Abstract
Alterations in trans-sarcolemmal and sarcoplasmic reticulum (SR) Ca2+ fluxes may contribute to impaired cardiomyocyte contraction and relaxation in heart failure. We investigated the mechanisms underlying heart failure progression in mice with conditional, cardiomyocyte-specific excision of the SR Ca2+-ATPase (SERCA) gene. At 4 weeks following gene deletion (4-week KO) cardiac function remained near normal values. However, end-stage heart failure developed by 7 weeks (7-week KO) as systolic and diastolic performance declined. Contractions in isolated myocytes were reduced between 4- and 7-week KO, and relaxation was slowed. Ca2+ transients were similarly altered. Reduction in Ca2+ transient magnitude resulted from complete loss of SR Ca2+ release between 4- and 7-week KO, due to loss of a small remaining pool of SERCA2. Declining SR Ca2+ release was partly offset by increased L-type Ca2+ current, which was facilitated by AP prolongation in 7-week KO. Ca2+ entry via reverse-mode Na+–Ca2+ exchange (NCX) was also enhanced. Up-regulation of NCX and plasma membrane Ca2+-ATPase increased Ca2+ extrusion rates in 4-week KO. Diastolic dysfunction in 7-week KO resulted from further SERCA2 loss, but also impaired NCX-mediated Ca2+ extrusion following Na+ accumulation. Reduced Na+-K+-ATPase activity contributed to the Na+ gain. Normalizing [Na+] by dialysis increased the Ca2+ decline rate in 7-week KO beyond 4-week values. Thus, while SERCA2 loss promotes both systolic and diastolic dysfunction, Na+ accumulation additionally impairs relaxation in this model. Our observations indicate that if cytosolic Na+ gain is prevented, up-regulated Ca2+ extrusion mechanisms can maintain near-normal diastolic function in the absence of SERCA2.
Introduction
Contraction in adult ventricular myocytes is triggered by a transient increase in intracellular [Ca2+] ([Ca2+]i) during the action potential (AP). This rise in [Ca2+]i results from Ca2+ influx through L-type Ca2+ channels, which in turn triggers release of a much larger amount of Ca2+ from the sarcoplasmic reticulum (SR) (Bers, 2001). Relaxation occurs as Ca2+ is recycled into the SR by the SR Ca2+-ATPase (SERCA) and as Ca2+ is extruded from the cell, primarily by the Na+–Ca2+ exchanger (NCX).
Altered cardiomyocyte Ca2+ homeostasis is believed to be an important mechanism underlying heart failure (HF), and may contribute to both systolic and diastolic dysfunction in this condition. Systolic dysfunction results, at least in part, from reduced magnitude of Ca2+ transients due to lower Ca2+ content of the SR (Bers, 2006). Reduced SR content is believed to be caused by depressed SERCA function and greater diastolic SR Ca2+ leak. Increased expression and function of the NCX may also impair SR Ca2+ reuptake since NCX competes with SERCA for extrusion of Ca2+ from the cytosol (Pogwizd et al. 2001). Less is known about the cellular mechanisms that underlie diastolic HF. However, Hasenfuss et al. (1999) observed that hearts from human HF patients with diastolic dysfunction had decreased SERCA expression but unchanged NCX. In contrast, patients with preserved diastolic function exhibited increased NCX levels. Thus, alterations in the relationship between trans-SR and trans-sarcolemmal Ca2+ fluxes may be critical in determining diastolic function in the failing heart, and progression to end-stage failure. The ability to increase transport of Ca2+ and Na+ across the sarcolemma may be an important compensatory mechanism for reduction in SR function, and a progressive decline in this ability may cause deterioration of diastolic function in HF patients.
We recently investigated the consequences of SERCA down-regulation in adult mice with cardiomyocyte-specific, conditional excision of the Serca2 gene (Andersson et al. 2009a). SERCA protein expression declined rapidly following gene deletion, and was below 5% of control values after 4 weeks. Surprisingly, we observed only moderately impaired cardiac performance with a relatively minor reduction in both systolic and diastolic function (Andersson et al. 2009a). Cardiomyocytes were observed to compensate for loss of SR function by increasing Ca2+ cycling across the sarcolemma. In the present study, we examine the cellular mechanisms underlying the eventual progression to end-stage HF in these mice. Our results indicate that while reduction of SR function following SERCA loss contributes to both systolic and diastolic dysfunction, impaired relaxation also results from slowing of NCX-mediated Ca2+ extrusion due to cytosolic Na+ accumulation. Importantly, we show that when such Na+ increases are prevented, up-regulated Ca2+ extrusion mechanisms can maintain near-normal diastolic function in the complete absence of a functional SR.
Methods
Ethical approval
The investigation was approved by the Norwegian National Committee for Animal Welfare under the Norwegian Animal Welfare Act, which conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and ethical guidelines from The Journal of Physiology (Drummond, 2009).
Animal care
The Serca2flox/flox Tg(αMHC-MerCreMer) mouse (KO) employed in our experiments allows for inducible, cardiomyocyte-specific disruption of the Serca2 gene in adult mice (Andersson et al. 2009a). Serca2 gene excision in 8- to 10-week-old mice was accomplished by inclusion of tamoxifen base powder (RM1 FG SQC, 811004, Scanbur BK) in the feed (100 mg (200 g)−1) for 7 days (Andersson et al. 2009c). Serca2flox/flox mice (FF) served as controls (Andersson et al. 2009a,b;) and were subjected to the same feeding regime. Tamoxifen administration results in Serca2 gene excision exclusively in the cardiomyocytes of KO animals (Andersson et al. 2009a). A total of 171 anaesthetized (∼2% isoflurane) mice were killed by cervical dislocation, and hearts were harvested at 4 and 7 weeks following tamoxifen administration. Echocardiographic characterization of heart function was performed on anaesthetised (∼2% isoflurane), self-breathing mice (Andersson et al. 2009a).
Histology
Following fixation in 4% formaldehyde, hearts were transected at the midventricular level, routinely processed and embedded in paraffin. Serial 3.5 μm sections were stained with haematoxylin and eosin, acid fuchsin orange G (AFOG) and van Gieson stains, and were assessed blindly by two investigators. The degree of fibrosis in each heart was given a score on a scale of 0–3.
Western blotting
Protein levels were determined by Western blot as described in Andersson et al. (2009a) but with images detected by a LAS-4000 Mini CCD detection system (Fujifilm, Tokyo, Japan). Antibodies used for protein detection were: SERCA2a (MA3-919), L-type Ca2+ channel α2δ1subunit (MA3-921), pan-plasmalemmal Ca2+-ATPase (PMCA, MA3-914) (all Affinity BioReagents, Golden, CO, USA), NCX1 (Thomas et al. 2003), Na+–K+-ATPase (NKA) α2 isoforms (Millipore, Billerica, MA, USA), and L-type Ca2+ channel α1C subunit (AAC-003, Alomone Laboratories, Jerusalem, Israel). SERCA2b antiserum (Campbell et al. 1993) was the kind gift of Frank Wuytack, Katholieke Universiteit Leuven, Leuven, Belgium. Calsequestrin was used as loading control.
Ca2+-ATPase content
Total left ventricular (LV) Ca2+-ATPase was quantified as thapsigargin-sensitive calcium-dependent incorporation of [32P] using whole-tissue homogenates and [γ32P]ATP (Everts et al. 1989; Andersson et al. 2009a).
Cardiomyocyte experiments
Cardiomyocytes were enzymatically isolated (Andersson et al. 2009a) and placed in a perfusion chamber on the stage of an inverted microscope. Experiments were performed at 37°C. Basic cell shortening and Ca2+ transient measurements were recorded during field stimulation (3 ms biphasic pulse, 25% above threshold) during superfusion with Hepes Tyrode solution containing (in mm): 140 NaCl, 1 CaCl2, 0.5 MgCl2, 5.0 Hepes, 5.5 glucose, 0.4 NaH2PO4 and 5.4 KCl (pH 7.4). Contractions were measured using a video edge detector (Crescent Electronics, Sandy, UT, USA) coupled to a television monitor. Ca2+ transients were recorded in fluo-4 AM (Invitrogen Molecular Probes, Eugene, OR, USA) loaded myocytes using an LSM 510 confocal microscope (Zeiss GmbH, Jena, Germany) in line-scan mode (Louch et al. 2006). Time constants (τ) and rate constants (defined as 1/τ) were calculated from single exponential fits of Ca2+ transient decays (Mork et al. 2009). Time to 50% decline was measured in experiments with very slow changes in [Ca2+]i which were difficult to fit exponentially.
Diastolic [Ca2+]i was determined by whole-cell photometry (Photon Technology International, Monmouth Junction, NJ, USA) in cells pipette-loaded with a solution containing (in mm): 0.1 fura-2 salt (Invitrogen Molecular Probes), 120 potassium aspartate, 0.5 MgCl2, 10 NaCl, 0.06 EGTA, 10 Hepes, 10 glucose, 25 KCl and 4 K2-ATP (pH 7.2). Fura-2 fluorescence ratios (F340/F380) were calibrated to [Ca2+]i, and calculated diastolic values were then used to calibrate Ca2+ transients recorded in fluo-4-loaded cells (Cheng et al. 1993; Andersson et al. 2009a).
Patch-clamp experiments were conducted with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA, USA), pCLAMP software (Axon Instruments) and low resistance pipettes (1–2 MΩ). AP recordings were made in bridge-mode, and representative tracings employed as voltage-clamp waveforms as described (Mork et al. 2009). L-type Ca2+ current was recorded during APs in Na+- and K+-free internal (in mm: 135 CsCl2, 3 Mg-ATP, 5 EGTA and 10 Hepes) and external (in mm: 136 TEA-Cl, 1.1 MgCl2, 10 glucose, 10 Hepes, 4 KCl and 1 CaCl2) solutions, and calculated as the current sensitive to 20 μm nifedipine (Mork et al. 2009). L-type Ca2+ current was also recorded during depolarizing 200 ms voltage steps from −50 mV, with an internal solution containing (in mm) 130 CsCl, 0.33 MgCl2, 4 Mg-ATP, 0.06 EGTA, 10 Hepes and 20 TEA, and an extracellular solution containing (in mm) 20 CsCl, 1 MgCl2, 135 NaCl, 10 Hepes, 10 d-glucose, 4 4-aminopyridine and 1 CaCl2 (Andersson et al. 2009a).
Intracellular Na+ concentration ([Na+]i) was assessed in myocytes loaded with SBFI AM as described previously (Swift et al. 2007 & Baartscheer et al. 1997). Total NKA current was obtained by rapidly elevating extracellular [K+] from 0 mm to 5.4 mm (Baartscheer et al. 1997; Swift et al. 2007). The α2 NKA isoform component of the total current was calculated as the difference current following treatment with 5 μm ouabain, which selectively blocks the α2 isoform (Berry et al. 2007). Remaining current was attributed to the α1 isoform.
Statistics
Data are expressed as mean values ±s.e.m. Statistical significance was calculated using paired or unpaired t tests. P values < 0.05 were considered significant.
Results
Development of end-stage HF
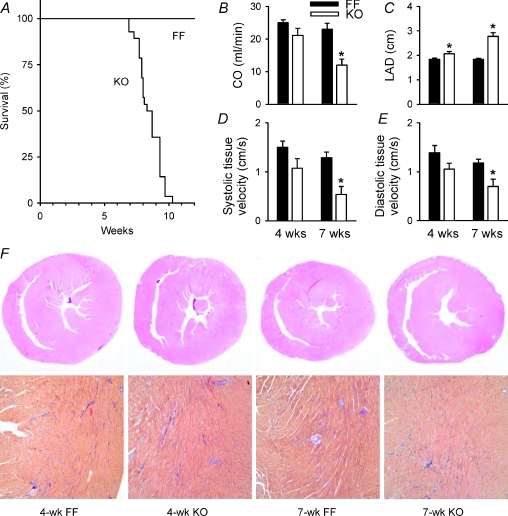
Kaplan Meyer survival curves (Fig. 1A) indicated that KO mice died over a period of 3.4 weeks, starting at 6.9 weeks following induction of Serca2 gene deletion by tamoxifen. KO mice were killed at 7 weeks (7-week KO) in further experiments due to ethical considerations. To examine the phenotype and mechanisms of HF development, data from 7-week KO were compared with the 4 week time point (4-week KO). Cardiac output was reduced in 7-week, but not 4-week KO (Fig. 1B), as we have observed previously (Andersson et al. 2009a). Similarly, left atrial diameter (LAD), a sensitive parameter for congestive HF in mice (Finsen et al. 2005), was increased between 4-week and 7-week KO (Fig. 1C, P < 0.05 within KO). Lung weight/body weight ratios also indicated greater pulmonary congestion in 7-week KO than 4-week KO (167%vs. 130% of FF values, P < 0.05, Table 1). Tissue Doppler measurements showed that HF development involved impairment of both systolic and diastolic function (Fig. 1D and E, respectively). We also observed depressed heart rate in KO mice (Table 1), which we believe resulted from Serca2 ablation in the sinoatrial node (Andersson et al. 2009a).
Figure 1. Progression to end-stage HF in KO mice involved impairment of systolic and diastolic function.
A, survival curves (FF, n= 26; KO, n= 27). Echocardiographic measurements of cardiac output (B), left atrial diameter (C), systolic tissue velocity (D), and diastolic tissue velocity (E) (FF, n= 6; KO, n= 5). F, whole heart histological sections at 20× (top panels, hematoxylin/eosin) and 100× magnification (bottom panels, AFOG, with blue staining indicating fibrosis). *P < 0.05 vs. equivalent FF.
Table 1.
Animal and cell characteristics
| 4-week FF | 4-week KO | 7-week FF | 7-week KO | |
|---|---|---|---|---|
| Age (weeks) | 14.0 ± 0.1 | 13.9 ± 0.2 | 17.1 ± 0.1 | 16.8 ± 0.2 |
| Heart rate (min−1) | 487 ± 18 | 433 ± 24* | 498 ± 19 | 395 ± 11* |
| BW (g) | 23.7 ± 0.6 | 26.4 ± 0.7* | 27.0 ± 0.7 | 24.6 ± 0.7* |
| LVW (mg) | 75.7 ± 2.4 | 87.5 ± 2.7* | 89.0 ± 3.0 | 83.5 ± 3.0 |
| LW (mg) | 134 ± 2 | 182 ± 6* | 153 ± 5 | 232 ± 9* |
| LVW/BW | 3.2 ± 0.1 | 3.3 ± 0.0 | 3.3 ± 0.0 | 3.4 ± 0.1 |
| LW/BW | 5.4 ± 0.3 | 7.0 ± 0.2* | 5.7 ± 0.2 | 9.5 ± 0.4* |
| Cell length (μm) | 129.6 ± 1.8 | 127.6 ± 1.8 | 127.6 ± 1.7 | 131.9 ± 1.9 |
| Cell width (μm) | 22.0 ± 0.4 | 21.8 ± 0.4 | 22.2 ± 0.4 | 21.5 ± 0.3 |
| Fract. short. at 6 Hz (%) | 3.76 ± 0.54 | 2.92 ± 0.43 | 2.98 ± 0.32 | 1.43 ± 0.11* |
| dl/dtmin at 6 Hz (μm ms−1) | 0.101 ± 0.020 | 0.048 ± 0.007* | 0.075 ± 0.010 | 0.019 ± 0.004* |
BW, body weight; LVW, left ventricular weight; LW, lung weight; Fract. short., fractional shortening, *P < 0.05 vs. FF; nanimals: 4-week FF = 29, 4-week KO = 32; 7-week FF = 35; 7-week KO = 25; ncells (length/width): 4-week FF = 115, 4-week KO = 121; 7-week FF = 139; 7-week KO = 143; ncells (6 Hz function): 4-week FF = 13, 4-week KO = 13, 7-week FF = 15, 7-week KO = 11.
LV weight/body weight ratios and LV myocyte dimensions were similar in FF and KO at both time points (Table 1), indicating that LV hypertrophy was not present in KO. Histological examination of heart sections showed no evidence of inflammatory cell infiltration, generalized myocyte degeneration or disarray in KO hearts (Fig. 1F). However, a modest degree of fibrosis was observed with no apparent progression between the two time points (mean score = 1.3 ± 0.5 in 4-week KO vs. 1.3 ± 0.8 in 7-week KO, P= NS). Since we could not detect time-dependent alterations in LV geometry or histology, we hypothesized that HF progression in KO resulted from declining systolic and diastolic cardiomyocyte function.
Isolated myocyte contractions and Ca2+ transients
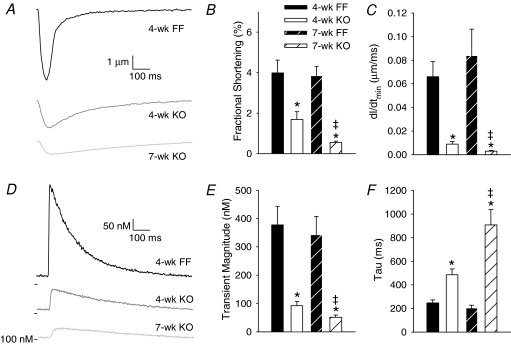
Representative myocyte contraction recordings during 1 Hz stimulation are shown in Fig. 2A. Contraction magnitude was reduced in 4-week KO from 4-week FF values, and further decreased in 7-week KO (Fig. 2B). Cardiomyocyte relaxation was also progressively slowed between 4-week and 7-week KO cells (Fig. 2C). Similar changes were observed in Ca2+ transients (Fig. 2D). Mean transient magnitude was markedly decreased in 4-week KO myocytes, and further decreased in 7-week KO (Fig. 2E). The declining phase of the Ca2+ transient was progressively slowed in KO cells (Fig. 2F).
Figure 2. Contractions and Ca2+ transients became progressively smaller and slower to decline in KO cardiomyocytes.
A, representative contraction traces, from isolated myocytes during 1 Hz field stimulation. Mean fractional shortening (B) and maximum relaxation velocity measurements (C). Representative Ca2+ transients recorded by fluo-4 fluorescence (D). Mean Ca2+ transient magnitudes (E) and decay kinetics (F). (n for panels B and C, E and F: 4-week FF = 13, 16; 4-week KO = 13, 20; 7-week FF = 15, 19; 7-week KO = 11, 19; *P < 0.05 vs. equivalent FF, ‡P < 0.05 vs. equivalent 4-week).
Increasing stimulation frequency to 6 Hz increased contraction magnitude to near-normal values in 4-week KO (Table 1). In 7-week KO, cell shortening was also improved by increasing the stimulation rate (1 Hz, 14% FF; 6 Hz, 48% FF, P < 0.05), but mean measurements remained significantly below control values. Similarly, relaxation kinetics were improved in both KO groups at higher stimulation frequency, but remained more markedly below FF values in 7-week KO (25% FF vs. 47% FF in 4-week KO, P < 0.05, Table 1). Thus, during stimulation in the physiological frequency range, myocyte contraction magnitude and relaxation kinetics roughly paralleled in vivo measurements of systolic and diastolic function. While we observed important differences in contraction amplitudes with frequency, Ca2+ transient amplitudes were similarly depressed in KO myocytes at 1 and 6 Hz (4-week KO: 25 ± 4%, 27 ± 4% FF, respectively; 7-week KO: 15 ± 2%, 16 ± 3% FF, P= NS for 1 Hz vs. 6 Hz, P < 0.05 for 4-week vs. 7-week KO). Diastolic [Ca2+]i in KO was not altered from FF values at either time point or frequency (data not shown).
Declining SR function
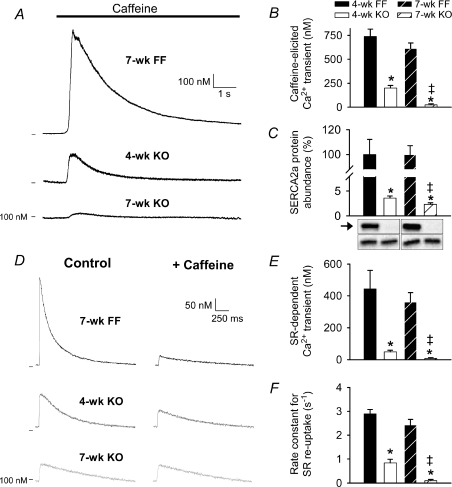
We examined changes in cellular Ca2+ fluxes underlying alterations in Ca2+ transients and contraction/relaxation in KO myocytes. Ca2+ release elicited by rapid application of 10 mm caffeine was reduced to 27% of FF values in 4-week KO and 4% in 7-week KO, indicating declining SR Ca2+ content (Fig. 3A and B). To estimate SR Ca2+ release during each beat, we compared steady-state Ca2+ transients in the presence and absence of caffeine (Andersson et al. 2009a) (Fig. 3D). In FF cells, Ca2+ transient amplitudes were markedly reduced during caffeine, confirming that the Ca2+ transient was predominantly derived from SR (Fig. 3E). In contrast, SR Ca2+ release was minute in 4-week KO (11% FF values), and was not detectable in 7-week KO. Similarly, calculated rate constants for SR Ca2+ re-uptake were markedly reduced in 4-week KO, and were not significantly different from zero in 7-week KO (Fig. 3F). Using the measurements of SR-dependent and caffeine-elicited Ca2+ transients, we estimated that fractional release was reduced to 21 ± 3% in 4-week KO compared with 51 ± 10% in 4-week FF controls (P < 0.05). Very low SERCA2a expression levels were observed in 4-week KO; however, a small but significant further down-regulation was observed in 7-week KO (Fig. 3C). SERCA2b expression was also markedly reduced in KO, but similar in 4-week and 7-week cells (10 ± 1% and 10 ± 1% FF values, respectively, P= NS). Although Western blot sensitivity is below optimal at low protein expression levels, a reduction in SERCA2a levels between 4- and 7-week KO could account for declining SR function. Indeed, we observed that thapsigargin-sensitive Ca2+-ATPase content in whole-heart homogenates was reduced from 15.8 ± 3.0% to 7.9 ± 1.6% of FF values between 4-week and 7-week KO (P < 0.05). Also, the small degree of SR function present in 4-week KO cells was blocked by 1 μm thapsigargin, as caffeine-elicited transients were reduced to 4 ± 4 nm (vs. 167 ± 42 nm in untreated, P < 0.05) and SR Ca2+ release did not occur (data not shown). Seven-week KO transient magnitudes were unaffected by thapsigargin application (23 ± 4 nmvs. 26 ± 4 nm in untreated, P= NS). Thus, very low SERCA levels appear capable of partially refilling the SR in 4-week KO myocytes, and this residual SERCA activity is lost by the 7-week time point.
Figure 3. Minute SR function was present in 4-week, but not 7-week KO.
Representative caffeine-elicited Ca2+ transients (A) and magnitude measurements (B). Expression levels of SERCA2a (arrow) vs. calsequestrin (lower bands) (C). SR Ca2+ release during each beat was assessed by comparing Ca2+ transients in the presence and absence of caffeine (D). SR Ca2+ release (E) and reuptake (F). (n for panels B and C, E and F: 4-week FF = 13, 5, 9; 4-week KO = 20, 6, 19; 7-week FF = 20, 6, 18; 7-week KO = 19, 7, 19; *P < 0.05 vs. equivalent FF, ‡P < 0.05 vs. equivalent 4-week).
Enhanced Ca2+ influx
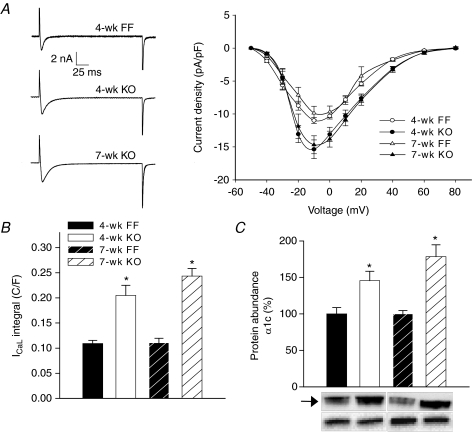
Representative recordings of L-type Ca2+ currents during voltage-clamp steps are shown in Fig. 4A. Mean measurements of peak current in 4-week and 7-week KO were 140% and 149% of FF values, respectively (P < 0.05 vs. FF, P= NS within KO). No marked shift in the current–voltage relationship was observed. Slower Ca2+ current decay kinetics in KO (half-decay time: 4-week FF = 4.7 ± 0.2 ms, 4-week KO = 8.5 ± 0.8 ms P < 0.05, 7-week FF = 5.6 ± 0.5 ms, 7-week KO = 8.6 ± 0.9 ms P < 0.05) resulted in very large integrated Ca2+ currents, especially in 7-week KO (4-week KO = 188% FF, 7-week KO = 220% FF, P= NS within KO, Fig. 4B). Parallel alterations were observed in L-type Ca2+ channel expression. Levels of the pore-forming subunit, α1C, were 146% of FF values in 4-week KO (Fig. 4C), and there was a strong tendency for further up-regulation in 7-week KO (178% FF). Levels of the α2/δ1 regulatory subunit were also increased to 133 ± 10% and 147 ± 10% of FF levels in 4-week and 7-week KO, respectively (data not shown; P= NS within KO).
Figure 4. Increased L-type Ca2+ current in KO myocytes.
A, representative L-type Ca2+ currents recorded during a voltage step from −50 mV to −10 mV (left panel). L-type current–voltage relationship (right panel). B, integrated current measurements during the step to –10 mV. C, protein levels of the Ca2+ channel α1C subunit (arrow) vs. calsequestrin (lower bands). (n for panels A and B, and C: 4-week FF = 14, 5; 4-week KO = 12, 6; 7-week FF = 8, 6; 7-week KO = 13, 7; *P < 0.05 vs. equivalent FF).
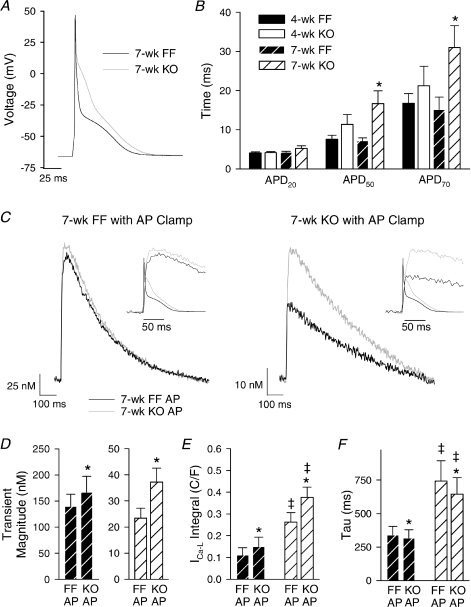
We examined whether alterations in AP configuration might additionally modify L-type Ca2+ influx and the Ca2+ transient. Representative recordings (Fig. 5A) and mean data (Fig. 5B) show that APs were prolonged at 50% and 70% repolarization in 7-week KO. Resting membrane potential and transient outward K+ current measurements were similar in all groups (data not shown), suggesting that AP prolongation in KO resulted primarily from augmented Ca2+ current. Also, AP prolongation was not observed in 7-week KO during treatment with 20 μm nifedipine (data not shown). The representative APs shown in Fig. 5A were employed as voltage-clamp waveforms in 7-week FF and 7-week KO cells. Figure 5C and D show that steady-state stimulation with the KO AP resulted in a larger Ca2+ transient than stimulation with the FF AP. This difference in amplitude was small in absolute terms (27 nm in 7-week FF cells, 14 nm in 7-week KO cells, P= NS), but proportionally very large in KO (% increase, 67%vs. 19% in FF, P < 0.05). In 7-week KO myocytes, where the entire transient results from Ca2+ influx, augmentation of transients during the KO AP predominantly resulted from a 43% increase in integrated L-type Ca2+ current (Fig. 5E). Thus, overall L-type Ca2+ entry in 7-week KO was dramatically increased from 7-week FF values by approximately 3- to 3.5-fold, when AP alterations were considered (Fig. 5E). This indicates that AP alterations can be an important compensatory mechanism for augmenting Ca2+ influx when SR function is inhibited.
Figure 5. AP prolongation facilitated Ca2+ entry in 7-week KO.
A, representative AP recordings at 1 Hz. B, times to 20%, 50% and 70% repolarization (APD20, APD50 and APD70) (n= 16, 17, 11, 16; *P < 0.05 vs. corresponding FF). C, representative Ca2+ transients recorded during voltage-clamp stimulation with AP waveforms from A. Insets illustrate an expanded time scale with APs superimposed. Transient magnitude (D), integrated L-type Ca2+ current (E), and decay kinetics of the Ca2+ transient (F). (n for panels D, E and F: 7-week FF = 9, 8, 9; 7-week KO = 12, 4, 12; *P < 0.05 vs. equivalent FF AP, ‡P < 0.05 vs. equivalent 7-week FF).
NCX-mediated Ca2+ entry was also examined in 7-week FF and KO, by treating myocytes with 5 μm KB-R7943 (Tocris/Bio Nuclear AB, Bromma, Sweden) to preferentially block reverse-mode function (Iwamoto et al. 1996; Satoh et al. 2000). KB-R7943 treatment did not alter Ca2+ transient magnitude in 7-week FF cells (134 ± 23 nmvs. 140 ± 23 nm in untreated, P= NS), but markedly reduced transient size in 7-week KO (14 ± 2 nmvs. 23 ± 4 nm in untreated, P < 0.05). This suggests that the Ca2+ transient in 7-week KO is supported by enhanced NCX-mediated Ca2+ entry, in addition to increased L-type Ca2+ current (described above). Reverse-mode NCX function might be expected to be further facilitated by AP prolongation. However, switching between the FF and KO AP stimulus during KB-R7943 treatment augmented Ca2+ transients (increase of 17 ± 8% in 7-week FF, 67 ± 10% in 7-week KO, P < 0.05) to a similar extent as was observed in untreated cells (Fig. 5D). Thus, the prolonged AP in 7-week KO predominantly amplifies Ca2+ transients by increasing L-type current.
Ca2+ extrusion: compensation and de-compensation
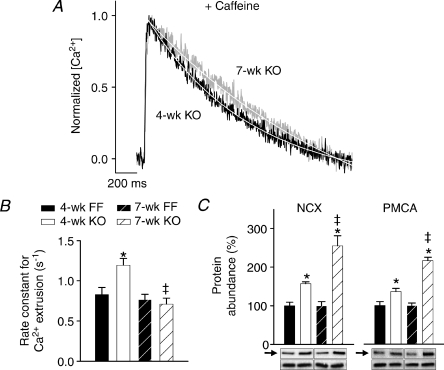
We investigated whether alterations in Ca2+ extrusion contributed to slowing of Ca2+ transient decline during development of diastolic dysfunction between 4- and 7-week KO. Decay kinetics were examined for transients recorded in the presence of caffeine (as in Fig. 3D), when Ca2+ removal is dependent solely on trans-sarcolemmal flux. Representative Ca2+ transients (Fig. 6A) and mean data (Fig. 6B) show that while Ca2+ extrusion was more rapid than FF in 4-week KO, extrusion was slowed to control values in 7-week KO. By comparing rate constants of Ca2+ reuptake and extrusion, we can calculate that 60% of the overall slowing of Ca2+ transient decline between 4-week and 7-week KO resulted from loss of SR re-uptake, while the remaining 40% resulted from slowing of Ca2+ extrusion. Importantly, slowing of extrusion in KO occurred despite a progressive up-regulation in NCX and PMCA protein levels between 4 and 7 weeks (Fig. 6C).
Figure 6. Ca2+ extrusion was enhanced in 4-week, but not 7-week KO.
A, steady-state Ca2+ transients recorded in the presence of 10 mm caffeine. B, decay rate measurements. C, protein levels of NCX and PMCA (arrows) vs. calsequestrin (lower bands). (n for panels B and C: 4-week FF = 16, 5; 4-week KO = 18, 6; 7-week FF = 20, 6; 7-week KO = 18, 7; *P < 0.05 vs. equivalent FF, ‡P < 0.05 vs. equivalent 4-week).
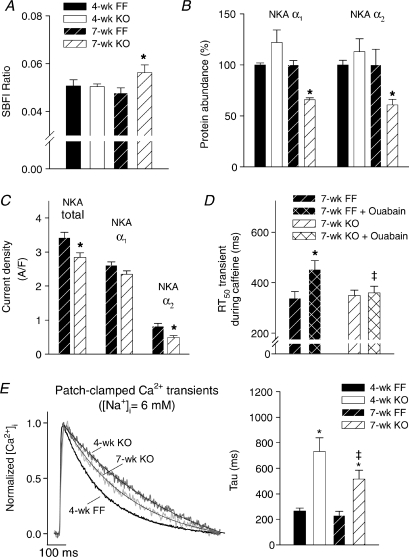
In AP clamp experiments, we observed that the 7-week KO AP did not alter Ca2+ transient decline (Fig. 5F), since even the prolonged AP was repolarized shortly after the transient peak (insets in Fig. 5C). We hypothesized that slowing of Ca2+ extrusion instead resulted from inhibition of forward-mode NCX function by elevation of [Na+]i. Indeed, SBFI ratios were increased in 7-week myocytes, indicating cytosolic Na+ accumulation (Fig. 7A). Interestingly, this was associated with reduced expression levels of NKA α1 and α2 isoforms (Fig. 7B). Total NKA current was also reduced in 7-week KO, which included a 40% reduction in α2 current and a tendency for decreased α1 current (Fig. 7C). We have previously shown that reduced NKA activity increases [Na+]i (Swift et al. 2007). As proof of principle that such changes can also impair Ca2+ extrusion, we examined Ca2+ removal during NKA blockade by ouabain. In 7-week FF cells, ouabain significantly slowed the half-decay time of Ca2+ transients recorded during caffeine (Fig. 7D). Ouabain treatment did not, however, slow Ca2+ extrusion in 7-week KO, suggesting that baseline elevations in [Na+]i may have maximally inhibited NCX function. Interestingly, Ca2+ extrusion during ouabain was more rapid in 7-week KO than FF, which probably resulted from the marked up-regulation of PMCA protein expression in KO.
Figure 7. Elevated [Na+]i impaired Ca2+ extrusion in 7-week KO.
A, SBFI F410/F590 ratios were increased in 7-week KO indicating cytosolic Na+ accumulation (n= 9, 9, 13, 10 (order as in key here and in E)). B, expression of NKA α1 and α2 isoforms (n= 6 all groups). C, NKA currents obtained by rapid elevation of extracellular [K+]i were subdivided into α1 and α2 components based on sensitivity to 5 μm ouabain (n for 7-week FF = 10, 7-week KO = 6). D, Ca2+ extrusion during NKA blockade by 1 mm ouabain, as estimated by half-decay time of Ca2+ transients during caffeine (n for 7-week FF = 10, 7-week KO = 10). E, Ca2+ transients following dialysis with 6 mm[Na+]i (n= 19, 11, 20, 19). (For panels A–C, *P < 0.05 vs. equivalent FF; panel D, *P < 0.05 vs. untreated; ‡P < 0.05 vs. equivalent 7-week FF; panel E, *P < 0.05 vs. equivalent FF, ‡P < 0.05 vs. equivalent 4-week).
To further investigate the importance of elevated [Na+]i for slowing of Ca2+ extrusion, we examined Ca2+ transients in cells dialysed with patch pipettes containing a low [Na+] (6 mm). Figure 7E shows that under such conditions the decay of Ca2+ transients was significantly enhanced in 7-week KO (compare Fig. 2F), and became even more rapid than in 4-week KO. These data support a detrimental role of Na+ accumulation in promoting diastolic dysfunction in 7-week KO.
Discussion
In this study, we observed that mice with cardiomyocyte-specific ablation of SERCA2 developed end-stage HF by 7 weeks following knockout. Declining systolic and diastolic function between 4-week and 7-week KO was associated with reduction in cardiomyocyte contraction magnitude and slowing of relaxation, caused by parallel changes in Ca2+ transients. Progressive reduction in SR Ca2+ release was partially offset by AP prolongation, which increased L-type Ca2+ current, and by greater Ca2+ entry via reverse-mode NCX function. Diastolic dysfunction in end-stage HF resulted from a combination of declining SERCA activity and accumulation of intracellular Na+ which impaired NCX-mediated Ca2+ extrusion. Increased [Na+]i in 7-week KO was caused, at least in part, by down-regulation of NKA.
Elevation of [Na+]i has been previously reported in both human HF (Pieske et al. 2002) and animal models (Despa et al. 2002; Baartscheer et al. 2003). The mechanisms proposed to underlie this Na+ accumulation include increased late Na+ current (Sossalla et al. 2008) and increased Na+–H+ exchange (Baartscheer et al. 2003). As well, in agreement with our observations, several previous studies have reported reduced NKA expression in failing myocytes (Schwinger et al. 1999; Muller-Ehmsen et al. 2001; Despa et al. 2002; Swift et al. 2008). Two of these studies have additionally reported decreased NKA activity (Schwinger et al. 1999; Swift et al. 2008). We observed reduced expression of both the α1 and α2 NKA isoforms. Based on our previous work (Swift et al. 2007), we believe that the α1 isoform plays a more important role in controlling global [Na+] than the α2 isoform. With a reduction in α1 expression, it might be hypothesized that remaining pumps could simply increase their activity to maintain normal [Na+]i. However, our preliminary data indicate that this is not the case, as we have observed that even partial blockade of the α1 isoform results in increased [Na+]i. With reduced NKA pumping capacity, we postulate that [Na+]i rises to a new steady-state level in 7-week KO, which stimulates remaining pumps, resulting in a normal rate of Na+ extrusion to balance Na+ influx. However, we actually measured reduced NKA current. This discrepancy is probably due to the fact that NKA current was measured in patch-clamped, dialysed cells which allowed comparison of pump current in KO and FF with [Na+]i normalized. Interestingly, we observed that the α2 component of the total NKA current was significantly decreased in 7-week KO. In our recent work, we have shown that the α2 isoform is preferentially localized to the T-tubules, where it regulates local [Na+] near NCX (Swift et al. 2007). Therefore, it follows that down-regulation of α2 in 7-week KO could impair NCX-mediated Ca2+ extrusion. Indeed, we observed that NKA blockade by ouabain treatment slowed Ca2+ extrusion in FF. Our finding that ouabain did not alter Ca2+ extrusion in KO suggests that Na+ accumulation in the dyad may have maximally impaired forward-mode NCX in these cells.
PMCA function is believed to normally play a minor role in Ca2+ removal. However, data from the NCX knockout mouse indicate that the PMCA can have a surprisingly large capacity to extrude Ca2+ when challenged (Henderson et al. 2004). We presently observed a striking up-regulation of PMCA expression by more than 200% in 7-week KO, which would be expected to increase the rate of Ca2+ extrusion. As well, the Ca2+ affinity of PMCA might theoretically also be increased in KO myocytes, due to the action of regulatory molecules such as calmodulin (Brini, 2009). Thus, with NCX-mediated Ca2+ extrusion inhibited in these cells, PMCA may become an important mechanism for Ca2+ removal. In support of this view, we observed that in the presence of ouabain and high [Na+]i (Swift et al. 2008), Ca2+ extrusion was significantly faster in 7-week KO than FF. Also, normalization of [Na+]i by dialysis increased the rate of decay of 7-week KO Ca2+ transients beyond 4-week KO values. Therefore, with NCX function uninhibited, Ca2+ extrusion via NCX and PMCA may be able to maintain diastolic function at near-normal levels in the complete absence of SERCA function.
The mechanisms that compensate for declining SR Ca2+ content and release are intriguing. We previously reported up-regulation of the L-type Ca2+ channel and increased Ca2+ current magnitude in 4-week KO myocytes (Andersson et al. 2009a). We presently confirmed these findings, and observed that increases in Ca2+ channel expression and current magnitude persist in 7-week KO. In fact, integrated currents tended to be even larger in 7-week than 4-week KO. Our AP clamp experiments showed that the longer AP in 7-week KO additionally facilitated L-type Ca2+ current, resulting in Ca2+ currents that were 3- to 3.5-fold larger than those recorded in FF cells during FF APs. Additional Ca2+ current during the prolonged AP substantially increased Ca2+ transient magnitude in 7-week KO. However, these effects were more modest in FF cells, which have functional SR, since AP prolongation is known to reduce the efficiency of Ca2+-induced Ca2+ release (Sah et al. 2003). Ca2+ currents may have been additionally enhanced in 7-week KO by Ca2+–calmodulin-dependent kinase II phosphorylation, as has been reported in other heart failure models (Wang et al. 2008).
AP prolongation might also be expected to enhance Ca2+ entry via reverse-mode NCX. However, we observed that the 7-week KO AP induced a similar increase in Ca2+ transient magnitude in the presence and absence of KB-R7943. It appears then that the benefits of AP prolongation in 7-week KO mice stem primarily from greater L-type Ca2+ current. Compared to the untreated condition, however, Ca2+ transient magnitude was decreased by KB-R7943 in 7-week KO, but unaltered in 7-week FF. This suggests that NCX-mediated Ca2+ entry is enhanced in KO on a beat-to-beat basis, as predicted when Na+ levels are increased and Ca2+ transients are reduced. At the tested dose of 5 μm, KB-R7943 is reported to block 90% of reverse-mode NCX function without reducing forward-mode activity (Satoh et al. 2000). Although some have suggested that KBR is not mode-selective, recent evidence indicates that bi-modal NCX blockade increases Ca2+ transient magnitude (Ozdemir et al. 2008). We observed that KBR reduced transient magnitude and did not alter decay kinetics (data not shown), indicating preferential reverse-mode blockade.
Despite markedly enhanced Ca2+ influx, declining SR function resulted in a progressive reduction in Ca2+ transient amplitude in KO cells, which was observed at both low and high stimulation frequency (1 Hz and 6 Hz). Four-week KO myocytes also exhibited reduced contraction magnitudes at 1 Hz, but contractile function was enhanced at high pacing rates as 6 Hz contractions were similar in magnitude to controls. This is in agreement with our previous observation that myofilament responsiveness to Ca2+ is increased in 4-week KO (Andersson et al. 2009a), which may become particularly pronounced at high stimulation rates when diastolic [Ca2+]i is elevated. In addition, the slowed Ca2+ transient kinetics in KO may also improve contractile function by prolonging myofilament activation. Such alterations may continue to aid contractile function in 7-week KO, as contraction magnitude was increased between 1 Hz and 6 Hz despite the presence of very small Ca2+ transients at both frequencies. Nevertheless, it appears that such adaptations are insufficient to maintain systolic function at normal levels at this late time point when SR function fails completely.
Although there are clearly important differences between end-stage heart failure in humans and SERCA2 KO mice, some cellular mechanisms may be conceptually similar. Data from human HF appear to support our observation that slowing of Ca2+ extrusion is an important mechanism underlying diastolic dysfunction. Hasenfuss et al. (1999) observed that HF patients with impaired diastolic performance exhibited decreased SERCA expression but unchanged NCX. In contrast, NCX levels were increased in patients with preserved diastolic function. The authors suggested that greater NCX activity may compensate for reduced SERCA function to maintain near-normal Ca2+ decay and relaxation rates. Our results also support the notion that reduction in SERCA levels can promote systolic dysfunction. In human HF, SERCA expression is often reported to be reduced by 30–40% (for review, see Hasenfuss & Pieske, 2002), and this reduction is generally believed sufficient to impair systolic performance. Mice are clearly very adept at compensating for loss of SERCA, since we observed near-normal systolic function in 4-week KO despite a more than 95% reduction in SERCA2a protein. We have previously shown that activation of the sympathetic nervous system contributes to this compensation (Andersson et al. 2009a). In the present study, we observed that loss of a small remaining pool of SERCA2a by 7-week KO was associated with development of systolic HF. Since the half-life for SERCA protein is approximately 2–3 days (Andersson et al. 2009a), it is surprising that a functional amount of SERCA would be present beyond the first few weeks following knockout. However, it may be that there is normally an excessive amount of SERCA protein, much of which is either not required or not accessible for normal function. Alternatively, there may be two different pools of SERCA protein: one pool which has a short half-life, and a second perhaps smaller pool which has a longer half-life. Theoretically, declining SERCA activity between 4- and 7-week KO could result from gradual loss of this second pool of SERCA protein.
Compensatory mechanisms present in KO myocytes may not be present in human HF. For example, Ca2+ current is generally reported to be unaltered in failing human myocytes (Bers, 2006). As well, more profound AP prolongation in human HF is believed to reduce, not augment, Ca2+ transient magnitude (Sah et al. 2003). Therefore, relatively moderate reductions in SERCA expression such as those reported in HF patients may be sufficient to impair systolic function if Ca2+ influx is not increased. Our findings suggest then that strategies aimed at enhancing Ca2+ influx may be beneficial in treating systolic dysfunction. Diastolic function, on the other hand, would probably benefit from augmentation of NKA activity and Na+ unloading. Such therapies may, however, have the opposite effect on systolic function, since elevated [Na+]i is known to increase Ca2+ transients by elevating SR Ca2+ load. Indeed, clinical trials have indicated that digoxin, a NKA inhibitor, is appropriate for treatment of systolic but not diastolic dysfunction in HF (Shammas et al. 2007). A therapeutic compromise may be attainable through agents such as istaroxime, a newly developed compound which simultaneously inhibits NKA and stimulates SERCA. Early clinical data suggest that istaroxime improves both contractility and diastolic relaxation in HF patients (Khan et al. 2009).
In conclusion, our data from the SERCA KO mouse indicate that declining SERCA function in end-stage HF contributes to the simultaneous development of both systolic and diastolic dysfunction. However, impaired relaxation also results from accumulation of intracellular Na+ following down-regulation of NKA. Our data indicate that Ca2+ extrusion mechanisms can dramatically compensate for SERCA loss to maintain diastolic function when Na+ gain is prevented.
Acknowledgments
We thank Annlaug Ødegård, Ulla Enger, Heidi Kvaløy, and Tove Norén for excellent technical assistance. This work was supported by The Research Council of Norway (W.E.L, H.K.M.); The South-Eastern Norway Regional Health Authority (F.S.); Anders Jahre's Fund for the Promotion of Science; Oslo University Hospital Ullevaal (I.S., K.B.A., G.C., O.M.S.); and the University of Oslo (G.C., O.M.S.).
Glossary
Abbreviations
- AP
action potential
- [Ca2+]i
intracellular [Ca2+]
- FF
Serca2flox/flox mice
- HF
heart failure
- KO
Serca2flox/flox Tg(αMHC-MerCreMer) mouse
- LAD
left atrial diameter
- LV
left ventricular
- [Na+]i
intracellular [Na+]
- NCX
Na+–Ca2+ exchanger
- NKA
Na+-K+-ATPase
- PMCA
plasmalemmal Ca2+-ATPase
- SERCA
SR Ca2+-ATPase
- SR
sarcoplasmic reticulum
Author contributions
Conception and design of this study was conducted by W.E.L, I.S., K.B.A., G.C. and O.M.S. Experiments examining cardiomyocyte size, contractile function, Ca2+ and Na+ homeostasis, and action potential configuration, were performed by W.E.L., H.K.M., F.S. and J.M.A. Cardiomyocyte isolation was conducted by K.H. Echocardiographic analysis was performed by K.H. and I.S. Morphological and histological examinations were conducted by K.B.A., H.M.R. and B.R. Kaplan Meyer curves and LV Ca2+-ATPase activity were determined by K.B.A. All authors contributed to writing of the manuscript.
References
- Andersson KB, Birkeland JA, Finsen AV, Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien KR, Sejersted OM, Christensen G. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol. 2009a;47:180–187. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Andersson KB, Finsen AV, Sjåland C, Winer LH, Sjaastad I, Ødegaard A, Louch WE, Wang Y, Chen J, Chien KR, Sejersted OM, Christensen G. Mice carrying a conditional Serca2flox allele for the generation of Ca2+ handling-deficient mouse models. Cell Calcium. 2009b;46:219–225. doi: 10.1016/j.ceca.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KB, Winer LH, Mork HK, Molkentin JD, Jaisser F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res. 2009c doi: 10.1007/s11248-009-9342-4. DOI 10.1007/s11248-009-9342-4. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Fiolet JW. Small changes of cytosolic sodium in rat ventricular myocytes measured with SBFI in emission ratio mode. J Mol Cell Cardiol. 1997;29:3375–3383. doi: 10.1006/jmcc.1997.0567. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, van Borren MM, Belterman CN, Coronel R, Fiolet JW. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res. 2003;57:1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase α1 and α2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res. 2007;73:92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- Brini M. Plasma membrane Ca2+-ATPase: from a housekeeping function to a versatile signalling role. Pflugers Arch. 2009;457:657–664. doi: 10.1007/s00424-008-0505-6. [DOI] [PubMed] [Google Scholar]
- Campbell AM, Wuytack F, Fambrough DM. Differential distribution of the alternative forms of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, SERCA2b and SERCA2a, in the avian brain. Brain Res. 1993;605:67–76. doi: 10.1016/0006-8993(93)91357-x. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts ME, Andersen JP, Clausen T, Hansen O. Quantitative determination of Ca2+-dependent Mg2+-ATPase from sarcoplasmic reticulum in muscle biopsies. Biochem J. 1989;260:443–448. doi: 10.1042/bj2600443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsen AV, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol. 2005;98:680–689. doi: 10.1152/japplphysiol.00924.2004. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99(5):641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res. 2004;95:604–611. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Khan H, Metra M, Blair JE, Vogel M, Harinstein ME, Filippatos GS, Sabbah HN, Porchet H, Valentini G, Gheorghiade M. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na-K-ATPase inhibition: a new promising treatment for acute heart failure syndromes? Heart Fail Rev. 2009;14:277–287. doi: 10.1007/s10741-009-9136-z. [DOI] [PubMed] [Google Scholar]
- Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mork HK, Sjaastad I, Sejersted OM, Louch WE. Slowing of cardiomyocyte Ca2+ release and contraction during heart failure progression in post-infarction mice. Am J Physiol Heart Circ Physiol. 2009;296:H1069–H1079. doi: 10.1152/ajpheart.01009.2008. [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen J, Wang J, Schwinger RH, McDonough AA. Region specific regulation of sodium pump isoform and Na,Ca-exchanger expression in the failing human heart – right atrium vs left ventricle. Cell Mol Biol (Noisy -le-grand) 2001;47:373–381. [PubMed] [Google Scholar]
- Ozdemir S, Bito V, Holemans P, Vinet L, Mercadier JJ, Varro A, Sipido KR. Pharmacological inhibition of Na/Ca exchange results in increased cellular Ca2+ load attributable to the predominance of forward mode block. Circ Res. 2008;102:1398–1405. doi: 10.1161/CIRCRESAHA.108.173922. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Piacentino V, III, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation–contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito. J Physiol. 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Ginsburg KS, Qing K, Terada H, Hayashi H, Bers DM. KB-R7943 block of Ca2+ influx via Na+/Ca2+ exchange does not alter twitches or glycoside inotropy but prevents Ca2+ overload in rat ventricular myocytes. Circulation. 2000;101:1441–1446. doi: 10.1161/01.cir.101.12.1441. [DOI] [PubMed] [Google Scholar]
- Schwinger RH, Wang J, Frank K, Muller-Ehmsen J, Brixius K, McDonough AA, Erdmann E. Reduced sodium pump α1, α3, and β1-isoform protein levels and Na+,K+-ATPase activity but unchanged Na+-Ca2+ exchanger protein levels in human heart failure. Circulation. 1999;99:2105–2112. doi: 10.1161/01.cir.99.16.2105. [DOI] [PubMed] [Google Scholar]
- Shammas RL, Khan NU, Nekkanti R, Movahed A. Diastolic heart failure and left ventricular diastolic dysfunction: what we know, and what we don't know! Int J Cardiol. 2007;115:284–292. doi: 10.1016/j.ijcard.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts – role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Swift F, Birkeland JA, Tovsrud N, Enger UH, Aronsen JM, Louch WE, Sjaastad I, Sejersted OM. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase α2-isoform in heart failure. Cardiovasc Res. 2008;78:71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- Swift F, Tovsrud N, Enger UH, Sjaastad I, Sejersted OM. The Na+/K+-ATPase α2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovasc Res. 2007;75:109–117. doi: 10.1016/j.cardiores.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Sjaastad I, Andersen K, Helm PJ, Wasserstrom JA, Sejersted OM, Ottersen OP. Localization and function of the Na+/Ca2+-exchanger in normal and detubulated rat cardiomyocytes. J Mol Cell Cardiol. 2003;35:1325–1337. doi: 10.1016/j.yjmcc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tandan S, Cheng J, Yang C, Nguyen L, Sugianto J, Johnstone JL, Sun Y, Hill JA. Ca2+/calmodulin-dependent protein kinase II-dependent remodelling of Ca2+ current in pressure overload heart failure. J Biol Chem. 2008;283:25524–25532. doi: 10.1074/jbc.M803043200. [DOI] [PMC free article] [PubMed] [Google Scholar]