: 5.9; NaHCO3: 15.5; MgCl2: 1.2; NaH2PO4: 1.2; glucose: 11.5; CaCl2: 2.5; pH: 7.4; 21°C); bubbled with 97% O2–3% CO2.
Several different preparations were used in this study. (1) To examine the effect of mechanical stimulation of the mucosa (brush strokes), the major part of the colon remained as an intact tube, and a small (10–15 mm) area towards the middle of the tissue was cut along the mesenteric border to allow that region to be pinned out flat (serosal side topmost). Both ends of the preparation were opened along the mesentery to expose the mucosa for mechanical stimulation. Strips of longitudinal muscle from the middle section were removed to expose the myenteric plexus (Fig. 1A and B). (2) To examine the effect of local stimulation the colon was cut along the mesenteric border and pinned flat, serosal side-up (upright) or serosal side down (inverted microscope) in a Sylgard-layered recording chamber and the serosa and longitudinal muscle were removed in a small window to allow for visualization of the myenteric plexus (Fig. 1B). Polyethylene tubing of 1 mm diameter was embedded along the length of the chamber using an upright microscope to allow for picospritzer stimulation (Picospitzer II: General Valve Corp., Fairfield, NJ, USA) (Fig. 1C). (3) In experiments to examine the role of the mucosa on neuronal activity, the preparation was opened along the mesenteric border and pinned as a flat sheet in 4°C KRB buffer to minimize the effect of substances released from the mucosa during microdissection being taken up into descending ‘5-hydroxytryptamine-accumulating neurones’ (Meedeniya et al. 1994). The mucosa was cut in the middle at the anal or oral end of the colon and gently peeled outwards along its length. This procedure removes the mucosa and submucosa without damaging the underlying circular muscle and myenteric plexus. Strips of longitudinal muscle were removed in a small area to expose the myenteric plexus. Following tissue dissection, preparations were equilibrated for approximately 60 min with oxygenated KRB at 37°C.
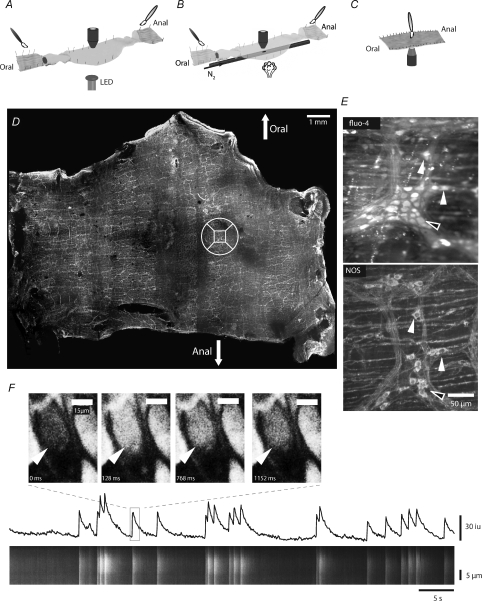
Figure 1. Correlating calcium activity in identified neurons.
A, colonic preparation used for stimulating the mucosa at the oral and anal cut ends. The colonic wall was opened and pinned in the middle with the longitudinal muscle (LM) uppermost. Strips of LM were peeled away to reveal the myenteric plexus. The colon was also opened at the oral and anal ends and pinned with the mucosa uppermost to allow stimulation of the mucosa with a brush (see symbol). The stimulus was registered by activating a light emitting diode (LED), which was located under the organ bath, at the beginning of the stimulus regime. Recordings were made in the middle of the preparation ∼2.5 cm from the sites of stimulation. B, to locally activate the mucosa, nitrogen was spritzed onto the mucosa via a polyethylene tube with a small hole under the recording site. C, to brush the mucosa directly over the imaging site, the preparation was pinned with the mucosa uppermost over a coverslip and imaged using an inverted microscope. D, following calcium imaging experiments the preparation was stained with an antibody to nitric oxide synthase (NOS) and the ganglia on which imaging was performed located (NOS +ve neurons, closed arrows; NOS −ve neurons, open arrows). The square in the circle locates the ganglion that was imaged in E. E, maximum Ca2+ induced fluorescence in myenteric neurons within the ganglion, and later NOS staining of the same ganglion. F, the calcium fluorescence in an individual neuron was converted into a spatio-temporal map (ST map; lower panel). Note that the ST map corresponds to the activity (see line trace) in the neuron. The width of the ST map corresponds to the length of the long axis of the neuron.
Fluorescent dye loading
Following tissue equilibration, perfusion was stopped, and the bath volume was minimized (∼5 ml). Tissues were then incubated with 50 nm Fluo-4 AM (Molecular Probes, Eugene, OR, USA), 0.02% dimethyl sulfoxide (DMSO), and 0.01% of the non-toxic detergent Chremophor EL (Sigma, St Louis, MO, USA) for 15 min at 21°C. Following loading, tissues were perfused with oxygenated KRB solution at 37°C for another 15 min to allow for de-esterification and dye-trapping within cells of the tissues (see Park et al. 2006; Lee et al. 2009).
In some experiments where local stimulation was used to study the activity of Dogiel type II neurons, probenecid (Molecular Probes) was added to the recording chamber immediately prior to dye loading (0.5–1 mm) to prevent dye expulsion (see Di Virgilio et al. 1990) from Type II neurons. Following incubation with fluo-4, probenecid was further perfused through the system for the duration of the experiment at the same concentration.
Mito Tracker Red CMXRos (Molecular Probes) was used at the conclusion of experiments to mark mitochondria in myenteric neurons (Vanden Berghe et al. 2002). Mitotracker was dissolved using 0.02% DMSO and 0.01% Chremophor EL, and was used in concentrations ranging between 0.2 and 0.4 μm. Mitotracker was allowed to penetrate the tissue for 15 min at 21°C, followed by perfusion with oxygenated KRB at 37°C for another 20 min before visualization.
Image acquisition
Recordings were made using a Nikon Eclipse E600FN upright fluorescence microscope and Nikon Fluor® water-immersion lenses (10–60×; Nikon, USA) for preparations in which tissues were pinned serosal side up, and a Nikon Eclipse TS 100 inverted fluorescence microscope using Modulation Optics air lenses (Modulation Optics Inc., Glen Cove, NY, USA) for preparations pinned serosal side down. Fluo-4 was excited at 488 nm and Mitotracker Red was excited at 594 nm using a Lambda DG-5 illumination system (Sutter Instrument Co., Novato, CA, USA), and image sequences were captured using a Photometrics Cascade 512-B camera (Roper Scientific Inc., Trenton, NJ, USA). Image sequences (1000 frames, captured at 15.67 Hz) were acquired on a Windows-based PC using Metamorph 6.1.6 (Molecular Devices, Sunnyvale, CA, USA). Acquisition of Ca2+ transients at this frequency, while providing us with lower temporal resolution when compared with potentiometric probe techniques, allowed us to increase the spatial resolution (512 × 512 pixels; 410 × 410 μm at ×20 magnification) and resolve activity in many individual neurons. Furthermore, unlike potentiometric dyes, which have been shown to be phototoxic to cells over relatively short periods of time (8–20 min; see Obaid et al. 2004), use of Ca2+ dyes has allowed us to visualize tissues over extended periods of time, often exceeding 60 min exposure between the first and last recordings. This, in turn has provided the ability to establish the effects of a number of pharmacological agents on the tissues.
Mucosal stimulation
Stimulation of the mucosa at the oral and anal ends of preparations was accomplished using a small (6 mm-wide, flat head) camel hair paintbrush, where each stimulation consisted of a sequence of five brush strokes in approximately 5 s (Fig. 1A; Smith et al. 1992; Heredia et al. 2009). Stimulation of the mucosa directly underneath the recording site was produced using a single 25 ms puff of N2 at 10–20 psi from a small (∼500 μm) hole in the tube embedded in the Sylgard base of the recording chamber (Fig. 1B). Local stimulations performed on preparations pinned serosal side down, were accomplished using a small (6 mm-wide, flat head) camel hair paintbrush, where stimuli consisted of one to five stroked directly over the site of recording (Fig. 1C).
Immunohistochemistry
Following Ca2+ imaging recordings, tissues were cut around the site of imaging and were re-pinned on a fixing dish lined with Sylgard, fixed using acetone at 4°C for 15 min, washed overnight in 0.01 m phosphate buffered saline (PBS, pH 7.2), followed by treatment with bovine serum albumin (BSA, 1%) for 60 min. Tissues were then incubated with an antibody against nitric oxide synthase (NOS) raised in sheep (1:500 dilution with 0.5% Triton X-100 in PBS; a generous gift from P. C. Emson, University of Cambridge, UK; Ward et al. 1994; Herbison et al. 1996) for 48 h at 4°C. Following incubation with primary antibody, tissues were washed in PBS for another 24 h, and were then incubated with Alexa Fluor® 594 donkey anti-sheep IgG (H+L) (1 : 500 dilution) secondary antibody (Molecular Probes) for 1 h at 21°C. Tissues were washed overnight in PBS at 4°C before being mounted on glass microscope slides (Fisher Scientific, Pittsburgh, PA, USA) with Aquamount (VWR International, West Chester, PA, USA).
For labelling of calbinbin +ve neurons, tissues were fixed in paraformaldehyde (4% in PBS, Sigma) at 4°C overnight. Following fixation, tissues were washed for 24 h in PBS, and were treated with BSA (1%) for 60 min. Tissues were then incubated for 48 h in rabbit anti-calbindin D-28k antibody (Swant, Bellizona, Switzerland; see Furness et al. 1988) at a dilution of 1 : 1600 in PBS with 0.5% Triton X-100. Following incubation, tissues were again washed in PBS for 24 h, and were then incubated with Alexa Fluor 488 goat anti-rabbit IgG (H+L) secondary antibody. Tissues were washed in PBS overnight, and were mounted on glass microscope slides.
Whole mounts were viewed and recorded from using a Zeiss LSM 510 META confocal microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) with 10–100× lenses.
Image processing and analysis
Raw Metamorph files (16-bit, .stk) were analysed on an Apple Powermac G5 desktop computer using in-house analysis software (Volumetry G6a, GWH). Tissue movements were tracked to allow stable measurements from individual regions of interest (ROI) as most preparations moved in the y-axis or circumferential axis (see Δy in Fig. 2; Park et al. 2006). Ca2+-induced fluorescence is reported as average intensity inside an ROI in 8-bit intensity units. Spatio-temporal maps (ST maps) for individual cells were constructed using rectangular ROIs over the cells, and averaging the fluorescence signal perpendicular to the long axis of the cell (Fig. 1E). Care was taken to avoid including fluorescence from nearby circular muscle cells in neuronal measurements. The delay between the brush stimulus to the first neuronal response was calculated from the last brush stroke.
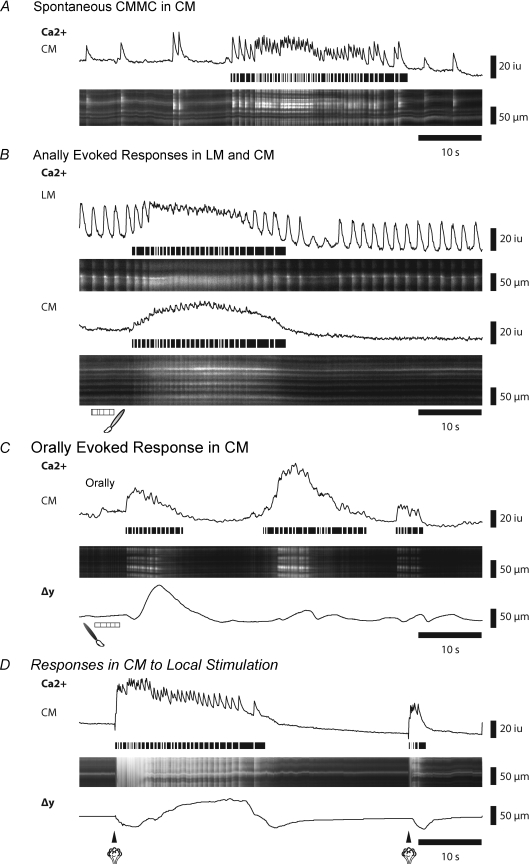
Figure 2. Spontaneous and evoked muscle responses during CMMCs.
A, Ca2+ activity in the circular muscle (CM) during a spontaneous CMMC. The line trace corresponds to activity in ST map. Horizontal lines below transients trace the occurrence of fast Ca2+ transients in the muscle during the CMMC. B, Ca2+ activity in both the longitudinal muscle (LM) and the CM following an evoked CMMC initiated by stimulating the mucosa at the anal end of the preparation. C, Ca2+ activity in the CM following an evoked CMMC initiated by stimulating the mucosa at the oral end of the preparation. Δy shows the displacement (contraction) of the tissue. D, Ca2+ activity in the CM after stimulating the mucosa with a puff of nitrogen directly under the recording site. Note that a second stimulus generates an aborted response.
Panoramas of NOS or calbindin labelled enteric ganglia were constructed by taking a montage of low resolution confocal images (10×), which were combined using Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA, USA). When ganglia in which Ca2+ activity was recorded were identified, high-resolution images (20–100×) were taken of neurons in those ganglia. Neurons recorded using Ca2+ imaging were compared to NOS or calbindin stained images to determine whether they contained that immunohistochemical marker (Fig. 1C and D).
At the conclusion of the Ca2+ imaging experiments, 50 mm KCl injected into the bath evoked large Ca2+ transients in >95% of neurons, indicating that most neurons were viable and are properly loaded with the Ca2+ indicator (data not shown). Neurons that exhibited no Ca2+ transients were not analysed.
Statistical analysis
Results are expressed as a means ±s.e.m. Student's unpaired t test and one-way ANOVA were used where appropriate. P < 0.05 was accepted as statistically significant. The n values represent the number of animals on which observations were made. ‘Cells’ is the number of neurons from which measurements were made.
Drugs used
Hexamethonium bromide, ondansetron hydrochloride, pyridoxal-phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt (PPADS), potassium chloride, Nω- nitro-l-argenine (l-NA) and tetrodotoxin (TTX) were all purchased from Sigma. Hexamethonium and l-NA were applied at 100 μm concentrations, ondansetron at 1 μm, and PPADS at 10 μm. Potassium chloride was applied directly into our recording chamber at a final concentration of 50 mm. Acetone was purchased from Fisher Scientific (Pittsburgh, PA, USA).
Results
Characteristics of spontaneous and evoked CMMCs
Examination of preparations using Ca2+ imaging of muscle activity in conjunction with displacement of the tissue within the field of view allowed the onset and duration of the colonic migrating motor complex (CMMC) to be determined. Spontaneous activity in the circular muscle (CM) consisted of CMMCs occurring every 2–3 min with little or no activity between complexes. Spontaneous CMMCs, which had a duration 22.72 ± 2.43 s (n= 8), consisted of a rapid series of Ca2+ transients (frequency: 1.99 ± 0.05 Hz; n= 7) in circular muscle (CM) layer, superimposed on a sustained increase in basal Ca2+ that resulted from tetanic firing of the muscle fibres (Fig. 2A). Contraction of the tissue, or displacement (Δy), accompanied the Ca2+ transients in the muscle. However, no spontaneous CMMCs were recorded in 35 colons where the mucosa had been removed, consistent with other studies (see below and Dickson et al. 2009a,b; Heredia et al. 2009).
As the timing of spontaneous CMMCs was variable, we evoked CMMCs by brushing the mucosa (5 strokes) at the anal end of the preparation (Fig. 1A). Evoked CMMCs were similar to spontaneous CMMCs (and Fig. 2A and B), as we have recently described (Heredia et al. 2009). Stimulation of the mucosa (3–5 strokes) at the anal end of the preparation almost always led to a CMMC response, save for instances when a spontaneous complex was occurring, or immediately following a spontaneous CMMC, when the tissue was refractory. In order to ensure that stimulation would result in a CMMC, stimulations were applied only between complexes, approximately 60 s following the last CMMC. The evoked CMMC muscle response occurred 6.26 ± 0.68 s (32 CMMCs; n= 16) following the last anal brush stimulus (duration of stimulation = 3.62 ± 0.08 s), and were similar in duration (27.86 ± 3.01 s; P≥ 0.05; n= 8) to spontaneous complexes. When longitudinal muscle (LM) fibres were within the field of view, simultaneous firing of both the CM and LM were observed during a complex (Fig. 2B).
It was difficult to elicit CMMCs by brushing the mucosa at the extreme oral end (last 5 mm; see Fig. 1A) of the preparation. Most stimulations applied within the oral-most 5 mm of the preparation failed to elicit a CMMC, though on several occurrences (3 of 34 recordings, n= 7) CMMCs were observed approximately 30 s following the stimulus. However, when oral brush stimulations were performed 9–12 mm away from the oral cut end of the colon (Fig. 2C), we observed CMMC Ca2+ activity in the muscle and contraction. Orally evoked CMMCs were, however, usually shorter in duration (12.6 ± 0.82 s; range 9—23 s; n= 7) than spontaneously occurring or anally evoked CMMCs (Fig. 2C). This discrepancy in the activation of the CMMC from the anal and oral ends suggests that the dominant nerve pathways required for generating the CMMC are likely to be the ascending excitatory nervous pathways.
While brush stimulations from either the oral or the anal ends of preparations allowed us to examine neuronal and muscle activity as the CMMC was propagating, we also wanted to examine the muscle responses at the origin of the stimulus. CMMCs evoked by a local stimulus (a 25 ms puff of nitrogen applied to the underlying mucosa; Fig. 1B) occurred after a latency of 1.9 ± 0.43 s (n= 6). These responses to local stimulation were variable, with approximately 27% producing a full CMMC (Fig. 2D), 45% producing shorter bursts of activity, and 27% producing no discernible activity in the muscle in response to the stimulation (33 stimulations, n= 7). Some of this variability was likely to be due to the overall refractory state of the tissue following several stimuli, since after local activation of a full complex, the tissue is refractory to a second stimulus and does not generate a CMMC but produces an aborted CMMC-like event (Fig. 2D).
Comparison of neural activity during an evoked and a spontaneous CMMC
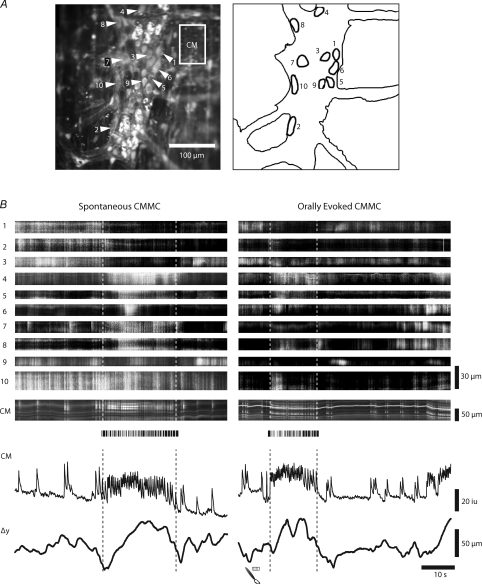
We investigated whether evoked CMMCs and spontaneous CMMCs, which originate at some undefined location within the colon (see Heredia et al. 2009), use the same neuronal circuitry (Fig. 3). During both the evoked and the spontaneous CMMC it is clear that the majority of myenteric neurons are activated during both types of CMMC. In the example shown in Fig. 3 some myenteric neurons (1–3, 9) decreased their activity, whereas others increased their activity throughout each complex (neurons 4, 5, 8, 10).
Figure 3. Comparison of neuronal responses during an evoked and a spontaneous CMMC.
A, average Ca2+ and silhouette showing neurons in a ganglion from which Ca2+ responses were measured. B, low power imaging (×20 objective) of Ca2+ activity in 10 neurons located in a single myenteric ganglion during an evoked and spontaneous CMMC, as indicated by increased activity in the CM and the associated contraction. Note that during both the spontaneous and evoked CMMC several neurons (1 to 3) decreased their activity, whereas other neurons increased in activity during the CMMC. Height of ST maps indicate long axis of neurons.
Activity in myenteric neurons between and during evoked CMMCs
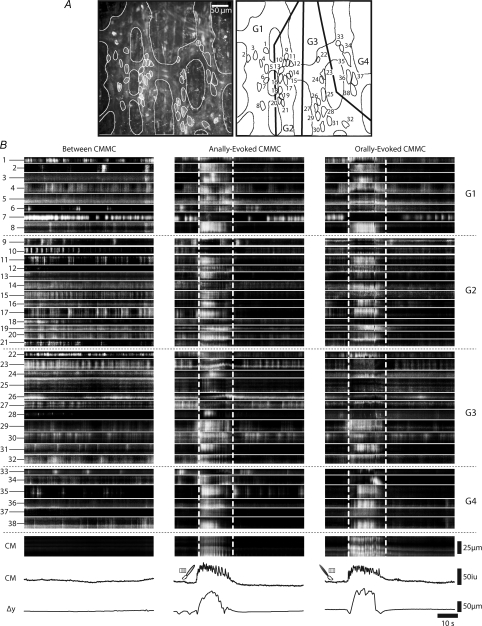
Using low power imaging (×20 objective) we examined the behaviour of myenteric neurons in several ganglia during a spontaneous and an evoked CMMC. A typical example of the complexity and range of activity in 38 neurons between and during an evoked CMMC is shown in Fig. 4.
Figure 4. Calcium responses in myenteric neurons between and during an evoked CMMC.
A, low power imaging (×20 objective) of Ca2+ activity in 38 myenteric neurons located in 4 ganglia (labelled G1-G4 on figure). Left hand panel (LHP) shows average Ca2+, whereas right hand panel (RHP) shows the location and the size of neurons. B, LHP shows the spontaneous activity in these neurons between CMMCs. The heights of the ST maps indicate the length of the long axis of the neurons. Lower ST map and line traces indicate Ca2+ activity in CM, and Δy shows the displacement (contraction) of the tissue at the bottom of each panel. Middle panel and right hand panel show the Ca2+ responses of these same neurons to anal and oral stimulation of the mucosa respectively. Note that both oral and anal stimulation evoked a similar CMMC response in the CM. Dotted horizontal lines indicate neurons in different ganglia and activity in the circular muscle. Vertical lines indicate the duration of muscle activity during the CMMC.
Neuronal activity between CMMCs
Ongoing Ca2+ transients occurred in most myenteric neurons between CMMCs despite the fact that the muscle usually exhibited little or no activity and a corresponding lack of movement of the tissue (Fig. 4B). There was little coordinated activity either between neurons within the same ganglia or between neurons in adjacent ganglia. Some neurons fired in regular bursts (see neuron 1, 6, 8–10, 16, 17, 19, 20, 22–24, 27, 35, 38); others fired continuously (see neurons 4, 7, 14, 15, 19, 20, 34, 38); others exhibited an occasional burst of activity (neurons 2, 3, 6, 33); and some neurons (4) were quiescent throughout the recording period (neurons 12, 28, 37). Presumably, many of these neurons are involved in neural circuits generating ongoing tonic inhibition of the circular muscle (Spencer et al. 1998; Powell et al. 2001; Dickson et al. 2009b).
Changes in neuronal activity during evoked CMMC
Oral and anal stimulation usually evoked similar responses in the same myenteric neurons across the several ganglia suggesting that modulation of neuronal firing was independent of whether ascending or descending nerve pathways were activated.
These increases in neuronal Ca2+ activity occurred after a variable latency of 5.03 ± 0.22 s (range 0.8 to 8.6 s; 75 neurons; n= 5) from the onset of the last stimulus (Fig. 4B). Increases in activity in some neurons preceded the onset of the response in the muscle (neurons 4, 17, 29; Fig. 5C), suggesting these may be interneurons that coordinate their activities during the CMMC (Spencer et al. 2005).
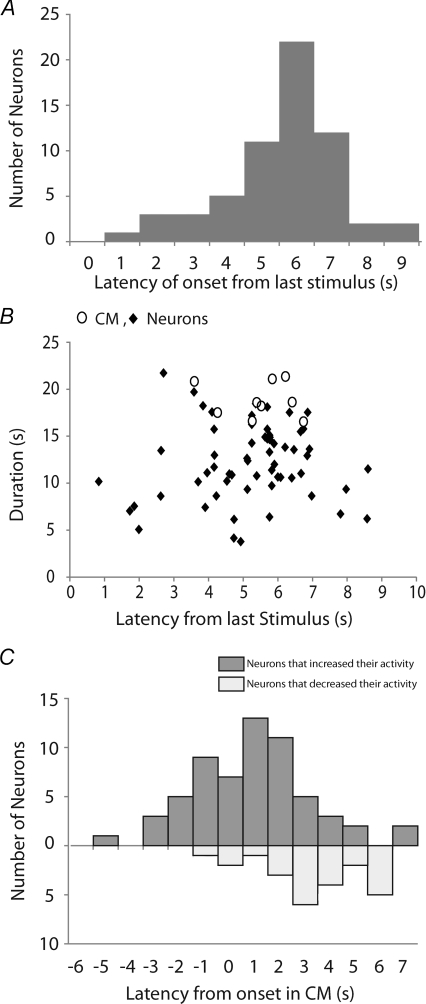
Figure 5. Latency and duration of activity in myenteric neurons at the onset of CMMCs.
A, the latency of onset of excited neurons following an anal mucosal stimulus (61 neurons; n= 4). B, the duration of Ca2+ transients in neurons (♦) and muscle (○) following evoked CMMCs (n= 6). C, the activation of excited neurons (upper bars) and inhibited neurons (lower bars) relative to the first rapid Ca2+ transient in the muscle (0 s) following an evoked CMMC (85 neurons, n= 6). Note that a number of neurons fired before the onset of the muscle, and that the neurons that ceased firing often occurred later during the CMMC.
In response to either stimulus, a large percentage of neurons exhibited a sustained increase in activity (duration 11.99 ± 0.47 s; range 4.15 to 21.73 s; n= 6). However, many neurons were activated at a similar time to the initiation of Ca2+ responses in the muscle (Fig. 5A and B) and their activity increased for the duration of the muscle response (neurons 2–4, 6, 8, 11, 12, 15–17, 19, 20–22, 24, 29, 30, 32, 36–38; Fig. 5B and online Supplementary Movie 1). These neurons increased their firing frequency from 0.79 ± 0.09 Hz (between complexes) to 1.38 ± 0.09 Hz during the CMMC, i.e. an increase of 206 ± 17% (24 neurons; P < 0.01; n= 3). This increase in frequency was similar to the frequency of the fast electrical oscillations in the muscle observed during a CMMC (Spencer et al. 2005; Dickson et al. 2009b), suggesting that many of these neurons that are activated at the same time as the muscle are likely to be excitatory muscle motor neurons (Dickson et al. 2009b). Interestingly, not all neurons exhibited identical firing patterns or latency of onset in response to anal and oral stimulation (e.g. neurons 11, 13–15, 25, 28, 34) despite the fact the CMMC response of the muscle was similar (Fig. 4B). This suggests that there is considerable neuronal integration within the myenteric plexus and that some neurons exhibit varying responses.
A ∼10 s delay was observed from the onset of the first stimulus to the onset of the CMMC, suggesting that we are dealing with a complex integrated motor pattern rather than a simple reflex arc (see Discussion).
Most interestingly, a proportion (∼13%, 24 of 186, n= 6) of neurons decreased or stopped their Ca2+ activity during the evoked CMMC (neurons 7, 9, 26, 27, 33; Fig. 4B middle and right hand panels and Supplementary Movie 1). Many of the neurons that ceased firing during the CMMC were inhibited after the response in the muscle had peaked (see Fig. 5C). This suggests that the turning off of these neurons, which are likely to be NOS +ve inhibitory motor neurons (see below), was unlikely to be the major contributor to the excitation of the muscle. Also, the inhibition of these neurons often lasted longer than the duration of the CMMC, some not returning to their pre-CMMC activity for the duration of the recording period (e.g. neurons 6, 10; see Fig. 4B). There was a 66.3 ± 7.0% reduction in the firing frequency of these neurons during the CMMC (between CMMCs 1.25 ± 0.19 Hz; during CMMCs 0.38 ± 0.09 Hz, 11 neurons; P < 0.01; n= 3).
Some of the larger neurons that responded during spontaneous and evoked CMMCs (see neurons 35, 37, 38; Fig. 4B) may have been Dogiel Type II sensory neurons (see below).
Effects of blockade of neural transmission and muscle activation
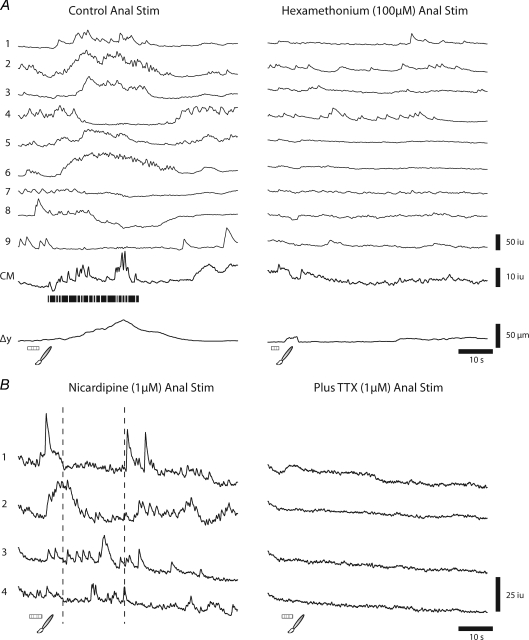
The CMMC in the murine colon is known to be blocked by the nicotinic antagonist hexamethonium (Spencer et al. 1998; Bywater et al. 1989; Heredia et al. 2009), so we were interested in determining whether this drug blocked the responses of neurons to mucosal stimulation. We found that hexamethonium (100 μm; n= 4) blocked the CMMC, the prolonged excitatory responses and inhibitory responses in neurons to mucosal stimulation, an example of which is shown in Fig. 6A.
Figure 6. Effects of blocking neurotransmission on anally evoked responses.
A, Ca2+ responses in 9 neurons to anal mucosal stimulation. Note that during the CMMC the prolonged excitatory responses (neurons 1, 2, 3, 5, and 6) were somewhat variable in both duration and onset, as were the inhibitory responses (neurons 4, 7, 8, 9). However, following hexamethonium (100 μm) the responses in all neurons to stimulation were blocked, although their prestimulus activity continued. B, anal stimulation of the mucosa evoked responses in several myenteric neurons after nicardipine (1 μm), which abolished responses in the muscle. Note: dotted lines indicate where CMMC is likely to be occurring. Following the further addition of TTX (1 μm) all spontaneous and evoked Ca2+ transients in these neurons were abolished.
Spontaneous CMMCs occur following muscle paralysis with L-type calcium channel blockers (Bywater et al. 1989; Spencer et al. 2005) and in flaccid tubes (Heredia et al. 2009), suggesting that the activation of mechanosensitive myenteric neurons that are dependent on muscle contraction or stretch is not essential for the generation of the CMMC (Smith et al. 2007). Following nicardipine (1 μm) we found that normal responses to mucosal stimulation occurred following either local or anal mucosal stimulation (Fig. 6B; n= 6). These responses to anal stimulation included inhibition of neuronal activity (neurons 1 and 2) and a range of excitatory responses (neurons 3–4; Fig. 6B). Furthermore, tetrodotoxin (TTX; 1 μm, n= 3), which blocks Na+ action potentials in most neurons and transmission along nerves (Smith et al. 2007), blocked the spontaneous and evoked Ca2+ transients in all myenteric neurons (Fig. 6B; n= 6).
In three other experiments we attempted to determine whether nitric oxide, which has been shown to generate inhibitory postsynaptic potentials in myenteric interneurons, was responsible for the decrease in activity of some neurons during the CMMC (Dickson et al. 2007). However, l-NA (100 μm; n= 3) had no significant effect on the duration of inhibition of activity of neurons during the evoked CMMC (before 15.46 ± 1.22 s; after l-NA 16.15 ± 1.28 s; P > 0.05; n= 3) suggesting that some other presynaptic or postsynaptic inhibitory mechanism was responsible for the decrease in activity during the CMMC (Galligan & North, 1991).
Spontaneous and evoked activity in NOS +ve neurons
Using subsequent NOS labelling we were able to determine the behaviour of NOS +ve neurons between and during the evoked CMMC. Previous immunohistochemical studies have demonstrated that neurons in the myenteric plexus that contain NOS include descending interneurons and inhibitory motor neurons to the circular and longitudinal muscle layers, and comprise approximately 35% of the myenteric neurons in the murine colon (Sang & Young, 1996). Post staining tissues in our experiments revealed that NOS +ve neurons were randomly scattered throughout ganglia (Fig. 1C and D and Fig. 7), and accounted for approximately 42% (152 of 357 neurons, n= 5) of all myenteric neurons loaded with Ca2+ indicator.
Figure 7. Ca2+ activity in NOS +ve neurons.
A, between CMMCs, the activity in NOS +ve neurons occurred in regular bursts (neurons 1–4; see left hand panel). Following anal mucosal stimulation there was a brief increase in burst activity in most of these neurons (see neurons 1, 2 and 4; right hand panel), except neuron 3 just before the onset of the CMMC. During the CMMC all these neurons decreased their activity. B–E, responses of NOS +ve neurons to anal mucosal stimulation in another preparation. B, left hand panel shows the location of NOS +ve neurons (arrows) in two ganglia. Right hand panel shows the average Ca2+ activity of neurons within these ganglia. C, NOS +ve neurons (1–5) decreased their activity during the CMMC following anal mucosal stimulation, whereas others (neurons 6–8) appeared to be unchanged by the stimulus. These neurons had complex morphology, round or oval cell bodies with lamina dendrites (see their corresponding morphologies in D). Surprisingly, some NOS +ve neurons increased their firing during the CMMC. These neurons had a simple morphology exhibiting a ‘dew drop’ appearance (see corresponding morphologies in D and E).
The majority of NOS +ve neurons (76%; 261 neurons, n= 5) remained relatively quiescent throughout the recordings, although robust Ca2+ transients were elicited in these quiescent neurons by the application high KCl (50 mm) to the bath, suggesting that they were viable and effectively loaded with indicator.
Two distinct morphologies of NOS +ve neurons were observed when we used 100× confocal imaging: some NOS +ve neurons had round somas with lamina dendrites (long axis 32.78 ± 4.18 μm, short axis 26.04 ± 3.4 μm; area = 402.5 ± 48.5 μm2; perimeter/area ratio: 0.45 ± 0.06, n= 3), whereas, the other NOS +ve neurons had simple ‘dew drop’ shaped somas (long axis of 23.1 ± 1.7 μm, a short axis of 15.4 ± 1.2 μm, an area = 231.3 ± 18.8 μm2 and a perimeter/area ratio of 0.33 ± 0.02; n= 3; Fig. 7B, D and E). We observed several distinctive behaviours in these different NOS +ve neurons between and during CMMCs.
A percentage of the NOS +ve neurons with lamina dendrites (24%; 87 neurons, n= 5) exhibited rhythmic, ongoing Ca2+ transients (frequency 0.51 ± 0.02 Hz; n= 5; Fig. 7A neurons 1–4) and (neurons 1–4, 7, 8, Fig. 7A), sometimes interrupted by brief reductions in activity (see neuron 3, Fig. 7A). The rhythmic behaviour was observed in all preparations, including ones where the mucosa and submucosa had been removed (see below). During evoked CMMCs, the reduction in activity in these NOS +ve neurons was more pronounced and coordinated (neurons 1–4, right hand panel of Fig. 7A, and neurons 1–5, Fig. 7C). Following the CMMC, their activity returned to their intrinsic firing frequencies between complexes. In several preparations (n= 3) these rhythmically firing NOS +ve neurons increased their activity immediately following the stimulus and then became quiescent during the development of the CMMC (neurons 1, 3 and 4; Fig. 7A right hand panel), suggesting they were responsible for the hyperpolarization of the muscle that sometimes precedes a CMMC (Bywater et al. 1989; Spencer et al. 2005; Dickson et al. 2009b). Other neurons with this morphology were relatively quiescent or exhibited sustained firing that was uninterrupted during the CMMC (neurons 6–8, Fig. 7C).
Some NOS +ve neurons with the ‘dew drop’ appearance were activated after a long latency from the stimulus (∼20 s) and exhibited a sustained (duration 25.3 ± 0.7 s) increase in activity during the CMMC (see neurons 9–12 in Fig. 7C–E).
Dogiel Type II neurons and the initiation of the CMMC
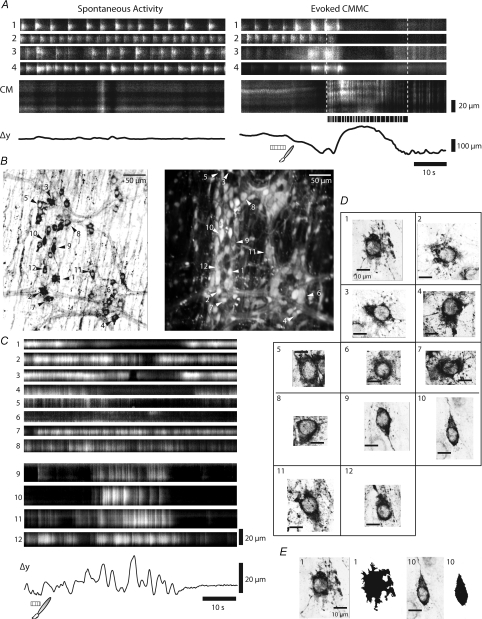
Since mucosal stimulation activates the CMMC we wanted to determine whether Dogiel Type II sensory neurons, which project to the mucosa (Furness et al. 1990), are responsible for the initiation of the CMMC (see Heredia et al. 2009). We mechanically stimulated the mucosa directly under the recording site with puffs of nitrogen (Fig. 1B; Kirchgessner et al. 1992; Smith, 1996) or by brushing the mucosa over the recording site (Fig. 1C) to determine the responses in these neurons.
Following application of a brief (∼25 ms) puffs of nitrogen to the mucosa, transient Ca2+ responses (duration 5.0 ± 0.3 s) occurred in the somas of several large neurons and the varicose nerve processes that appeared to emanate from these neurons after a latency of 1.4 ± 0.2 s (n= 4). These neurons were likely to be Dogiel Type II, since they had large oval cell somas (area = 341.45 ± 32.8 μm2; Fig. 8A; n= 4).
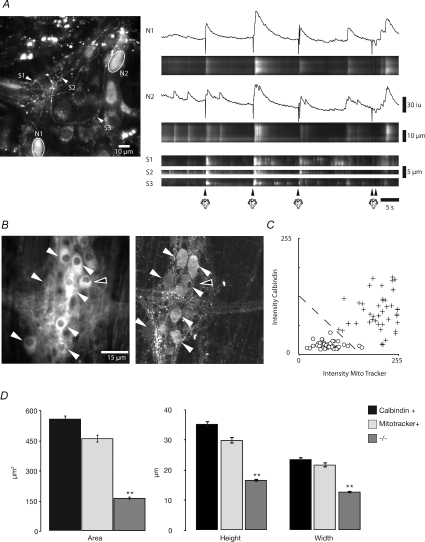
Figure 8. Ca2+ transients in and characteristics of Dogiel Type II neurons.
High power imaging (×40 and 60 objective) of Ca2+ activity in Dogiel Type II neurons. A, local stimulation (puffs of nitrogen applied to the mucosa) evoked a brief Ca2+ transient in two neurons (N1 and N2) that appeared to have Dogiel Type II morphology. These responses were mimicked in the processes (S1, S2 and S3) that ramified throughout the ganglia and appeared to emanate from these neurons. B, most mitotracker +ve neurons (left hand panel) appeared to also contain calbindin (right hand panel). Neurons were considered positive for either mitotracker or calbindin if they had an average pixel intensity greater than 150 (8-bit scale). Mitotracker +ve and calbindin +ve neurons had a similar area and shape and were longer and wider than neurons that were considered to be negative for these labels. White arrows indicate mitotracker stained neurons and calbindin +ve neurons; open arrows indicate mitotracker stained neurons but calbindin −ve neurons. C, analysis of the average intensities of calbindin +ve versus mitotracker +ve labelled neurons reveals that positive neurons are readily identifiable, and, given the two labels, are statistically more likely to be either co-labelled or negative for both substances. Dotted line respresents the threshold for a labelled neuron. D, analysis of the size of calbindin +ve, mitotracker +ve, and −/− neurons demonstrates that neurons that label positive for either or both substances have significantly larger average soma areas (558 μm2, to 462 μm2, to 165 μm2, respectively), height (35 μm, to 30 μm, to 16 μm, respectively), and width (24 μm, to 22 μm, to 13 μm, respectively).
To determine whether these larger neurons were indeed Dogiel Type II sensory neurons we post-stained the preparation with mitotracker (Vanden Berghe et al. 2002), as these particular neurons contain a greater number of mitochondria compared to other myenteric neurons (Fig. 8B; Pompolo & Furness, 1988; Vanden Berghe et al. 2002). We found that mitotracker densely stained a number of the larger neurons in a ganglia (4 to 6), which were similar in size (long axis = 29.87 ± 1.07 μm; short axis 21.73 ± 0.85 μm; n= 6) and morphology to Dogiel Type II neurons observed in the guinea-pig ileum and murine colon (Fig. 8B–D; Iyer et al. 1988; Pompolo & Furness, 1988; Furness et al. 1990, 2004; Vanden Berghe et al. 2002; Nurgali et al. 2004). Also, mitotracker +ve neurons were larger than the mitotracker –ve neurons (Fig. 8D). In fact, 81% (48 of 59 neurons; n= 4) of the mitotracker +ve neurons were also co-labelled for the calcium binding protein calbindin (Fig. 8B and C; n= 4), which has been shown to be a characteristic marker for 80% of Dogiel type II sensory neurons in the guinea-pig ileum (Iyer et al. 1988; Pompolo & Furness, 1988; Furness et al. 1990; Vanden Berghe et al. 2002). The calbindin +ve neurons in the murine colon were similar in size to the mitotracker neurons (long axis = 35.3 ± 0.97 μm; short axis 23.6 ± 0.76 μm; n= 4). Slight differences in their sizes were due to the fact that mitotracker +ve neurons had a more indefinite outline compared to calbindin +ve neurons.
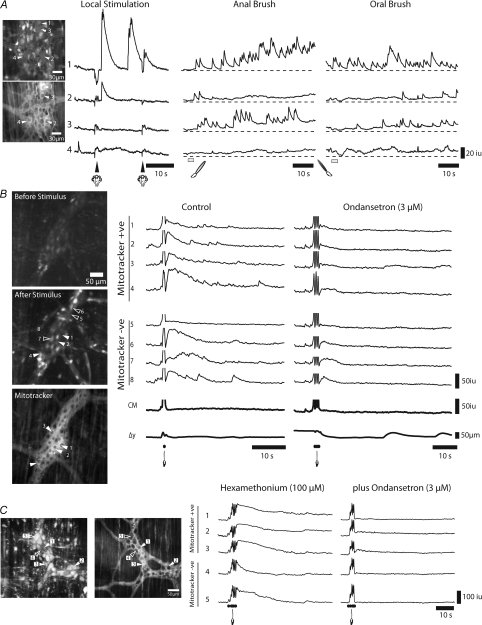
Many mitotracker +ve neurons, unlike other neurons, did not respond to either local (nitrogen puff), or anal or oral mucosal stimulation and appeared to have low basal levels of Ca2+ (n= 5). We reasoned that their lack of response was probably due to their failure to take up or effectively maintain the Ca2+ indicator. Previously, it has been shown that anion transporters can cause indicator extrusion, so in several experiments (n= 3) we applied probenecid (0.5–1 mm; anion transport inhibitor) to the Krebs solution (see Di Virgilio et al. 1990). Following probenecid, we found that some mitotracker +ve neurons exhibited short duration Ca2+ responses (duration 5.43 ± 0.55 s; n= 6) to local mucosal stimulation after a latency of 1.43 ± 0.31 s (see neurons 1 and 2; Fig. 9A), similar to the short latency response of the large neurons that responded without probenecid (compare Fig. 8A with 9A). Also, the majority of mitotracker +ve neurons (neurons 1–3; Fig. 9A; n= 3) responded with a prolonged response to both anal and oral mucosal stimulation (Fig. 9A).
Figure 9. Responses of mitotracker +ve neurons to local stimulation.
High power imaging (×60) was used to record responses in neurons to local stimulation. A, upper panel shows average calcium in neurons and location of selected neurons. Lower panel shows location of neurons stained with mitotracker (filled arrows) in the same ganglia. Following local mucosal stimulation (a puff of nitrogen administered to the mucosa under the recording site) two of the 4 mitotracker +ve neurons (left hand panel; e.g. neurons 1 and 2) responded to local stimulation with a brief Ca2+ transient; whereas, two other mitotracker +ve neurons did not respond (see left hand panel, neurons 3 and 4). However, 3 of these 4 neurons (1–3) exhibited a sustained response following anal and oral mucosal stimulation. B, upper panel: shows average calcium in neurons before stimulation, and location of selected neurons; middle panel shows average calcium immediately following stimulation, and lower panel shows location of neurons stained with mitotracker (filled arrows). A single brush stroke applied to the mucosa over the recording site evoked Ca2+ transients in both mitotracker +ve neurons (1–4) and in mitotracker −ve neurons (5–8), although neuron 5 failed to respond. Ondansetron (3 μm) significantly reduced the responses in these neurons (1–8) to mucosal stimulation (3 strokes). Apparent changes in activity in CM and displacement are stimulus artifacts. C, left hand panel: average calcium in ganglia and location of selected neurons; right hand panel: location of mitotracker +ve (filled arrows) and mitotracker −ve (open arrows) neurons. In the presence of hexamethonium (100 μm), brushing the mucosa (5 strokes) over the stimulation site evoked a sustained Ca2+ response in both mitotracker +ve neurons (1–3) and in mitotracker −ve neurons (4 and 5). Ondansetron (3 μm) significantly reduced the responses to stroking the mucosa in these neurons, leaving mostly the stimulus artifact. In these experiments, probenecid (0.5 mm; anion transport pump inhibitor) was added to the Krebs solution bathing the tissue.
Brushing the mucosa directly over the recording site (Fig. 1C) was a more effective stimulus than a brief puff of nitrogen onto the underlying mucosa. Brushing the mucosa activated synchronized Ca2+ transients in all Dogiel Type II (mitotracker +ve; neurons 1–4, Fig. 9B) neurons and some mitotracker −ve neurons within the field of view (neurons 5 and 6, Fig. 9B). Subsequent stimulations caused a facilitation of the response in these neurons, eventually leading to a CMMC response that was difficult to capture since several strokes produced pronounced movement and overwhelmed neuronal Ca2+ transients by activity in the muscle. However, the application of ondansetron (3 μm; 5-HT3 receptor antagonist, n= 4), which significantly reduces CMMCs evoked by mucosal stimulation (Heredia et al. 2009), significantly reduced the responses in all neurons by approximately 82% (area under the curve intensity unit-seconds): control: 151.5 ± 27.8 iu-s; ondansetron: 27.3 ± 8.2 iu-s, P < 0.01, n= 3).
We also performed these experiments in the presence of hexamethonium (100 μm; Fig. 9C, n= 3). In the example shown, brushing the mucosa (5 strokes) produced a large and prolonged Ca2+ transient in three mitotracker positive neurons (1–3) and shorter transients in mitotracker –ve neurons (neurons 4 and 5). No CMMCs were evoked in hexamethonium by local stimulation of the mucosa. Similarly, the further addition of ondansetron (3 μm; 5-HT3 receptor antagonist, n= 4) substantially reduced the responses to stroking the mucosa.
Effect of removing the mucosa
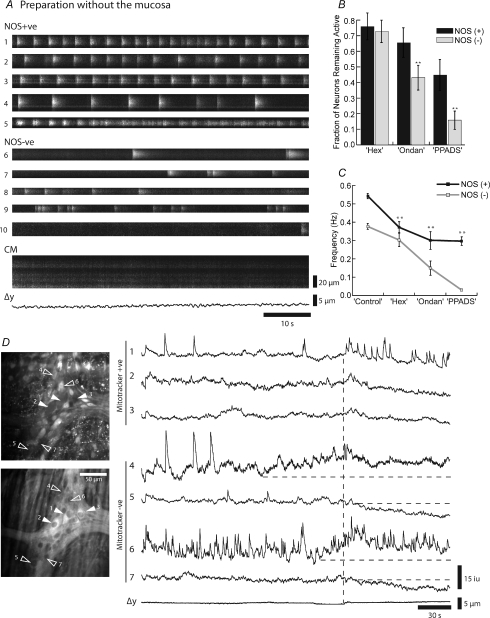
We observed no spontaneous CMMCs in 35 colonic preparations from which the mucosa had been removed (Heredia et al. 2009; Dickson et al. 2009a,b;). In these preparations we observed rhythmic bursts of activity in both NOS +ve (29 neurons; frequency 0.54 ± 0.01 Hz, n= 5) and NOS −ve (44 neurons; frequency 0.37 ± 0.02 Hz, n= 5) neurons (Fig. 10A). The number of spontaneously firing NOS +ve and NOS −ve neurons in any given field of view was decreased by the sequential addition of hexamethonium (100 μm; nicotinic antagonist); ondansetron (1 μm; 5-HT3 receptor antagonist) and PPADs (10 μm; P2X receptor antagonist), which have been shown to reduce the evoked fast excitatory postsynaptic potential in myenteric neurons (see Galligan, 2003; Nurgali et al. 2003). This suggests that most NOS +ve neurons are activated by fast synaptic input rather than being stretch sensitive (Fig. 10B and C).
Figure 10. Neural activity in preparations without the mucosa.
A, both NOS +ve neurons and NOS −ve neurons usually exhibited ongoing bursts of activity, even though the CM was quiescent. Note that the fifth NOS +ve neuron went from burst activity to more continuous firing pattern B, following the sequential addition of blockers of fast synaptic transmission, hexamethonium (Hex, 100 μm), ondansetron (Ondan, 1 μm) and pyridoxal-phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt (PPADs, 10 μm), there was a decrease in the number of both NOS +ve and NOS −ve neurons within the field of view that exhibited spontaneous Ca2+ transients. C, following the addition of these drugs the frequency of firing also decreased, which was more pronounced in NOS +ve neurons. Data from 73 neurons, 29 NOS +ve, 44 NOS −ve; n= 5; **P < 0.01. D, upper panel, average Ca2+ intensity and location of mitotracker +ve neurons (1–3, filled arrows) and mitotracker −ve neurons (4–7, open arrows). Lower panel, mitotracker staining of same ganglion. Note that neurons 1–3 are mitotracker +ve (filled arrows) and 4–7 are negative. Spontaneous activity in a preparation without the mucosa. Vertical dotted line shows synchronized activity in the 3 mitotracker +ve neurons that appears to correlate with an increase in activity in neurons 4 and 7, and the onset of a reduction in Ca2+ in neurons 5 and 7.
In addition, when we poststained these preparations with mitotracker we found that mitotracker +ve, Dogiel Type II neurons were still active (neurons 1–3, Fig. 10D; n= 4). In this example, when Dogiel Type II neurons were observed to synchronize their activity there was a decrease in activity in two mitotracker −ve neurons, which presumably were NOS +ve motor neurons (see neurons 5 and 7). It appeared that the synchronized activity in the Dogiel Type II neurons may have been produced by increased activity in mitotracker −ve neurons (neurons 4 and 6) that fired continually, suggesting they may have been interneurons.
Discussion
There have been previous attempts to record enteric neural activity in ganglia from more or less intact preparations using either calcium imaging (Vanden Berghe et al. 2001) or voltage sensitive dyes (Neunlist et al. 1999), yet this study is the first attempt to describe the behaviour of neurons in the enteric nervous system and correlate their behaviour to the motor output to the muscle during a major motor pattern, namely the colonic migrating motor complex (CMMC). The fluorescent imaging techniques used allowed us to record Ca2+ transients in many myenteric neurons, in several ganglia at the same time and correlate their activity with Ca2+ transients in the muscle and contraction. All the Ca2+ transients in myenteric neurons were abolished by TTX, suggesting they resulted largely from action potential firing in neurons. Previously it has been shown that both S and AH myenteric neurons in the intestine exhibit action potential-dependent Ca2+ transients (Tatsumi et al. 1988; Shuttleworth & Smith, 1999; Hillsley et al. 2000; Vanden Berghe et al. 2001, 2002).
What is clear is that neuronal integration during the CMMC is complex and the colonic migrating motor complex is aptly named. Nonetheless, we were able to make a number of important observations that would have been difficult, if not impossible, using other current technologies.
Proposed mechanisms underlying the CMMC
Electrical recordings from the circular muscle of the murine colon have shown that the CMMC consists of a brief hyperpolarization followed by fast oscillations with action potentials superimposed on a slow depolarization of the circular muscle (Bywater et al. 1989; Spencer et al. 2005; Dickson et al. 2009a,b; Heredia et al. 2009). The mechanisms underlying the generation of the CMMC were proposed to largely involve a turning off of ongoing inhibitory nerve activity (disinhibition) to the muscle (Christensen et al. 1978; Spencer et al. 1998; Spencer 2001). This hypothesis was later modified, since the fast oscillations were found to be due to the release of acetylcholine (ACh), with the atropine sensitive slow depolarization being due to disinhibition (Bywater et al. 1989; Spencer et al. 1998; Spencer, 2001). However, recent evidence suggests that both the fast oscillations and the slow depolarization are generated by the release of excitatory (ACh and tachykinins) neurotransmitters, rather than disinhibition (Brierley et al. 2001; Dickson et al. 2009b).
Circuitry underlying spontaneous and evoked CMMCs
Both spontaneous and evoked (local and distant mucosal stimulation) CMMCs gave rise to similar rapid calcium transients in the muscle, suggesting that we were recording the same neural event, regardless of how the CMMCs were generated. Also, many of the same myenteric neurons were active during both a spontaneous and an evoked CMMC, suggesting they were in the same neural circuit. Similar responses were also observed in the same neurons regardless of whether the stimulus was applied orally or anally, suggesting that distinct or converging nerve pathways may exist which generate CMMCs. The CMMC was readily evoked from the anal end of the preparation but rarely from the extreme oral end. Presumably, ascending excitatory interneuronal pathways are important for activating excitatory motor neuron responses to the muscle (Spencer et al. 2005; Dickson et al. 2009b), whereas descending nerve pathways are likely to be involved in the preceding inhibition and the activation of AH neurons at distant sites (Dickson et al. 2009a,b;). Activation of descending seroternergic interneurons, which also contain ACh, in the guinea-pig ileum generates a slow excitatory postsynaptic potential in AH/Dogiel Type II neurons mediated by activation of 5-HT7 receptors (Monro et al. 2005). These pathways appear to be important in the murine colon as well, since a 5-HT7 receptor antagonist blocks the CMMC (Dickson et al. 2009a). It is also likely that ascending interneurons and descending interneurons synapse with one another (Spencer et al. 2005; Smith et al. 2007).
Hexamethonium blocks spontaneous CMMCs (Bywater et al. 1989; Bush et al. 2000; Heredia et al. 2009) and CMMCs evoked by electric field stimulation in preparations without the mucosa (Dickson & Smith, unpublished results). In the current study, Dogiel Type II neurons could still be activated in hexamethonium by local mucosal stimulation, but they failed to generate a CMMC. Hexamethonium did, however, block both the prolonged excitatory and the inhibitory responses in neurons to mucosal stimulation applied at a distance from the recording site. Therefore, nicotinic synapses must be located at critical points within the neural circuitry underlying the CMMC. The ascending excitatory nervous pathway is particularly sensitive to nicotinic receptor blockade suggesting it may activate these neurons (Smith & Furness, 1988; Smith et al. 1992), although most other interneurons in the colon contain ACh (Lomax & Furness, 2000), suggesting they can also activate other interneurons or motor neurons via nicotinic receptors. Also, some interneurons may be directly activated by ACh released from Dogiel Type II neurons (Galligan & North, 1991).
Ca2+ transients in myenteric neurons
Various patterns of activity were recorded in myenteric neurons both between and during the CMMC. Both NOS +ve and NOS −ve neurons exhibited ongoing Ca2+ transients between CMMCs, as well as in preparations without the mucosa. The NOS +ve neurons with lamina dendrites are likely to be inhibitory motor neurons (Sang & Young, 1996; Lomax & Furness, 2000) that are activated by neuronal circuits involved in maintaining the colonic smooth muscle in a state of tonic inhibition (Lyster et al. 1995; Bywater et al. 1989; Spencer et al. 1998; Powell et al. 2001; Dickson et al. 2009b; Heredia et al. 2009). A number of these particular NOS +ve motor neurons sometimes exhibited an initial transient burst of activity before they became inhibited, suggesting that they are responsible for the initial hyperpolarization of the CM that is sometimes observed to precede the CMMC (Bywater et al. 1989; Dickson et al. 2009b). Why these NOS +ve motor neurons usually reduced their firing after the initial CMMC response in the muscle is unclear, since their decreased activity is unlikely to contribute significantly to the initiation of the CMMC, although this reduction may facilitate the action of excitatory neurotransmitters by disinhibiting the muscle (Spencer et al. 1998; Dickson et al. 2009b). The other distinct group of NOS +ve neurons with the ‘dew drop’ appearance exhibited prolonged bursts of activity during the CMMC and are probably a subclass of descending cholinergic interneurons (Sang & Young, 1996; Lomax & Furness, 2000). Some neurons that increased their activity prior to the onset of the CMMC may be interneurons. We have previously shown that interneurons appear to synchronize their activity during the CMMC (Spencer et al. 2005). Other neurons, which fired throughout the response in the muscle, are likely to be excitatory motor neurons that release ACh and tachykinins to produce muscle contraction (Brierley et al. 2001; Dickson et al. 2009b).
Initiation and propagation of the CMMC
Our results are consistent with the notion that a major component of the neural circuitry required for generating CMMC is within the myenteric plexus (Heredia et al. 2009; Keating & Spencer, 2009). We, however, found that the generation of the spontaneous CMMC, or a CMMC evoked by mechanically stimulating the mucosa, requires 5-HT release from enterochromaffin cells (ECC) in the mucosa, as we recently reported (see Dickson et al. 2009a,b; Heredia et al. 2009). The released 5-HT appears to activate 5-HT3 receptors on the mucosal endings of Dogiel Type II sensory (mitotracker +ve) neurons within the myenteric plexus, which are likely to be the first responders (Bertrand et al. 2000). In support of this hypothesis, Dogiel Type II neurons responded to local stimulation of the mucosa, even in the presence of hexamethonium, and ondansetron significantly attenuated these responses. Keating & Spencer (2009) demonstrated that ‘high concentrations of 5-HT were being secreted, at least in vitro, from the mucosa’ and that ‘most CMMCs (93%) could be temporally correlated with the release of 5-HT’. Whether the CMMC is activated by spontaneous (Heredia et al. 2009) or ‘dynamically’ mediated (Keating & Spencer, 2009) release of 5-HT from the mucosa is unclear and needs further examination.
While not all Dogiel Type II neurons within the same ganglia were activated by brief puffs of nitrogen to the underlying mucosa, when the mucosa was stroked with a brush over the recording site all Dogiel Type II neurons responded to the stimulus with synchronized activity. These results suggest that although Dogiel Type II neurons may have different thresholds for activation, a stronger and broader stimulus can synchronize their activity and bring them to threshold for the generation of a CMMC. Presumably, since several successive stimuli lead to a potentiated response, this facilitation is likely to entail synaptic interactions between Dogiel Type II/AH neurons involving slow excitatory post synaptic potentials (SEPSPs; Bertrand et al. 2000; Hillsley et al. 2000; Monro et al. 2005).
Although Dogiel Type II neurons and the CMMC respond to local stimulation after ∼1–2 s, it can take about 6–10 s for a CMMC to arrive at the recording site in response to mucosal stimulation applied some distance (∼25 mm) away. This long latency appears to be a characteristic feature of the CMMC, regardless of whether it is generated by mucosal stimulation (Heredia et al. 2009) or transmural nerve stimulation (Spencer & Bywater, 2002). In contrast, the latency of onset of excitatory or inhibitory junction potentials in the muscle evoked by mucosal stimulation or distension is ∼500–1200 ms (Smith & Furness, 1988; Smith et al. 1992; Spencer & Smith, 2001). Unlike reflex responses in the guinea-pig ileum and colon, which are graded according to the strength of the stimulus (Smith & Furness, 1988; Smith et al. 1992; Spencer & Smith, 2001), the evoked CMMC appears to be an ‘all-or-none’ event (Heredia et al. 2009). Furthermore, the conduction velocity of activity through the nerve pathways during a reflex response is fast (∼75 mm s−1; Smith et al. 1992; guinea-pig colon) compared to the conduction velocity of ∼0.8 mm s−1 for a CMMC, which is similar to the velocity of a faecal pellet (Heredia et al. 2009). Therefore, the long latency and slow conduction velocity suggest that the CMMC is a motor pattern involving more complex neural circuitry than a simple reflex arc (Levitan & Kaczmarek, 2002). As surmised above, the generation and propagation of the CMMC is likely to involve the development of SEPSPs in Dogiel Type II/AH neurons at many sites along the colon that are integrated into a ‘recurrent’ network, similar to the computer model of the MMC in the small bowel (see Thomas et al. 2004).
Preparations without the mucosa
In the present study we recorded from a large number of preparations without the mucosa and never observed a single spontaneous CMMC, though in preparations without the mucosa CMMCs can be readily evoked by transmural nerve stimulation (Dickson et al. 2009a,b; Heredia et al. 2009). In contrast, Keating & Spencer (2009) observed ‘spontaneous’ CMMCs (albeit at a lower frequency) in preparations without the mucosa. However, from the size of their CMMC contractions, we have argued that they are unlikely to be recording normal spontaneous CMMCs, but CMMCs activated by excessive amounts of circumferential stretch (see Heredia et al. 2009) that bear little or no relationship to the normal physiology of the murine colon (Smith et al. 2009).
The lack of spontaneous CMMCs in these preparations was unlikely to have been due to damage to the myenteric plexus (see Keating & Spencer, 2009), for a number of reasons: (1) Ongoing Ca2+ transients were observed in both NOS +ve, NOS −ve neurons and Dogiel Type II neurons (mitotracker positive). Their patterns of neuronal activity had many features in common with preparations with the mucosa intact such as rhythmically or continuously firing NOS +ve and NOS −ve neurons. (2) Our pharmacological experiments suggest that many of these neurons were driven by other neurons, suggesting that the nerve pathways were intact. (3) Also, we have previously shown that CMMCs can be readily evoked by electric field stimulation in preparations without the mucosa, and that the low resting membrane potential, which is driven by spontaneous firing of inhibitory motor nerves, is the same as in preparations with the mucosa intact (Dickson et al. 2009b; Heredia et al. 2009). This is not surprising since most electrophysiological studies of myenteric neurons, including Dogiel Type II neurons, remove the mucosa (and underlying circular muscle) without damaging the myenteric plexus (Hillsley et al. 2000; Nurgali et al. 2003, 2004).
In summary, using Ca2+ imaging, in conjunction with post-labelling techniques, we have attempted to define aspects of the neuronal circuitry in the enteric nervous system during a well defined motor behaviour. To do this we have recorded the simultaneous activities of several classes of myenteric neurons in the mouse colon at physiological temperatures, and have described the function of a large number of neurons, within several ganglia, during the initiation of the CMMC. The advantages of this technique over traditional electrophysiological and mechanical recordings lie not only in the ability to concurrently measure activities in a large number of cells, but also to relate these activities to a larger physiological event. Here we have used this method to demonstrate that myenteric neurons, which generate a CMMC, do so regardless of the origin of the stimulus, suggesting that pathways responsible for such responses are intrinsically linked. We have also shown that many of the observed responses in both neurons and smooth muscle cells are the result of interneuronal linking via nicotinic neurotransmission, as hexamethonium abolished these events. Post-staining of myenteric ganglia has allowed us to describe the activities of several varying classes of neurons. We have identified two morphologically distinct classes of NOS-containing neurons, and have described their activities between and during CMMCs. Moreover, we have reported the activities of Dogiel type-II neurons in response to both local and mucosal brush stimulations, and have shown them to be potential triggers for the generation of the CMMC. This study, albeit focusing squarely on the neuronal interworking of the myenteric plexus in the generation and maintenance of a well-described motor event in the colon, presents an important step in our understanding of how integrated neuronal networks in mammalian systems function. Here we have described in detail the complex interaction of sensory, motor and interneurons that occurs in response to a physiological stimulus. This study has presented methods by which the actions of individual classes of neurons can be examined in other complex neuronal networks, and allows the study of neuronal function from a more integrated perspective.
Acknowledgments
The authors would like to thank Dr Bill Shuttleworth for helpful suggestions. This study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases: RO1 DK45713. Imaging was performed in a Core laboratory supported by NIH grant P20 RR-18751.
Glossary
Abbreviations
- CM
circular muscle
- CMMC
colonic migrating motor complex
- LM
longitudinal muscle
- NOS
nitric oxide synthase
- ST map
spatio-temporal map
Author contributions
P.O.B. performed Ca2+ imaging experiments, immunohistochemistry, and analysis. G.W.H. wrote algorithms used in analysis and was involved in study design. T.K.S. was involved in study design, interpretation of results, and manuscript construction. Experiments were performed in a Core laboratory in the Department of Physiology and Cell Biology at the University of Nevada, Reno.
Supplemental material
Supplementary Movie 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Nichols K, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol. 2001;132:507–517. doi: 10.1038/sj.bjp.0703814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarisations and rapid oscillations in circular muscle of mouse colon. J Physiol. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000;84:162–168. doi: 10.1016/S1566-0702(00)00201-0. [DOI] [PubMed] [Google Scholar]
- Christensen J, Anuras S, Arthur C. Influence of intrinsic nerves on electromyogram of cat colon in vitro. Am J Physiol Endocrinol Metab. 1978;234:E641–647. doi: 10.1152/ajpendo.1978.234.6.E641. [DOI] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, Hennig GW, Smith TK. The importance of 5-HT 1A and 5-HT 7 receptors during murine colonic migrating motor complex. Neurogastroenterol Motil. 2009a;21(Suppl 1):48. Abs 151. [Google Scholar]
- Dickson EJ, Heredia DJ, McCann CJ, Hennig GW, Smith TK. The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol. 2009b doi: 10.1152/ajpgi.00399.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912–1924. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Dining PG, Szczesniak MM, Cook IJ. Twenty-four hour spatiotemporal mapping of colonic propagating sequences provides pathophysiological insight into constipation. Neurogastroenterol Motil. 2008;20:1017–1021. doi: 10.1111/j.1365-2982.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Steinberg TH, Silverstein SC. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Furness JB, Keast JR, Pompolo S, Bornstein JC, Costa M, Emson PC, Lawson DE. Immunohistochemical evidence for the presence of calcium-binding proteins in enteric neurons. Cell Tissue Res. 1988;252(1):79–87. doi: 10.1007/BF00213828. [DOI] [PubMed] [Google Scholar]
- Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res. 2004;317:1–12. doi: 10.1007/s00441-004-0895-5. [DOI] [PubMed] [Google Scholar]
- Furness JB, Trussell DC, Pompolo S, Bornstein JC, Smith TK. Calbindin neurons of the guinea-pig small intestine: quantitative analysis of their numbers and projections. Cell Tissue Res. 1990;260:261–272. doi: 10.1007/BF00318629. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2003;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, North RA. Opioid, 5-HT1A and α2 receptors localized to subsets of guinea-pig myenteric neurons. J Auton Nerv Syst. 1991;32:1–11. doi: 10.1016/0165-1838(91)90229-v. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov P, Hennig GW, Smith TK. Localized release of 5-HT by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kenyon J, Smith TK. Ryanodine sensitive stores regulate the excitability of myenteric AH neurons. J Neurophysiolgy. 2000;84:2777–2785. doi: 10.1152/jn.2000.84.6.2777. [DOI] [PubMed] [Google Scholar]
- Iyer V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T. Electrophysiology of guinea-pig myenteric neurons correlated with immunoreactivity for calcium binding proteins. J Auton Nerv Syst. 1988;22:141–150. doi: 10.1016/0165-1838(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.020. (in press) [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, Smith TK. Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology. 2009;136(7):2226–2236. doi: 10.1053/j.gastro.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB, Kaczmarek LK. The Neuron: Cell and Molecular Biology. 3rd edn. New York: Oxford University Press; 2002. Neural networks and behavior; pp. 507–536. Ch 19. [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Lyster DJ, Bywater RA, Taylor GS. Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology. 1995;108:371–1378. doi: 10.1016/0016-5085(95)90684-3. [DOI] [PubMed] [Google Scholar]
- Meedeniya ACB, Brookes SJH, Costa M. Sources of 5-hydroxytryptamine immunoreactivity in the myenteric plexus of the guinea-pig small intestine. Proc Aust Neurosci Soc. 1994;5:176. [Google Scholar]
- Monro RL, Bornstein JC, Bertrand PP. Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience. 2005;134:975–986. doi: 10.1016/j.neuroscience.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Peters S, Schemann M. Multisite optical recording of excitability in the enteric nervous system. Neurogastroenterol Motil. 1999;11:393–402. doi: 10.1046/j.1365-2982.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Nicolls JG, Martin AR, Wallace BG, Fuchs PA. From Neuron to Brain. 4th edn. Sunderland, MA, USA: Sinauer Associates, Inc; 2001. Cellular mechanisms of integration and behaviour in leeches, ants and bees; pp. 291–314. Ch 15. [Google Scholar]
- Nurgali K, Furness JB, Stebbing MJ. Analysis of purinergic and cholinergic fast synaptic transmission to identified myenteric neurons. Neuroscience. 2003;16:335–347. doi: 10.1016/s0306-4522(02)00749-2. [DOI] [PubMed] [Google Scholar]
- Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol. 2004;468:112–124. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- Obaid AL, Loew LM, Wuskell JP, Salzberg BM. Novel naphthylstyryl-pyridium potentiometric dyes offer advantages for neural network analysis. J Neurosci Methods. 2004;134(2):179–190. doi: 10.1016/j.jneumeth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Park KJ, Henning GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290(5):C1411–C1427. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Furness JB. Ultrastructure and synaptic relationships of calbindin-reactive, Dogiel type II neurons, in myenteric ganglia of guinea-pig small intestine. J Neurocytol. 1988;17:771–782. doi: 10.1007/BF01216705. [DOI] [PubMed] [Google Scholar]
- Powell AK, Fida R, Bywater RA. Ongoing nicotinic and non-nicotinic inputs to inhibitory neurons in the mouse colon. Clin Exp Pharmacol Physiol. 2001;28:792–798. doi: 10.1046/j.1440-1681.2001.03524.x. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985;89(4):894–913. doi: 10.1016/0016-5085(85)90589-x. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CWR, Smith TK. Action potential-dependent calcium transients in myenteric S neurons of the guinea-pig ileum. Neuroscience. 1999;92:751–762. doi: 10.1016/s0306-4522(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Smith TK. An electrophysiological identification of intrinsic sensory neurons responsive to 5-HT applied to the mucosa that underlie peristalsis in the guinea-pig proximal colon. J Physiol. 1996;495.P:102P. [Google Scholar]
- Smith TK, Bywater RA, Taylor GS, Holman ME. Electrical responses of the muscularis externa to distension of the isolated guinea-pig distal colon. J Gastrointest Mot. 1992;4:145–156. [Google Scholar]
- Smith TK, Dickson EJ, Heredia DJ, Hennig GW, Bayguinov PO. Controversies involving the role of 5-hydroxytryptamine (5-HT) in generating colonic migrating motor complexes: what is spontaneous? Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J Auton Nerv Syst. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007;19:869–78. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ. Control of the migrating motor activity in the colon. Curr Opin Pharmacol. 2001;1:604–610. doi: 10.1016/s1471-4892(01)00103-5. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bayguinov P, Hennig GW, Park KJ, Lee HT, Sanders KM, Smith TK. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G546–555. doi: 10.1152/ajpgi.00352.2006. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RA. Enteric nerve stimulation evokes a premature colonic migrating motor complex in mouse. Neurogastroenterol Motil. 2002;14:657–665. doi: 10.1046/j.1365-2982.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst. 1998;71:37–47. doi: 10.1016/s0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol. 2005;564:829–847. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Publicover NG, Smith TK. Induction and regulation of Ca2+waves by enteric neural reflexes. Nature. 1999;399:62–66. doi: 10.1038/19973. [DOI] [PubMed] [Google Scholar]
- Tatsumi H, Hirai K, Katayama Y. Measurement of the intracellular calcium concentration in guinea-pig myenteric neurons by using fura-2. Brain Res. 1988;451:371–375. doi: 10.1016/0006-8993(88)90787-1. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Sjövall H, Bornstein JC. Computational model of the migrating motor complex of the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G564–572. doi: 10.1152/ajpgi.00369.2003. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe P, Bisschops R, Tack J. Imaging of neuronal activity in the gut. Curr Opin Pharmacol. 2001;1:563–567. doi: 10.1016/s1471-4892(01)00097-2. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe P, Kenyon JL, Smith TK. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J Neuroscience. 2002;22:6962–6971. doi: 10.1523/JNEUROSCI.22-16-06962.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Xue C, Sanders KM. Localization of nitric oxide synthase in canine ileocolonic and pyloric sphincters. Cell Tissue Res. 1994;275(3):513–527. doi: 10.1007/BF00318820. [DOI] [PubMed] [Google Scholar]
- Wood JD. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Dig Dis. 1973;18:477–487. doi: 10.1007/BF01076598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.