Abstract
Aim
Exercise is associated with an increased risk of hypoglycemic or hyperglycemic events. The aim of this study was to assess glucose changes during and after physical exercise in patients with type 1 diabetes managed by continuous subcutaneous insulin infusion before and after a 14-day moderate or intense exercise program.
Methods
Sixteen male patients [hemoglobin A1c 7.3 ± 0.8% (mean ± standard deviation), age 39 ± 11 years, body mass index 26.0 ± 2.7 kg/m2] were enrolled in this single-center, randomized, open-label study. They underwent exercise challenges before and after a 14-day moderate (group A, n = 8) and intense (group B) exercise program. Changes in glucose levels were monitored continuously by means of a microdialysis technique.
Results
Patients in group A trained less intensively than the patients in group B. The treadmill exercise led to a comparable level of challenge in both patient groups. Neither heart rate nor energy consumption differed within the groups or between the groups. Patients in both groups had a comparable basal insulin infusion rate. Prandial insulin doses were higher pretraining than posttraining in both groups. Identical amounts of additional carbohydrates were consumed by the patients in both groups during the 21 h after the exercise challenge. Glucose profiles recorded showed a wide variability. No differences in the glucose profiles with respect to the training intensity could be observed within and between the groups. Patients in group A tended to spend a shorter period of time in hypoglycemia after the exercise challenge posttraining compared to pretraining, but not the patients in group B. The number of hypoglycemic episodes was not different between the groups.
Conclusions
The patients with type 1 diabetes exhibit the expected wide variability in glucose profiles before, during, and after physical exercise. Use of continuous glucose monitoring allows handling of this situation without running into the risk of acute metabolic deteriorations.
Keywords: carbohydrate intake, continuous glucose monitoring, continuous subcutaneous insulin infusion, exercise, fitness index, hypoglycemia
Introduction
Hypoglycemia can occur during, immediately after, or even many hours after exercise, as it is difficult to determine the optimal balance between meals, insulin dose, and level and duration of exercise to prevent acute blood glucose (BG) deteriorations. Usually, patients with diabetes measure capillary BG levels [self-monitoring of blood glucose (SMBG)] frequently to obtain information about glucose changes after exercise to achieve such a balance. However, the information provided by such spot measurements is limited, even if as many as 12 measurements are done per day. Consequently, many patients with type 1 diabetes have negative feelings toward physical activity and abstain from sport.1
Since the late 1990s, it has been possible to monitor glucose changes continuously [continuous glucose monitoring (CGM)] by means of different systems. Interestingly, the number of clinical-experimental studies and clinical studies with CGM is small when it comes to exercise.2–6 The aim of this (observational) clinical study was to evaluate the impact of exercise on glucose profiles measured continuously for 21 hours following physical activity in patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) without any interventions with respect to insulin infusion rates. This was evaluated twice, once before and once after a 14-day training program. Half of the patients participated in a moderate training program and the other half in an intense one.
Materials and Methods
Patients
Sixteen male patients with type 1 diabetes [hemoglobin A1c (HbA1c) 7.3 ± 0.8% (6.1−8.8%), age 38 ± 11 years (25−57), body mass index (BMI) 26.0 ± 2.7 kg/m2 (20.5–30.7), fitness index (discussed later) 91.4 ± 7.8 (83−107), mean ± standard deviation (SD) (range)] currently managed by CSII for at least 6 months were enrolled in this study. They performed regular physical activities 2–3 h weekly during the previous 6 months and had no profound diabetes-related late complications or any other severe problems that prevented them from performing intense exercise. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and of good clinical practice. The protocol was approved by the local ethical committee, and all patients provided written informed consent.
Study Design and Procedures
This study was a single-center, randomized, open-label “noninterventional” study in which the patients were randomized to one of two groups (A or B), each consisting of eight patients. The patients underwent exercise challenges before and after a 14-day moderate (group A) or intense (group B) training period. Each patient participated in four study visits. Visit 1 was a screening visit. On visit 2 (day -1), patients came to the institute in the morning before day 0 on an outpatient basis to get microdialysis catheters inserted for CGM, and the CGM system was started. They returned to the investigational site the next day (day 0), after an overnight fast of at least 8 h, for 50 h (first in-house period with two overnight stays) followed by a 14-day outpatient period. Changes in glucose profiles were monitored continuously during the whole in-house period. The measurement results were not displayed to the patients. During visit 3 (day 17), microdialysis catheters were inserted again. The second in-house period with an identical experimental procedure started with day 18. The final examination was performed at visit 4 (day 20). Patients were encouraged to maintain their regular exercise; however, they were also instructed not to undertake vigorous or prolonged exercise within the 24 h prior to days 0 and 18.
During the two in-house periods, patients received standardized meals: breakfast at 09:00 h, lunch at 13:00 h, dinner at 18:00 h. A 13-point BG profile was performed by collecting capillary blood samples at the fingertips at 08:00 h fasting (immediately before breakfast), 1 and 2 h postbreakfast, 13:00 h (immediately before lunch), 1 and 2 h postlunch, 18:00 h (immediately before dinner), 1 and 2 h postdinner, 23:00 h, 01:00 h, 03:00 h, and 06:00 h on every in-house study day. Blood glucose levels were measured with a glucose meter built into the data manager of the subcutaneous glucose monitoring system (SCGM) system (discussed later).
For insulin therapy, patients were instructed to keep the basal insulin infusion rate at their usual dose. Doses of prandial insulin boluses were determined by the patients according to their usual doses taken for meals according to carbohydrate (CHO) content of the meals and self-measured preprandial BG values. Snacks were allowed only in case of hypoglycemic events, i.e., if measured BG values were <60 mg/dl or symptoms occurred. The patients were not informed about low BG values by the SCGM 1 system, but if capillary BG measurements showed such low values, the patients were informed about this for ethical reasons.
At about 11:00 h on the second day of the in-house periods (days 1 and 19), patients began to perform exercise on a treadmill for 45 min. The exercise challenge should be sufficient to induce a sustained heart rate between 65% and 85% of the individual maximum heart rate (calculated as 220 minus age) and measured by a heart rate monitor. Three additional BG measurements were performed: immediately before, halfway through, and immediately after exercise. If symptoms of hypoglycemia occurred or if BG values <60 mg/dl were measured, patients ate/drank one CHO unit. This procedure was repeated until hypoglycemic symptoms disappeared or BG remained stable >60 mg/dl. Four hours after the exercise, an impedance measurement was performed with a body fat analyzer (Model BF 906, Maltron International, Rayleigh, Essex, UK) to determine body composition (lean body mass, fat mass, and body water). The patients were instructed to stay with their typical insulin therapy, i.e., no standardization of basal insulin therapy and such was encouraged, as it was the aim of this study to observe the clinical reality in these patients.
The microdialysis catheters were removed on the morning of days 2 and 20, 1 h after breakfast. The patients were released from the site, provided there were no safety concerns as judged by the investigator.
During the training period between the two in-house periods, the patients performed either moderate exercise (for 30 min every second day) in group A or intense exercise (>60 min every day) in group B. During training, heart rate (monitored by a heart rate monitor, discussed later) should be within the individual limits as defined earlier. The time spent within the target heart rate zone was recorded as well as mean heart rate and total energy consumption. Training could be walking, jogging, or biking, either outdoors or on a cardio-machine (treadmill, ergometer, stepper, or cross-trainer), if patients had such a machine at home or were clients of a gym. During the 14 days that training was performed, the timing of the training could be selected by the patients according to their daily routine. No specific instructions for diabetes treatment were given.
Blood glucose measurements were performed every day at the following time points: fasting, 2 h postbreakfast, prelunch, 2 h postlunch, predinner, 2 h postdinner, and 23:00 and 03:00 h. Additional BG measurements were to be performed before, halfway through, and after exercise. All patients used the same BG meter (Accu-Chek® Compact Plus, Roche Diagnostics, Mannheim, Germany) for SMBG throughout the training period. The data stored in the meter were downloaded after the study. Patients were instructed to enter in a diary the level and duration of their daily physical activity, self-measured BG values, meals (CHO units), insulin treatment (basal rate and bolus dose), as well as hypoglycemic events (severe hypoglycemia was defined as an episode requiring assistance from a third party) and other adverse events (AEs). The diary entries were discussed with the investigator by phone contacts at least twice weekly in order to optimize the diabetes treatment. Patients were instructed to contact the investigational site on their part in case of AEs, especially hypoglycemia, or if problems with their diabetes treatment occurred.
By means of a self-walk test the patients' fitness level before and after the training period was determined.7,8 Patients received a Polar F4™ fitness heart rate monitor (Polar Electro Oy, Kempele, Finland), which could be programmed with individual data (http://www.polarusa.com/service_repair). If the fitness level of a patient was below or above “average” or “good,” this patient was considered not eligible to participate in the study (this was the case for three screened patients).
Subcutaneous Glucose Monitoring System 1 for Continuous Glucose Monitoring
Glucose profiles of the patients were monitored by a continuous monitoring system, the so-called SCGM 1 system (Roche Diagnostics).2,3 The microdialysis technique employed in this system for glucose monitoring offers the possibility to extract glucose from the interstitial fluid, e.g., from the subcutaneous adipose tissue. The SCGM system was composed of (a) the data manager and (b) the sensor unit. The data manager contained a BG meter (using Accu-Chek Active test strips), which was used for calibration and for control measurements during in-house periods. It further included a built-in comprehensive data management and display system. The sensor unit contained a consumable part (cartridge), which was composed of a CMA-60 microdialysis catheter (CMA Microdialysis AB, Stockholm, Sweden), tube connections, required consumable solutions (glucose oxidase solution for analysis and Ringer's solution as perfusion fluid), a waste container, and the electro-chemical sensor. It also housed the perfusate pump, electronic controls, and data memory. The dual-lumen plastics catheter was surrounded by a dialysis tube of small diameter (approximately 0.5 mm) and was inserted into the subcutaneous adipose tissue of the abdomen by means of a puncture needle. The catheters could be inserted on either side of the umbilicus. The sensor unit was hooked up to a flexible belt system.
Data Analysis and Statistics
Glycemic control was assessed by analyzing the 50 h glucose profiles registered during in-house periods. In addition, the coefficients of variation (CV) were determined for the glucose profiles.
The primary objective of this study was to assess the impact of physical exercise on metabolic control in the 21 h thereafter in patients with type 1 diabetes on CSII before and after a 14-day moderate or intense training program. Areas under the curve (AUCs) were calculated with the trapezoidal rule for the glucose levels on a minute-to-minute basis for total AUC from 06:00 h day 0 until 09:30 h day 2 and from 06:00 h day 18 until 09:30 h day 20, fractional AUCs on days 0 and 18 (6:00–12:00 h), days 1 and 19 (0:00–12:00 h), and days 2 and 20 (0:00–09:30 h), and the AUCs 6 and 21 h following the start of the exercise challenge on days 1 and 19, respectively. All AUCs were compared between the groups and in-house periods.
The SCGM 1 recordings were calibrated retrospectively, taking linear drift compensation and all reference measurements into account. This approach was used to avoid bias of calibrated glucose profiles introduced by selection of single calibration points. It was not the aim of this study to evaluate the performance of the SCGM system but to analyze the registered glucose profiles. Hypoglycemic events during in-house periods were evaluated by three different approaches: (1) The number of hypoglycemic readings (i.e., calibrated BG <60 mg/dl) was determined for the same time intervals as mentioned earlier. The time spent in hypoglycemia (in minutes) corresponds to the number of these readings. (2) Hypoglycemic episodes were also evaluated with regard to their incidence during the time intervals. To be counted as separate episodes, a normoglycemic interval for at least 30 min had to occur between two hypoglycemic BG readings. Allocation to a particular time interval depended on the start time of the episode. (3) Hypoglycemia episodes (symptomatic and/or confirmed by BG measurements) as reported by the patients and documented as AEs were assessed.
Basal, bolus, and total insulin administration was evaluated with regard to the amount of units administered during the in-house periods, and for bolus administrations, the number of all boluses was also considered. Additional CHO administrations were evaluated regarding the amount (g) administered as well as the number of administrations.
All results were analyzed exploratively by means of descriptive statistics. Within-group and between-group pretraining versus posttraining period comparisons were made using standard two-sided statistical tests. Reported AEs were analyzed descriptively.
Results
Patient Demography and Disposition
All 16 male patients included completed the study. In 1 patient, glucose profiles were not evaluable due to malfunction of the SCGM 1 system. Therefore, only 15 profiles [8 in group A with moderate training (HbA1c 7.2 ± 0.8%, age 39 ± 12 years, BMI 24.6 ± 2.6 kg/m2, fitness index 94.9 ± 9.6) and 7 in group B with intense training (HbA1c 7.4 ± 0.9%, age 37 ± 11 years, BMI 27.3 ± 2.3 kg/m2, 8 fitness index 8.4 ± 3.2)] were available for data analysis. Demographics and baseline data of all included patients were comparable between both groups; however, the BMI of the patients in group A tended to be lower than in group B (p = .052). In group A, five patients used insulin lispro for CSII, two used human insulin, and one patient insulin aspart. In group B, six patients used insulin lispro, one patient used human insulin, and another insulin aspart.
Training Period
According to the study protocol, patients in group A trained less intensively than the patients in group B; the average training workload was more than three times higher in the latter group (Table 1). However, impedance measurements showed that the training had no measurable effect on body composition and body weight. Interestingly, the fitness level of the patients with moderate training remained constant but decreased in the patients on more intense training. This is in line with the decrease in heart rate in group A and the increase in group B during this test. At the same time, the energy consumption in group A remained constant, but increased in group B.
Table 1.
Training Period, Level of Exercise, and Related Effects (Mean ± SD)
| Parameter | Group A (moderate exercise, n = 8) | Group B (intense exercise, n = 7) | p value a |
|---|---|---|---|
| Training period | |||
| Number of training sessions | 7.6 ± 0.5 | 14.4 ± 0.8 | <.0001 |
| Total exercise time (min) | 281 ± 49 | 1046 ± 234 | <.0001 |
| Time within heart rate limits (min) | 234 ± 50 | 853 ± 100 | <.0001 |
| % time within heart rate limits | 83.4 ± 11.6 | 83.8 ± 14.7 | .959 |
| Heart rate (bpm) | 135.9 ± 11.8 | 132.7 ± 10.0 | .582 |
| Total energy consumption (kcal) | 3062 ± 614 | 10,564 ± 1838 | <.0001 |
| Pretraining | Posttraining | Difference pre–post | p valueb | Pretraining | Posttraining | Difference pre–post | p valueb | p valuec | |
| Impedance measurementsd | |||||||||
| BFM (kg) | 19.6 ± 7.4 | 20.0 ± 7.6 | −0.4 ± 2.8 | 0.711 | 22.2 ± 5.7 | 22.5 ± 4.0 | −0.3 ± 3.4 | .832 | .589 |
| BFMP (%) | 22.6 ± 6.7 | 23.2 ± 6.9 | −0.6 ± 3.2 | 0.605 | 25.5 ± 4.8 | 25.9 ± 2.2 | −0.3 ± 4.1 | .830 | .548 |
| LBM (kg) | 64.9 ± 5.1 | 20.0 ± 7.7 | 0.8 ± 3.1 | 0.469 | 64.0 ± 5.6 | 63.7 ± 4.7 | 0.3 ± 3.1 | .835 | .997 |
| LBM (%) | 77.4 ± 6.7 | 23.2 ± 6.9 | 0.6 ± 3.1 | 0.578 | 74.5 ± 4.8 | 74.1 ± 2.2 | 0.3 ± 4.1 | .830 | .521 |
| H2O (kg) | 47.5 ± 3.8 | 64.0 ± 5.9 | 0.8 ± 2.2 | 0.340 | 46.9 ± 4.1 | 46.6 ± 3.4 | 0.2 ± 2.3 | .800 | .922 |
| H2O (%) | 56.7 ± 4.9 | 76.8 ± 6.9 | 0.4 ± 2.3 | 0.600 | 54.5 ± 3.6 | 54.2 ± 1.6 | 0.3 ± 3.0 | .789 | .531 |
| Self-walk test | |||||||||
| Duration (min) | 16.6 ± 0.5 | 16.0 ± 0.6 | 0.0 ± 0.8 | 0.993 | 16.7 ± 0.5 | 16.9 ± 1.1 | −0.2 ± 1.5 | .711 | .082 |
| Average heart rate (bpm) | 135.9 ± 13.1 | 132.5 ± 11.4 | 3.4 ± 9.5 | 0.349 | 131.9 ± 19.6 | 135.7 ± 15.7 | −3.9 ± 14.7 | .513 | .282 |
| Energy consumption (kcal) | 233 ± 35 | 234 ± 36 | −0.5 ± 7.6 | 0.858 | 242 ± 33 | 245 ± 24 | −3.7 ± 17.7 | .599 | .043e |
| VO2max (ml/min/kg) | 42.0 ± 5.9 | 42.7 ± 6.5 | −0.7 ± 3.6 | 0.593 | 39.9 ± 3.1 | 37.7 ± 3.5 | 2.2 ± 4.9 | .268 | .449 |
| Fitness index | 94.9 ± 9.6 | 96.5 ± 14.8 | −1.6 ± 8.4 | 0.603 | 88.4 ± 3.2 | 83.6 ± 13.7 | 4.9 ± 12.6 | .349 | .313 |
t test for between-group difference.
t test for within-group change (pre-post).
f test for between-group differences in change.
BFM, body fat mass; BFMP, body fat mass profile; LBM, lean body mass.
p value indicating statistically significant difference (< .05).
Exercise Challenge
The treadmill exercise of about 45 min duration led to a comparable level of challenge in both patient groups (Table 2). Neither heart rate nor energy consumption differed within the groups comparing pretraining results versus posttraining results or between the groups. Patients in both groups also had a comparable basal insulin infusion rate and number of insulin boluses during the 21 h after start of the exercise challenge. However, prandial insulin doses were higher pretraining than posttraining in both groups. Similar amounts of additional CHO were consumed by the patients in both groups during the 21 h after the exercise challenge.
Table 2.
Summary Statistics of Exercise Challenge and Parameters 21 h Postchallenge Before and After the 14-Day Training Period (Mean ± SD)
| Parameter | Group A (moderate exercise, n = 8) | Group B (intense exercise, n = 7) | p valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretraining | Posttraining | Difference pre–post | p value a | Pretraining | Posttraining | Difference pre–post | p valuea | ||
| Exercise challenge | |||||||||
| Duration (min) | 43.1 ± 5.3 | 44.3 ± 2.1 | −1.1 ± 6.0 | .612 | 44.3 ± 2.4 | 45.0 ± 0.0 | −0.7 ± 2.4 | .454 | .037c |
| Average heart rate (bpm) | 125.4 ± 9.7 | 126.8 ± 13.7 | −1.4 ± 10.5 | .722 | 128.3 ± 12.1 | 126.6 ± 7.6 | 1.7 ± 10.2 | .673 | .965 |
| Time within heart rate limits (min) | 35.9 ± 8.9 | 37.1 ± 6.3 | −1.2 ± 8.7 | .714 | 35.4 ± 4.2 | 36.6 ± 4.4 | −1.2 ± 4.8 | .534 | .177 |
| Energy consumption (kcal) | 428 ± 86 | 458 ± 105 | −30.4 ± 92.7 | .385 | 430 ± 55 | 444 ± 32 | −14.0 ± 65.4 | .591 | .412 |
| Insulin treatment | |||||||||
| Basal insulin (IU) | 17.5 ± 3.7 | 17.2 ± 3.2 | 0.3 ± 0.6 | .229 | 18.3 ± 4.5 | 17.9 ± 4.6 | 0.5 ± 0.7 | .124 | .638 |
| Bolus insulin (IU) | 23.3 ± 8.9 | 19.9 ± 5.4 | 3.4 ± 6.3 | .168 | 25.9 ± 5.7 | 23.1 ± 5.4 | 2.8 ± 8.5 | .413 | .452 |
| Number of bolus administrations | 4.5 ± 1.6 | 4.3 ± 1.0 | 0.3 ± 1.3 | .598 | 2.9 ± 0.9 | 3.6 ± 1.5 | −0.7 ± 1.5 | .253 | .690 |
| Glucose (SCGM 1) | |||||||||
| AUC (mg/dl*h) | 2716 ± 270 | 2726 ± 211 | −9.5 ± 341 | .940 | 2729 ± 368 | 2846 ± 456 | −117 ± 575 | .610 | .199 |
| CV (%) | 34.7 ± 9.8 | 34.8 ± 9.9 | −0.1 ± 12.1 | .988 | 32.3 ± 8.4 | 36.0 ± 10.1 | −3.7 ± 11.8 | .434 | .962 |
| Hypoglycemia | |||||||||
| Time in hypoglycemia (min) | 60.9 ± 54.8 | 28.6 ± 29.3 | 32.3 ± 42.7 | .070 | 56.4 ± 70.0 | 57.4 ± 47.3 | −1.0 ± 57.5 | .965 | .457 |
| Number of hypoglycemic episodes | 1.0 ± 1.1 | 1.4 ± 1.2 | −0.4 ± 1.6 | .528 | 1.0 ± 1.2 | 1.1 ± 0.9 | −0.1 ± 0.9 | .689 | .183 |
| CHO | |||||||||
| Additional CHO (g) | 42.8 ± 34.8 (n = 7) | 39.2 ± 55.4 | −2.0 ± 57.8 (n = 7) | .929 | 48.7 ± 67.0 | 52.5 ± 57.8 | −3.7 ± 60.2 | .875 | .925 |
| Number of additional CHO administrations | 2.6 ± 1.9 | 2.0 ± 2.9 | 0.6 ± 3.3 | .608 | 2.4 ± 3.0 | 2.6 ± 2.7 | −0.1 ± 2.0 | .859 | .262 |
t test for within-group change (pre-post).
F test for between-group differences in change.
p value indicating statistically significant difference (< .05).
Glucose Profiles
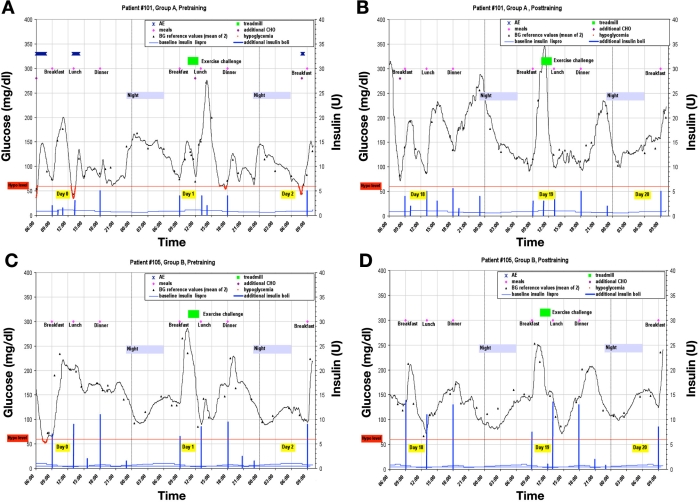
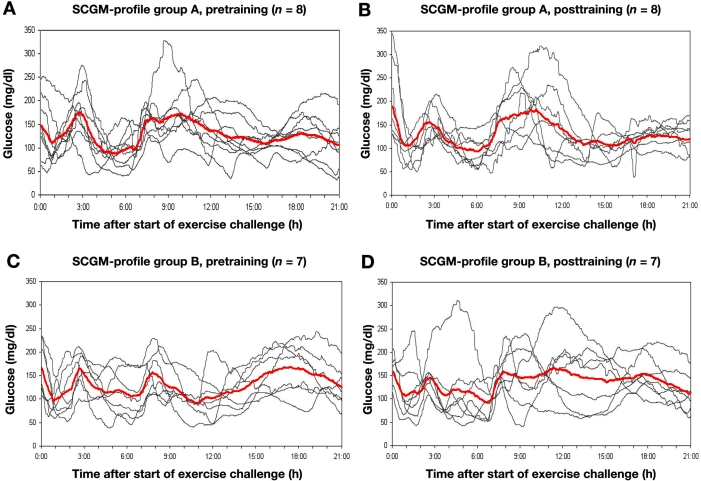
As an example, the individual recordings from a patient in group A and B are shown in Figure 1. The in-house day prior to the study day with the exercise challenge is also displayed, showing all the parameters relevant for changes in metabolic control. The mean glucose profiles of the 21 h after the challenges, together with all individual profiles, are shown in Figure 2. No differences in the glucose profiles with respect to the exercise challenge could be observed. It is of interest to note that, between 0:00 and 03:00 h, there was an increase in glycemia in a number of patients (without an additional snack). Also, the changes in the AUCs from the pretraining period to the posttraining period do not differ within and between the groups. The variability of the glucose profiles was also not different.
Figure 1.
Glucose profiles of one patient (#101) of Group A (a) pretraining and (b) posttraining and one patient (#105) of Group B (c) pretraining and (d) posttraining.
Figure 2.
Subcutaneous glucose monitoring system 1 profiles of Group A (a) pretraining and (b) posttraining and Group B (c) pretraining and (d) posttraining. Mean SCGM 1 profile highlighted in red.
Patients in group A tended to spend a shorter period of time in hypoglycemia (BG <60 mg/dl) during the 21 h after exercise challenge posttraining compared to pretraining, but for the patients in group B, the time in hypoglycemia remained constant. Also, the number of hypoglycemic episodes was not different between the groups.
Safety
In total, 310 AEs were reported during this study, and the most common AEs (278, 90%) were hypoglycemic events, which were experienced several times by each of the 16 patients included in the study. The vast majority of the hypoglycemic events were of mild intensity, and no cases of severe hypoglycemia were observed.
Discussion
The objective of this study was to assess the impact of (moderate) physical exercise on metabolic control in the hours thereafter in patients with type 1 diabetes managed by CSII before and after an intensive and a moderate training phase. Our aim was first to evaluate if the approach chosen has a relevant effect or not. If a “dramatic” difference would have been seen, a more sound study (with respect to evaluation of the training effect) would have been justified. The major outcome of this study, which was a mixture of an observational study and a clinical-experimental study, was that the glucose profiles of the patients were highly variable, not a surprising outcome. This strongly argues for the use of CGM under such circumstances, as no general recommendation can be given to the patients. It appears that the interaction of all parameters that are of relevance for driving glucose changes after an exercise challenge are difficult to foresee. In view of this unpredictability, use of CGM can provide an important safety net for patients with diabetes. In practice, patients will not perform SMBG frequently enough to achieve the same level of glucose monitoring. The CGM system that is used must provide reliable recordings also under the conditions of exercise (i.e., sweat) and with low glucose levels.
Interestingly, participation of the patients in a training program induced only moderate differences between the pretraining to the posttraining situation and between the groups. Also, the impact of the 14-day training period on the fitness level and metabolic control was lower, and not in the way that was expected when the study was designed. Probably our assessment of the fitness level of the patients before/after the training phase was not adequate and/or the duration of the training phase was too short. However, that the training led to a reduction in prandial insulin requirements—with comparable glucose profiles—is in line with the expected (rapid) increase in insulin sensitivity after an intensification of physical exercise.
In this study, patients were blinded to the measurements results, i.e., the current glucose levels measured by the CGM system were not displayed. Therefore, they performed their diabetes therapy as usual. With an online display of the current glucose values and provision of alarms once BG tended to decline to too low values or increase to too high levels, the outcome of such a study can be expected to differ massively. In daily life, patients on CSII would, e.g., reduce basal insulin infusion rates to reduce the risk of hypoglycemic events or eat at the appropriate point in time.
It is of note that use of CGM during exercise has received relatively little attention thus far, at least, the number of respective studies published is very small. In addition, such studies investigated the metabolic effect of exercise under highly standardized clinical-experimental conditions.2–6 It appears as if no study has been published thus far that evaluates the impact of exercise on glycemic profiles under more daily life conditions. However, analysis of such glucose profiles— like they were obtained in our study—show the typical “mistakes” of patients in terms of CHO intake and insulin therapy.
If one is interested in the impact of a given therapeutic intervention or a specific exercise challenge on glucose profiles in a typical clinical-experimental study, one would try to standardize the experimental conditions on the different study days as far as possible. In practice, it is challenging to establish comparable levels of glycemia and circulating insulin and is somewhat artificial. Despite all these efforts to keep as many variables constant as possible, one cannot be sure that the outcome is predictable. This is clearly also the clinical experience of patients with diabetes. As one example for the complexity of exercise-related studies, one has to acknowledge that the counter-regulatory response to a hypoglycemic event some hours after an exercise challenge is reduced in patients with type 1 diabetes.11 Most probably these challenges are the reason why the number of studies about exercise and CGM is so small. Unfortunately, this is in sharp contrast to the need of patients with diabetes.
In summary, this study showed how variable glucose profiles are in patients with type 1 diabetes after exercise if they also treat themselves with CSII. It also indicates how the use of real-time CGM will support patients to optimize their metabolic control under such conditions. It should help patients to reduce the risk of acute metabolic deteriorations at the same time, and it should be studied if this helps patients with diabetes to reduce glycemic variability during exercise. An interesting study would be to have a design that allowed for the comparison between blinded and unblinded CGM and training. A higher degree of safety during exercise would enable patients with diabetes to profit from the physiological and psychological benefits associated with physical activity, despite their disease.
Acknowledgments
We thank Dr. Andreas Thomas, Medtronic, Düsseldorf, and Prof. Dr. W. Kern, Ulm, for his helpful suggestions with respect to literature.
Abbreviations
- AE
adverse event
- AUC
area under the curve
- BG
blood glucose
- BMI
body mass index
- CGM
continuous glucose monitoring
- CHO
carbohydrate
- CSII
continuous subcutaneous insulin infusion
- CV
coefficients of variation
- HbA1c
hemoglobin A1c
- SCGM
subcutaneous glucose monitoring system
- SD
standard deviation
- SMBG
self-monitoring of blood glucose
References
- 1.Ludvigsson J, Larsson Y, Svensson PG. Attitudes towards physical exercise in juvenile diabetics. Acta Paediatr Scand Suppl. 1980;283:106–111. doi: 10.1111/j.1651-2227.1980.tb15331.x. [DOI] [PubMed] [Google Scholar]
- 2.Kapitza C, Lodwig V, Obermaier K, Wientjes KJ, Hoogenberg K, Jungheim K, Heinemann L Glucose Monitoring Study Group. Continuous glucose monitoring: reliable measurements for up to 4 days with the SCGM1 system. Diabetes Technol Ther. 2003;5(4):609–614. doi: 10.1089/152091503322250622. [DOI] [PubMed] [Google Scholar]
- 3.Schoemaker M, Andreis E, Röper J, Kotulla R, Lodwig V, Obermaier K, Stephan P, Reuschling W, Rutschmann M, Schwaninger R, Wittmann U, Rinne H, Kontschieder H, Strohmeier W. The SCGM1 system: subcutaneous continuous glucose monitoring based on microdialysis technique. Diabetes Technol Ther. 2003;5(4):599–608. doi: 10.1089/152091503322250613. [DOI] [PubMed] [Google Scholar]
- 4.Fayolle C, Brun JF, Bringer J, Mercier J, Renard E. Accuracy of continuous subcutaneous glucose monitoring with the GlucoDay in type 1 diabetic patients treated by subcutaneous insulin infusion during exercise of low versus high intensity. Diabetes Metab. 2006;32(4):313–320. doi: 10.1016/s1262-3636(07)70285-9. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald AL, Philip A, Harrison M, Bone AJ, Watt PW. Monitoring exercise-induced changes in glycemic control in type 2 diabetes. Med Sci Sports Exerc. 2006;38(2):201–207. doi: 10.1249/01.mss.0000183852.31164.5a. [DOI] [PubMed] [Google Scholar]
- 6.Cauza E, Hanusch-Enserer U, Strasser B, Kostner K, Dunky A, Haber P. Strength and endurance training lead to different post exercise glucose profiles in diabetic participants using a continuous subcutaneous glucose monitoring system. Eur J Clin Invest. 2005;35(12):745–751. doi: 10.1111/j.1365-2362.2005.01573.x. [DOI] [PubMed] [Google Scholar]
- 7.Praet SF, Manders RJ, Lieverse AG, Kuipers H, Stehouwer CD, Keizer HA, van Loon LJ. Influence of acute exercise on hyper-glycemia in insulin-treated type 2 diabetes. Med Sci Sports Exerc. 2006;38(12):2037–2044. doi: 10.1249/01.mss.0000235352.09061.1d. [DOI] [PubMed] [Google Scholar]
- 8.Iscoe KE, Campbell JE, Jamnik V, Perkins BA, Riddell MC. Efficacy of continuous real-time blood glucose monitoring during and after prolonged high-intensity cycling exercise: spinning with a continuous glucose monitoring system. Diabetes Technol Ther. 2006;8(6):627–635. doi: 10.1089/dia.2006.8.627. [DOI] [PubMed] [Google Scholar]
- 9.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61(1):3–11. [PubMed] [Google Scholar]
- 10.Laukkanen R. Vol. 23. Kuopio: Kuopio University Publications D. Medical Sciences; 1993. Development and evaluation of a 2-km walking test for assessing maximal aerobic power of adults in field conditions. Doctoral thesis. [Google Scholar]
- 11.Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Acute, same-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab. 2006;290(6):E1331–E1338. doi: 10.1152/ajpendo.00283.2005. [DOI] [PubMed] [Google Scholar]