Abstract
Noncommunicable diseases, of which coronary artery disease (CAD) and diabetes top the list, have overtaken communicable diseases with respect to overall mortality, even in developing countries like India. High prevalence rates of diabetes and CAD are seen not only in affluent migrant Indians, but also in those living within the subcontinent. Indeed the epidemic of diabetes and CAD is now spreading to the middle- and lower-income groups in India. The risk for CAD is two to four times higher in diabetic subjects, and in Indians, CAD occurs prematurely, i.e., one to two decades earlier than in the West. Thus there is an urgent need for studies on CAD in diabetic and nondiabetic subjects in India.
The Chennai Urban Population Study, a population-based study in Chennai, in South India, showed a prevalence of CAD of 11%, which is 10 times more than what it was in 1970. Clustering of risk factors for CAD such as hyperglycemia, central body obesity, dyslipidemia, and hypertension tends to occur, and interplay of these risk factors could explain the enhanced CAD risk in Indians. Additionally, low-grade inflammation and a possible inherent genetic susceptibility are other contributing factors. Preventive measures such as lifestyle modification with healthy diet, adequate physical activity, and decrease in stress could help prevent the twin epidemics of diabetes and CAD.
Keywords: Asian Indians, cardiovascular risk factors, coronary artery disease, South Asians, type 2 diabetes
Introduction
Type 2 diabetes is on the verge of becoming a pandemic in India.1 As type 2 diabetes shares several risk factors in common with coronary artery disease (CAD), such as age, hypertension, dyslipidemia, obesity, physical inactivity, and stress, an increase in the prevalence of diabetes indirectly implicates an escalating risk of CAD as well.2,3 Diabetic subjects are known to have a two to four times increased CAD risk, and CAD has been reported to occur two to three decades earlier in diabetic subjects as opposed to their nondiabetic counterparts.2 The life expectancy of people with diabetes is reduced by nearly eight years due to increased mortality.4 Coronary artery disease accounts for more than 80% of all deaths and 75% of all hospitalizations in diabetic subjects.5,6 It is also reported that plaques are more vulnerable to rupture among patients with diabetes.7
The association between CAD and diabetes is strong despite the fact that there are wide ethnic and geographic variations in their prevalence. The protective female gender effect is lost in diabetic subjects, and indeed, women with diabetes are possibly more prone to develop CAD than men with diabetes.8 It was established in the Organization to Assess Strategies for Ischaemic Syndromes study that diabetic subjects without prior CAD had a similar risk of CAD as nondiabetic subjects with prior CAD, with poorer prognosis seen in diabetic subjects than nondiabetic subjects after a clinical event.5 Diabetes has been categorized as a cardiovascular risk equivalent according to the current National Cholesterol Education Program (NCEP) guidelines.9 Metabolic abnormalities due to diabetes have been found to predispose to vascular changes, leading to atherosclerotic end points.10 In addition to cardiovascular risk factors seen in nondiabetic subjects, diabetes-specific cardiovascular risk factors also contribute to CAD in diabetic subjects.
Health Burden Due to Coronary Artery Disease and Diabetes Worldwide
Since 1990, CAD has been the leading cause of death worldwide, and this trend is expected to continue until 2020.11 Cardiovascular diseases accounted for 30.9% of all deaths in 1998 and 10.3% of disability adjusted life year loss.11 Most of the developing countries have witnessed a dramatic increase in the prevalence of CAD, while the developed countries have followed a reverse trend.12 By 2020, 85% of the global cardiovascular disease burden is expected to be borne by developing nations, and the increase in CAD mortality in developing countries between 1990 to 2020 is projected to be 120% in women and 137% in men.13,14 As these projections are based on conservative figures and changes in demography of the population, the actual numbers of CAD-related deaths would be more alarming if increases in all cardiovascular risk factors are taken into account.
Currently holding the 15th place in the list of causes of death worldwide, diabetes is expected to affect 300 million people globally by the year 2025 compared to 135 million in 1995, according to recent statistics from the World Health Organization.1 Moreover, the increase in prevalence of diabetes in developing countries is projected to be 170% compared to 42% in developed countries. Thus, developing nations would contribute to more than 75% of the global diabetes burden by the year 2025.
Epidemiology of Coronary Artery Disease in Indians
India is predicted to bear the greatest CAD burden, according to the estimates from the Global Burden of Disease Study.11 Of the more than 9 million deaths due to CAD in 1990 in developing countries, 2.4 million (25%) occurred in India.11,14 In the same year, mortality rates in India due to acute myocardial infarction (MI) were 141 per 100,000 in males and 136 per 100,000 in females, which was much higher than in China (66 per 100,000 in males and 69 per 100,000 in females) and Latin American countries (81 per 100,000 in males and 76 per 100,000 in females). The overall cardiovascular mortality in Indians is predicted to rise by 103% in men and 90% in women between 1985 and 2015. A matter of serious concern is that 52% of the CAD deaths in India occurred in people aged below 70 years, while the same was just 22% in developed countries.14,15
In 1959, Shaper and Jones demonstrated the predilection to CAD among Asian Indians in Uganda.16 Later studies from various parts of the world not only confirmed that migrant Indians have much higher prevalence of CAD and CAD mortality rates compared to the host populations of those countries but also showed that Asian Indians develop CAD at least a decade earlier.17–22 Higher prevalence of cardiovascular disease in South Asians living in Canada as compared to Europeans and Chinese was demonstrated by the Study of Health Assessment and Risk in Ethnic groups study.22
Evidence indicates that the prevalence of CAD is rapidly increasing in India, particularly in the urban areas. In the 1970s, prevalence of CAD was 1.0% in urban India.14 By 1990, the prevalence of CAD reported by Chadha et al. in Delhi was 9.7%.23 Remarkably high prevalence of both CAD and cardiovascular risk factors was also shown in the Jaipur Heart Watch study.24 A meta-analysis of the CAD prevalence based on the surveys conducted since 1990 suggested that the increase in prevalence of CAD in the urban and rural populations were nine-fold and two-fold, respectively.25 Thus, in the next 15 years, a phenomenal increase in the prevalence of CAD is expected in India, adding to the health burden due to CAD among Indians.15,25
Epidemiology of Diabetes in Indians
There are currently 135 million people with diabetes in the world, and India leads the world with 40.9 million people in diabetes in 2007.26 Moreover, it is projected that, by the year 2025, 80.9 million will have diabetes in India. The prevalence of diabetes in urban Indians has steadily increased from 2.1% in the 1970s27 to 8.2% in the 1980s,28 later climbing to 12–16%.29,30 Thus the phenomenon of high prevalence of diabetes reported among migrant Asian Indians31 has now spread to urban India and is rapidly moving to rural areas as well.32 There is still inadequate population-based data on the prevalence of CAD in India, particularly comparing diabetic and nondiabetic subjects.
Chennai Urban Population Study
The Chennai Urban Population Study (CUPS) is a population-based study involving two residential areas representing the lower- and middle-income groups in Chennai in South India.33 All inhabitants in these two colonies aged above 20 years were requested to participate in the study, and 90.2% responded to the study. The subjects were classified as normal glucose tolerance (NGT), impaired glucose tolerance (IGT), or diabetes, based on oral glucose tolerance test. The prevalence of diabetes in this study population was 12%, while that of IGT was 5.9%.34
Techniques and Methods Used to Assess CAD and Pre-clinical Atherosclerotic Markers in CUPS
In the CUPS, CAD was diagnosed based on a past history of documented MI or electrocardiogram changes suggestive of ST-segment depression (Minnesota codes 1-1-1 to 1-1-7) or Q-wave changes (Minnesota codes 4-1 to 4-2) or T-wave changes (Minnesota codes 5-1 to 5-3).35
Structural and functional preclinical atherosclerotic markers were also assessed in diabetic and nondiabetic subjects in the CUPS population. Carotid intimal medial thickness (IMT) was studied to assess the structural changes in the arteries, and functional changes were assessed by studying endothelial dysfunction by flow-mediated dilatation and arterial stiffness studies.36–38
Measurement of the Carotid Intimal Medial Thickness
Measurement of the carotid IMT is being used increasingly as a noninvasive marker of atherosclerosis.38 In the CUPS, the intimal plus medial thickness of the carotid arteries was determined using a high-resolution B mode ultrasonography system (Logic 400 GE, Milwaukee, WI) having an electrical linear transducer midfrequency of 7.5 MHz. The axial resolution of the system was 0.3 mm. The images were recorded as well as photographed. The scanning was done for an average of 20 min. Intimal medial thickness, as defined earlier,39 was measured as the distance from the leading edge of the first echogenic line to the second echogenic line. The first echogenic line represents the lumen intimal interface, and the second line is produced by the collagen-containing upper layer of the intimal adventitia. At each longitudinal projection, determinations of IMT were conducted at the side of greatest thickness and at two points 1 cm upstream and 1 cm downstream from the side of greatest thickness, as described previously.40 The mean of the six IMT measurements (three from the left and three from the right) was used as the representative value for each subject.
Arterial Stiffness Assessment
Arterial stiffness was assessed by measuring the augmentation index (AI) using the Sphygmocor machine (Sphygmocor BPAS–1; PWV Medical, Sydney, Australia). In brief, a high-fidelity micromanometer (SPC-301; Millar Instruments, Houston, Texas) was used to flatten but not occlude the right radial artery using gentle pressure. When the two surfaces are flattened, circumferential pressures are equalized and an accurate pressure waveform can be recorded. Data were collected directly into a portable microcomputer. The system software allowed online recording of the peripheral waveform, which was assessed visually to ensure that the best possible recording was obtained and that artifacts from movement were minimized. After 20 sequential waveforms had been acquired, the integral software was used to generate an averaged peripheral and corresponding central waveform that was used for the determination of the AI. The AI was defined as the difference between the first and second peaks of the central arterial waveform, expressed as a percentage of the pulse pressure.39
Endothelial Dysfunction
Endothelial dysfunction was measured as flow-mediated dilatation (FMD), and FMD of the brachial artery was determined using a high-resolution B-mode ultrasono-graphic system (Logic 400 GE) with an electrical linear transducer midfrequency of 7.5 MHz, using the technique described by Celermajer and associates.38 Briefly, each subject was requested to lie at rest for more than 10 min before the procedure began, and the first scan at rest was then taken. This was followed by inflation of the pneumatic tourniquet of the standard sphygmomanometer (Diamond BP Apparatus) placed around the forearm to a pressure of 300 mm Hg followed by deflation after 4.5 min. The second scan was taken 30 s before and 90 s after cuff deflation. Fifteen minutes was then allowed for vessel recovery, and a further scan at rest was then recorded. Sublingual glyceryl trinitrate spray (400 μg) was administered, and 3 to 4 min later, the last scan was performed. Electrocardiography was monitored continuously throughout the study. Flow-mediated dilatation was calculated using the following ratio: diameter of brachial artery after cuff deflation to the diameter measured at rest.38
In the CUPS study, all three preclinical atherosclerotic markers were assessed on the same day on the right side (right common carotid artery, right brachial artery, and right radial artery).
Coronary Artery Disease in Diabetic and Nondiabetic Subjects in the Chennai Urban Population Study
The prevalence of CAD in the CUPS study was 11% in the total population, with 1.2% patients having had a MI, 1.3% with Q-wave changes, 1.5% with ST-segment changes, and 7.0% with T-wave abnormalities.41,42 This 11% represents a 10-fold increase in CAD prevalence in urban India since 1970,41,43 now approaching those reported in migrant Indians. In the same study, the prevalence of CAD among diabetic subjects was 21.4% (known diabetes, 25.3%, and newly diagnosed diabetes, 13.1%), which was much higher than the figure of 14.9% among subjects with IGT and 9.1% among those with NGT (Table 1).41 Prevalence of known MI was three times higher in diabetic subjects. However, this study showed that the risk for CAD increased even at the stage of IGT itself.
Table 1.
Prevalence of Coronary Artery Disease in South Indian Subjects With and Without Glucose Intolerance41
| Subjects | |||
|---|---|---|---|
| NGT | IGT | Diabetes | |
| Documented MI (%) | 0.9% | — | 3.4% |
| Overall Q waves (%) | 1.2% | 1.4% | 8.2% |
| ST-segment depression (%) | 1.1% | 5.4% | 2.8% |
| T-wave abnormalities (%) | 6.6% | 8.1% | 9.0% |
| Total CAD prevalence (%) | 9.1% | 14.9% | 21.4% |
Preclinical Atherosclerotic Markers in Diabetic and Nondiabetic Subjects in the Chennai Urban Population Study
In the CUPS, the mean IMT values among diabetic subjects were significantly higher (0.95 ± 0.31 mm) compared to normal subjects (0.74 ± 0.14 mm) (p < .001). The range of IMT values in nondiabetic subjects was 0.5–1.2 mm, whereas it was 0.4–3.0 mm in patients with diabetes. An IMT value ≥ 1.1 mm was used as a cut off for defining carotid atherosclerosis, and using this definition, 20% of diabetic subjects had carotid atherosclerosis compared to 1% of nondiabetic subjects.44
Endothelial dysfunction, measured as FMD, was found to be reduced in diabetes patients compared to age- and sex-matched nondiabetic subjects.45 Arterial stiffness was also found to be significantly greater among diabetic subjects compared to age- and sex-matched nondiabetic subjects. Pearson correlation analysis of AI and FMD was done with the risk factors for CAD, which revealed age, fasting plasma glucose, and glycated hemoglobin to be positively associated with AI and negatively associated with FMD. These studies confirm that Asian Indian diabetic subjects have an increased tendency to develop premature atherosclerosis compared to their nondiabetic counterparts.
Diabetes–Coronary Artery Disease Link
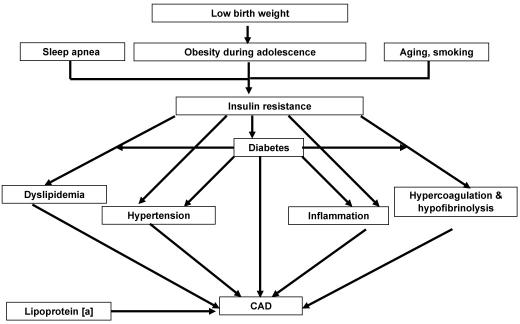
The identified risk factors for CAD include aging, smoking, and a family history of CAD. Type 2 diabetes is a part of the insulin resistance cluster or metabolic syndrome, which is a combination of hyperglycemia, central body obesity, dyslipidemia, and hypertension (Figure 1).41 Each of these can contribute independently to the CAD risk or may cluster to increase the risk (Figure 2).46 The role of some CAD risk factors are discussed here, particularly in relation to studies from India.
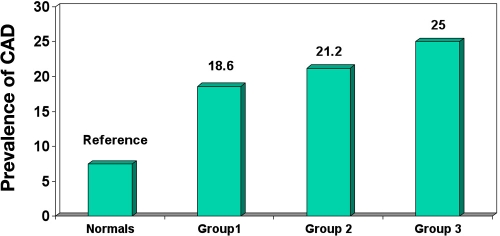
Figure 1.
Prevalence of CAD among subjects with multiple risk factors.41 Group 1, glucose intolerance (IGT + diabetes); group 2, glucose intolerance ([IGT + diabetes] + hypertension); group 3, glucose intolerance ([IGT + diabetes) + hypertension + dyslipidemia). Dyslipidemia = serum cholesterol ≥ 200 mg/dl or triglycerides ≥ 140 mg/dl.
Figure 2.
The interaction of cardiovascular risk factors.46
Plasma Glucose Levels and Coronary Artery Disease
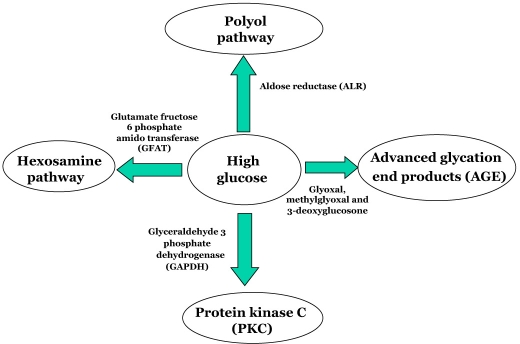
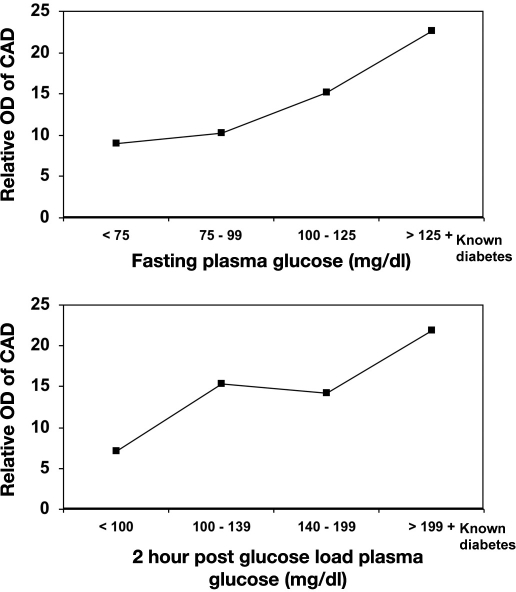
Multiple biochemical alterations have been reported in diabetes, and several metabolic pathways seem to be involved in glucose toxicity, with a possible redundancy in their mechanisms.47 Insulin deficiency and hyperglycemia enhance glucose metabolism through multiple pathways, including the polyol pathway, advanced glycation end products, protein kinase C, and hexosamine pathway (Figure 3).48 Increased plasma glucose concentration leads to increased glycosylation of proteins, particularly lipoproteins. Glycosylation of low-density lipoprotein (LDL) has been shown to enhance its susceptibility to oxidation, which triggers the atherosclerotic processes. The Honolulu Heart Study, the Bedford Study, and the Pathological Determinants of Atherosclerosis in Youth Study are some of the studies that have demonstrated the association of hyperglycemia with CAD.49–51 Reduction of CAD events with intensive glycemic control using insulin was shown in the randomized trial of insulin–glucose infusion followed by subcutaneous insulin treatment in diabetes patients with acute MI,52 which indirectly proves the association of hyperglycemia with CAD. In the CUPS, prevalence of CAD was seen to increase with increase in fasting plasma glucose levels, even among nondiabetic subjects. The odds ratio (OR) for CAD increased with increase in quartiles of fasting plasma glucose and 2 h postglucose load plasma glucose, indicating a strong association of plasma glucose levels with CAD (Figure 4), which also means that in Indians, as shown in the West, the clock for CAD starts “ticking” even at the IGT stage itself. It also indicates that the plasma glucose– CAD relationship is a continuum and that there is no threshold value of risk.53
Figure 3.
Multiple biochemical aberrations based on high glucose.
Figure 4.
Odds ratio of CAD in relation to plasma glucose (CUPS).
Sleep Apnea and Coronary Artery Disease
Sleep-related breathing disorders are highly prevalent in patients with established cardiovascular disease.54 Obstructive sleep apnea (OSA) has been linked to increased cardiovascular morbidity and mortality from both coronary heart disease and stroke,55,56 but whether this risk is due to coexistent known cardiovascular risk factors or specific effects of OSA remains to be established. Udwadia and coworkers57 have shown that higher prevalence of OSA in urban middle-aged Indian men is striking and may have major public health implications in a developing country with limited health resources.
Blood Pressure and Coronary Artery Disease
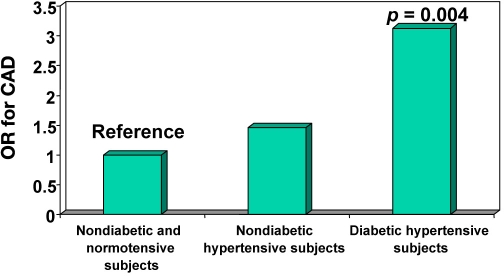
The high risk for CAD among hypertensive subjects has been documented in several studies. Further, intervention studies using antihypertensive medications have shown significant decrease in CAD risk.58,59 The overall prevalence of hypertension in the CUPS was 22.1%, of which 8.2% had “known” hypertension. Coronary artery disease was much more prevalent among hypertensives than normotensives. The CAD risk was even higher among subjects who both had diabetes and were hypertensive (OR 3.13, p = .004) (Figure 5). Both systolic and diastolic blood pressure showed a strong correlation with CAD on a univariate analysis in the CUPS study.41
Figure 5.
Odds ratio of CAD among subjects with diabetes and hypertension (CUPS).
Dyslipidemia and Coronary Artery Disease
Dyslipidemias, which include high serum cholesterol, high serum triglycerides, high LDL cholesterol and low high-density lipoprotein (HDL) cholesterol, are known to be associated with diabetes. Several intervention studies have clearly shown reduction in CAD mortality through reduction of serum cholesterol and triglyceride levels.60,61 However, the association of isolated hypertriglyceridemia with CAD is still a matter of debate.61
A LDL cholesterol level less than 100 mg/dl has been proposed as the treatment goal in diabetes patients by the recent NCEP guidelines.62 A recent meta-analysis on the effect of statins on LDL cholesterol recommended using statins to lower LDL cholesterol markedly and suggested that the reduction of CAD risk was possible by almost 60%.63 High-density lipoprotein cholesterol, in contrast to LDL cholesterol, is a protective lipoprotein with anti-atherogenic potential. It is also believed to reduce peroxidation, as it carries enzymes like paraoxanases.64
The prevalence of CAD in the CUPS increased with an increase in total cholesterol (trend χ2 26.2, p < .001), low-density lipoprotein cholesterol (trend χ2 24.5, p < .001), triglycerides (trend χ2 9.96, p = .002), and total cholesterol/HDL ratio (trend χ2 6.14, p = .0132). Age (OR 1.05, p < .001) and LDL cholesterol (OR 1.009, p = .051) were identified as the main risk factors for CAD by multiple logistic regression analysis.41 In a large clinic-based study carried out on 17,855 type 2 diabetes subjects, the association of isolated hypertriglyceridemia and isolated hypercholesterolemia with CAD was assessed. The prevalence of CAD was significantly higher among patients with isolated hypercholesterolemia, isolated high LDL, and isolated low HDL cholesterol compared to normolipidemic individuals, but not in those with isolated hypertriglyceridemia.65 Regression analysis revealed LDL cholesterol to be associated with CAD.41
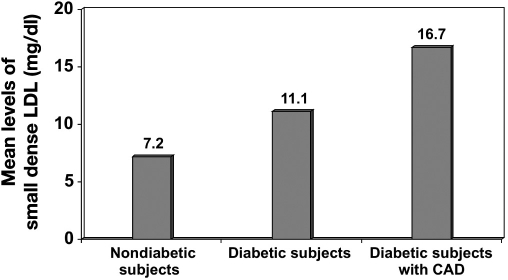
It is important to note that, in CUPS, subjects with CAD had lipid levels that were much lower than the high-risk category described by NCEP guidelines.9 For instance, the mean total cholesterol and LDL cholesterol in the nondiabetic groups with CAD were 182 ± 36 mg/dl and 118 ± 30 mg/dl, respectively. Indians are known to have much lower HDL cholesterol levels, and hence the total cholesterol/HDL cholesterol and LDL/HDL cholesterol rates are higher in Indians.41 The OR for CAD was calculated in the CUPS in relation to total cholesterol/ HDL ratio. A ratio of ≤ 4.0 was taken as the reference, and the OR for CAD for a total cholesterol/HDL ratio of 4.1–4.5 was 1.82, which increased further with further increase in this ratio. Recent studies have emphasized the role of small dense LDL in atherogenesis and have shown that diabetic subjects have higher levels of small dense LDL compared to nondiabetic subjects. A study in Birmingham, Alabama, revealed that migrant Indians have higher small dense LDL compared to their white counterparts.66 The Chennai Urban Rural Epidemiology Study (CURES) conducted in South Indians showed that small dense LDL levels were higher in diabetic patients and even higher in diabetic subjects with CAD, as shown in Figure 6.67
Figure 6.
Mean levels of small dense LDL (CURES).67
Clustering of Metabolic Risk Factors and Insulin Resistance Syndrome
Type 2 diabetes is a combination of several metabolic abnormalities, and most of these are preceded by insulin resistance. Fasting insulin levels have been found, in prospective studies, to be a surrogate marker of insulin resistance and a predictor of CAD.68 Most of the cardiovascular risk factors like dyslipidemia, hypertension, obesity, central obesity, and glucose intolerance have been shown to be associated with insulin resistance, and a combination of these abnormalities could lead to CAD. The term “syndrome X” was first coined by Reaven69 to denote this cluster, which is also called “metabolic syndrome” or “insulin resistance syndrome” (IRS). The metabolic cluster seems to explain a major part of the pathogenesis of CAD. In CUPS, the CAD risk increased with increase in the number of metabolic abnormalities. The same study also assessed the prevalence of IRS using the European Group of Insulin Resistance (EGIR) criteria and found that IRS was present in 11.2% of urban South Indians. It is to be noted, however, that this figure of 11.2% was based on the higher cutoff points that the EGIR recommends for dyslipidemia, i.e., serum triglyceride levels more than 200 mg/dl and/or serum cholesterol levels greater than 200 mg/dl. If the Adult Treatment Panel III guidelines, which have much lower cutoffs, had been used, the prevalence of IRS would have been obviously much higher. Clustering of these metabolic parameters was evident even among young individuals.70 Misra and Vikram71 described this clustering effect and suggested that body fat, dietary modification, physical inactivity, and stress are important contributory factors for high prevalence of metabolic syndrome in Indians, and the term “cardio-metabolic syndrome” is used for this entity.72
Asian Indians have higher prevalence of hyperinsulinemia, insulin resistance, and other components of metabolic syndrome. Obesity, particularly abdominal obesity, is considered to contribute to the increased insulin resistance in Indians. Though Indians have low rates of generalized obesity, the prevalence of abdominal obesity is higher compared to other ethnic groups.73,74 Further, for any given degree of obesity, Indians also have higher body fat than other ethnic groups, and for any given body mass index, the waist-to-hip ratio was higher among Indians.75 Finally, for any given body fat, Indians have higher insulin resistance compared to other ethnic groups.73
Low Birth Weight
Low birth weight has been shown to contribute to insulin resistance among Indians.76 It has been hypothesized that low birth weight followed by a tendency for obesity in childhood or adolescence could lead to IRS during adulthood (Figure 2). Indians have insulin resistance and adiposity even at birth when compared to Caucasians.76 Barker and colleagues postulated that this was a result of fetal adaptation to inadequate intrauterine nutrition.77 An alternative hypothesis is that CAD and low birth weight could share a common genetic predisposition. In India, according to the National Health Survey, the prevalence of low birth weight among neonates is 28%.78 A strong association for low birth weight with insulin resistance has been shown in Indian children. A study of a cohort of 1492 subjects followed starting in 1969 revealed that the prevalence of diabetes was highest among subjects with lowest weight at age 2 and highest weight at age 12.79
Fibrinolytic Factors
Many of the new risk factors like plasminogen activator inhibitor-1 (PAI-1), fibrinogen, and inflammatory markers like C-reactive protein (CRP) and interleukins have been included in the list of abnormalities under the insulin resistance syndrome.80 Some of the comparative studies on migrant Indians have suggested that the excess risk for CAD seen among Indians could be partly explained by these risk factors.81–83 Fibrinogen and PAI-1 levels have been found to be associated with angiographically proven CAD, and the relative ORs for CAD increased with increase in quartiles of fibrinogen and PAI-1.82 A study from South India also showed a weak association of PAI-1 with CAD,83 while another study on native Indians showed that PAI-1 correlated well with triglycerides.84
Lipoprotein (a)
Lipoprotein (a) [Lp(a)], an atherothrombogenic moiety, is a complex of apolipoprotein (a) [Apo(a)] and LDL, which is determined genetically.85 It can competitively inhibit plasminogen activity, leading to impaired fibrinolysis. Lipoprotein (a) has also been implicated in enhanced oxidation and foam cell formation. The smaller the Apo(a), the higher are the Lp(a) levels and the risk for CAD. Lipoprotein (a) levels above 20 mg/dl are reported to be associated with a high risk of CAD.86 In a South Indian study on 300 subjects, Lp(a) had an independent association with CAD in type 2 diabetes patients. An increase in Lp(a) was found to be associated with increase in carotid IMT, a preclinical atherosclerotic marker.87 This suggests that Lp(a) is associated with CAD even at an early stage of atherosclerosis.
Homocysteine
Homocysteine, a sulfur-containing amino acid, is an atherothrombogenic moiety that triggers platelet adhesion in cell culture.88 Homocysteine has been shown to be strongly associated with CAD in several studies.89 Migrant Indian studies have shown higher levels of homocysteine compared to the native population.90,91 However, studies on its association with CAD among native Indians have been consistently negative.92,93 Yet these studies should be interpreted with caution, as they were based on small sample sizes, and moreover, oral methionine-loaded homocysteine levels were not assessed.
Inflammatory Markers
There is increasing evidence that inflammatory processes and specific immune mechanisms are involved in atherogenesis, and inflammatory markers are reported to be higher among subjects with insulin resistance and diabetes.94 Inflammation is considered to be a part of insulin resistance syndrome,95 and this, at least partly, explains the high risk for CAD among diabetic subjects. Inflammatory changes could take place near the rupture of the plaque, leading to instability in the fibrous tissue in the plaque. Studies on pro-inflammatory markers have revealed that cytokines like tumor necrosis factor α (TNF-α), CRP, and interleukin-6 are strongly associated with CAD. Studies suggest that TNF-α plays a key role in mediating insulin resistance as a result of obesity.96 C-reactive protein levels seem to be higher in migrant Indians compared to other ethnic groups.97,98In a large study of 1025 subjects, CRP levels were 17% higher in Asian Indians compared with white Europeans. C-reactive protein also had a strong association with cardiovascular risk factors like obesity, insulin resistance, and lipids.97 In another age-matched study on 82 Asian Indian men and 55 Caucasian men with similar body fat content and truncal skin-fold thickness, Asian Indians were shown to have elevated CRP levels, suggesting that pro-inflammatory factors may contribute to increased risk for diabetes and CAD.98 Asian Indian children were also shown to have 104% higher levels of CRP compared to Europeans.99 However, there have been very few studies on native Indians. One study showed that CRP correlated significantly with body fat.100 In our study on 150 subjects, which included nondiabetic subjects without CAD and diabetic subjects with and without CAD, CRP levels were higher among diabetic subjects with and without CAD compared to nondiabetic subjects without CAD.100 A review on the relevance of CRP in young individuals associates high CRP in Indians with excess body fat, subcutaneous fat, and physical inactivity.101
Other markers of CAD are endothelin 1, adhesion molecules like vascular cell adhesion molecule, intercellular adhesion molecule 1, E-selectin, and P-selectin, elevated levels of which have been shown to be associated with serious coronary events and angiographically documented CAD102–105 in Western populations. Inhibiting the action of adhesion molecules has been a focus to prevent atherosclerosis.106 Owing to ethnic differences in the prevalence of CAD, ethnic-specific studies on the association of these parameters with CAD are required.
Prevention of Coronary Artery Disease
India is now facing a double epidemic of diabetes and CAD. Research studies indicate that diabetes plays a contributory role for CAD in Indians by increasing the risk for hypertension, hypercholesterolemia, hyper-triglyceridemia, low HDL cholesterol, and increasing PAI-1 and fibrinogen levels. Prospective longitudinal cohort studies for evaluation of coronary risk factors in India are the need of the day, as most of the current data are cross sectional in nature.
Pharmacological Intervention
Despite the fact that many of the risk factors for CAD are genetically inherited, pharmacological intervention has been found by prospective studies to reduce the incidence of CAD. Statin trials have demonstrated that reducing LDL cholesterol could be very beneficial in reducing cardiovascular mortality.60,107,108 Trials using fibrates have shown that reduced triglycerides and moderate elevation of HDL can prevent cardiovascular events.109,110 The United Kingdom Prospective Diabetes Study (UKPDS) and Microalbuminuria, Cardiovascular, and Renal Outcomes in the Heart Outcomes Prevention Evaluation have shown that intensive hypertension control is beneficial in reducing cardiovascular events, even among diabetic subjects. However, in the UKPDS, good control of blood glucose by itself was not sufficient to significantly reduce risk of cardiovascular disease, although there was a 16% reduction. A multifactorial approach by good control of blood glucose, blood pressure, and serum lipids appears to be necessary to prevent CAD in diabetes patients.111,112
Lifestyle Changes
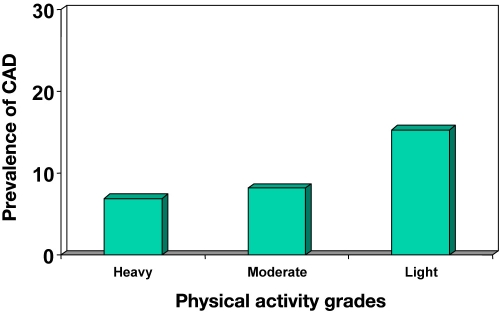
Dietary modification, regular physical activity, weight reduction, and cessation of smoking have been proven to be beneficial in preventing CAD. The Harvard Alumni Study documented that physical inactivity plays a role in CAD.113 The CUPS revealed that subjects who performed light-grade activity had increased prevalence of not only CAD but also all cardiovascular risk factors compared to subjects who performed heavy-grade activity (Figure 7).114 Though there are very few studies on the role of exercise in prevention of CAD in diabetes patients, there is ample evidence to support that exercise does reduce cardiovascular risk factors and thus can potentially be of great help in reducing CAD itself.115–118
Figure 7.
Prevalence of CAD in relation to grades of physical activity (CUPS).114
In summary, given the explosion of diabetes and CAD in India, increased emphasis on lifestyle modification, including diet, exercise, weight reduction, and, whenever relevant, stress reduction, is urgently needed. A comprehensive surveillance system of risk factors and CAD will be an invaluable public health research tool for monitoring population health status, guiding resource allocation and policy, identifying and prioritizing interventions for subpopulations at particular risk, identifying disparities in outcomes, and planning and evaluating health programs. Carefully planned prevention programs with intervention strategies could also be taken up in different parts of the country to prevent the double epidemic of diabetes and CAD, as both have common causative factors, and prevention strategies could also be combined judiciously to prevent both disorders, as this would make it more cost-effective.
Acknowledgment
We are grateful to the epidemiology team and the study participants. This is the 20th publication from Chennai Urban Population study (CUPS 20).
Abbreviations
- AI
augmentation index, [Apo(a)] apolipoprotein (a)
- CAD
coronary artery disease
- CRP
C-reactive protein
- CUPS
Chennai Urban Population Study
- CURES
Chennai Urban Rural Epidemiology Study
- EGIR
European Group of Insulin Resistance
- FMD
flow-mediated dilatation
- HDL
high-density lipoprotein
- IGT
impaired glucose tolerance
- IMT
intimal medial thickness
- IRS
insulin resistance syndrome
- LDL
low-density lipoprotein, [Lp(a)] lipoprotein (a)
- MI
myocardial infarction
- NCEP
National Cholesterol Education Program
- NGT
normal glucose tolerance
- OR
odds ratio
- OSA
obstructive sleep apnea
- PAI-1
plasminogen activator inhibitor-1
- TNF-α
tumor necrosis factor α
- UKPDS
United Kingdom Prospective Diabetes Study
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with Type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114(2):413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 4.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br Med J. 1983;287(6396):867–870. doi: 10.1136/bmj.287.6396.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102(9):1014–1019. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 6.Nesto RW, Rutter MK. Impact of the atherosclerotic process in patients with diabetes. Acta Diabetol. 2002;39(Suppl 2):22–28. doi: 10.1007/s005920200022. [DOI] [PubMed] [Google Scholar]
- 7.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102(18):2180–2184. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 8.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281(14):1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann Intern Med. 2003;139(10):824–834. doi: 10.7326/0003-4819-139-10-200311180-00010. [DOI] [PubMed] [Google Scholar]
- 11.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 13.Bulato RA, Stephens PW. Global estimates and projections of mortality by cause. Washington DC: Population, Health and Nutrition Department: World Bank. 1992;1007 [Google Scholar]
- 14.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97(6):596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 15.Bahl VK, Prabhakaran D, Karthikeyan G. Coronary artery disease in Indians. Indian Heart J. 2001;53(6):707–713. [PubMed] [Google Scholar]
- 16.Shaper AG, Jones KW. Serum cholesterol, diet and coronary heart-disease in Africans and Asian in Uganda. Lancet. 1959;2(7102):534–537. doi: 10.1016/s0140-6736(59)91777-5. [DOI] [PubMed] [Google Scholar]
- 17.Miller GJ, Beckles GL, Alexis SD, Byam NT, Price SG. Serum lipoproteins and susceptibility of men of Indian descent to coronary heart disease. The St James Survey, Trinidad. Lancet. 1982;2(8291):200–203. doi: 10.1016/s0140-6736(82)91041-8. [DOI] [PubMed] [Google Scholar]
- 18.Hughes K, Yeo PP, Lun KC, Sothy SP, Thai AC, Wang KW, Cheah JS. Ischaemic heart disease and its risk factors in Singapore in comparison with other countries. Ann Acad Med Singapore. 1989;18(3):245–249. [PubMed] [Google Scholar]
- 19.Balarajan R. Ethinic differences in mortality from ischemic heart disease and cerebrovascular disease in England and Wales. BMJ. 1991;302(6776):560–564. doi: 10.1136/bmj.302.6776.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckles GL, Miller GJ, Kirkwood BR, Alexis SD, Carson DC, Byam NT. High total and cardiovascular disease mortality in adults of Indian descent in Trinidad, unexplained by major coronary risk factors. Lancet. 1986;1(8493):1298–1301. doi: 10.1016/s0140-6736(86)91221-3. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackness MI, Creed F, Tomenson B, Chandrashekhar Y, Winterbotham M, Britt RP, Keil JE, Sutton GC. Coronary risk factors in people from the Indian subcontinent living in west London and their siblings in India. Lancet. 1995;345(8947):405–409. doi: 10.1016/s0140-6736(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 22.Anand SS, Yusuf S, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356(9226):279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 23.Chadha SL, Radhakrishnan S, Ramachandran K, Kaul U, Gopinath N. Epidemiological study of coronary heart disease in urban population of Delhi. Indian J Med Res. 1990;92:424–430. [PubMed] [Google Scholar]
- 24.Gupta R, Gupta VP, Sarna M, Bhatnagar S, Thanvi J, Sharma V, Singh AK, Gupta JB, Kaul V. Prevalence of coronary heart disease and risk factors in an urban Indian population: Jaipur Heart Watch-2. Indian Heart J. 2002;54(1):59–66. [PubMed] [Google Scholar]
- 25.Gupta R, Gupta VP. Meta-analysis of coronary heart disease prevalence in India. Indian Heart J. 1996;48(3):241–245. [PubMed] [Google Scholar]
- 26.Sicree R, Shaw J, Zimmet P. Diabetes and impaired glucose tolerance. In: Gan D, editor. Diabetes Atlas. Third Ed. Belgium: International Diabetes Federation; 2006. 15 pp.103 pp. [Google Scholar]
- 27.Ahuja MMS. Epidemiological studies on diabetes mellitus in India. In: Ahuja MMS, editor. Epidimiology of diabetes in developing countries. New Delhi: Interprint; 1979. pp. 29–38. [Google Scholar]
- 28.Ramachandran A, Jali MV, Mohan V, Snehalatha C, Viswanathan M. High prevalence of diabetes in an urban population in South India. BMJ. 1988;297(6648):587–590. doi: 10.1136/bmj.297.6648.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, Rao PV, Yajnik CS, Prasanna Kumar KM, Nair JD Diabetes Epidemiology Study Group in India (DESI) High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44(9):1094–1101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 30.Mohan V, Deepa M, Deepa R, Shanthirani CS, Farooq S, Ganesan A, Datta M. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India— the Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49(6):1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 31.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337(8738):382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 32.Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, Anand K, Desai NG, Joshi PP, Mahanta J, Thankappan KR, Shah B. Urban rural differences in prevalence of self-reported diabetes in India—the WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80(1):159–168. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Shanthi Rani CS, Rema M, Deepa R, Premalatha G, Ravikumar R, Anjana Mohan, Sastry NG, Ramu M, Saroja R, Kayalvizhi G, Mohan V. The Chennai Urban Population Study (CUPS)— methodological details—(CUPS Paper No. 1) Int J Diab Dev Countries. 1999;19:149–157. [Google Scholar]
- 34.Mohan V, Shanthirani S, Deepa R, Premalatha G, Sastry NG, Saroja R. Chennai Urban Population Study (CUPS No. 4). Intra-urban differences in the prevalence of the metabolic syndrome in southern India—the Chennai Urban Population Study (CUPS No. 4) Diabet Med. 2001;18(4):280–287. doi: 10.1046/j.1464-5491.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 35.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular survey methods. 2nd Ed. Geneva: World Health Organisation; 1982. [Google Scholar]
- 36.Pignoli P, Longo T. Ultrasound evaluation of atherosclerosis. Methodological problems and technological developments. Eur Surg Res. 1986;18(3-4):238–253. doi: 10.1159/000128532. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16(12 Pt 2):2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 38.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 39.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki Y, Mohan V, Kodama M, Kajimoto Y, Morishima T, Kamada T. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994;43(5):634–639. doi: 10.2337/diab.43.5.634. [DOI] [PubMed] [Google Scholar]
- 41.Mohan V, Deepa R, Rani SS, Premalatha G Chennai Urban Population Study (CUPS No. 5) Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: the Chennai Urban Population Study (CUPS No. 5) J Am Coll Cardiol. 2001;38(3):682–687. doi: 10.1016/s0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 42.Arvind K, Pradeepa R, Deepa R, Mohan V. Diabetes and coronary artery disease. Indian J Med Res. 2002;116:163–176. [PubMed] [Google Scholar]
- 43.Padmavati S, Gupta S, Pantulu GV. Dietary fats, serum cholesterol levels and incidence of atherosclerosis in Delhi. Circulation. 1959;19(6):849–855. doi: 10.1161/01.cir.19.6.849. [DOI] [PubMed] [Google Scholar]
- 44.Mohan V, Ravikumar R, Shanthi Rani S, Deepa R. Intimal medial thickness of the carotid artery in South Indian diabetic and non-diabetic subjects: the Chennai Urban Population Study (CUPS) Diabetologia. 2000;43(4):494–499. doi: 10.1007/s001250051334. [DOI] [PubMed] [Google Scholar]
- 45.Ravikumar R, Deepa R, Shanthirani C, Mohan V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow medicated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]) Am J Cardiol. 2002;90(7):702–707. doi: 10.1016/s0002-9149(02)02593-6. [DOI] [PubMed] [Google Scholar]
- 46.Mohan V, Deepa R. Coronary artery disease and diabetes—Indian Scenario. Indian J Endocrinol Metab. 2005;7:72–85. [Google Scholar]
- 47.Dutour A. Mechanisms of glucose toxicity. New hope for prevention of diabetic complications? Eur J Endocrinol. 1997;136(1):39–40. doi: 10.1530/eje.0.1360039. [DOI] [PubMed] [Google Scholar]
- 48.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 49.Donahue RP, Abbott RD, Reed DM, Yano K. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36(6):689–692. doi: 10.2337/diab.36.6.689. [DOI] [PubMed] [Google Scholar]
- 50.Jarrett RJ, McCartney PM, Keen H. The Bedford Survey: ten year mortality rates in newly diagnosed diabetics, borderline diabetics and normoglycaemic controls and risk indices for coronary heart disease in borderline diabetics. Diabetologia. 1982;22(2):79–84. doi: 10.1007/BF00254833. [DOI] [PubMed] [Google Scholar]
- 51.McGill HC Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Pathological Determinants of Atherosclerosis in Youth (PADY) Research Group. Arterioscler Thromb Vasc Biol. 1995;15(4):431–440. doi: 10.1161/01.atv.15.4.431. [DOI] [PubMed] [Google Scholar]
- 52.Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenström A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 53.Balkau B, Shipley M, Jarrett RJ, Pyörälä K, Pyörälä M, Forhan A, Eschwège E. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21(3):360–367. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- 54.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 55.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94(1):9–14. [PubMed] [Google Scholar]
- 56.Partinen M, Guilleminault C. Daytime sleepiness and vascular morbidity at seven-year follow-up in obstructive sleep apnea patients. Chest. 1990;97(1):27–32. doi: 10.1378/chest.97.1.27. [DOI] [PubMed] [Google Scholar]
- 57.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169(2):168–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 58.Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338(10):645–652. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 59.Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S LIFE Study Group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 60.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 61.Gotto AM Jr. Triglyceride: the forgotten risk factor. Circulation. 1998;97(11):1027–1028. doi: 10.1161/01.cir.97.11.1027. [DOI] [PubMed] [Google Scholar]
- 62.Durrington P. Dyslipidaemia. Lancet. 2003;362(9385):717–731. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 63.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326(7404):1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Libby P. Managing the risk of atherosclerosis: the role of high-density lipoprotein. Am J Cardiol. 2001;88(12A):3N–8N. doi: 10.1016/s0002-9149(01)02145-2. [DOI] [PubMed] [Google Scholar]
- 65.Rajmohan L, Deepa R, Mohan A, Mohan V. Association between isolated hypercholesterolemia, isolated hypertriglycerideia and coronary artery disease in South Indian type 2 diabetic patients. Indian Heart J. 2000;52(4):400–406. [PubMed] [Google Scholar]
- 66.Kulkarni KR, Markovitz JH, Nanda NC, Segrest JP. Increased prevalence of smaller and denser LDL particles in Asian Indians. Arterioscler Thromb Vasc Biol. 1999;19(11):2749–2755. doi: 10.1161/01.atv.19.11.2749. [DOI] [PubMed] [Google Scholar]
- 67.Mohan V, Deepa R, Velmurugan K, Gokulakrishnan K. Association of small dense LDL with coronary artery disease and diabetes in urban Asian Indians—the Chennai Rural Epidemiology Study (CURES 8) J Assoc Physicians India. 2005;53:95–100. [PubMed] [Google Scholar]
- 68.Pyörälä M, Miettinen H, Laakso M, Pyörälä K. Plasma insulin and all-cause, cardiovascular, and noncardiovascular mortality: the 22-year follow-up results of the Helsinki Policemen Study. Diabetes Care. 2000;23(8):1097–1102. doi: 10.2337/diacare.23.8.1097. [DOI] [PubMed] [Google Scholar]
- 69.Reaven GM. A syndrome of resistance to insulin stimulated uptake (Syndrome X). Definitions and implications. Cardiovasc Risk Factors. 1993;3:2–11. [Google Scholar]
- 70.Misra A, Reddy RB, Reddy KS, Mohan A, Bajaj JS. Clustering of impaired glucose tolerance, hyperinsulinemia and dyslipidemia in young north Indian patients with coronary heart disease: a preliminary case-control study. Indian Heart J. 1999;51(3):275–280. [PubMed] [Google Scholar]
- 71.Misra A, Vikram NK. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition. 2004;20(5):482–491. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Joshi SR. Cardio-metabolic burden of native Asian Indian—India the global capital. J Assoc Physicians India. 2004;52:359–361. [PubMed] [Google Scholar]
- 73.Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84(7):2329–2335. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 74.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84(1):137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 75.Ramachandran A, Snehalatha C, Viswanathan V, Viswanathan M, Haffner SM. Risk of noninsulin dependent diabetes mellitus conferred by obesity and central adiposity in different ethnic groups: a comparative analysis between Asian Indians, Mexican Americans and Whites. Diabetes Res Clin Pract. 1997;36(2):121–125. doi: 10.1016/s0168-8227(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 76.Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, Joglekar CV, Yudkin JS. Adiposity and hyper-insulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87(12):5575–5580. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- 77.Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 78.Popkin BM, Horton S, Kim S, Mahal A, Shuigao J. Trends in diet, nutritional status, and diet-related noncommunicable disease in China and India: the economic costs of the nutrition transition. Nutr Rev. 2001;59(12):379–390. doi: 10.1111/j.1753-4887.2001.tb06967.x. [DOI] [PubMed] [Google Scholar]
- 79.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2(2):82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 81.Swahn E, von Schenck H, Wallentin L. Plasma fibrinogen in unstable coronary artery disease. Scand J Clin Lab Invest. 1989;49(1):49–54. doi: 10.3109/00365518909089077. [DOI] [PubMed] [Google Scholar]
- 82.Deepa R, Velmurugan K, Saravanan G, Dwarakanath V, Agarwal S, Mohan V. Relationship of tissue plasminogen activator, plasminogen activator inhibitor-1 and fibrinogen with coronary artery disease in South Indian male subjects. J Assoc Physicians India. 2002;50:901–906. [PubMed] [Google Scholar]
- 83.Ramachandran A, Sathyamurthy I, Snehalatha C, Satyavani K, Sivasankari S, Misra J, Girinath MR, Viswanathan V. Risk variables for coronary artery disease in Asian Indians. Am J Cardiol. 2001;87(3):267–271. doi: 10.1016/s0002-9149(00)01356-4. [DOI] [PubMed] [Google Scholar]
- 84.Sarkar R, Misra A, Saxena R, Pandey RM, Chaudhary D. Plasma plasminogen activator inhibitor-1 activity in normoglycemic hypertriglyceridemic north Asian Indian subjects: a preliminary case-control study. Indian Heart J. 2001;53(1):61–65. [PubMed] [Google Scholar]
- 85.Mohan A, Srinivasan V, Deepa R, Mohan V. Lipoprotein (a): role in diabetes and its vascular complications. J Assoc Physicians India. 2001;49:1100–1105. [PubMed] [Google Scholar]
- 86.von Eckardstein A, Schulte H, Cullen P, Assmann G. Lipoprotein(a) further increases the risk of coronary events in men with high global cardiovascular risk. J Am Coll Cardiol. 2001;37(2):434–439. doi: 10.1016/s0735-1097(00)01126-8. [DOI] [PubMed] [Google Scholar]
- 87.Velmurugan K, Deepa R, Ravikumar R, Lawrence JB, Anshoo H, Senthilvelmurugan M, Enas EA, Mohan V. Relationship of lipoprotein (a) with initmal medial thickness of the carotid artery in type 2 diabetic patients in south India. Diabet Med. 2003;20(6):455–461. doi: 10.1046/j.1464-5491.2003.00976.x. [DOI] [PubMed] [Google Scholar]
- 88.Dardik R, Varon D, Tamarin I, Zivelin A, Salomon O, Shenkman B, Savion N. Homocysteine and oxidized low density lipoprotein enhanced platelet adhesion to endothelial cells under flow conditions: distinct mechanisms of thrombogenic modulation. Thromb Haemost. 2000;83(2):338–344. [PubMed] [Google Scholar]
- 89.Moghadasian MH, McManus BM, Frohlich JJ. Homocyst(e)ine and coronary artery disease. Clinical evidence and genetic and metabolic background. Arch Intern Med. 1997;157(20):2299–2308. [PubMed] [Google Scholar]
- 90.Chandalia M, Abate N, Cabo-Chan AV, Jr, Devaraj S, Jialal I, Grundy SM. Hyperhomocysteinemia in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88(3):1089–1095. doi: 10.1210/jc.2002-021133. [DOI] [PubMed] [Google Scholar]
- 91.Chambers JC, Obeid OA, Refsum H, Ueland P, Hackett D, Hooper J, Turner RM, Thompson SG, Kooner JS. Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian Asian and European men. Lancet. 2000;355(9203):523–527. doi: 10.1016/S0140-6736(99)93019-2. [DOI] [PubMed] [Google Scholar]
- 92.Chacko KA. Plasma homocysteine levels in patients with coronary heart disease. Indian Heart J. 1998;50(3):295–299. [PubMed] [Google Scholar]
- 93.Deepa R, Velmurugan K, Saravanan G, Karkuzhali K, Dwarakanath V, Mohan V. Absence of association between serum homocysteine levels and coronary artery disease in south Indian males. Indian Heart J. 2001;53(1):44–47. [PubMed] [Google Scholar]
- 94.Jialal I, Devaraj S. Inflammation and atherosclerosis: the value of the high-sensitivity C-reactive protein assay as a risk marker. Am J Clin Pathol. 2001;116(Suppl):S108–S115. doi: 10.1309/J63V-5LTH-WYFC-VDR5. [DOI] [PubMed] [Google Scholar]
- 95.Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 96.Ledochowski M, Murr C, Widner B, Fuchs D. Association between insulin resistance, body mass and neopterin concentrations. Clin Chim Acta. 1999;282(1-2):115–123. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 97.Chambers JC, Eda S, Bassett P, Karim Y, Thompson SG, Gallimore JR, Pepys MB, Kooner JS. C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in Indian Asians from the United Kingdom compared with European whites. Circulation. 2001;104(2):145–150. doi: 10.1161/01.cir.104.2.145. [DOI] [PubMed] [Google Scholar]
- 98.Chandalia M, Cabo-Chan AV, Jr, Devaraj S, Jialal I, Grundy SM, Abate N. Elevated plasma high-sensitivity C-reactive protein concentrations in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88(8):3773–3776. doi: 10.1210/jc.2003-030301. [DOI] [PubMed] [Google Scholar]
- 99.Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, Miller GJ, Strachan DP. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149(1):139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 100.Mohan V, Deepa R, Velmurugan K, Premalatha G. Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-6) Diabet Med. 2005;22(7):863–870. doi: 10.1111/j.1464-5491.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 101.Vikram NK, Misra A, Dwivedi M, Sharma R, Pandey RM, Luthra K, Chatterjee A, Dhingra V, Jailkhani BL, Talwar KK, Guleria R. Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis. 2003;168(2):305–313. doi: 10.1016/s0021-9150(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 102.Misra A. C-reactive protein in young individuals: problems and implications for Asian Indians. Nutrition. 2004;20(5):478–481. doi: 10.1016/j.nut.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 103.Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard JM, Elkhalil L, Fruchart JC, Ducimetiere P PRIME Study Group. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170(1):169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 104.Semaan HB, Gurbel PA, Anderson JL, Muhlestein JB, Carlquist JF, Horne BD, Serebruany VL. Soluble VCAM-1 and E-selectin, but not ICAM-1 discriminate endothelial injury in patients with documented coronary artery disease. Cardiology. 2000;93(1-2):7–10. doi: 10.1159/000006995. [DOI] [PubMed] [Google Scholar]
- 105.Bousette N, Giaid A. Endothelin-1 in atherosclerosis and other vasculopathies. Can J Physiol Pharmacol. 2003;81(6):578–587. doi: 10.1139/y03-010. [DOI] [PubMed] [Google Scholar]
- 106.Bláha M, Krejsek J, Bláha V, Andrýs J, Vokurková D, Malý J, Blazek M, Skorepová M. Selectins and monocyte chemotactic peptide as the markers of atherosclerosis activity. Physiol Res. 2004;53(3):273–278. [PubMed] [Google Scholar]
- 107.Lutters BC, Leeuwenburgh MA, Appeldoorn CC, Molenaar TJ, Van Berkel TJ, Biessen EA. Blocking endothelial adhesion molecules: a potential therapeutic strategy to combat atherogenesis. Curr Opin Lipidol. 2004;15(5):545–552. doi: 10.1097/00041433-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 108.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 109.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 110.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB VA-HIT Study Group. Veterans Affairs High-Density Lipoprotein Intervention Trial. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285(12):1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 111.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomized study. Lancet. 2001;357(9260):905–910. [PubMed] [Google Scholar]
- 112.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 113.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326(7404):1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohan V, Gokulakrishnan K, Deepa R, Shanthirani CS, Datta M. Association of physical inactivity with components of metabolic syndrome and coronary artery disease—the Chennai Urban Population Study (CUPS no. 15) Diabet Med. 2005;22(9):1206–1211. doi: 10.1111/j.1464-5491.2005.01616.x. [DOI] [PubMed] [Google Scholar]
- 115.Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and coronary heart disease in men: the Harvard Alumni Health Study. Circulation. 2000;102(9):975–980. doi: 10.1161/01.cir.102.9.975. [DOI] [PubMed] [Google Scholar]
- 116.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 117.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]