Abstract
Osteosarcoma (OS) is the most common nonhematologic malignancy of bone in children and adults. Although dysregulation of tumor suppressor genes and oncogenes, such as Rb, p53, and the genes critical to cell cycle control, genetic stability, and apoptosis have been identified in OS, consensus genetic changes that lead to OS development are poorly understood. Disruption of the osteogenic differentiation pathway may be at least in part responsible for OS tumorigenesis. Current OS management involves chemotherapy and surgery. Peroxisome proliferator-activated receptor (PPAR) agonists and/or retinoids can inhibit OS proliferation and induce apoptosis and may inhibit OS growth by promoting osteoblastic terminal differentiation. Thus, safe and effective PPAR agonists and/or retinoid derivatives can be then used as adjuvant therapeutic drugs for OS therapy. Furthermore, these agents have the potential to be used as chemopreventive agents for the OS patients who undergo the resection of the primary bone tumors in order to prevent local recurrence and/or distal pulmonary metastasis.
1. Introduction
Osteosarcoma (OS) is the most common nonhematologic malignant tumor of bone in adults and children, with the peak incidence in early childhood [1, 2]. It is associated with a poor prognosis due to its high grade at presentation, resistance to chemotherapy, and propensity to metastasize to the lungs [3, 4]. Furthermore, while 80% of OS patients are believed to have micrometastatic disease, only 10%–15% present as radiographically detectable lesions [5, 6]. Herein lies the challenge in identifying the 20% OS patients without micrometastases and modifying medical and surgical management accordingly. Genetic markers associated with metastatic disease could potentially spare those patients that need for chemotherapeutic agents, such as adriamycin, cisplatin, or methotrexate, and experience its severe toxicities ranging from cardiotoxicity to renal dysfunction.
It has been shown that OS cells are similar to undifferentiated osteoblasts, and increasing evidence suggests that osteogenic differentiation defects may be responsible for OS tumorigenesis [2, 7–10]. Osteoblasts are derived from mesenchymal stem cells (MSCs), and osteoblastic differentiation is a tightly regulated process by numerous growth and differentiation factors, such as bone morphogenetic proteins (BMPs) and Wnts [2] (Figure 1). It is conceivable that any disruption of osteogenic terminal differentiation may result in the development of OS. The aggressiveness of OS may depend on the stage of disruption; that is, more aggressive OS phenotypes may be developed from mutant early osteoblast progenitors, whereas benign tumors may arise from disruptions of late stage osteoblasts [2, 7] (Figure 2).
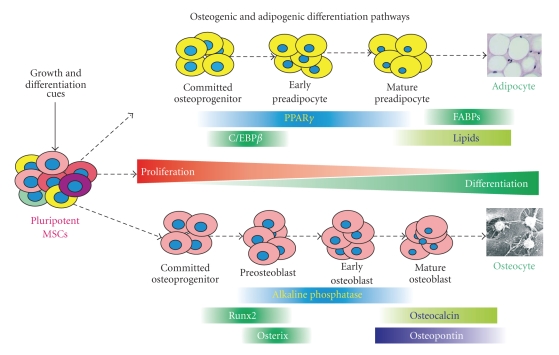
Figure 1.
Osteogenic and adipogenic differentiation pathways in mesenchymal stem cells (MSCs). MSCs are pluripotent progenitor cells that are able to differentiate into several lineages, including osteogenic and adipogenic lineages, upon the stimulation with distinct growth and differentiation cues. The lineage-specific differentiation is a multiple-stage and well-coordinated process regulated by master regulators, such as PPARγ and C/EBPβ for adipogenesis and Runx2 and Osterix for osteogenesis. Osteogenic differentiation can be staged by measuring alkaline phosphatase (early marker) and osteocalcin and osteopontin (late markers). Expression of FABPs and production of lipids are indicators of terminal adipogenic differentiation.
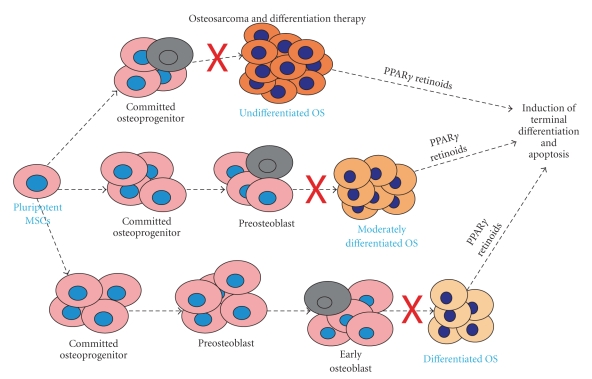
Figure 2.
Osteosarcoma (OS) development and nuclear receptor agonist-mediated differentiation therapy. OS can be regarded as a differentiation disease, which is caused by disruptions of the terminal osteogenic differentiation. The stage and nature of differentiation defects may determine the aggressiveness of OS tumors. PPARγ agonists and/or retinoids have been shown to inhibit OS proliferation, induce apoptosis, and promote osteogenic differentiation. Thus, these agents can be used as differentiation therapy, in combination with conventional chemotherapy, for OS treatment.
Current cancer therapies primarily target the proliferative compartment of tumor cells. While effective in initial treatment, these strategies are often nullified by subsequent drug resistance. An attractive alternative is to overcome the uncontrolled cell proliferation through promoting terminal differentiation [8, 9, 11–13]. One possibility deals with the use of agonists or antagonists of the nuclear receptor superfamily, including vitamin D3, thyroid hormone, glucocorticoids, sex hormones, retinoids, and orphan receptors [14–18]. One interesting subgroup of the nuclear receptor superfamily is peroxisome proliferator-activated receptors (PPARs), which play a role in both promoting tumorigenesis and inducing terminal differentiation and apoptosis. Antitumor activity of PPARγ agonists has been shown in tumor cells derived from liposarcoma, colon cancer, breast cancer, leukemia, gastric cancer, nonsmall cell lung cancer, and prostate cancer [19–31]. Furthermore, PPARγ agonists have the potential to induce terminal differentiation in osteosarcoma cells [2, 9, 32]. In this review, we focus on the functional role of PPARs and their cross-talk with other nuclear receptors in osteogenic differentiation and tumorigenesis and the potential use of PPARγ agonists as chemotherapeutic and/or chemopreventive agents for human OS.
2. PPARs and Their Ligands
PPARs are ligand-activated transcription factors that achieve functionality after forming a heterodimer with the 9-cis retinoid X receptor (RXR). The subsequent transcriptional activity is modulated by nuclear receptor coactivators and corepressors [33], such as C/EBP PGC1 (α/β), PRIP, N-Cor, SRC-1, p300, Hsp-72, and PBP [34–39]. The ligands include the synthetic thiazolinediones and fibrates and endogenous fatty acids and eicosanoids [40, 41]. Upon ligand binding and heterodimerization with RXR, PPARs recognize PPAR response elements (PPREs) containing the direct repeat sequence (DR-1) AGGTCA [33, 34]. PPARs can also repress gene transcription through interfering with NFκB, STAT, and AP-1 signaling pathways [41–43]. Three subtypes of PPARs have been identified, PPARα, PPARβ, and PPARγ [16, 44]. PPARα is found in liver, brown fat, kidney, heart, and skeletal muscle. PPARβ (also known as PPARδ) is expressed in the gut, kidney, brain, skeletal muscle, and heart [34, 45]. PPARγ is expressed primarily in adipose tissue and to a lesser extent in large intestine, kidney, prostate, cartilage, osteoblasts, epithelial cells, and monocytes [46].
PPARs play a role in diabetes, atherosclerosis, obesity, the inflammatory response, and cancer [47]. PPARs regulate the expression of many genes associated with lipid storage, β-oxidation of fatty acids, terminal differentiation of preadipocytes, and modulation of the body's response to insulin and glucocorticoids [16, 47–50]. PPARα functions primarily in lipid catabolism, lipoprotein metabolism, and inflammation, as its expression increases with stress, glucocorticoid expression, exercise, and fasting [47, 51, 52]. PPARα knockouts develop normally, however, exhibit hepatomegaly from lipid accumulation and liver tumors, impaired wound healing, prolonged inflammatory responses, and increased adipose tissue [53]. PPARβ has a relatively diverse range of functions, including β-oxidation of fats, tumorigenesis [54], vascular integrity [55], and bone metabolism [16]. PPARβ knockouts have fatal placental defects secondary to abnormal vascular development, and those that survive become small, but healthy adults [53]. PPARγ affects the storage of fatty acids in adipose tissue, while also opposing TNFα and IL-6 production in inflammatory responses and insulin sensitization [47, 49, 50, 56, 57]. PPARγ knockouts are embryo-lethal as the placenta fails to implant and develop properly, and those that survive display severe metabolic, intestinal, hepatic, and adipogenic abnormalities [53].
3. Formation of PPAR and RXR Heterodimeric Receptor Complexes
PPARs consist of 4 domains, AB, C, D, and E. The AB domain function has not been clearly elucidated. The C domain represents the DNA binding domain (DBD), whereas the D domain contains the DBD carboxyl group along with the hinge connecting the C and E domains. The E domain (LBD) has a variety of functions, including ligand binding, hormone transactivation, and dimerization interface [16, 34, 45]. The three-dimensional structure of the LBD domain is very well conserved amongst the thyroid hormone receptor (TRα1), retinoic acid receptor (RAR), and retinoid X receptor (RXR) [58–60]. RXR is a promiscuous receptor and able to heterodimerize with RAR, PPAR, VDR, TR, and orphan receptors. This enables the competition among multiple hormones and other ligands to exert a variety of effects within the same tissue. It has been demonstrated that high concentrations of thyroid hormone inhibit the ability of PPARγ to heterodimerize with RXR and, therefore, blocks transcriptional activation (Figure 3) [61]. Transrepression occurs through sequestration of the coactivators CBP and SRC-1 by the PPAR/RXR heterodimers, preventing their utilization in other signaling pathways [62].
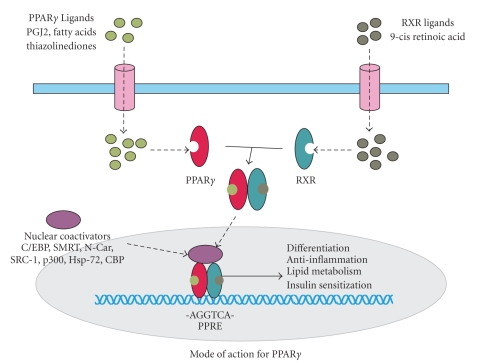
Figure 3.
Current mode of action for PPARγ. PPARγ and RXR form a heterodimer, which is activated by the respective ligands. The activated PPARγ/RXR heterodimer will be translocated into nucleus and regulates downstream target genes in concert with nuclear receptor coactivators.
A unique association with PPAR and RXR is the direct repeat responsive elements (DR) and the PPAR response elements (PPRE) associated with the heterodimer. DR1 is a repetition of 2 core motifs consisting of AGGTCA spaced apart from one another by one nucleotide in the promoter of multiple target genes (Figure 3) [63]. These motifs are recognized by two zinc finger-like motifs in the DBD region of PPAR. These PPREs produced by the PPAR : RXR heterodimer are different from those recognized by the vitamin D receptor (DR3), thyroid hormone receptor (DR4), and retinoic acid receptor (DR2, DR5) [34, 64, 65]. The importance of PPARγ : RXR interaction is seen in Familial Partial Lipodystrophy, an autosomal dominant condition associated with metabolic syndrome, characterized by dyslipidemia, abnormal adipose tissue distribution, and a number of metabolic abnormalities [66]. This syndrome is associated with multiple missense and nonsense mutations of PPARγ that affect its ability to dimerize with RXR and bind coactivators.
4. Diverse Functions of PPARγ
PPARγ is of particular interest because of its roles in adipogenesis, atherosclerosis, inflammation, proliferation, differentiation, and apoptosis [67]. The PPARγ gene contains 3 promoters, producing 2 different proteins, PPARγ1 and PPARγ2, which likely contributes to this diverse range of functions [55]. PPARγ expression in vascular endothelium and smooth muscle cells leads to an inhibition of MMPs, downregulation of Angiotensin II type 1 receptor, and alteration of macrophage invasion [68–71]. In inflammatory responses, its strongest association occurs with the ligand prostaglandin J2 (PGJ2) [16, 42, 72]. This eicosanoid metabolite binds directly to PPARγ, leading to its activation in the inflammatory response cascade. Furthermore, the insulin sensitizing effects of PPARγ are demonstrated using the synthetic antidiabetic therapy thiazolinediones [42, 72, 73]. Thus, the promiscuity of its ligand binding and a variety of associated nuclear proteins enables the diversity of PPARγ functions in many tissues.
For adipogenesis, PPARγ binds to fatty acids and their derivatives, such as linoleic acid and docosahaxaenoic acid (DHA) [34, 43]. These ligands activate PPARγ, stimulating preadipocytes to differentiate and initiate the steps required in lipid storage. PPARγ effects are carried out through target genes, such as aP2, lipoprotein lipase (LPL), acyl-Coa synthetase (ACS), and CD36 [74, 75]. Overexpression of PPARγ in fibroblasts initiates the adipogenic cascade, while PPARγ knockout mice are unable to form adipocytes or adipose tissue [48, 49, 76]. In humans, an activating mutation in PPARγ leads to increased adipogenesis and obesity [77]. Another mutation decreasing PPARγ activity results in lower body mass index [78, 79].
Adipose tissue targets of PPARγ include LPL and fatty acid transporter FATP in stimulating fatty acid uptake, malic enzyme in NADPH synthesis for lipogenesis, phosphoenolpyruvate carboxykinase in gluconeogenesis, and ACS in FA esterification [16, 80–82]. PPARγ promotes adipocyte differentiation of MSCs through various signaling pathways [83–85]. MSCs can differentiate into either osteogenic or adipogenic lineage, depending on the differentiation cues (Figure 1). It has been reported that osteogenesis and its signaling cascade are inhibited by PPARγ activation [86–89]. However, the extent of PPARγ-associated adipogenic stimulation or osteogenic inhibition depends on the nature of the ligands [90]. Such ligand-dependent regulatory functions of differentiation may explain the shift away from osteogenesis in the aging process, due to an increase in the number of bone marrow adipocytes, oxidized LDL metabolites, and fatty acids metabolites.
5. Role of PPARγ in Osteogenesis and Adipogenesis of MSCs
Osteogenesis and adipogenesis appear to originate from the same progenitor bone marrow mesenchymal stem cells (MSCs) [91–93]. MSC differentiation into osteoblast or preadipocytes occurs through a complex regulation of events [91–94] (Figure 1). Bone morphogenetic proteins (BMPs) play an important role in this differentiation process and subsequent bone formation [2, 93, 95–101]. BMP-2, BMP-6, and BMP-9 regulate targets associated with osteoblast differentiation, while BMP-2, BMP-4, and BMP-7 appear to be associated with preadipocyte differentiation [93, 101–110]. Mice with BMP2-regulated Schnurri-2 knockout showed a reduction in white fat mass [111].
PPARγ can stimulate adipocyte differentiation of MSCs. PPAR+ blasts (progenitor cells) have been noted in hematopoeitic lineages, and PPARγ is able to induce terminal differentiation in monocytes and adipocytes [48, 50, 112, 113]. Overexpression of PPARγ in fibroblastic cells initiates the adipogenic cascade, while PPARγ knockout mice were unable to form adipocytes or adipose tissue [48, 49, 76]. In humans, an activating mutation in PPARγ leads to increased adipogenesis and obesity, while inactivating mutation results in a lower body mass [77–79]. To demonstrate its importance, no factor has been shown to be able to induce adipogenesis in the absence of PPARγ and almost all pathways involved in adipogenesis involve regulation of PPARγ [114].
Furthermore, in a review by Giaginis et al. PPARγ agonists were found to have a remarkable role regulating bone turnover [115]. However, while PPARγ seems to shift the differentiation pathway away from osteoblastogenesis and towards osteoclastogenesis, this is not always the case. Giaginis et al. reviewed studies focusing on both the synthetic and natural PPARγ ligand effects on osteoblast and osteoclast formation, as well as apoptosis and overall bone formation. They found divergent results, as it appears there are other factors that contribute to bone turnover regulated by PPARγ. For example, since natural ligands are found in both the diet and the inflammatory cascade, perhaps these processes determine the final outcome of PPARγ-regulated bone turnover. Its effect in clinical studies poses a similar paradox, while some patients receiving synthetic PPARγ agonists for Diabetes Mellitus type II experienced bone loss, others were noted to have a decrease in bone resorption markers [115].
PPARγ plays an intriguing role in both adipogenesis and osteogenesis. Earlier reports indicate that homozygous PPARγ deficient progenitor cells spontaneously differentiate into osteoblasts via increased osteoblastogenic factors in vitro, and heterozygous PPARγ deficiency results in increased in vivo bone formation [87]. However, recent studies have demonstrated that osteogenic BMPs can effectively induce adipogenic differentiation [101, 116, 117]. PPARγ has been shown to be significantly upregulated by osteogenic BMPs [101, 118]. Overexpression of PPARγ2 promotes the osteogenic BMP-induced osteogenesis and adipogenesis [101]. Silencing PPARγ2 expression leads to an inhibition of adipogenic differentiation as well as stimulation of osteogenic differentiation and osteoid matrix mineralization [101]. However, it remains to be elucidated how BMP-induced MSC differentiation into osteogenesis and adipogenesis diverges.
The regulation underlying these effects could be secondary to the nuclear competition between PPARγ and other members of its nuclear receptor superfamily. Regulation of the osteogenic promoter, osteocalcin, by glucocorticoids, vitamin D, and thyroid hormone, occurs through the same nuclear pathway as PPARγ [119–121]. In addition, PPARγ activation by fatty acids and their derivatives might lead to a slowing of osteoblast differentiation, which would explain the tendency to shift to adipogenesis. These findings are intriguing as recent studies have indicated that aging activates adipogenesis and suppresses osteogenesis, possibly through the increased availability of these fatty acids and a decrease in metabolic production of many nuclear hormones. These would shift the signaling towards PPARγ, which might explain part of the mechanism underlying osteoporosis [122, 123].
5.1. Side Effects of PPARγ Ligands
In the treatment of Diabetes Mellitus (DM) type II with synthetic PPARγ ligands, the most common side effects observed have been headaches, gastrointestinal symptoms (nausea, diarrhea), and susceptibility to infections. Troglitazone has been withdrawn from the market secondary to its hepatic toxicity; however, this appears to be drug specific and not universal amongst PPAR agonists.
In a review by Mudaliar and Henry about the clinical use of glitazones, side effects include edema, weight gain, and mild drops in hematocrit. Rarely, increases in liver enzymes are observed. These synthetic PPARγ agonists induce the cytochrome P450 isoform CYP3A4 in the liver, affecting the metabolism of many other drugs. These drugs have been shown to increase plasma volume, thus, leading to edema and a dilutional drop in hematocrit. While the mechanism has not been elicited, PPARγ agonists antagonize the vasoconstriction induced by hyperinsulinemia, by sensitizing cells to the effects of insulin. Therefore, they relax vascular smooth muscles and decrease peripheral blood pressure. While there is no mention of increased peripheral adipose tissue, the propensity of PPARγ to induce lipid storage might underlie the observed weight gain [124].
Theoretically, a shift away from osteogenesis and towards adipogenesis might also promote osteoporosis and increase fracture risk. This has been demonstrated in a prospective study of over 80,000 patients being treated for DM type II [125]. Furthermore, competition for the RXR heterodimer might decrease the effects of other nuclear receptors in the superfamily, having a variety of effects on many different tissues. While recently there was a report suggesting increased fracture risk in patients receiving PPARγ agonists, there has been relatively little other evidence supporting any of these notions [122].
6. Molecular Biology of Osteosarcoma
The molecular pathogenesis underlying OS development is poorly understood. OS is associated with aberrations in p53 and Rb expression [1, 2, 126–128]. Other genetic alterations associated with OS development include p16INK4a, c-Myc, Fos-Jun, MDM2, CDK4, and cyclin D [1, 2, 126]. Altered cell signaling pathways in OS include Wnt, sonic hedgehog, TGFβ/BMP families, and IGF2 [1, 2, 126]. Mutations in DNA helicase increase OS risk and MMP expression leads to a more aggressive OS tumor, while lack of telomerase activity is associated with a favorable prognosis [129–131]. The identifiable risks associated with OS include exposure to the FBJ or SV40 virus, beryllium oxide chemical, and radiation [132–134].
Disruption of the osteogenic differentiation pathway from MSCs is thought at least in part to be responsible for OS tumorigenesis [2, 7, 9, 10] (Figure 2). By preventing the differentiation of MSCs, the proliferative capability of preosteoblasts increases the risk for malignant transformation. It has been well established that early progenitor cells have similar characteristics to a variety of tumor cells. For example, progenitor cells of the hematopoietic system share similar leukocyte receptors to leukemic cells. Furthermore, tumor cells share many of the antiapoptotic and self renewal machinery with stem cells [135]. It has been proposed that a small subset of cancer cells, known as cancer stem cells, act in a similar manner to adult stem cells, with proliferative and regenerative capabilities that enable tumors to survive and grow [135]. In colon cancer cells, preneoplasia and neoplasia have the same nuclear morphotypes that 5–7-week-old fetal gut stem cells possess but are not found in the adult colonic crypt cells [136]. Osteosarcoma cells display similar characteristics to undifferentiated osteoblasts [7–10]. In OS cell lines, early osteogenic markers, such as CTGF, are high while late markers such as Runx2, Alkaline Phosphatase, Osteopontin, and Osteocalcin are low [7].
Therefore, investigation into the induction of terminal differentiation in cells with such differentiation defects has increased in recent years. A similar process has been reported in Ewing's Sarcoma, in which silencing the EWS/FLI-1 in Ewing's sarcoma cells leads to their recovery of MSC capability to differentiate into osteogenic lineages [137]. Accordingly, overexpression of this oncogene causes MSCs to remain in an undifferentiated state and promotes tumor growth [138]. Rb mutations occur in many OS tumors [1, 2, 126–128]. Rb coactivates the osteoblast differentiating agent Runx2 and loss of function of Rb stalls terminal osteoblast differentiation [139]. Furthermore, it has been recently shown that OS cells are refractory to BMP-induced osteogenic differentiation, whereas osteogenic BMPs promote OS growth in vivo, which can be overcome by introducing key osteogenic regulator Runx2 [7]. These findings suggest that the late stages of osteogenic differentiation may be preserved [7, 8]. Therefore, anti-OS therapies may be developed by promoting osteogenic terminal differentiation [2, 7]. Differentiation agents would add another dimension to the current chemotherapeutic cocktails focusing on the inhibition of the cell cycle.
6.1. Molecular Biology Relating to the Differentiation Status in Tumors
It appears that the differentiation status not only is responsible for the development of OS but also may predict its malignant potential. From the early 1970s when the idea of differentiation was first proposed, to more recently when differentiation agents are used for certain cancer phenotypes, it has been observed that this process is associated with many morphological changes in the respective cells. These changes leading to a well-differentiated cell include repression of responsiveness to growth factors, withdrawal from the cell cycle into a state of quiescence, and a decreased ability to re-initiate proliferation [140]. For example, as adipocytes differentiate, they progressively become less responsive to mitogenic growth factors MIX and PDGF, eventually repressing the expression of proto-oncogenes c-jun and junB [141, 142]. The more differentiated the adipocyte, the less responsive it is to growth factors. Terminal adipocyte differentiation is accompanied by expression of proteins that repress RNA expression, along with induction of p21, leading to irreversible loss of proliferative potential [140, 143]. When breast cancer's estrogen receptors were first discovered and evaluated, the notion was proposed that the cancers with estrogen receptors represent a well-differentiated class of tumors that undergo clonal evolution and eventually lose their receptor status when they become poorly differentiated [144]. Another example occurs when Simian Virus 40 large T antigen transforms cells to increase their responsiveness to growth factors and become undifferentiated [145].
The fundamental idea behind differentiation therapy for tumors is that by inducing terminal differentiation, the tumor cells lose their proliferative phenotypes. Differentiation causes cells to lose their proliferative potential and repress their responsiveness to growth factors, while at the same time possibly increasing their susceptibility to apoptosis, induction of tumor suppressors, repression of oncogenes, inhibition of angiogenesis, and induction of cytotoxic agents. As cells become more differentiated, these changes make them less aggressive and more responsive to other chemotherapeutic agents.
6.2. Clinical Examples of Therapeutic Success by Induction of Terminal Differentiation
Inhibition of tumor growth through differentiation therapy has been demonstrated in clinical cases of hematologic and breast tumors. Induction of terminal differentiation was first shown to be successful in treating AML with low-dosage araC [146]. Recently, there have been many more therapeutic interventions that have focused on overcoming the uncontrolled cell proliferation through terminal differentiation [8, 9, 11–13]. One possibility deals with the nuclear receptor superfamily associated with vitamin D3, thyroid hormone, glucocorticoids, sex hormones, retinoids, and orphan receptors [14–17]. Treatment focusing on counteracting hormone dependent activation of these nuclear receptors is seen in therapies such as tamoxifen for breast cancer [18]. In this and other examples, regulation of the nuclear receptor leads to differentiation, causing the cells to lose their proliferative properties and antiapoptotic tendencies.
7. Role of PPARs in Tumorigenesis and Differentiation
PPARs play an important role in tumorigenesis and differentiation (Table 1). PPARα is responsible for hepatocarcinogenic effects in rodents [147]. PPARβ was identified as a downstream target of the APC/β-catenin pathway, associated with human colon tumors [54]. PPARβ has been shown as a target for nonsteroidal anti-inflammatory drug- (NSAID-) induced chemopreventive effects in colon cancer [45, 54]. High dose of NSAIDs, such as sulindac and indomethacin, displays chemopreventive effects in the familial adenomatous polyposis mouse model. It downregulates Cox-2 expression in humans, leading to a decrease in intestinal polyps, inhibition of cell cycle progression, and induction of apoptosis in colorectal tumor cells [45, 148, 149]. NSAIDs can disrupt the ability of PPARβ to bind to its peroxisome proliferator response elements (PPREs) in vitro, while PPARβ overexpression was able to rescue NSAID induced apoptosis in colon tumor cells [45]. These findings may explain the correlation between dietary fat consumption and colon cancer incidence, since fatty acids can serve as ligands for PPARβ.
Table 1.
Basic features of the three PPAR isoforms.
| Location | Ligands | Coactivators | Primary function | Knockout | |
|---|---|---|---|---|---|
| PPARα | liver, brown fat, kidney, heart, skeletal muscle | fibrates fatty acids (e.g., oleic acid, palmitic acid) eicosanoids (e.g., arachidonic acid) | p300, c/EBP, SRC-1, PBP, PGC-1, PRIP | lipid catabolism, inflammatory responses, lipoprotein metabolism | hepatomegaly, liver tumors, impaired wound healing, prolonged inflammatory responses, increased adipose tissue |
|
| |||||
| PPARδ | gut, kidney, brain, heart, skeletal muscle | fatty acids, NSAIDS (antagonist) | SRC-1, PBP | fatty acid β-oxidation, bone metabolism, tumorigenesis, vascular integrity | fatal placental defects from abnormal vasculature, small healthy adults |
|
| |||||
| PPARγ | adipose tissue, cartilage, osteoblasts, epithelial cells, prostate, large intestine, monocytes, kidney | thiazolinediones eicosanoids (e.g., 15d-PGJ2, 15-HETE) fatty acids (e.g., DHA, linoleic acid) | p300, c/EBP, SRC-1, PBP, PGC-1, PRIP | adipogenesis, inflammatory response, insulin sensitization, differentiation | embryo-fatal, placenta fails to implant and develop, severe metabolic, hepatic, intestinal, adipogenic, abnormalities |
PPARγ has shown promise in therapy promoting terminal differentiation and apoptosis in a variety of malignancies, including liposarcoma, breast cancer, leukemia, gastric cancer, non‐small cell lung cancer, and prostate cancer [19–27]. In humans, the treatment of end stage prostate cancer with PPARγ synthetic ligand troglitazone leads to prostate specific antigen (PSA) stabilization [150]. Although these results are promising, there are many examples of contradictory roles of PPARγ in tumorigenesis. It has been observed that while PPARγ agonists inhibit growth and induce apoptosis in both breast tumor cells and leukemic cells, administration of PPARγ antagonists enhanced this tumor growth inhibitory effect [151, 152]. The fusion protein EWSRI/NR4A3 in extraskeletal chondrosarcomas activates PPARγ expression [153]. In fibrosarcoma cells, the synthetic PPARγ agonist ciglitazone induces tumor cell invasion through the generation of ROS and ERK [154].
This controversy is best exemplified by the role of PPARγ in colorectal tumorigenesis. PPARγ agonists have been shown to promote mouse intestinal tumors, while loss of function mutations of PPARγ has been identified in human colon tumors [30, 45, 150]. Synthetic PPARγ agonists promote the development of colon tumors in mice with a mutation in the tumor suppressor APC [29, 30]. This leads to increased levels of B-Catenin. Furthermore, mice diets high in saturated fats promotes tumorigenesis [155]. PPARγ activation by Fas could explain the link between high fat diets and colon cancer. However, one study disputed the PPARγ agonist role in tumor promotion, as it showed that PPARγ agonists were able to induce differentiation and inhibit human tumors from growing in nude mice [31]. Furthermore, PPARγ agonists are able to induce differentiation, cell cycle arrest, and apoptosis in human colon cancer cell lines [31]. The anti-inflammatory effects of PPARγ lead to a reduced number of cancer precursor foci in inflammatory bowel disease [156]. One possible explanation for the antagonistic role of PPARβ and PPARγ in colon tumorigenesis may be competition for RXR heterodimerization. The cellular proliferation caused by PPARβ, induced by specific ligands, may lead to overexpression and inhibition of PPARγ heterodimerization, and subsequently contribution to human colon cancer development.
These contradictory results might be explained by species specific effects, where a combination of the different factors within each animal leads to different PPARγ-associated signaling outcomes. Another example of the differences between species occurs in hepatic cancers. PPARγ agonists are seen as potent carcinogens in rodents, but not seen in humans or primates [157, 158]. Furthermore, PPARγ agonist rosiglitazone seems to enhance carcinogenic effects of the urinary bladder in rodents, while treatment of diabetes with pioglitazone in humans does not seem to increase the incidence of these or any other tumors [159].
While the mechanisms underlying PPARγ action are not fully established, it has been shown that PPARγ can inhibit the cell cycle, which is accomplished at least in part through downregulating the protein phosphatase PP2A upon PPARγ activation [160]. The PPARγ ligands can also inhibit the G1/S transition by inhibiting Rb phosphorylation [161]. Furthermore, PPARγ upregulates the CDK inhibitors p18 and p21 [162]. PPARγ ligand PGJ2 induces both CDK p21 and the proapoptotic Bax but downregulates the anti-apoptotic Bcl-xL [163]. Synthetic PPARγ agonist treatment in human pancreatic cancer and bladder cancer cell lines resulted in G1 cell cycle arrest secondary to p21 induction [164, 165]. Further insight into the cross-talk between these different mechanisms will guide future antitumor therapies.
8. Antitumor Activity of PPARγ Agonists in Osteosarcoma
Increasing evidence suggests that activation of PPARγ may be explored as a possible intervention in osteosarcoma (Table 2). PPARγ agonists are thought to induce terminal differentiation in adipogenesis. OS cells share many characteristics to undifferentiated osteoblasts [7–10]. Therefore, modulators that are able to promote the differentiation of these immature osteoblasts should have similar effects on the OS cells. PPARγ agonist rosiglitazone has been shown to inhibit osteoblast proliferation, leading to decreased osteogenesis [88, 166]. A recent study showed PPARγ to be a critical mediator underlying doxorubicin resistance in OS cell lines [167]. The chemoresistant OS cell lines were shown to have an increased expression of IL-8, which induces the antiapoptotic KLF2 [167, 168]. KLF2 is thought to negatively regulate the PPARγ-induced expression of C/EBP and ADD1/SREBP, suggesting that the drug resistance may occur through the inhibition of PPARγ-induced apoptosis. Furthermore, the NSAID-Associated Gene 1 (NAG-1), which is associated with NSAID-induced apoptosis, has been upregulated by PPARγ in canine OS cell lines [169].
Table 2.
Potential effects of nuclear receptor ligands on osteosarcoma tumors.
| Ligand | Experimental Setup | Effect | Mechanism | Reference | |
|---|---|---|---|---|---|
| PPARγ | Troglitazone | in vitro proliferation and apoptosis assays | inhibited proliferation | promotion of apoptosis, induce differentiation (alkaline phosphatase) | Haydon 2002, 2007 [9, 32] |
| Ciglitazone | in vitro proliferation and apoptosis assays | minimal effect | unknown | Haydon2002 [32] | |
| Troglitazone | in vitro MTT proliferation and apoptosis assays | increased proliferation | inhibition of apoptosis | Lucarelli 2002 [170] | |
| Pioglitazone and PG J(2) | in vitro MTT and apoptosis assays of Chondrosarcoma Cells | inhibition of proliferation | promoted apoptosis | Nishida [171] | |
|
| |||||
| Retinoic Acid | 9-cis retinoic acid | in vitro proliferation and apoptosis assays | inhibited proliferation | promotion of apoptosis | Haydon 2002, 2007 [9, 32] |
|
| |||||
| Estrogen | 17-beta estradiol, Ospemifene | U2OS expressing ER, in vitro apoptosis assays | opposed Etoposide-induced cell death | oppose increases in IL-6 and decreases in OPG, preventing osteoclast activation | Kallio 2008 [172] |
| Tamoxifen, Raloxifene | U2OS expressing ER, in vitro apoptosis assays | no effect on Etoposide-induced cell death | unknown | Kallio 2008 [172] | |
| 17-beta estradiol, SERMS (genistein, daidzein) | U2OS expressing ER, in vitro cell cycle, proliferation, apoptosis assays | inhibit proliferation, promote apoptosis | decrease EGFR, increased osteoblast maturation markers | Salvatori 2009 [173] | |
|
| |||||
| Vitamin D | 1-alpha hydroxyvitamin D3 | oral administration in Dunn murine OS model | inhibits tumor growth and metastasis | increased necrosis, no cell cycle mitotic index effects | Hara 2001 [174] |
| Prolactin + 1,25 (OH)2 Vitamin D3 | RT-PCR, western blots | PRL inhibits VDR expression in response to 1,25 (OH)2 Vitamin D3 | VDR expression is dependent on BRCA1 expression | Deng [175] | |
| calcitriol | OS cells with increased RXR degradation, treated with calcitriol | increased expression of RXR restores anti-proliferative effects of calcitriol | calcitriol induced degradation is dependent on RXR expression | Prufer 2002 [176] | |
PPARγ agonists and 9-cis-retinoic acid have the capability to induce osteoblastic differentiation of OS cells and inhibit OS proliferation [9, 32]. After exposure to these agents, not only did the OS cells show decreased proliferative capabilities and susceptibility to apoptosis but they also expressed increased differentiation markers, such as alkaline phosphatase. Therefore, it appears that such agents would be useful in preventing recurrence and metastasis after surgical removal of osteosarcoma. The results are further supported by the ability of PPARγ to induce apoptosis in chondrosarcoma cells [177]. However, one study by Lucarelli et al. showed that treatment of human osteosarcoma cells with the PPARγ agonist troglitazone promotes the in vitro survival via reduction in apoptosis of the malignant cells [170]. Although the mechanisms accounting for this difference are not known, it is likely that this molecular complexity results from the nuclear cross-talk and interplays between PPARγ and other nuclear receptor hormones. It is plausible that the effects of PPARγ agonists on OS are largely dependent on which step in the differentiation pathway the defect has occurred (Figure 2). Downstream defects may be resistant to PPARγ agonists-induced terminal differentiation of its upstream counterparts. The specific factors that participate in the nuclear signaling and transcriptional regulation, along with the differentiation molecules associated with OS tumorigenesis, have yet to be fully elicited.
To the best of our knowledge, there have been no studies examining the effects of PPARγ on the metastatic potential in OS. However, our notion of the potential for PPARγ to reduce metastatic potential of OS is supported by examples in other tumors. Rosiglitazone has been shown to decrease the number of lung metastasis of mammary tumors in mice [178]. Dietary administration of PPARγ ligands linoleic acid and conjugated linoleic acid inhibited peritoneal metastasis of colorectal tumors in nude mice [179]. Furthermore, Pioglitazone inhibited colon tumor liver metastasis in mice, possibly by downregulating Cox-2 and cyclin D1 [180]. These anti-inflammatory and other possible antiangiogenic effects of PPARγ extend its potential as a chemotherapeutic agent beyond differentiation. Overexpressing PPARγ in nonsmall cell lung cancer cells inhibited tumor number and metastasis [181]. Due to its ability to inhibit angiogenesis, tumor cell invasion, and inflammation, it therefore follows that PPARγ would inhibit metastasis. In an analysis of primary breast tumors, PPARγ expression was more often in low-grade than high-grade tumors, associated with a more favorable survival, and decreased in tumor relapses [182]. PPARγ ligand troglitazone inhibited growth and liver metastasis of papillary thyroid tumors [183].
9. Synergistic Antitumor Activity between PPARγ Agonists and Retinoids in Osteosarcoma
Receptors for retinoids include retinoic acid receptors (RARs) and retinoid X receptors (RXRs). RARs are activated by all-trans retinoic acid, a vitamin A metabolite, and heterodimerizes with RXR after ligand binding. RARs have been implicated in early embryonic morphogenesis, including development of the forebrain, hindbrain, and body axis, as well as, early signaling associated with the pancreas, heart, eye, lung, and genitourinary tracts [184–186]. Furthermore, it is able to induce the differentiation of many cancer cells and is used as an effective therapy in the treatment of acute promyelocytic leukemia by differentiating cells that express the PML-RARα fusion protein [9, 32, 45, 187–189].
RXRs are activated by 9-cis retinoic acid. Beyond the ability of RXR to heterodimerize with many members of the nuclear receptor superfamily, such as PPARs, RXRs can form homodimers. RXRs play important roles in signaling pathways associated with development and carcinogenesis. We have recently demonstrated that exogenous expression of RARα induces ligand-independent myogenic differentiation from progenitor cells [190]. We have also found that all-trans retinoic acid and 9-cis retinoid acid can effectively induce the differentiation of mouse fetal liver-derived hepatic progenitor cells [191]. RXRs are overexpressed in breast ductal carcinomas, its ablation leads to prostate and skin hyperplasia, and its overexpression sensitizes tumors to rexinoid family differentiation agents [192–195]. A synthetic rexinoid bexatrone has been developed for use in chemotherapeutic cocktails in mice mammary tumors, as well as human cutaneous T-cell lymphoma, with partial responsiveness in nonsmall cell lung cancer [196–198].
A comprehensive analysis of the possible synergistic effects between PPARγ and retinoids has been carried out in a panel of OS cell lines (Table 2) [9, 32]. As a single agent, PPARγ ligand troglitazone was shown to be the most effective in inducing cell death, followed by 9-cis retinoic acid [9, 32]. The strong synergistic effect on the induction of cell death was observed when both troglitazone and 9-cis retinoic acid or ciglitazone and 9-cis retinoic acid were administered to osteosarcoma cells [9, 32]. Troglitazone was shown to effectively induce alkaline phosphatase activity, a well-characterized hallmark for osteoblastic differentiation [9, 32]. These findings suggest that PPARγ and/or RXR ligands may be used as efficacious adjuvant therapeutic agents for osteosarcoma as well as potential chemopreventive agents for preventing the recurrence and metastasis of osteosarcoma after the surgical removal of the primary tumors.
10. Other Nuclear Receptors in Osteosarcoma
Except for PPARs and retinoid receptors, several members of the nuclear receptor superfamily are also involved in the cell signaling and differentiation processes associated with OS (Table 2). Estrogens and selective estrogen receptor modulators (SERMs) are able to induce terminal differentiation in osteosarcoma cell lines through the downregulation of EGFR [173]. EGFR is a critical mediator of cell proliferation and differentiation, whose expression decreases over the course of osteoblast differentiation and maturation [199, 200]. The stimulation of estrogen receptors leads to the downregulation of EGFR in OS, resulting in cell cycle inhibition and apoptosis. Alternatively, the estrogen 17 β-estradiol protected osteosarcoma cells expressing estrogen receptors from etoposide-induced apoptosis [172]. However, the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene have no antiapoptosis effects.
Vitamin D receptor (VDR) has also been shown to play a role in OS cell lines and their responsiveness to therapeutic interventions. VDR is overexpressed on some OS cell lines and administration of 1, 25-dihydroxyvitamin D3 inhibits tumor growth and metastasis, while promoting terminal differentiation [174, 201]. Interestingly, it has been reported that the upregulation of VDR in OS cell lines is dependent on the tumor suppressor BRCA1 [202]. Although a synthetic VDR ligand calcitriol is able to exert antiproliferative effects in OS cells, this process is dependent on the expression of RXRs. Degradation or downregulation of RXRs causes OS resistance to the antitumor effect of calcitriol [176]. Thus, the anti-OS activity of VDR is dependent on many other nuclear proteins, including BRCA1 and RXRs.
11. Concluding Remarks
OS is the most frequent primary bone sarcoma, comprising approximately 20% of all bone tumors and about 5% of pediatric tumors overall. OS tumors display a broad range of genetic and molecular alterations, including the gains, losses, or arrangements of chromosomal regions, inactivation of tumor suppressor genes, and the deregulation of major signaling pathways. However, except for p53 and/or RB mutations, most alterations are not constantly detected in the majority of osteosarcoma tumors. Recent studies strongly suggest that OS may be regarded as a differentiation disease that is caused by genetic and epigenetic disruptions of osteoblast terminal differentiation. It has been well established that PPARs and retinoids play an important role in regulating osteogenic differentiation of MSCs. Increasing evidence indicates that PPAR agonists and/or retinoids can inhibit cell proliferation and induce apoptosis in cancer cells, including OS cells. PPAR agonists and/or retinoids may also inhibit OS growth by promoting osteoblastic terminal differentiation. One of the future directions is to develop safe and effective PPAR agonists and/or retinoid derivatives. These agents can be then used as adjuvant therapeutic drugs for OS therapy. Meanwhile, more thorough investigations will be needed to examine the potentially beneficial and/or adverse effects of PPARγ ligands on different cells in bone and bone marrow microenvironment. Furthermore, these agents can be used as chemopreventive agents for the patients with OS who undergo the resection of the primary bone tumors in order to prevent local recurrence and/or distal pulmonary metastasis.
Acknowledgments
The authors apologize to the authors whose original work was not cited due to space constraints. The reported work was supported in part by research grants from The Brinson Foundation (TCH), Musculoskeletal Transplant Foundation (RCH), National Institutes of Health (RCH, TCH, and HHL), and Orthopaedic Research and Education Foundation (RCH and HHL).
References
- 1.Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genetics and Cytogenetics. 2003;145(1):1–30. [PubMed] [Google Scholar]
- 2.Tang N, Song W-X, Luo J, et al. Osteosarcoma development and stem cell differentiation. Clinical Orthopaedics and Related Research. 2008;466(9):2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unni KK, Dahlin DC. Osteosarcoma: pathology and classification. Seminars in Roentgenology. 1989;24(3):143–152. doi: 10.1016/0037-198x(89)90010-2. [DOI] [PubMed] [Google Scholar]
- 4.Burns BS, Edin ML, Lester GE, et al. Selective drug resistant human osteosarcoma cell lines. Clinical Orthopaedics and Related Research. 2001;(383):259–267. doi: 10.1097/00003086-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Kaste SC, Pratt CB, Cain AM, et al. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86(8):1602–1608. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Yonemoto T, Tatezaki S-I, Ishii T, et al. Prognosis of osteosarcoma with pulmonary metastases at initial presentation is not dismal. Clinical Orthopaedics and Related Research. 1998;(349):194–199. doi: 10.1097/00003086-199804000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Chen J, Song W-X, et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Laboratory Investigation. 2008;88(12):1264–1277. doi: 10.1038/labinvest.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DM, Johnson SA, Sims NA, et al. Terminal osteoblast differentiation, mediated by runx2 and p27 KIP1, is disrupted in osteosarcoma. The Journal of Cell Biology. 2004;167(5):925–934. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haydon RC, Luu HH, He T-C. Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clinical Orthopaedics and Related Research. 2007;(454):237–246. doi: 10.1097/BLO.0b013e31802b683c. [DOI] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 11.Carpio L, Gladu J, Goltzman D, et al. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. American Journal of Physiology. 2001;281(3):E489–E499. doi: 10.1152/ajpendo.2001.281.3.E489. [DOI] [PubMed] [Google Scholar]
- 12.Nozaki K, Kadosawa T, Nishimura R, et al. 1,25-dihydroxyvitamin D3, recombinant human transforming growth factor-β 1, and recombinant human bone morphogenetic protein-2 induce in vitro differentiation of canine osteosarcoma cells. The Journal of Veterinary Medical Science. 1999;61(6):649–656. doi: 10.1292/jvms.61.649. [DOI] [PubMed] [Google Scholar]
- 13.Postiglione L, Di Domenico G, Montagnani S, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the osteoblastic differentiation of the human osteosarcoma cell line SaOS-2. Calcified Tissue International. 2003;72(1):85–97. doi: 10.1007/s00223-001-2088-5. [DOI] [PubMed] [Google Scholar]
- 14.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83(6):859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 16.Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annual Review of Cell and Developmental Biology. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 17.Chawta A, Repa JJ, Evans RM, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 18.Osborne MP. Breast cancer prevention by antiestrogens. Annals of the New York Academy of Sciences. 1999;889:146–151. doi: 10.1111/j.1749-6632.1999.tb08732.x. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RG, Williamson E, Patel MK, et al. A ligand of peroxisome proliferator-activated receptor γ, retinoids, and prevention of preneoplastic mammary lesions. Journal of the National Cancer Institute. 2000;92(5):418–423. doi: 10.1093/jnci/92.5.418. [DOI] [PubMed] [Google Scholar]
- 20.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi N, Okumura T, Motomura W, et al. Activation of PPARγ inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Letters. 1999;455(1-2):135–139. doi: 10.1016/s0014-5793(99)00871-6. [DOI] [PubMed] [Google Scholar]
- 22.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Research. 1998;58(15):3344–3352. [PubMed] [Google Scholar]
- 23.Asou H, Verbeek W, Williamson E, et al. Growth inhibition of myeloid leukemia cells by troglitazone, a ligand for peroxisome proliferator activated receptor gamma, and retinoids. International Journal of Oncology. 1999;15(5):1027–1031. doi: 10.3892/ijo.15.5.1027. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura S, Miyazaki Y, Shinomura Y, et al. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Japanese Journal of Cancer Research: Gann. 1999;90(1):75–80. doi: 10.1111/j.1349-7006.1999.tb00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elstner E, Muller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller E, Sarraf P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPAR gamma. Molecular Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 27.Chang T-H, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Research. 2000;60(4):1129–1138. [PubMed] [Google Scholar]
- 28.Warrell RP, Jr., de The H, Wang ZY, et al. Acute promyelocytic leukemia. The New England Journal of Medicine. 1993;329(3):177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre AM, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 30.Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Medicine. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 31.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 32.Haydon RC, Zhou L, Feng T, et al. Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clinical Cancer Research. 2002;8(5):1288–1294. [PMC free article] [PubMed] [Google Scholar]
- 33.Torchia J, Glass C, Rosenfeld MG. Co-activators and co-repressors in the integration of transcriptional responses. Current Opinion in Cell Biology. 1998;10(3):373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 34.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 35.Berger J, Leibowitz MD, Doebber TW, et al. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. The Journal of Biological Chemistry. 1999;274(10):6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- 36.Krey G, Braissant O, L’Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11(6):779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 37.Huang Q, Alvares K, Chu R, et al. Association of peroxisome proliferator-activated receptor and Hsp72. The Journal of Biological Chemistry. 1994;269(11):8493–8497. [PubMed] [Google Scholar]
- 38.Puigserver P, Adelmant G, Wu Z, et al. Activation of PPARγ coactivator-1 through transcription factor docking. Science. 1999;286(5443):1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 39.Yu S, Reddy JK. Transcription coactivators for peroxisome proliferator-activated receptors. Biochimica et Biophysica Acta. 2007;1771(8):936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Keller H, Dreyer C, Medin J, et al. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forman BM, Tontonoz P, Chen J, et al. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR γ . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 43.Yu K, Bayona W, Kallen CB, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. The Journal of Biological Chemistry. 1995;270(41):23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt A, Endo N, Rutledge SJ, et al. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Molecular Endocrinology. 1992;6(10):1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- 45.Park BH, Breyer B, He T-C. Peroxisome proliferator-activated receptors: roles in tumorigenesis and chemoprevention in human cancer. Current Opinion in Oncology. 2001;13(1):78–83. doi: 10.1097/00001622-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Theocharis S, Margeli A, Vielh P, et al. Peroxisome proliferator-activated receptor-γ ligands as cell-cycle modulators. Cancer Treatment Reviews. 2004;30(6):545–554. doi: 10.1016/j.ctrv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Kersten S, Desvergne B, Wahli W. Roles of PPARS in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 48.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR γ2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 49.Rosen ED, Sarraf P, Troy AE, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Molecular Cell. 1999;4(4):611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 50.Greene ME, Pitts J, McCarville MA, et al. PPARγ: observations in the hematopoietic system. Prostaglandins & Other Lipid Mediators. 2000;62(1):45–73. doi: 10.1016/s0090-6980(00)00075-7. [DOI] [PubMed] [Google Scholar]
- 51.Horowitz JF, Leone TC, Feng W, et al. Effect of endurance training on lipid metabolism in women: a potential role for PPARα in the metabolic response to training. American Journal of Physiology. 2000;279(2):E348–E355. doi: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- 52.Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008;8(4):329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPARα), beta (PPARβ), and gamma (PPARγ) in rodent and human development. Reproductive Toxicology. 2009;27(3-4):246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 54.He T-C, Chan TA, Vogelstein B, et al. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kota BP, Huang TH-W, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacological Research. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. The Journal of Biological Chemistry. 1998;273(40):25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 57.Gelman L, Fruchart J-C, Auwerx J. An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer. Cellular and Molecular Life Sciences. 1999;55(6-7):932–943. doi: 10.1007/s000180050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourguet W, Ruff M, Chambon P, et al. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-α . Nature. 1995;375(6530):377–382. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- 59.Renaud J-P, Rochel N, Ruff M, et al. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378(6558):681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 60.Wagner RL, Apriletti JW, McGrath ME, et al. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378(6558):690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 61.Qi J-S, Desai-Yajnik V, Greene ME, et al. The ligand-binding domains of the thyroid hormone/retinoid receptor gene subfamily function in vivo to mediate heterodimerization, gene silencing, and transactivation. Molecular and Cellular Biology. 1995;15(3):1817–1825. doi: 10.1128/mcb.15.3.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. The Lancet Oncology. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 63.Dreyer C, Krey G, Keller H, et al. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 64.Osada S, Tsukamoto T, Takiguchi M, et al. Identification of an extended half-site motif required for the function of peroxisome proliferator-activated receptor α . Genes to Cells. 1997;2(5):315–327. doi: 10.1046/j.1365-2443.1997.1220319.x. [DOI] [PubMed] [Google Scholar]
- 65.Juge-Aubry C, Pernin A, Favez T, et al. DNA binding properties of peroxisome proliferator-activated receptor subtypes on various natural peroxisome proliferator response elements: importance of the 5′-flanking region. The Journal of Biological Chemistry. 1997;272(40):25252–25259. doi: 10.1074/jbc.272.40.25252. [DOI] [PubMed] [Google Scholar]
- 66.Jeninga EH, van Beekum O, van Dijk ADJ, et al. Impaired peroxisome proliferator-activated receptor γ function through mutation of a conserved salt bridge (R425C) in familial partial lipodystrophy. Molecular Endocrinology. 2007;21(5):1049–1065. doi: 10.1210/me.2006-0485. [DOI] [PubMed] [Google Scholar]
- 67.Heikkinen S, Auwerx J, Argmann CA. PPARγ in human and mouse physiology. Biochimica et Biophysica Acta. 2007;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keen HL, Ryan MJ, Beyer A, et al. Gene expression profiling of potential PPARγ target genes in mouse aorta. Physiological Genomics. 2004;18(1):33–42. doi: 10.1152/physiolgenomics.00027.2004. [DOI] [PubMed] [Google Scholar]
- 69.Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 70.Marx N, Schönbeck U, Lazar MA, et al. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circulation Research. 1998;83(11):1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeda K, Ichiki T, Tokunou T, et al. Peroxisome proliferator-activated receptor γ activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102(15):1834–1839. doi: 10.1161/01.cir.102.15.1834. [DOI] [PubMed] [Google Scholar]
- 72.Kliewer SA, Lenhard JM, Willson TM, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 73.Lehmann JM, Moore LB, Smith-Oliver TA, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) The Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 74.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. The Journal of Biological Chemistry. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 75.Allen T, Zhang F, Moodie SA, et al. Halofenate is a selective peroxisome proliferator-activated receptor γ modulator with antidiabetic activity. Diabetes. 2006;55(9):2523–2533. doi: 10.2337/db06-0618. [DOI] [PubMed] [Google Scholar]
- 76.Barak Y, Nelson MC, Ong ES, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 77.Ristow M, Müller-Wieland D, Pfeiffer A, et al. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. The New England Journal of Medicine. 1998;339(14):953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 78.Beamer BA, Yen CJ, Andersen RE, et al. Association of the Pro12Ala variant in the peroxisome proliferator-activated receptor-γ2 gene with obesity in two Caucasian populations. Diabetes. 1998;47(11):1806–1808. doi: 10.2337/diabetes.47.11.1806. [DOI] [PubMed] [Google Scholar]
- 79.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nature Genetics. 1998;20(3):284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 80.Castelein H, Gulick T, Declercq PE, et al. The peroxisome proliferator activated receptor regulates malic enzyme gene expression. The Journal of Biological Chemistry. 1994;269(43):26754–26758. [PubMed] [Google Scholar]
- 81.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ . Current Opinion in Genetics & Development. 1995;5(5):571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 82.Schoonjans K, Watanabe M, Suzuki H, et al. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. The Journal of Biological Chemistry. 1995;270(33):19269–19276. doi: 10.1074/jbc.270.33.19269. [DOI] [PubMed] [Google Scholar]
- 83.Diascro DD, Jr., Vogel RL, Johnson TE, et al. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. Journal of Bone and Mineral Research. 1998;13(1):96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- 84.Johnson TE, Vogel R, Rutledge SJ, et al. Thiazolidinedione effects on glucocorticoid receptor-mediated gene transcription and differentiation in osteoblastic cells. Endocrinology. 1999;140(7):3245–3254. doi: 10.1210/endo.140.7.6797. [DOI] [PubMed] [Google Scholar]
- 85.Lecka-Czernik B, Gubrij I, Moerman EJ, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. Journal of Cellular Biochemistry. 1999;74(3):357–371. [PubMed] [Google Scholar]
- 86.Jeon MJ, Kim JA, Kwon SH, et al. Activation of peroxisome proliferator-activated receptor-γ inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. Journal of Biological Chemistry. 2003;278(26):23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 87.Akune T, Ohba S, Kamekura S, et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. Journal of Clinical Investigation. 2004;113(6):846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ali AA, Weinstein RS, Stewart SA, et al. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 89.Soroceanu MA, Miao D, Bai X-Y, et al. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. Journal of Endocrinology. 2004;183(1):203–216. doi: 10.1677/joe.1.05723. [DOI] [PubMed] [Google Scholar]
- 90.Lecka-Czernik B, Moerman EJ, Grant DF, et al. Divergent effects of selective peroxisome proliferator-activated receptor-γ2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143(6):2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 91.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289(5484):1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 92.He T-C. Distinct osteogenic activity of BMPs and their orthopaedic applications. Journal of Musculoskeletal & Neuronal Interactions. 2005;5(4):363–366. [PubMed] [Google Scholar]
- 93.Luu HH, Song W-X, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. Journal of Orthopaedic Research. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 94.Aubin JE. Regulation of osteoblast formation and function. Reviews in Endocrine & Metabolic Disorders. 2001;2(1):81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 95.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 96.Attisano L, Wrana JL. Signal transduction by the TGF-β superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 97.Luo J, Sun MH, Kang Q, et al. Gene therapy for bone regeneration. Current Gene Therapy. 2005;5(2):167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 98.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nature Biotechnology. 1998;16(3):247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 99.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney International. 2000;57(6):2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 100.Deng Z-L, Sharff KA, Tang N, et al. Regulation of osteogenic differentiation during skeletal development. Frontiers in Bioscience. 2008;13(6):2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 101.Kang Q, Song W-X, Luo Q, et al. A Comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells and Development. 2009;18(4):545–558. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peng Y, Kang Q, Luo Q, et al. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. Journal of Biological Chemistry. 2004;279(31):32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 103.Luo Q, Kang Q, Si W, et al. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. Journal of Biological Chemistry. 2004;279(53):55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 104.Si W, Kang Q, Luu HH, et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Molecular and Cellular Biology. 2006;26(8):2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen TL, Shen W-J, Kraemer FB. Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. Journal of Cellular Biochemistry. 2001;82(2):187–199. doi: 10.1002/jcb.1145. [DOI] [PubMed] [Google Scholar]
- 106.Sottile V, Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Letters. 2000;475(3):201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 107.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):13022–13027. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9(1):57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 109.Mie M, Ohgushi H, Yanagida Y, Haruyama T, Kobatake E, Aizawa M. Osteogenesis coordinated in C3H10T1/2 cells by adipogenesis-dependent BMP-2 expression system. Tissue Engineering. 2000;6(1):9–18. doi: 10.1089/107632700320847. [DOI] [PubMed] [Google Scholar]
- 110.Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T 1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA and Cell Biology. 1993;12(10):871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- 111.Jin W, Takagi T, Kanesashi S-N, et al. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Developmental Cell. 2006;10(4):461–471. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 112.Okuno A, Tamemoto H, Tobe K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Journal of Clinical Investigation. 1998;101(6):1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 114.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cellular and Molecular Life Sciences. 2009;66(2):236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundamental and Clinical Pharmacology. 2007;21(3):231–244. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 116.Tseng Y-H, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang H, Song T-J, Li X, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng Y, Kang Q, Cheng H, et al. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. Journal of Cellular Biochemistry. 2003;90(6):1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 119.Demay MB, Roth DA, Kronenberg HM. Regions of the rat osteocalcin gene which mediate the effect of 1,25-dihydroxyvitamin D3 on gene transcription. Journal of Biological Chemistry. 1989;264(4):2279–2282. [PubMed] [Google Scholar]
- 120.Morrison NA, Shine J, Fragonas J-C, Verkest V, McMenemy ML, Eisman JA. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989;246(4934):1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- 121.Gouveia CH, Schultz JJ, Bianco AC, Brent GA. Thyroid hormone stimulation of osteocalcin gene expression in ROS 17/2.8 cells is mediated by transcriptional and post-transcriptional mechanisms. Journal of Endocrinology. 2001;170(3):667–675. doi: 10.1677/joe.0.1700667. [DOI] [PubMed] [Google Scholar]
- 122.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell. 2004;3(6):379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 124.Mudaliar S, Henry RR. New oral therapies for type 2 diabetes mellitus: the glitazones or insulin sensitizers. Annual Review of Medicine. 2001;52:239–257. doi: 10.1146/annurev.med.52.1.239. [DOI] [PubMed] [Google Scholar]
- 125.Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM. Thiazolidinediones and fractures in men and women. Archives of Internal Medicine. 2009;169(15):1395–1402. doi: 10.1001/archinternmed.2009.214. [DOI] [PubMed] [Google Scholar]
- 126.Gorlick R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clinical Cancer Research. 2003;9(15):5442–5453. [PubMed] [Google Scholar]
- 127.Feugeas O, Guriec N, Babin-Boilletot A, et al. Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. Journal of Clinical Oncology. 1996;14(2):467–472. doi: 10.1200/JCO.1996.14.2.467. [DOI] [PubMed] [Google Scholar]
- 128.Gamberi G, Benassi MS, Bohling T, et al. C-myc and c-fos in human osteosarcoma: prognostic value of mRNA and protein expression. Oncology. 1998;55(6):556–563. doi: 10.1159/000011912. [DOI] [PubMed] [Google Scholar]
- 129.Wang LL. Biology of osteogenic sarcoma. Cancer Journal. 2005;11(4):294–305. doi: 10.1097/00130404-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 130.German J. Bloom syndrome: a mendelian prototype of somatic mutational disease. Medicine. 1993;72(6):393–406. [PubMed] [Google Scholar]
- 131.Hickson ID. RecQ helicases: caretakers of the genome. Nature Reviews Cancer. 2003;3(3):169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 132.Tucker MA, D’Angio GJ, Boice JD, Jr., et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. The New England Journal of Medicine. 1987;317(10):588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 133.Weatherby RP, Dahlin DC, Ivins JC. Postradiation sarcoma of bone: review of 78 Mayo Clinic cases. Mayo Clinic Proceedings. 1981;56(5):294–306. [PubMed] [Google Scholar]
- 134.Mark RJ, Poen J, Tran LM, Fu YS, Selch MT, Parker RG. Postirradiation sarcomas: a single-institution study and review of the literature. Cancer. 1994;73(10):2653–2662. doi: 10.1002/1097-0142(19940515)73:10<2653::aid-cncr2820731030>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 135.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 136.Gostjeva EV, Thilly WG. Stem cell stages and the origins of colon cancer: a multidisciplinary perspective. Stem Cell Reviews. 2005;1(3):243–252. doi: 10.1385/SCR:1:3:243. [DOI] [PubMed] [Google Scholar]
- 137.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11(5):421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 138.Castillero-Trejo Y, Eliazer S, Xiang L, Richardson JA, Ilaria RL., Jr. Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells results in EWS/FLI-1-dependent, Ewing sarcoma-like tumors. Cancer Research. 2005;65(19):8698–8705. doi: 10.1158/0008-5472.CAN-05-1704. [DOI] [PubMed] [Google Scholar]
- 139.Thomas DM, Carty SA, Piscopo DM, et al. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Molecular Cell. 2001;8(2):303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 140.Scott RE. Differentiation, differentiation/gene therapy and cancer. Pharmacology and Therapeutics. 1997;73(1):51–65. doi: 10.1016/s0163-7258(96)00120-9. [DOI] [PubMed] [Google Scholar]
- 141.Hoerl BJ, Scott RE. Nonterminally differentiated cells express decreased growth factor responsiveness. Journal of Cellular Physiology. 1989;139(1):68–75. doi: 10.1002/jcp.1041390111. [DOI] [PubMed] [Google Scholar]
- 142.Wang H, Scott RE. Adipocyte differentiation selectively represses the serum inducibility of c-jun and junB by reversible transcription-dependent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(11):4649–4653. doi: 10.1073/pnas.91.11.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 144.Stanford JL, Szklo M, Brinton LA. Estrogen receptors and breast cancer. Epidemiologic Reviews. 1986;8:42–59. doi: 10.1093/oxfordjournals.epirev.a036295. [DOI] [PubMed] [Google Scholar]
- 145.Wang H, Scott RE. Insulin-induced mitogenesis associated with transformation by the SV40 large T antigen. Journal of Cellular Physiology. 1991;147(1):102–110. doi: 10.1002/jcp.1041470114. [DOI] [PubMed] [Google Scholar]
- 146.Housset M, Daniel MT, Degos L. Small doses of ARA-C in the treatment of acute myeloid leukaemia: differentiation of myeloid leukaemia cells? British Journal of Haematology. 1982;51(1):125–129. doi: 10.1111/j.1365-2141.1982.tb07297.x. [DOI] [PubMed] [Google Scholar]
- 147.Youssef JA, Badr MZ. Aging and enhanced hepatocarcinogenicity by peroxisome proliferator-activated receptor alpha agonists. Ageing Research Reviews. 2005;4(1):103–118. doi: 10.1016/j.arr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 148.Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Advances in Pharmacology. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- 149.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apcδ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 150.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 151.Lea MA, Sura M, Desbordes C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) gamma agonists and antagonists. Anticancer Research. 2004;24(5A):2765–2771. [PubMed] [Google Scholar]
- 152.Seargent JM, Yates EA, Gill JH. GW9662, a potent antagonist of PPARγ, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARγ agonist rosiglitazone, independently of PPARγ activation. British Journal of Pharmacology. 2004;143(8):933–937. doi: 10.1038/sj.bjp.0705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Filion C, Motoi T, Olshen AB, et al. The EWSRI/NR4A3 fusion protein of extraskeletal myxoid chondrosarcoma activates the PPARG nuclear receptor gene. Journal of Pathology. 2009;217(1):83–93. doi: 10.1002/path.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim K-H, Cho YS, Park J-M, Yoon S-O, Kim K-W, Chung A-S. Pro-MMP-2 activation by the PPARγ agonist, ciglitazone, induces cell invasion through the generation of ROS and the activation of ERK. FEBS Letters. 2007;581(17):3303–3310. doi: 10.1016/j.febslet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 155.Wasan HS, Novelli M, Bee J, Bodmer WF. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(7):3308–3313. doi: 10.1073/pnas.94.7.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tanaka T, Kohno H, Yoshitani S-I, et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Research. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 157.Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annual Review of Pharmacology and Toxicology. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- 158.Corton JC, Lapinskas PJ, Gonzalez FJ. Central role of PPARα in the mechanism of action of hepatocarcinogenic peroxisome proliferators. Mutation Research. 2000;448(2):139–151. doi: 10.1016/s0027-5107(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 159.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial. The Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 160.Altiok S, Xu M, Spiegelman BM. PPARγ induces cell cycle withdrawal: inhibition of E2f/DP DNA-binding activity via down-regulation of PP2A. Genes and Development. 1997;11(15):1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA, Law RE. Peroxisome proliferator-activated receptor γ ligands inhibit retinoblastoma phosphorylation and G1 → S transition in vascular smooth muscle cells. Journal of Biological Chemistry. 2000;275(29):22435–22441. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- 162.Morrison RF, Farmer SR. Role of PPARγ in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. Journal of Biological Chemistry. 1999;274(24):17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 163.Shen Z-N, Nishida K, Doi H, et al. Suppression of chondrosarcoma cells by 15-deoxy-Δ12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochemical and Biophysical Research Communications. 2005;328(2):375–382. doi: 10.1016/j.bbrc.2004.12.186. [DOI] [PubMed] [Google Scholar]
- 164.Eibl G, Wente MN, Reber HA, Hines OJ. Peroxisome proliferator-activated receptor γ induces pancreatic cancer cell apoptosis. Biochemical and Biophysical Research Communications. 2001;287(2):522–529. doi: 10.1006/bbrc.2001.5619. [DOI] [PubMed] [Google Scholar]
- 165.Yin F, Wakino S, Liu Z, et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochemical and Biophysical Research Communications. 2001;286(5):916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- 166.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rajkumar T, Yamuna M. Multiple pathways are involved in drug resistance to doxorubicin in an osteosarcoma cell line. Anti-Cancer Drugs. 2008;19(3):257–265. doi: 10.1097/cad.0b013e3282f435b6. [DOI] [PubMed] [Google Scholar]
- 168.Schober SL, Kuo CT, Schluns KS, Lefrancois L, Leiden JM, Jameson SC. Expression of the transcription factor lung Kruppel-like factor is regulated by cytokines and correlates with survival memory T cells in vitro and in vivo. Journal of Immunology. 1999;163(7):3662–3667. [PubMed] [Google Scholar]
- 169.Yamaguchi K, Whitlock NC, Liggett JL, Legendre AM, Fry MM, Baek SJ. Molecular characterisation of canine nonsteroidal anti-inflammatory drug-activated gene (NAG-1) Veterinary Journal. 2008;175(1):89–95. doi: 10.1016/j.tvjl.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lucarelli E, Sangiorgi L, Maini V, et al. Troglitazione affects survival of human osteosarcoma cells. International Journal of Cancer. 2002;98(3):344–351. doi: 10.1002/ijc.10203. [DOI] [PubMed] [Google Scholar]
- 171.Nishida K, Kunisada T, Shen ZN, Kadota Y, Hashizume K, Ozaki T. Chondrosarcoma and peroxisome proliferator-activated receptor. PPAR Research. 2008;2008:p. 7. doi: 10.1155/2008/250568. Article ID 250568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kallio A, Guo T, Lamminen E, et al. Estrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cells. Molecular and Cellular Endocrinology. 2008;289(1-2):38–48. doi: 10.1016/j.mce.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 173.Salvatori L, Caporuscio F, Coroniti G, et al. Down-regulation of epidermal growth factor receptor induced by estrogens and phytoestrogens promotes the differentiation of U2OS human osteosarcoma cells. Journal of Cellular Physiology. 2009;220(1):35–44. doi: 10.1002/jcp.21724. [DOI] [PubMed] [Google Scholar]
- 174.Hara K, Kusuzaki K, Takeshita H, et al. Oral administration of 1 alpha hydroxyvitamin D3 inhibits tumor growth and metastasis of a murine osteosarcoma model. Anticancer Research. 2001;21(1):321–324. [PubMed] [Google Scholar]
- 175.Deng C, Ueda E, Chen KE, et al. Prolactin blocks nuclear translocation of VDR by regulating its interaction with BRCA1 in osteosarcoma cells. Molecular Endocrinology. 2009;23(2):226–236. doi: 10.1210/me.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prufer K, Schroder C, Hegyi K, Barsony J. Degradation of RXRs influences sensitivity of rat osteosarcoma cells to the antiproliferative effects of calcitriol. Molecular Endocrinology. 2002;16(5):961–976. doi: 10.1210/mend.16.5.0821. [DOI] [PubMed] [Google Scholar]
- 177.Nishida K, Furumatsu T, Takada I, et al. Inhibition of human chondrosarcoma cell growth via apoptosis by peroxisome proliferator-activated receptor-γ . British Journal of Cancer. 2002;86(8):1303–1309. doi: 10.1038/sj.bjc.6600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Magenta G, Borenstein X, Rolando R, Jasnis MA. Rosiglitazone inhibits metastasis development of a murine mammary tumor cell line LMM3. BMC Cancer. 2008;8, article 47 doi: 10.1186/1471-2407-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kuniyasu H. The roles of dietary PPARγ ligands for metastasis in colorectal cancer. PPAR Research. 2008;2008:7 pages. doi: 10.1155/2008/529720. Article ID 529720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takano S, Kubota T, Nishibori H, et al. Pioglitazone, a ligand for peroxisome proliferator-activated receptor-γ acts as an inhibitor of colon cancer liver metastasis. Anticancer Research. 2008;28(6A):3593–3599. [PubMed] [Google Scholar]
- 181.Bren-Mattison Y, Van Putten V, Chan D, Winn R, Geraci MW, Nemenoff RA. Peroxisome proliferator-activated receptor-γ (PPARγ) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC) Oncogene. 2005;24(8):1412–1422. doi: 10.1038/sj.onc.1208333. [DOI] [PubMed] [Google Scholar]
- 182.Papadaki I, Mylona E, Giannopoulou I, Markaki S, Keramopoulos A, Nakopoulou L. PPARγ expression in breast cancer: clinical value and correlation with ERβ . Histopathology. 2005;46(1):37–42. doi: 10.1111/j.1365-2559.2005.02056.x. [DOI] [PubMed] [Google Scholar]
- 183.Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor γ inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. Journal of Clinical Endocrinology and Metabolism. 2001;86(5):2170–2177. doi: 10.1210/jcem.86.5.7493. [DOI] [PubMed] [Google Scholar]
- 184.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134(6):921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nature Reviews Genetics. 2008;9(7):541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 186.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annual Review of Pharmacology and Toxicology. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 187.Hansen LA, Sigman CC, Andreola F, Ross SA, Kelloff GJ, De Luca LM. Retinoids in chemoprevention and differentiation therapy. Carcinogenesis. 2000;21(7):1271–1279. [PubMed] [Google Scholar]
- 188.Gee MFW, Tsuchida R, Eichler-Jonsson C, Das B, Baruchel S, Malkin D. Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene. 2005;24(54):8025–8037. doi: 10.1038/sj.onc.1208939. [DOI] [PubMed] [Google Scholar]
- 189.Ricaud S, Vernus B, Bonnieu A. Response of human rhabdomyosarcoma cell lines to retinoic acid: relationship with induction of differentiation and retinoic acid sensitivity. Experimental Cell Research. 2005;311(2):192–204. doi: 10.1016/j.yexcr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 190.Zhu G-H, Huang J, Bi Y, et al. Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells. Differentiation. 2009;78(4):195–204. doi: 10.1016/j.diff.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang J, Bi Y, Zhu G, et al. Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver International. 2009;29(10):1569–1581. doi: 10.1111/j.1478-3231.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 192.Lawrence JA, Merino MJ, Simpson JF, Manrow RE, Page DL, Steeg PS. A high-risk lesion for invasive breast cancer, ductal carcinoma in situ, exhibits frequent overexpression of retinoid X receptor. Cancer Epidemiology Biomarkers and Prevention. 1998;7(1):29–35. [PubMed] [Google Scholar]
- 193.Li M, Chiba H, Warot X, et al. RXRα ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128(5):675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- 194.Huang J, Powell WC, Khodavirdi AC, et al. Prostatic intraepithelial neoplasia in mice with conditional disruption of the retinoid X receptor α allele in the prostate epithelium. Cancer Research. 2002;62(16):4812–4819. [PubMed] [Google Scholar]
- 195.Tanaka T, Suh KS, Lo AM, De Luca LM. p21WAF1/CIP1 is a common transcriptional target of retinoid receptors: pleiotropic regulatory mechanism through retinoic acid receptor (RAR)/retinoid X receptor (RXR) heterodimer and RXR/RXR homodimer. Journal of Biological Chemistry. 2007;282(41):29987–29997. doi: 10.1074/jbc.M701700200. [DOI] [PubMed] [Google Scholar]
- 196.Duvic M, Martin AG, Kim Y, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Archives of Dermatology. 2001;137(5):581–593. [PubMed] [Google Scholar]
- 197.Blumenschein GR, Jr., Khuri FR, von Pawel J, et al. Phase III trial comparing carboplatin, paclitaxel, and bexarotene with carboplatin and paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT II. Journal of Clinical Oncology. 2008;26(11):1879–1885. doi: 10.1200/JCO.2007.12.2689. [DOI] [PubMed] [Google Scholar]
- 198.Wu K, Kim H-T, Rodriquez JL, et al. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiology Biomarkers and Prevention. 2002;11(5):467–474. [PubMed] [Google Scholar]
- 199.Matsuda N, Yokoyama K, Takeshita S, Watanabe M. Role of epidermal growth factor and its receptor in mechanical stress-induced differentiation of human periodontal ligament cells in vitro. Archives of Oral Biology. 1998;43(12):987–997. doi: 10.1016/s0003-9969(98)00079-x. [DOI] [PubMed] [Google Scholar]
- 200.Chien H-H, Lin W-L, Cho M-I. Down-regulation of osteoblastic cell differentiation by epidermal growth factor receptor. Calcified Tissue International. 2000;67(2):141–150. doi: 10.1007/s00223001128. [DOI] [PubMed] [Google Scholar]
- 201.Tokuumi Y. Correlation between the concentration of 1,25α dihydroxyvitamin D3 receptors and growth inhibition, and differentiation of human osteosarcoma cells induced by vitamin D3. Nippon Seikeigeka Gakkai Zasshi. 1995;69(4):181–190. [PubMed] [Google Scholar]
- 202.Deng C, Ueda E, Chen KE, et al. Prolactin blocks nuclear translocation of VDR by regulating its interaction with BRCA1 in osteosarcoma cells. Molecular Endocrinology. 2009;23(2):226–236. doi: 10.1210/me.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]