Abstract
Background
Studies have shown that controlling blood glucose can reduce the onset and progression of the long-term microvascular and neuropathic complications associated with the chronic course of diabetes mellitus. Improved glycemic control can be achieved by frequent testing combined with changes in medication, exercise, and diet. Technological advancements have enabled improvements in analytical accuracy of meters, and this paper explores two such parameters to which that accuracy can be attributed.
Methods
Four blood glucose monitoring systems (with or without dynamic electrochemistry algorithms, codeless or requiring coding prior to testing) were evaluated and compared with respect to their accuracy.
Results
Altogether, 108 blood glucose values were obtained for each system from 54 study participants and compared with the reference values. The analysis depicted in the International Organization for Standardization table format indicates that the devices with dynamic electrochemistry and the codeless feature had the highest proportion of acceptable results overall (System A, 101/103). Results were significant when compared at the 10% bias level with meters that were codeless and utilized static electrochemistry (p = .017) or systems that had static electrochemistry but needed coding (p = .008).
Conclusions
Analytical performance of these blood glucose meters differed significantly depending on their technologic features. Meters that utilized dynamic electrochemistry and did not require coding were more accurate than meters that used static electrochemistry or required coding.
Keywords: accuracy, autocode, blood glucose, blood glucose meter, coded, dynamic electrochemistry, self-monitoring of blood glucose
Introduction
Diabetes mellitus is a public health concern of epidemic proportions around the world. In the United States alone, there are an estimated 20 million individuals with diabetes. Diabetes is also one of the leading causes of multisystem morbidity and mortality. The American Diabetes Association (ADA) estimates that the total annual economic burden of diabetes is approximately $174 billion. At least one-third of this cost can be attributed to treating diabetes-related chronic complications such as renal disease, neuropathy, retinopathy, and cardiovascular disease.1 Vigilant self-care behavior is advocated as an integrated approach for managing diabetes. This includes healthy eating, being active, monitoring blood glucose levels, taking oral medications and insulin, problem solving, reducing risks, and healthy coping.2 One cornerstone of self-care behavior that has utility for both patients and health care professionals (HCPs) is self-monitoring of blood glucose (SMBG). In 1996, the ADA recommended that HCPs should use SMBG results to make clinical decisions pertaining to nutritional and pharmacologic management of patients with diabetes. Although SMBG has been encouraged widely, there is no single testing protocol recommended. For the SMBG results to have direct and objective utility, it is imperative that the results obtained be accurate. The current standard for evaluating accuracy of blood glucose meters (BGMs) was established by the International Organization for Standardization (ISO). This standard, ISO 15197, states that 95% of BGM readings should fall within ±15mg/dl for reference values <75 mg/dl and within 20% of the reference value for reference values ≥75 mg/dl.3 The Food and Drug Administration has requested the ISO standard be revised to recommend more stringent accuracy criteria.4 The ADA's consensus statement on SMBG recommends that SMBG results be within 5% of the reference value.5
Accuracy depends on multiple parameters: the user, the instrument, and other aspects of the evaluation, including how accuracy is defined.6 Technological innovation in strip electrochemistry and measurement techniques have led to improved analytical parameters such as increased test result accuracy and enhanced user experience such as faster test time. This paper explores two such system parameters: dynamic electrochemistry and meters that do not require coding (i.e., codeless meters) in the context of technological innovation that has permitted these advancements.
Subjects and Methods
Subjects
The study was performed with the participation of 54 subjects who were recruited from a diabetes clinic. All subjects were diagnosed with either type 1 or type 2 diabetes and spanned a wide range of demographic characteristics (i.e., gender, age, years with diabetes, and frequency of SMBG). The study protocol was approved by an Institutional Review Board, and informed consent was obtained from each subject.
Blood Glucose Meters and Reference Method
Four different BGM systems, three of which offered improved features aimed at reducing user and/or system errors, were evaluated in a clinic setting. Calibration of the BGMs and the electrochemistry algorithms utilized in the meter were the key meter features that were evaluated.
Prior to use, a BGM must be calibrated. This can be done manually by the user or automatically by the blood glucose monitoring system, which minimizes the risk of calibration error by the user. The autocalibration or codeless feature reduces the number of variables that impact accuracy.
Static electrochemistry is a fixed input signal (such as an applied voltage) that results in an output signal that correlates to the glucose concentration in the sample (Figure 1). Dynamic electrochemistry is a time-varying input signal that induces an output that is more information rich than a static signal. This signal can be exploited using digital signal processing algorithms to give a more accurate glucose reading by correcting for variables such as temperature, hematocrit, and strip-to-strip variations (Figure 2).
Figure 1.
Graphic representation of static electrochemistry.
Figure 2.
Graphic representation of dynamic electrochemistry.
Table 1 summarizes the four different BGM systems evaluated. Systems B and D require manual calibration (coded), and systems A and C do not require calibration by the user (codeless). Systems A and B utilize dynamic electrochemistry to produce a signal that is translated into a glucose reading, and systems C and D use static electrochemistry.
Table 1.
Features of the Blood Glucose Monitoring Systems that Were Evaluated
| Calibration | Electrochemistry | |
|---|---|---|
| System A | Codeless | Dynamic |
| System B | Coded | Dynamic |
| System C | Codeless | Static |
| System D | Coded | Static |
To obtain unbiased results, three different test strip lots from each meter system were alternated between study subjects. The reference method used in this study was a Yellow Springs Instruments (YSI) 2300 Stat Plus (Yellow Springs, OH) glucose laboratory analyzer. To ensure all four meter systems and the reference method were operating properly, quality control checks were done at the beginning and end of each day as instructed by each system's manufacturer.
Study Procedure
The study was conducted under the observation of a trained HCP. Under the same testing conditions, a total of eight meters (two meters from each system, A, B, C, and D) were used by each study subject to obtain blood glucose readings. Study subjects were provided with the meters, vials of test strips, and owner's guides for each meter system. Subjects were urged to read the instructions accompanying the corresponding meter on how to correctly prepare the system to obtain their blood glucose readings. After the subjects were comfortable with the meter and test strip operation, the HCP cleaned and lanced the subject's fingertip. The subjects proceeded to test their blood glucose on each of two meters from the four blood glucose systems (eight readings in total). The HCP collected a capillary blood sample from the same finger stick, which was spun in a microhematocrit centrifuge (LW Scientific, Inc., LWS-M24) for 2 min. Each subject's hematocrit was measured, and the plasma was extracted and run on the YSI glucose laboratory analyzer to obtain a reference blood glucose value.
Statistical Analysis
The data were compiled and evaluated for possible data exclusions. A subject's data were excluded from analysis if their hematocrit fell outside the systems' operating range or if a YSI laboratory reference value was not obtained due to insufficient plasma volume. Individual meter readings that resulted in an error message on the screen's display were also excluded from analysis.
Accuracy was determined by comparing each system's meter readings with the corresponding YSI reference values. Linear regression analysis was performed, and the slope and intercept with 95% confidence intervals were calculated as shown in Table 2. The number and percentage of system results that were within ±15, ±10, and ±5 mg/dl of their respective YSI reference values for glucose concentrations <75 mg/dl and within ±20%, ±15%, ±10%, and ±5% of their respective YSI reference values for glucose concentrations ≥75 mg/dl were calculated and presented in ISO 15197 table format in Table 3.
Table 2.
Linear Regression Analysis of the Four Blood Glucose Meter Systems
| n | y | Slope | Intercept (mg/dl) | r | |
|---|---|---|---|---|---|
| System A | 103 | 0.99x − 1.7 | 0.96 to 1.02 | −7.3 to 3.9 | 0.9872 |
| System B | 101 | 0.96x + 10.8 | 0.93 to 0.99 | 5.3 to 16.3 | 0.9871 |
| System C | 102 | 1.01x − 11.3 | 0.97 to 1.05 | −18.3 to −4.4 | 0.9816 |
| System D | 88 | 1.07x − 15.2 | 1.03 to 1.11 | −22.4 to −8.0 | 0.9847 |
Table 3.
Accuracy of Blood Glucose Meters as Compared to YSI Reference Values in ISO 15197 Table Format
| YSI reference value <75 mg/dl | YSI reference value ≥75 mg/dl | Overall acceptable results (within ±15 mg/dl and ±20%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Within ±5 mg/dl | Within ±10 mg/dl | Within ±15 mg/dl | Within ±5% | Within ±10% | Within ±15% | Within ±20% | ||
| System A | (2/4) 50% | (3/4) 75% | (4/4) 100% | (56/99) 56.6% | (83/99) 83.8% | (94/99) 94.9% | (97/99) 98.0 % | (101/103) 98.1% |
| System B | (0/4) 0% | (2/4) 50% | (4/4) 100% | (54/97) 55.7% | (77/97) 79.4% | (89/97) 91.7% | (93/97) 95.9% | (97/101) 96.0% |
| System C | (2/4) 50% | (2/4) 50% | (4/4) 100% | (34/98) 34.7% | (68/98) 69.4% | (90/98) 91.8% | (95/98) 96.9% | (99/102) 97.1% |
| System D | (1/3) 33.3% | (2/3) 66.7% | (3/3) 100% | (38/85) 44.7% | (57/85) 67.1% | (74/85) 87.1% | (80/85) 94.1% | (83/88) 94.3% |
A chi-squared test was performed to determine if there was a significant difference in accuracy among systems. Analysis was performed with the number of observed blood glucose readings within 10% of the corresponding YSI reference value if the YSI reference value was ≥75 mg/dl.
Results
Of the 108 blood glucose results obtained from each of the four meter systems, 4 data points (two subjects) for each system were excluded because there was no YSI reference value for comparison, 10 data points were excluded from system D because the subjects' hematocrit fell outside the stated operating range, and 12 data points were excluded due to meter errors (system A, one error; system B, three errors; system C, two errors; and system D, six errors).
Subjects prepared each blood glucose system for obtaining a blood glucose reading. A trained HCP observed the subjects and recorded whether or not they properly coded the two types of BGMs that required manual calibration (systems B and D). The majority of the participants (42 out of 54) failed to follow the systems' instructions for use, and many blood glucose tests performed on systems B and D were conducted with the incorrect calibration code.
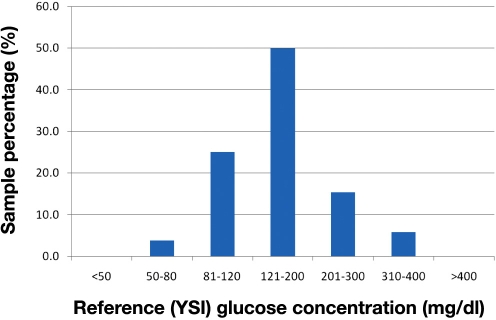
The glucose concentrations of subject samples as determined by the YSI 2300 ranged from 55.4 to 393.5 mg/dl. This distribution of samples is shown in Figure 3. Accuracy results from the linear regression analysis are summarized in Table 2. The regression results indicate there was no statistically significant difference between the meter readings and reference values for system A (95% confidence intervals for slope and intercept included 1 and 0, respectively). The linear regression analysis for system C indicates that the 95% confidence interval for slope included 1, but the 95% confidence interval for intercept did not include 0. The 95% confidence intervals for slope and intercept for systems B and D did not include 1 and 0, respectively.
Figure 3.
Distribution of subject blood glucose concentrations as determined by the YSI 2300.
The meter readings were compared to their corresponding YSI reference values based on the accuracy analysis format presented in ISO 15197. The number and percentage of meter results that are within ±15, ±10, and ±5 mg/dl of their respective YSI reference values for glucose concentrations <75 mg/dl and within ±20%, ±15%, ±10%, and ±5% of their respective YSI reference values for glucose concentrations ≥75 mg/dl were calculated, and the results are presented in ISO 15197 table format in Table 3.
The chi-squared analysis was performed with the number of observed blood glucose readings within 10% of the corresponding YSI reference value if the YSI reference value was ≥75 mg/dl. The analysis indicates a statistically significant difference in the accuracy meter readings for system A when compared to systems C and D (p = .017 and p = .008, respectively) and no statistically significant difference when system A was compared to system B (p = .420).
Discussion
Effective inter pretation of SMBG readings is dependent on obtaining frequent and accurate glucose readings. While frequency of testing depends on patient motivation, education, and less-defined elements, measurement accuracy is influenced by a variety of known factors. These factors include user-generated errors such as miscoding of a strip, system errors attributable to sample characteristics (hematocrit), testing environment (ambient temperature, humidity), and manufac turing errors (lot-to-lot variability in reagent strips). In addition, lay users may interpret the test instructions and handle the meter in a variable manner, which may lead to a decline in measurement accuracy. Routine testing at the HCP's office could potentially minimize errors but is burdensome and impractical. Therefore, to offer the best possible patient care, it is desirable to have a BGM that does not compromise on accuracy of the reading despite unpredictable user or system variables.
Several BGMs have provided improved features to minimize user and/or system errors. Technological advances allow for codeless systems that minimize user errors resulting from a mismatch between the code on the test strip vial and the code entered into the meter. A simulation model developed by Raine and colleagues7 demonstrated that autocoded (codeless) meters performed better than coded meters, even when the latter were correctly coded.7 In addition, dynamic electrochemistry, a set of autocorrecting algorithms to minimize system errors, has been added to some BGMs. This study was designed to evaluate the accuracy in the hands of the lay user for four BGMs that included these features in different combinations.
Miscoding can lead to inaccurate results and, therefore, insulin dosing errors as reported by Raine and colleagues.7 Baum and associates reported that miscoded meters resulted in readings with greater than 30% median difference when compared to correctly coded meters.8 Previously reported studies have concluded that incorrect coding occurred 16% to 25% of the time.9,10 Boyd and Bruns have theoretically estimated that meters with 5% and 10% total error could lead to insulin dosing errors 8–23% and 16–45% of the time, respectively.11
The results of this study show the importance of features that will enable more accurate results. There was a statistically significant difference in the meter readings for both coded meter systems in this study (systems B and D) and their corresponding YSI values based on linear regression analysis. In contrast, linear regression analysis showed that system C (codeless) (not significant for slope) and system A (codeless) glucose meter readings did not differ significantly from the YSI reference values.
The percentage of meter readings within 20% of the YSI reference value for concentrations ≥75 mg/dl was similar for all four meter systems. However, at the levels of meter readings within 15%, 10%, and 5% of the YSI reference, the two systems that utilize dynamic electrochemistry were more accurate. System A, which is codeless and utilizes dynamic electrochemistry, had the best overall accuracy performance, with 94.9%, 83.8%, and 56.6% of meter readings within 15%, 10%, and 5% of the YSI values, respectively. System B also showed improved accuracy as compared to systems that utilize static electrochemistry, with results at 91.7%, 79.4%, and 55.7% within 15%, 10%, and 5% of the YSI values, respectively.
The number of observed blood glucose readings within 10% of the corresponding YSI reference value if the YSI reference value was ≥75 mg/dl was also used to determine system accuracy. In a 1-to-1 comparison, system A (codeless and with dynamic electrochemistry) performed better when compared with systems that measure glucose via static electrochemistry (system C, p = .017, and system D, p = .008). There was no statistical difference in the 1-to-1 comparison with system B (p = .420), which also utilized dynamic electrochemistry.
Conclusion
Linear regression analysis and percentage difference between system readings ≥75 mg/dl at 10% and the corresponding YSI value indicate that individuals achieved the highest accuracy when the meter did not require coding and was embedded with dynamic electrochemistry algorithms (system A).
Abbreviations
- ADA
American Diabetes Association
- BGM
blood glucose meter
- HCP
health care professional
- ISO
International Organization for Standardization
- SMBG
self-monitoring of blood glucose
- YSI
Yellow Springs Instruments
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.American Association of Diabetes Educators. AADE position statement. Individualization of diabetes self-management education. Diabetes Educ. 2007;33(1):45–49. doi: 10.1177/0145721706298308. [DOI] [PubMed] [Google Scholar]
- 3.International Organization for Standardization. Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. Geneva: International Organization for Standardization; 2003. In vitro diagnostic test systems. ISO 15197:2003 (E) [Google Scholar]
- 4.Harris G. Standards might rise on monitors for diabetes. The New York Times. 2009. http://www.nytimes.com/2009/07/19/health/policy/19monitor.html?_r=1&scp=1&sq=Standards%20might%20rise%20 on%20monitors%20for%20diabetes&st=cse. July 19, Accessed November 12, 2009.
- 5.American Diabetes Association. Clinical practice recommendations 1996. Diabetes Care. 1996;19(Suppl 1):S1–S118. [PubMed] [Google Scholar]
- 6.Bergenstal R, Pearson J, Cembrowski GS, Bina D, Davidson J, List S. Identifying variables associated with inaccurate self-monitoring of blood glucose: proposed guidelines to improve accuracy. Diabetes Educ. 2000;26(6):981–989. doi: 10.1177/014572170002600610. [DOI] [PubMed] [Google Scholar]
- 7.Raine CH, Schrock LE, Edelman SV, Mudaliar SR, Zhong W, Proud LJ, Parkes JL. Significant insulin dose errors may occur if blood glucose results are obtained from miscoded meters. J Diabetes Sci Technol. 2007;1(2):205–210. doi: 10.1177/193229680700100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum JM, Monhaut NM, Parker DR, Price CP. Improving the quality of self-monitoring blood glucose measurement: a study in reducing calibration errors. Diabetes Technol Ther. 2006;8(3):347–357. doi: 10.1089/dia.2006.8.347. [DOI] [PubMed] [Google Scholar]
- 9.Schrock LE. Miscoding and other user errors: importance of ongoing education for proper blood glucose monitoring procedures. J Diabetes Sci Technol. 2008;2(4):563–567. doi: 10.1177/193229680800200405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raine CH III. Self-monitored blood glucose: a common pitfall. Endocr Pract. 2003;9(2):137–139. doi: 10.4158/EP.9.2.137. [DOI] [PubMed] [Google Scholar]
- 11.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001;47(2):209–214. [PubMed] [Google Scholar]